Abstract
Animal models of osteoarthritis (OA) are essential tools for investigating the development of the disease on a more rapid timeline than human OA. Mice are particularly useful due to the plethora of genetically modified or inbred mouse strains available. The majority of available mouse models of OA use a joint injury or other acute insult to initiate joint degeneration, representing post-traumatic osteoarthritis (PTOA). However, no consensus exists on which injury methods are most translatable to human OA. Currently, surgical injury methods are most commonly used for studies of OA in mice; however, these methods may have confounding effects due to the surgical/invasive injury procedure itself, rather than the targeted joint injury. Non-invasive injury methods avoid this complication by mechanically inducing a joint injury externally, without breaking the skin or disrupting the joint. In this regard, non-invasive injury models may be crucial for investigating early adaptive processes initiated at the time of injury, and may be more representative of human OA in which injury is induced mechanically. A small number of non-invasive mouse models of PTOA have been described within the last few years, including intra-articular fracture of tibial subchondral bone, cyclic tibial compression loading of articular cartilage, and anterior cruciate ligament rupture via tibial compression overