Abstract
Objective
The prevalence of radiographic osteoarthritis (OA) after anterior cruciate ligament reconstruction (ACLR) approaches 50%, yet the prevalence of significant knee pain is unknown. We applied three different models of Knee injury and Osteoarthritis Outcome Score (KOOS) thresholds for significant knee pain to an ACLR cohort to identify prevalence and risk factors.
Design
Multicenter Orthopaedic Outcomes Network (MOON) prospective cohort patients with a unilateral primary ACLR and normal contralateral knee were assessed at 2 and 6 years. Independent variables included patient demographics, validated Patient Reported Outcomes (PRO; Marx activity score, KOOS), and surgical characteristics. Models included: (1) KOOS criteria for a painful knee = quality of life subscale <87.5 and ≥2 of: KOOSpain <86.1, KOOSsymptoms <85.7, KOOSADL <86.8, or KOOSsports/rec <85.0; (2) KOOSpain subscale score ≤72 (≥2 standard deviations below population mean); (3) 10-point KOOSpain drop from 2 to 6 years. Proportional odds models (alpha≤0.05) were used.
Results
1,761 patients of median age 23 years, median BMI 24.8 kg/m2 and 56% male met inclusion, with 87% (1530/1761) and 86% (1506/1761) follow-up at 2 and 6 years, respectively. At 6 years, n=592 (39%), n=131 (9%) and n=169 (12%) met criteria for models #1 through #3, respectively. The most consistent and strongest independent risk factor at both time-points was subsequent ipsilateral knee surgery. Low 2-year Marx activity score increased the odds of a painful knee at 6 years.
Conclusions
Significant knee pain is prevalent after ACLR; with those who undergo subsequent ipsilateral surgery at greatest risk. The relationship between pain and structural OA warrants further study.
Keywords: Symptomatic osteoarthritis, Anterior cruciate ligament reconstruction, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee pain
INTRODUCTION
Anterior Cruciate Ligament (ACL) reconstruction (ACLR) is the most effective and reproducible treatment for ACL injured patients who want to return to cutting and pivoting sports1,2,3. More than half of patients undergoing ACLR will have concomitant pathology, including injuries to the articular cartilage in more than 20%, lateral meniscal tears in up to 46% and medial meniscal tears in 38% 4.
An ACL tear is a known risk factor for the development of osteoarthritis (OA)5. Intermediate and long-term follow-up of ACLR patients has demonstrated a high prevalence of radiographic findings consistent with post-traumatic OA6,7,8. Which factors, including concomitant pathology, the original injury, surgical techniques, or other as yet unidentified factors, are most responsible for the development of radiographic changes is unknown. A systematic review of studies including patients 5- to 10- years after ACLR6, found radiographic joint space narrowing in 0–13% of patients with intact menisci, and 21–48% in those who had undergone either meniscectomy or repair. The meniscal status was also demonstrated to be important in a systematic review of non-reconstructed ACL injured patients7. Most studies, however, are limited by poor follow-up and significant heterogeneity in the classification systems utilized to describe radiographic OA.
Although the definitions can be challenging9,10, a systematic review in 2011 demonstrated a relationship between structural OA and symptomatic OA among high quality studies11. Studies using Osteoarthritis Initiative (OAI) data have yielded further insight. Oak et al.12 found a correlation between joint space narrowing at study entry, and greater progression of narrowing over the course of the study, with worse patient reported outcomes (PRO) at 4 years. Others have found weak correlations between PRO and MRI confirmation of joint space narrowing13, but these correlations were highest for the knee pain subscale of the Knee injury and Osteoarthritis Outcome Score (KOOS).
A consensus expert panel developed a definition of patients with a symptomatic knee significant enough to seek medical attention. This definition, based on threshold levels of KOOS subscale scores14, was based on the long-term follow-up of patients who previously underwent isolated partial meniscectomy with intact cruciate ligaments. Other criteria for clinically significant knee pain that have been developed based on PRO, include the KOOS Minimally Clinically Important Difference (MCID) of 8–10 points15, and the Osteoarthritis Research Society International (OARSI) Standing Committee criteria for interventions of osteoarthritis of the knee (“OARSI responder criteria”) of 20 points16,17.
Given that many patients who undergo ACLR develop radiographic OA, the main objective of this study was to identify the prevalence of significant knee pain by PRO after ACLR, using published definitions and cut-offs for either symptomatic OA or clinically significant knee pain. The second objective was to identify risk factors for developing a painful knee from patient, injury, and surgical characteristics 6 years following an ACL reconstruction.
METHODS
Study design
Longitudinal prospective cohort (prognostic): The Multicenter Orthopaedic Outcomes Network (MOON) cohort18. MOON is a prospective, longitudinal, multicenter cohort study based in the United States, and designed to examine short and long-term prognosis after ACL reconstruction using validated patient-reported outcomes. MOON was also designed to generate hypotheses surrounding novel methods for improving outcomes after ACL injury.
Data sources
Participants completed a 13-page questionnaire providing patient demographics, a description of their injury, sports participation history, comorbidities and past medical history. Each participant also completed validated general and knee specific instruments, including the Knee injury and Osteoarthritis Outcome Score (KOOS)19 and the Marx activity rating scale20. Contained within the KOOS is the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) 21. All were completed within 2 weeks of the surgery date.
Surgeons completed a standardized questionnaire, which included detailed information regarding surgical technique, graft choice, and concomitant meniscal and articular cartilage pathology and treatment. The inter-rater reliability of grading systems for articular cartilage (modified Outerbridge) and meniscal lesions were previously validated among participating surgeons and found to be high22, 23. Meniscal pathology was classified by size, location, partial versus complete tears and treatment (not treated, repaired, resection and extent of resection).
Cohort design
All patients (n=2222) who had undergone a unilateral primary ACLR at a participating MOON institution (Vanderbilt University, The Ohio State University, Washington University at St. Louis, University of Iowa, the Cleveland Clinic, and the Hospital for Special Surgery) between 2002 and 2005 were eligible for inclusion into this study. All patients provided informed consent from their respective institution. A prior exclusion criteria included previous contralateral ACL reconstruction, simultaneous bilateral ACL reconstruction, ACL repair, or a revision ACL reconstruction as the index (enrollment) event. ACL revision patients report worse PRO than primary ACL reconstruction patients4,27–9, and so were excluded. ACL repair is an atypical treatment and was excluded. Patients with a contralateral ACL reconstruction prior to initial enrollment into the MOON cohort, or performed concurrently, were excluded on the basis that this study’s objective included understanding how a subsequent contralateral reconstruction would influence PRO for significant knee pain. No patients were excluded from analysis due to incomplete baseline data (all n=2222 completed baseline PRO).
Outcomes - Definitions of a ‘painful or symptomatic knee’
We utilized previously published definitions of KOOS thresholds for a symptomatic knee as described in the introduction. We built three models, as follows:
Model #1. The primary model was defined as the operational definition of Englund et al.14 (“Englund model”) to distinguish patients with sufficient knee symptoms to seek medical care. The Englund model is defined as having a KOOS knee-related quality of life (QoL) subscale ≤ 87.5 AND two or more of the other subscales: KOOS pain ≤ 86.1, KOOS symptoms ≤ 85.7, KOOS activities of daily living (ADL) ≤ 86.8, or KOOS sports and recreation (“sport/rec”) ≤85.0.
Model #2. The KOOS knee pain subscale has been shown to have the highest correlation with structural OA changes13, and is a direct measure of knee pain. Therefore, we defined a secondary model for significant knee pain as a KOOS pain subscale two standard deviations lower than the reported normal mean value in athletic populations with a history of (any) knee ligament injury. This value was 92.3 ± 10.0 24, which translated into a cut-off score of ≤72 points (“KOOS pain ≤72 model”). This definition also qualified as a 20-point change, consistent with OARSI responder criteria for effective interventions in OA16.
Model #3. The reported Minimal Clinically Important Difference (MCID) for the KOOS pain subscale is 6.1 points in athletes after ACL reconstruction25, to between 8 and 10 points for patients with OA15, 26. To utilize a more conservative estimate of the MCID, we selected a drop of 10 points in the KOOS pain subscale from 2 years to 6 years follow-up as an additional secondary definition of patients with a painful knee after ACL reconstruction (“KOOS pain MCID model”). This model attempted to identify patients who had a clinically significant worsening of knee pain.
Model variables
Variables included all those from the original MOON cohort. They included patient demographics (age, sex, body mass index [BMI], smoking status, education level, main sport played at the time of injury, enrollment year), validated PRO (KOOS, WOMAC, Marx activity), surgical characteristics (graft type, meniscal pathology/treatment, articular cartilage pathology), and incidence of subsequent surgery on either knee (Table 1). The Marx score is a measure of the frequency and intensity of cutting and pivoting sports. The inclusion of variables in our models was based on substantive knowledge of the clinical or epidemiological association between them and patient reported outcomes after ACL reconstruction surgery. These relationships have been established by our own work with this cohort4,7,18,27 and have been derived from literature review18. They extend to include baseline PRO scores, patient demographic factors and surgical variables.
Table 1.
List of Modeling Variables
| Category | Levels | |
|---|---|---|
|
| ||
| Baseline Outcome Scores | KOOS (5 subscales); | continuous |
| WOMAC (pain, stiffness subscales) | ||
|
| ||
| Patient Demographics | Age (years) | continuous |
| Gender | male, female | |
| BMI | continuous | |
| Smoking status | never, quit, current | |
| Education level (years) | 1 – 16 (continuous) | |
| Baseline activity level (Marx) | continuous | |
| Main sport played last 2 yrs | basketball, football, soccer, other, none | |
|
| ||
| Surgical Variables | Graft type | Autograft (BTB), autograft (soft tissue), allograft (BTB), allograft (soft tissue) |
| Meniscal pathology | ||
| * previous | no, yes | |
| * medial | normal, no tx for tear, repair, excised, other | |
| * lateral | normal, no tx for tear, repair, excised, other | |
| Articular cartilage pathology | ||
| * previous | no, yes | |
| * medial femoral condyle (MFC) | normal/grade 1, grade 2, grade 3, grade 4 | |
| * lateral femoral condyle (LFC) | normal/grade 1, grade 2, grade 3, grade 4 | |
| * medial tibial plateau (MTP) | normal/grade 1, grade 2, grades 3/4 | |
| * lateral tibial plateau (LTP) | normal/grade 1, grade 2, grades 3/4 | |
| * patella | normal/grade 1, grade 2, grades 3/4 | |
| * trochlea | normal/grade 1, grade 2, grades 3/4 | |
|
| ||
| Miscellaneous Variables | Year of surgery (enrollment) | 2002, 2003, 2004, 2005 |
| Subsequent ipsilateral surgery | no, arthroscopic procedure, revision ACL reconstruction, total knee arthroplasty (TKA) | |
| Subsequent contralateral surgery | no, arthroscopic procedure, ACL reconstruction, total knee arthroplasty (TKA) | |
Statistical analysis
To describe our patient sample, we summarized continuous variables as percentiles (i.e., 25th, 50th, and 75th) with their mean and standard deviation, and categorical variables with frequencies and percentages. Multivariable regression analyses were constructed to examine which baseline risk factors were independently associated with each outcome variable. An a priori determined list of variables to be included in all models were given by: age, gender, BMI, smoking status, education level, main sport played the last 2 years, baseline KOOS, WOMAC, and Marx activity levels, graft type, previous meniscal pathology, current meniscal pathology/treatment, previous articular cartilage pathology, current articular cartilage pathology, subsequent surgery on the ipsilateral and contralateral knee, and enrollment year. We assumed independence of all covariates because we compared between subjects and not within, and when fitting the multivariable regression models, we measured each covariate’s independent adjusted association with the outcome. For binary outcome variables a multivariable logistic regression model was fit to the data, parameter estimates were exponentiated to obtain odds ratios (OR) and 95% confidence intervals (CI), based on a dichotomous outcome (yes/no). We did not assume a linear relationship between continuous covariates (independent variables) and each outcome in order to avoid underestimating the true relationship, instead utilized a restricted cubic regression splines technique that assumes smooth relationships (i.e., they are linearly related to the log odds). To avoid case-wise deletion of records with missing covariates, we employed multiple imputation via predictive mean matching. All model assumptions (as listed above) were met. Statistical analysis was performed using open source R statistical software (www.r-project.org; Version 3.0.3).
Post hoc analysis
Preliminary findings demonstrated that a low Marx activity score at 2 years increased the odds of reporting a painful knee in both the Englund and KOOS pain ≤72 models. Therefore, in order to further understand the interaction of pain and activity, we performed a post hoc analysis to identify the proportion of patients reporting a high level of sport/activity-related knee pain, and to understand which factors modified that outcome. This model utilized responses from a 5-point Likert question on the IKDC: “What is the highest level of activity that you can perform without significant knee pain?” Patients were classified based on their answer to this question as high activity tolerance (“very strenuous activities” or “strenuous activities” or “moderate activities”) or low activity tolerance (“light activities” or “no described activities”). Models were built for this outcome (Model #4: “Activity tolerance model”) at 6 years based on the response to the question at 2 years.
After determining that subsequent ipsilateral surgery was a risk factor, we performed a second post-hoc analysis to identify the number of patients who underwent a second surgery within 1 and 3 months prior to the 2- and 6-year time-points. This was performed due to concern that recent surgery may be the cause of higher reported pain. Furthermore, we re-analyzed each of the 4 models after excluding the patients with surgery within 3 months of the 2- and 6-year time-points.
RESULTS
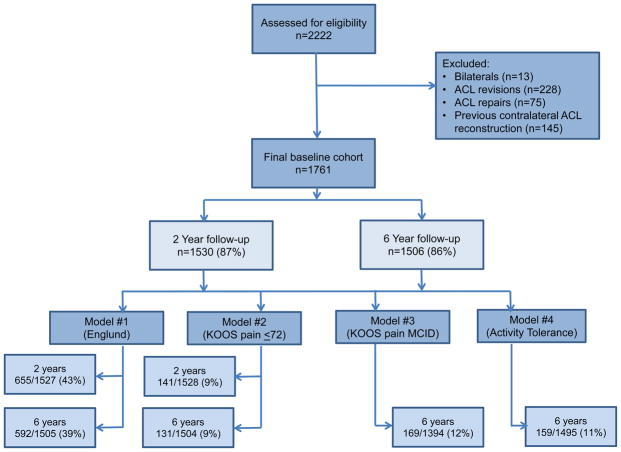
Figure 1 illustrates the cohort inclusion/exclusion criteria. There were 1761 subjects who fit the inclusion criteria and were included in this analysis. The median age of our cohort was 23 years, median BMI 24.8 kg/m2 and the cohort was 56% male. Patient follow-up was obtained on 87% (1530/1761) and 86% (1506/1761) at 2 and 6 years, respectively. The proportion of patients who met each of the three model criteria was calculated (see Table 3). At 2 years, n=46 patients fit both the Englund and KOOS pain ≤72 points models, out of a total n=141 possible patients (32.6%). At 6 years, n=67 patients fit all four models, out of a total n=131 possible (51%). Full baseline demographics are supplied in Table 2 alongside the list of model variables and levels.
Figure 1.
Flowchart showing the inclusion of participants in the study.
Table 3.
Summary of Independent Variables [reported as Odds Ratios (95% CI); bolded and p-value included when significant]
| Category | Variable Comparison | Variable (worse outcome, if significant) | Model 1 (Englund) | Model 2 (KOOS Pain ≤ 72) | Model 3 (KOOS Pain MCID) | Model 4 (Activity Tolerance) | |||
|---|---|---|---|---|---|---|---|---|---|
| 2 Years | 6 Years | 2 Years | 6 Years | 6 Years | 6 Years | ||||
| Number of patients who satisfied criteria for each model | 655/1527 (43%) | 592/1505 (39%) | 141/1528 (9%) | 131/1504 (9%) | 169/1394 (12%) | 159/1495 (11%) | |||
| Baseline Outcome Scores | KOOS | Symptoms | 0.90 (0.69, 1.18) | 0.83 (0.69, 1.09) | 0.78 (0.45, 1.36) | 0.81 (0.45, 1.49) | 0.91 (0.59, 1.40) | 1.27 (0.78, 2.07) | |
| Pain | 0.66 (0.40, 1.07) | 0.78 (0.46, 1.32) | 0.48 (0.17, 1.36) | 0.87 (0.32, 2.37) | 1.45 (0.66, 3.18) | 1.00 (0.40, 2.51) | |||
| ADL | ADL (low) |
0.53 (0.36, 0.79) p<0.001 |
0.67*
(0.45, 0.99) p=0.117 |
0.78 (0.37, 1.62) | 0.89 (0.42, 1.89) | 1.13 (0.61, 2.07) | 0.67 (0.32, 1.41) | ||
| Sports/Rec | Sports/Rec (high) | 1.04 (0.82, 1.32) | 1.01 (0.77, 1.33) |
1.63 (1.13, 2.34) p=0.016 |
0.78 (0.53, 1.16) | 0.90 (0.63, 1.30) | 0.88 (0.60, 1.29) | ||
| Quality of Life | 0.90 (0.76, 1.06) | 0.97 (0.82, 1.15) | 0.96 (0.73, 1.27) | 1.00 (0.73, 1.36) | 0.82 (0.64, 1.04) | 0.79 (0.60, 1.04) | |||
| WOMAC | Pain | 1.18 (0.77, 1.81) | 1.09 (0.69, 1.72) | 0.68 (0.31, 1.47) | 0.46 (0.20, 1.05) | 0.63 (0.29, 1.35) | 1.03 (0.50, 2.13) | ||
| Stiffness | 0.92 (0.74, 1.15) | 0.92 (0.72, 1.16) | 1.06 (0.70, 1.59) | 1.57 (1.00, 2.47) | 1.07 (0.73, 1.58) | 0.92 (0.62, 1.36) | |||
| Patient Characteristics | Age (yrs) | Age | 0.91 (0.55, 1.51) | 0.76 (0.42, 1.37) | 1.65 (0.73, 3.71) | 1.21 (0.48, 3.05) | 0.96 (0.42, 2.17) | 1.43 (0.62, 3.28) | |
| Gender | Females : Males | Males | 1.10 (0.86, 1.40) | 1.00 (0.75, 1.33) | 0.85 (0.54, 1.34) | 0.90 (0.54, 1.51) |
0.67 (0.47, 0.96) p=0.028 |
1.46 (0.89, 2.39) | |
| BMI | BMI | Higher BMI |
1.24 (1.01, 1.53) p=0.004 |
1.28*
(1.01, 1.61) p=0.071 |
1.52 (1.04, 2.20) p=0.003 |
1.30 (0.84, 2.02) | 1.00 (0.71, 1.39) | 1.27 (0.89, 1.83) | |
| Smoking status | Current : Never | Current (compared to never) | 1.45 (0.96, 2.19) | 1.22 (0.81, 1.85) | 1.59 (0.92, 2.75) |
2.83 (1.46, 5.49) p=0.002 |
1.13 (0.62, 2.08) | 1.78 (1.00, 3.18) | |
| Quit : Never | Quitting (compared to never) |
1.66 (1.12, 2.45) p=0.011 |
0.84 (0.54, 1.32) | 0.96 (0.50, 1.83) |
1.96 (1.02, 3.75) p=0.042 |
0.59 (0.32, 1.12) | 1.03 (0.57, 1.89) | ||
| Education (years) | Years of education | Less education years | 1.04 (0.77, 1.41) | 0.80 (0.56, 1.14) | 0.63 (0.38, 1.03) |
0.43 (0.23, 0.79) p=0.022 |
0.72 (0.44, 1.16) |
0.61*
(0.39, 0.98) p=0.126 |
|
| Baseline activity level (Marx) | Marx activity score | 1.31 (0.92, 1.87) | 1.16 (0.80, 1.69) | 1.38 (0.72, 2.65) | 1.39 (0.66, 2.92) | 0.93 (0.53, 1.63) | 1.26 (0.60, 2.67) | ||
| 2 Year activity level (Marx) | Marx activity score | Low score (low activity) |
0.62 (0.48, 0.82) p=0.001 |
0.53 (0.33, 0.86) p=0.032 |
0.91 (0.64, 1.31) |
0.41 (0.26, 0.63) p<0.001 |
|||
| Surgical Factors | Previous meniscal pathology | Yes : No | No (compared to ‘yes’) | 1.48 (0.91, 2.39) | 1.23 (0.76, 1.98) | 1.11 (0.57, 2.16) | 1.13 (0.51, 2.52) |
0.43 (0.19, 0.97) p=0.041 |
1.78 (0.90, 3.51) |
| Previous articular cartilage pathology | Yes : No | 3.98 (0.95, 16.73) | 0.70 (0.20, 2.50) | 2.50 (0.70, 8.86) | 1.79 (0.46, 6.93) | 0.53 (0.08, 3.55) | 3.47 (0.58, 20.63) | ||
| Current Meniscal pathology | |||||||||
| * medial | Repair : Normal | 1.34 (0.94, 1.90) | 1.11 (0.79, 1.55) | 1.53 (0.90, 2.60) | 1.61 (0.91, 2.86) | 0.96 (0.59, 1.59) | 1.21 (0.65, 2.25) | ||
| * lateral | No tear for treatment : Normal | Normal (compared to no tx for tear) |
0.59 (0.39, 0.89) p=0.012 |
0.74 (0.50, 1.10) | 0.97 (0.50, 1.89) | 0.62 (0.28, 1.40) | 0.93 (0.52, 1.67) | 1.22 (0.65, 2.30) | |
| Current Articular cartilage pathology | |||||||||
| * medial femoral condyle (MFC) | Grade 4 : Normal/grade 1 | Grade 4 (compared with normal/grade 1) | 1.62 (0.69, 3.76) | 1.43 (0.65, 3.14) | 1.07 (0.35, 3.32) | 1.28 (0.46, 3.61) | 1.42 (0.50, 3.98) |
2.67 (1.05, 6.80) p=0.040 |
|
| * lateral femoral condyle (LFC) | Grade 2 : Normal/grade 1 | Normal/grade 1 (compared to grade 2) | 0.70 (0.47, 1.05) | 0.73 (0.48, 1.09) | 0.75 (0.36, 1.57) |
0.34 (0.14, 0.82) p=0.016 |
0.83 (0.46, 1.49) | 1.01 (0.53, 1.92) | |
| Grade 3 : Normal/grade 1 | Grade 3 (compared with normal/grade 1) | 1.78 (0.85, 3.73) |
2.58 (1.21, 5.50) p=0.014 |
1.40 (0.54, 3.62) | 1.37 (0.44, 4.29) | 1.89 (0.83, 4.32) | 1.56 (0.47, 5.19) | ||
| * medial tibial plateau (MTP) | Grades 3/4 : Normal/grade 1 | Grades 3/4 (compared with normal/grade 1) | 0.73 (0.24, 2.27) | 2.98 (0.93, 9.50) | 1.26 (0.35, 4.53) | 3.20 (0.76, 13.48) |
4.20 (1.33, 13.25) p=0.015 |
0.86 (0.17, 4.25) | |
| * lateral tibial plateau (LTP) | Grade 2 : Normal/grade 1 | Normal/grade 1 (compared to grade 2) | 0.91 (0.57, 1.46) |
0.46 (0.26, 0.82) p=0.008 |
0.41 (0.15, 1.14) | 1.24 (0.53, 2.93) | 1.15 (0.60, 2.22) | 0.56 (0.24, 1.35) | |
| * patella | Grade 2 : Normal/grade 1 | Grade 2 (compared with normal/grade 1) | 0.95 (0.63, 1.42) |
1.65 (1.06, 2.58) p=0.028 |
1.81 (0.96, 3.39) | 1.59 (0.74, 3.43) | 1.30 (0.66, 2.59) |
3.06 (1.68, 5.56) p<0.001 |
|
| Grades 3/4 : Normal/grade 1 | Grades 3/4 (compared with normal/grade 1) |
1.71 (1.04, 2.80) p=0.033 |
1.55 (0.94, 2.55) | 1.28 (0.60, 2.70) | 2.29 (0.92, 5.68) |
2.10 (1.09, 4.03) p=0.026 |
2.14 (1.07, 4.30) p=0.032 |
||
| * trochlea | Grade 2 : Normal/grade 1 | Normal/grade 1 (compared to grade 2) | 0.93 (0.52, 1.67) | 0.96 (0.54, 1.73) | 0.88 (0.37, 2.10) | 0.44 (0.14, 1.37) | 0.57 (0.22, 1.48) |
0.31 (0.12, 0.82) p=0.017 |
|
| Subsequent Surgery | Ipsilateral knee | Yes : No | yes (compared to ‘no’) |
2.31 (1.35, 3.96) p<0.001 |
2.66 (1.77, 4.01) p<0.001 |
2.20 (1.07, 4.55) p<0.001 |
3.41 (1.72, 6.75) p<0.001 |
1.94*
(1.11, 3.39) p=0.060 |
3.03 (1.59, 5.79) p<0.001 |
| Contralateral knee | Yes : No | 0.62 (0.27, 1.41) | 0.92 (0.50, 1.68) | 0.23 (0.03, 1.88) | 0.90 (0.29, 2.76) | 1.55 (0.54, 4.45) | 0.47 (0.14, 1.64) | ||
Key:
Significant values are depicted in bold
Gray shading indicates outcome is counterintuitive to what one would think
Although odds ratio did not cross 1, indicating significance, the p value did not reach 0.05 level of significance.
For Continuous variables, where the Odds ratio < 1.0 --- variables are inversely related.
For Categorical variables, where the Odds ratio > 1.0 --- 1st variable listed is worse than 2nd variable listed
For Categorical variables, where the Odds ratio < 1.0 --- 2nd variable listed is worse than 1st variable listed
Table 2.
Baseline data for the included cohort and the patients lost to follow-up
| Category | Variable (N) | Level | Overall Cohort (n=1761) n (%) or median (25th–75th) | Lost to Follow-up @ 2 yrs (n=231) n (%) or median (25th–75th) | Lost to Follow-up @ 6 yrs (n=255) n (%) or median (25th–75th) |
|---|---|---|---|---|---|
| Patient demographics | Sex | Male | 980 (56%) | 146 (63%) | 169 (66%) |
| Female | 781 (44%) | 85 (37%) | 86 (34%) | ||
| Age | Continuous | 23 years (17–35) | 22 years (17–30) | 22 years (17–32) | |
| BMI | Continuous | 24.8 kg/m2 (22.3–27.9) | 25.4 kg/m2 (22.9–29.2) | 26.4 kg/m2 (23.2–29.9) | |
| Smoking status | Current | 167 (10%) | 32 (15%) | 44 (18%) | |
| Quit smoking (>6 months) | 172 (10%) | 15 (7%) | 16 (6%) | ||
| Never smoker | 1354 (80%) | 171 (78%) | 186 (76%) | ||
| Education level | Continuous | 14.0 years (11.0–16.0) | 13.0 years (11.0–16.0) | 12.0 years (10.0–16.0) | |
| Main sport | Basketball | 393 (23%) | 61 (27%) | 69 (27%) | |
| Football | 191 (11%) | 38 (17%) | 35 (14%) | ||
| Soccer | 230 (13%) | 17 (7%) | 20 (8%) | ||
| Other | 793 (46%) | 99 (43%) | 106 (42%) | ||
| None | 134 (8%) | 14 (6%) | 22 (9%) | ||
| Patient Reported Outcomes (PRO) | Marx activity | Baseline | 12 (8–16) | 13 (9–16) | 13 (8–16) |
| 2 Years | 9 (4–13) | N/A | 8 (1–12) | ||
| KOOS symptoms | Baseline | 68 (57–82) | 68 (50–79) | 68 (50–82) | |
| KOOS pain | Baseline | 75 (64–89) | 69 (58–86) | 72 (58–86) | |
| KOOS ADL | Baseline | 88 (74–96) | 83 (68–94) | 82 (65–94) | |
| KOOS Sports & rec | Baseline | 50 (30–75) | 50 (25–75) | 50 (25–75) | |
| KOOS QoL | Baseline | 38 (25–50) | 31 (19–50) | 31 (19–44) | |
| WOMAC stiffness | Baseline | 75 (62–88) | 75 (50–88) | 75 (50–88) | |
| WOMAC pain | Baseline | 90 (75–95) | 85 (70–95) | 85 (65–95) | |
| Surgical/Injury factors | Previous meniscal pathology | No | 1632 (93%) | 210 (91%) | 230 (90%) |
| Yes | 129 (7%) | 21 (9%) | 25 (10%) | ||
| Previous articular cartilage pathology | No | 1739 (99%) | 228 (99%) | 249 (98%) | |
| Yes | 22 (1%) | 3 (1%) | 6 (2%) | ||
| Graft type | Allograft (BTB) | 121 (7%) | 16 (7%) | 27 (11%) | |
| Allograft (soft tissue) | 299 (17%) | 39 (17%) | 35 (14%) | ||
| Autograft (BTB) | 832 (47%) | 116 (51%) | 120 (47%) | ||
| Autograft (soft tissue) | 509 (29%) | 60 (26%) | 73 (29%) | ||
| Medial meniscus treatment | Normal/none | 1106 (63%) | 149 (65%) | 173 (68%) | |
| Tear/no treatment | 94 (5%) | 10 (4%) | 10 (4%) | ||
| Repair | 229 (13%) | 39 (17%) | 35 (14%) | ||
| Partial excision | 317 (18%) | 33 (14%) | 37 (15%) | ||
| Other | 15 (1%) | 0 (0%) | 0 (0%) | ||
| Lateral meniscus treatment | Normal/none | 927 (53%) | 118 (51%) | 127 (50%) | |
| Tear/no treatment | 197 (11%) | 25 (11%) | 25 (10%) | ||
| Repair | 128 (7%) | 16 (7%) | 22 (9%) | ||
| Partial excision | 497 (28%) | 72 (31%) | 80 (31%) | ||
| Other | 12 (1%) | 0 (0%) | 1 (<1%) | ||
| Medial femoral condyle | Normal/grade 1 | 1381 (78%) | 189 (82%) | 202 (79%) | |
| Grade 2 | 225 (13%) | 30 (13%) | 31 (12%) | ||
| Grade 3 | 117 (7%) | 8 (3%) | 15 (6%) | ||
| Grade 4 | 38 (2%) | 4 (2%) | 7 (3%) | ||
| Lateral femoral condyle | Normal/grade 1 | 1498 (85%) | 197 (85%) | 209 (82%) | |
| Grade 2 | 186 (11%) | 24 (10%) | 32 (13%) | ||
| Grade 3 | 59 (3%) | 10 (4%) | 11 (4%) | ||
| Grade 4 | 18 (1%) | 0 (0%) | 3 (1%) | ||
| Medial tibial plateau | Normal/grade 1 | 1694 (96%) | 228 (99%) | 250 (99%) | |
| Grade 2 | 45 (3%) | 1 (<1%) | 1 (<1%) | ||
| Grades 3–4 | 22 (1%) | 0 (0%) | 0 (0%) | ||
| Lateral tibial plateau | Normal/grade 1 | 1611 (91%) | 215 (94%) | 230 (92%) | |
| Grade 2 | 122 (7%) | 13 (6%) | 20 (8%) | ||
| Grades 3–4 | 28 (2%) | 0 (0%) | 0 (0%) | ||
| Patella | Normal/grade 1 | 1470 (83%) | 199 (90%) | 213 (88%) | |
| Grade 2 | 181 (10%) | 21 (10%) | 29 (12%) | ||
| Grades 3–4 | 110 (6%) | 0 (0%) | 0 (0%) | ||
| Trochlea | Normal/grade 1 | 1641 (93%) | 219 (96%) | 238 (96%) | |
| Grade 2 | 82 (5%) | 10 (4%) | 11 (4%) | ||
| Grades 3–4 | 38 (2%) | 0 (0%) | 0 (0%) |
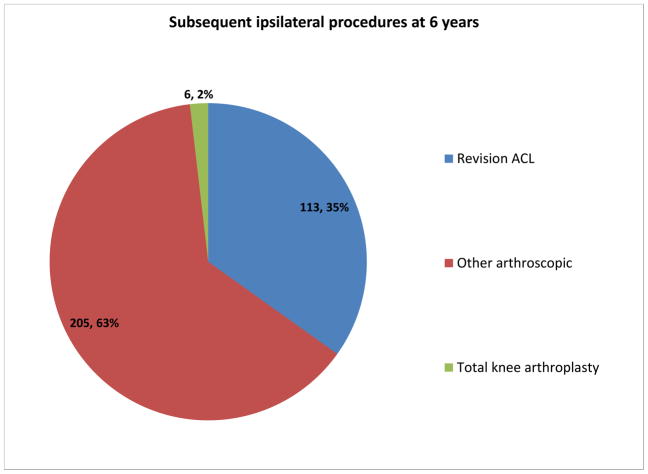
Table 3 depicts the significant independent risk factors identified in each model. Subsequent ipsilateral surgery was the most consistent and strongest predictor of increased symptoms at both 2 and 6 years post-ACL reconstruction (broken down by type in Figure 2). Subsequent surgeries were common, occurring at a rate of 16% (239/1530) at 2 years and 21.5% (324/1506) at 6 years. The majority of subsequent surgeries other than total knee replacement took place more than a year prior to the 6 year outcome measurement. The mean time to revision ACLR was 2.4 ± 1.9 years, total knee replacement 5.3 ± 2.2 years and other arthroscopic surgical procedures 2.1 ± 1.9 years. Revision ACLR was the single most common subsequent procedure. The vast majority of subsequent procedures took place remote from sampled time-points: only 1.3% (3/239) and 0% (0/324) of patients had a subsequent surgery within one month of filling out the KOOS forms at 2 years and 6 years, respectively. Furthermore, only 3.8% (9/239) and 1.2% (4/324) of patients had a subsequent surgery within 3 months of filling out the KOOS forms at 2 years and 6 years, respectively. When all 4 models were re-run with patients who had undergone subsequent surgery within 3 months removed, no changes were noted in the significance or magnitude of any statistically significant risk factors. One risk factor in model #4 which previously approached significance then became significant (current vs. never smoker: OR 1.82 (1.02, 3.27); p=0.043).
Figure 2.
Subsequent ipsilateral surgical procedures at 6 years (“Other arthroscopic” includes hardware removal, meniscal and articular cartilage surgery, infection, arthrolysis/manipulation).
Other independent risk factors that were found to be significant (although inconsistent) of increased symptoms at 2 and/or 6 years post-ACL reconstruction included higher BMI, smokers, less years of education, lower baseline KOOS ADL and higher baseline KOOS sports/rec subscale scores, and lower 2-year Marx activity levels (for predicting the 6-year models).
Potential prognostic factors that did not alter the risk of reporting a painful knee or having significant activity-related pain included age, pre-operative/baseline activity level, pre-operative WOMAC (pain, stiffness) or KOOS (symptoms, pain, and quality of life) baseline scores, graft type, medial meniscal pathology/treatment, and subsequent contralateral knee surgery. The grade of chondral damage at initial arthroscopy was an inconsistent predictor in the patellofemoral, medial and lateral compartments. In general, when chondral damage influenced the odds of reporting either a painful knee or significant activity-related pain, the tendency was for the effect to be driven by grade 3/4 change.
DISCUSSION
The prevalence of significant patient-reported knee pain 6 years after ACLR was high, including 39% by the Englund definition, 9% for KOOS pain score ≤72 (drop ≥20 points) and 12% for KOOS pain MCID definition (drop ≥10 points). A similar proportion of patients (11%) reported significant activity-related knee pain at 6 years. This study is the first to apply these definitions to characterize this patient population and represents an important first step in identifying at-risk patients for the development of significant knee pain after ACLR.
The most consistent risk factor across all definitions of significant knee pain also carried the largest impact – subsequent ipsilateral knee surgery. We utilized interactions of age and subsequent surgery in our statistical modeling because of our previous findings4 that demonstrated younger age increased the risk of subsequent surgery at 2 and 6 year follow-up. This limited the degrees of freedom we could use to identify which of the subsequent procedures had the most influence. At 6 years, ipsilateral re-operation was dominated by revision ACLR, further meniscus/articular cartilage surgery and surgical interventions for stiffness. Revision ACLR has been associated with worse PRO4,27,28,29, and subsequent meniscal or articular cartilage surgery is a recognized risk for radiographic OA changes in ACL reconstructed patients6. The identification of subsequent surgery as a risk factor for reporting a painful knee was also a robust enough finding that it held even with the removal of patients who had surgery within 3 months of the 2- and 6-year time-points from statistical analysis. That contralateral knee surgery did not increase the odds of reporting a painful knee, places further importance on subsequent surgeries as a marker for additional trauma or joint degeneration as a driver of poor outcomes. Better resolution of the type of procedure in subsequent investigations will be helpful, as some are potentially preventable through improved surgical technique, timing of surgery, or rehabilitation.
Many ACLR patients exhibited activity-related knee pain in follow-up. We assessed this using model #4, and determined that 11% of patients met these criteria. This included approximately half who also met criteria for models #2 and #3 – both KOOS pain models. KOOS pain assesses both activity-related and non-activity related pain and contains the questions from the validated hip and knee osteoarthritis tool – Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)15. Furthermore, we noted that a low Marx score at 2 years increased the odds of a patient meeting the Englund criteria (model #1), KOOS pain ≤72 criteria (model #2), and IKDC activity-related pain threshold (model #4). Whether simply being less active is a risk factor for reporting significant knee pain, or whether patients already developing significant knee pain become less active, is not known.
There is no consensus definition for symptomatic OA or significant knee pain using Patient Reported Outcomes. This is further complicated by the heterogeneity of diagnoses/definitions reported in the literature. While we found the prevalence of significant knee pain was high, it varied considerably based on our definitions. The Englund et al.14 criteria were the least stringent, but also the broadest including pain, symptoms and quality of life reporting. The KOOS pain threshold in that model was 86 points, which corresponded to the 25th percentile of KOOS pain scores in the MOON cohort 4. Accordingly, this model identified the most patients. Few cues are available from the literature for prevalence of pain based on the Englund model in similar patients, with only two small published studies. In a cohort of 84 female soccer players with an ACL injury treated with either rehabilitation or surgery30, 75% met the Englund criteria at 15 years follow-up. In contrast, 51% met criteria for knee OA on radiographs, and 42% met both. In a purely non-operative cohort31 of 67 ACL injured patients, the 15-year KOOS pain scores were all 85 points or greater.
The 20-point drop in KOOS pain score that we selected corresponded to the OARSI responder criteria16 and 2 standard deviations below the mean of KOOS scores of athletes with a history of knee ligament injury24. Even fewer comparative studies exist in the literature for this definition. Paradowski et al.17 applied the OARSI responder criteria, developed for use in OA interventional studies to identify therapies that produce significant knee pain reduction, to identify mild OA patients with significantly increased knee symptoms post-meniscectomy. Those with radiographic changes had a larger drop in KOOS pain score (11 points), and by six years they determined that 7% of patients had a ≥20 point KOOS pain drop. Another study of older, post-meniscectomy patients with intact ligaments demonstrated a mean baseline KOOS pain of 84 points32, but with high individual variation. Seven years later the same patients reported a further 6 point drop in KOOS pain on average which was worse in females and those with radiographic changes.
A 20-point KOOS pain drop that is 2 standard deviations below population norms24 should theoretically include only 2.5% of our cohort. In fact, however, the distribution of KOOS pain was skewed to the left at 6 years with more than 9% of patients having a score below this cut-off. This finding offers both clinical and statistical significance and reinforces the role of subsequent injury, joint degeneration, or concomitant pathology at the primary reconstruction in the identification of patients at-risk for high levels of self-reported knee pain. Furthermore, a significant proportion of these patients reported high levels of activity-related pain according to our IKDC model #4 definition.
Limitations
There are some challenges in comparing our cohort with previous studies that have attempted to develop and characterize the prevalence of significant knee pain. Prior studies have examined patients with a different primary surgical intervention – namely meniscectomy14, 17, 32, 33. The etiology of meniscal tears in those cohorts included both traumatic and atraumatic mechanisms, whereas our cohort had sustained a traumatic rupture of the ACL. Secondly, the meniscectomy cohorts have an older mean age than our cohort (mean age typically 46–56 years, compared to mean age <30 years at follow-up in our study). How a degenerative process and traumatic process modify the risk of developing knee OA is unknown.
Loss to follow-up in our study was 13% (2 year) or 14% (6 year). Although there is no consensus on the introduction of bias based on follow-up, most estimates suggest that <5% loss will have no effect, while >20% may pose serious threats to validity34. Yet the direction and approximate magnitude of some covariates, such as socioeconomic markers, do not change with attrition approaching even 50% 35,36. MOON investigators go to considerable length to contact enrolled patients, including repeated mailings and phone calls. As noted in Table 3, the proportion of males lost to follow-up was slightly higher (63% vs 56%), and some minor differences were seen for BMI and smoking status. We don’t think this will have had a large effect on the study conclusions, as sex was not associated with outcome, smoking was inconsistently associated with only a couple outcomes and BMI was only a predictor in model #1.
Our study was not designed to identify the best definition for a symptomatic knee. Accordingly, we utilized various definitions, each with advantages and disadvantages as well as mixed support in the literature. The agreement between models was reasonable at 6 years after ACLR, as exemplified by identifying approximately half of the patients (n=67) from the most stringent model (KOOS pain ≤72; n=131) in the remaining models. The identification of which outcomes (pain, function, or ADL) remain most important to post-ACLR patients, and the establishment of cut-off scores using the PASS (Patient Acceptable Symptomatic State) concept for ACLR will be important steps in further defining this subset of patients.
Finally, we did not have radiographs available in follow-up of these patients to correlate structural change with symptomatic findings, as has been done in smaller series post isolated meniscectomy17,30,32,37,38. The interaction of structural changes with symptoms is an important area for future research. This is heightened by the discordance between our study and systematic reviews of post-ACL reconstruction patients6 that suggests meniscal pathology at the time of injury/surgery moderates radiographic OA risk. It would appear that while meniscal loss initiates joint space changes, it may be a weaker mediator of symptoms compared to other factors we have identified such as chondral damage and subsequent injury/surgery. There is some support for this notion based upon weak associations demonstrated between joint space narrowing and poor PRO in Osteoarthritis Initiative cohort studies12, 13. A second explanation is that the follow-up in our study is not yet long enough for meniscal status at the time of surgery to have the same influence on PRO. Exploring these interactions in future work is of critical importance to define the patients truly at-risk for clinically relevant OA after ACL reconstruction.
Summary
Significant knee pain and symptoms is prevalent among 9–39% of first-time ACL reconstruction patients at 6 years. Patient-reported pain is affected to some degree by demographic factors and higher grades of concurrent cartilage damage at the index procedure, however, those who undergo second surgeries (e.g., revision, repeat arthroscopy) are at greatest risk. Whether this patient report of significant knee pain relates to structural arthritic changes requires further study.
Acknowledgments
We thank all our funding sources for supporting this work. These include National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant numbers: 5R01 AR053684; 5K23 AR052392), Center for Education and Research on Therapeutics/Agency of Health Research and Quality (Grant number: 5U18-HS016075). This project received partial support from the Orthopaedic Research and Education Foundation, DonJoy Orthopaedics, Smith and Nephew Endoscopy, Vanderbilt Sports Medicine Research Fund, and by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. The contents of this study are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
We thank the research coordinators, analysts and support staff from the MOON sites, whose efforts related to regulatory, data collection, subject follow-up, data quality control, analyses, and manuscript preparation make this consortium possible.
We also thank all the subjects who generously enrolled and participated in this study.
FUNDING DECLARATION
The MOON cohort has obtained internal and external funding from numerous peer reviewed and non-peer reviewed sources. These include National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant numbers: 5R01 AR053684; 5K23 AR052392), Center for Education and Research on Therapeutics/Agency of Health Research and Quality (Grant number: 5U18-HS016075). This project received partial support from the Orthopaedic Research and Education Foundation, DonJoy Orthopaedics, Smith and Nephew Endoscopy, Vanderbilt Sports Medicine Research Fund, and by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. The funding sources have partially supported the development and maintenance of the database and provided funding for a percent of effort for some of the research personnel.
AUTHOR CONTRIBUTIONS
| Factor | Contributor |
|---|---|
| Study design/conception | DW, LJH, KPS |
| Acquisition of data | LJH |
| Analysis and interpretation of data | DW, LJH, SN, KPS |
| Drafting of manuscript | DW |
| Critical revision for intellectual content | DW, LJH, KPS |
| Final approval | DW, LJH, KPS |
| Provision of study materials or patients | AA, JTA, WRD, CCK, RM, ECM, RDP, KPS, MLW, BRW, RWW |
| Statistical expertise | SN |
| Obtaining of funding | KPS |
| Administrative, technical or logistic support | ? |
| Collection and assembly of data | AA, JTA, WRD, LJH, CCK, RM, ECM, RDP, KPS, MLW, BRW, RWW |
Responsible for integrity of the work:
Kurt P. Spindler (spindlk@ccf.org)
Laura J Huston (laura.huston@vanderbilt.edu)
David Wasserstein (david.wasserstein@mail.utoronto.ca)
COMPETING INTEREST STATEMENT
David Wasserstein, Laura Huston, Samuel Nwosu, Kurt Spindler, and Warren Dunn report institutional funding by NIH/NIAMS grant #5R01 AR053684 (PI—Spindler) and #5K23 AR052392 (PI—Dunn). These same authors report institutional funding via Unrestricted Educational Gifts from Smith and Nephew Endoscopy and DonJoy Orthopaedics.
Robert Marx has received funds outside the submitted work from the Journal of Bone and Joint Surgery (American) as Associate Editor; royalties from Demos Health for The ACL Solution; and royalties from Springer for Revision ACL Reconstruction.
Annunziato Amendola has received personal funds outside the submitted work from Arthrex, Inc.; Arthrosurface, Inc.; and MTP Solutions.
Brian Wolf has received personal funds outside the submitted work from United Health Care for participation on the Scientific Advisory Board.
Rick W. Wright has received personal funds outside the submitted work for Board Membership on the American Board of Orthopaedic Surgery Board of Directors and from the American Orthopaedic Association as Treasurer-Elect. Additionally, he has received personal funds outside the submitted work from the NIA/NIAMS in the form of a research grant, and book royalties from Wolters Kluwer Lippincott Williams & Wilkins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
D Wasserstein, Email: david.wasserstein@mail.utoronto.ca.
LJ Huston, Email: laura.huston@vanderbilt.edu.
S Nwosu, Email: sam.nwosu@vanderbilt.edu.
MOON Group, Email: laura.huston@vanderbilt.edu.
KP Spindler, Email: spindlk@ccf.org.
References
- 1.Engebretsen L, Benum P, Fasting O, Molster A, Strand T. A prospective, randomized study of three surgical techniques for treatment of acute ruptures of the anterior cruciate ligament. Am J Sports Med. 1990;18(6):585–90. doi: 10.1177/036354659001800605. [DOI] [PubMed] [Google Scholar]
- 2.Grontvedt T, Engebretsen L, Bredland T. Arthroscopic reconstruction of the anterior cruciate ligament using bone-patellar tendon-bone grafts with and without augmentation. A prospective randomized study. J Bone Joint Surg Br. 1996;78(5):817–22. [PubMed] [Google Scholar]
- 3.Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J ed. 2008;359(20):2135–42. doi: 10.1056/NEJMcp0804745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox CL, Huston LJ, Dunn WR, Reinke EK, Nwosu SK, Parker RD, et al. Are articular cartilage lesions and meniscus tears predictive of IKDC, KOOS, and Marx activity level outcomes after ACL reconstruction? A 6-year MOON cohort study. Am J Sports Med. 2014;42(5):1058–67. doi: 10.1177/0363546514525910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muthuri SG, McWilliams DF, Doherty M, Zhang W. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis and Cartilage. 2011;19:1286–93. doi: 10.1016/j.joca.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury. Am J Sports Med. 2009;37(7):1434–43. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 7.Magnussen RA, Mansour AA, Carey JL, Spindler KP. Meniscus status at anterior cruciate ligament reconstruction associated with radiographic signs of osteoarthritis at 5- to 10-year follow-up. J Knee Surg. 2009;22(4):347–56. doi: 10.1055/s-0030-1247773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalmers PN, Mall NA, Moric M, Sherman SL, Paletta GP, Cole BJ, et al. Does ACL reconstruction alter natural history? A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96(4):292–300. doi: 10.2106/JBJS.L.01713. [DOI] [PubMed] [Google Scholar]
- 9.Pereira D, Peleteiro B, Araujo J, Branco J, Santosk RA, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis and Cartilage. 2011;19:1270–85. doi: 10.1016/j.joca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Guermazi A, Roemer F, Felson D, Brandt KD. Motion for debate: osteoarthritis clinical trials have not identified efficacious therapies because traditional imaging outcome measures are inadequate. Arthritis & Rheumatism. 2013;65(11):2748–58. doi: 10.1002/art.38086. [DOI] [PubMed] [Google Scholar]
- 11.Kinds MB, Welsing PM, Vignon EP, Bjilsma JW, Viergever MA, Marjinissen AC, et al. A systematic review of the association between radiographic and clinical osteoarthritis of hip and knee. Osteoarthritis and Cartilage. 2011;19(7):768–78. doi: 10.1016/j.joca.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Oak SR, Ghodadra A, Winalski CS, Miniaci A, Jones MH. Radiographic joint space width is correlated with 4-year clinical outcomes in patients with knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis and Cartilage. 2013;21(9):1185–90. doi: 10.1016/j.joca.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Illingworth KD, Bitar YE, Seiwert K, Scaife SL, El-Amin S, Saleh KJ. Correlation of WOMAC and KOOS scores to tibiofemoral cartilage loss on plain radiography and 3 Tesla MRI: data from the osteoarthritis initiative. Knee Surg Sports Traumatol Arthrosc. 2013 Jan 23; doi: 10.1007/s00167-013-2402-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis. Arthritis Rheum. 2003;48(8):2178–87. doi: 10.1002/art.11088. [DOI] [PubMed] [Google Scholar]
- 15.Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS) – validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17. doi: 10.1186/1477-7525-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougados M, LeClaire P, van der Heijde D, Bloch DA, Bellamy N, Altman RD. Response criteria for clinical trials on osteoarthritis of the knee and hip. A report of the osteoarthritis research society international standing committee for clinical trials response criteria initiative. Osteoarthritis and Cartilage. 2000;8:395–403. doi: 10.1053/joca.2000.0361. [DOI] [PubMed] [Google Scholar]
- 17.Paradowski PT, Englund M, Roos EM, Lohmander LS. Similar group mean scores, but large individual variations, in patient-relevant outcomes over 2 years in meniscectomized subjects with and without radiographic knee osteoarthritis. Health Qual Life Outcomes. 2004;2:38. doi: 10.1186/1477-7525-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spindler KP, Parker RD, Andrish JT, Kaeding CC, Wright RW, Marx RG, et al. Prognosis and predictors of ACL reconstructions using the MOON cohort: A model for comparative effectiveness studies. J Orthop Res. 2013;31(1):2–9. doi: 10.1002/jor.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee injury and Osteoarthritis Outcome Score (KOOS): development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 20.Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213–18. doi: 10.1177/03635465010290021601. [DOI] [PubMed] [Google Scholar]
- 21.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 22.Marx RG, Connor J, Lyman S, Amendola A, Andrish JT, Kaeding CK, et al. Multirater agreement of arthroscopic grading of knee articular cartilage. Am J Sports Med. 2005;33(11):1654–57. doi: 10.1177/0363546505275129. [DOI] [PubMed] [Google Scholar]
- 23.Dunn WR, Wolf BR, Amendola A, Andrish JT, Kaeding CK, Marx RG, et al. Multirater agreement of arthroscopic meniscal lesions. Am J Sports Med. 2004;32(8):1937–40. doi: 10.1177/0363546504264586. [DOI] [PubMed] [Google Scholar]
- 24.Cameron KL, Thompson BS, Peck KY, Owens BD, Marshall SW, Svoboda SJ. Normative values for the KOOS and WOMAC in a young athletic population: history of knee ligament injury is associated with lower scores. Am J Sports Med. 2013;41:582–89. doi: 10.1177/0363546512472330. [DOI] [PubMed] [Google Scholar]
- 25.Salavati M, Akbhari B, Mohammadi F, Mazaheri M, Khorrami M. Knee injury and Osteoarthritis Outcome Score (KOOS); reliability and validity in competitive athletes after anterior cruciate ligament reconstruction. Osteoarthritis and Cartilage. 2011;19:406–10. doi: 10.1016/j.joca.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Out-come Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright R, Spindler K, Huston L, Amendola A, Andrish J, Brophy R, et al. Revision ACL reconstruction outcomes: MOON cohort. J Knee Surg. 2011;24(4):289–94. doi: 10.1055/s-0031-1292650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright RW, Gill CS, Chen L, Brophy RH, Matava MJ, Smith MV, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531–36. doi: 10.2106/JBJS.K.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551–7. doi: 10.1177/0363546512446000. [DOI] [PubMed] [Google Scholar]
- 30.Lohmander L, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–52. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 31.Kostogiannis I, Ageberg E, Neuman P, Dahlberg L, Friden T, Roos H. Activity level and subjective knee function 15 years after anterior cruciate ligament injury: a prospective, longitudinal study of nonreconstructed patients. Am J Sports Med. 2007;35(7):1135–43. doi: 10.1177/0363546507299238. [DOI] [PubMed] [Google Scholar]
- 32.Roos EM, Bremander AB, Englund M, Lohmander LS. Change in self-reported outcomes and objective physical function over 7 years in middle-aged subjects with or at high risk of knee osteoarthritis. Ann Rheum Dis. 2008;67(4):505–10. doi: 10.1136/ard.2007.074088. [DOI] [PubMed] [Google Scholar]
- 33.Paradowski PT, Englund M, Lohmander S, Roos EM. The effect of patient characteristics on variability in pain and function over two years in early knee osteoarthritis. Health Qual Life Outcomes. 2005;3:59. doi: 10.1186/1477-7525-3-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacket DL, Richardson WS, Rosenberg W. Evidence-Based Medicine: How to Practice and Teach EBM. New York: Churchill Livingstone; 1997. [Google Scholar]
- 35.Howe LD, Tilling K, Galobardes B, Lawlor DA. Loss to Follow-up in Cohort Studies: Bias in Estimates of Socioeconomic Inequalities. Epidemiology. 24(1):1–9. doi: 10.1097/EDE.0b013e31827623b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winding TN, Andersen JH, Labriola M, Nohr EA. Initial non-participation and loss to follow-up in a Danish youth cohort: implications for relative risk estimates. J Epidemiol Community Health. 2014;68(2):137–44. doi: 10.1136/jech-2013-202707. [DOI] [PubMed] [Google Scholar]
- 37.Larsson S, Englund M, Struglics A, Lohmander LS. The association between changes in synovial fluid levels of ARGS-aggrecan fragments, progression of radiographic osteoarthritis and self-reported outcomes: a cohort study. Osteoarthritis Cart. 2012;20:388–95. doi: 10.1016/j.joca.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Oiestad BE, Holm I, Engebretsen, Risberg MA. The association between radiographic knee osteoarthritis and knee symptoms, function and quality of life 10–15 years after anterior cruciate ligament reconstruction. Br J Sports Med. 2011;45:583–8. doi: 10.1136/bjsm.2010.073130. [DOI] [PubMed] [Google Scholar]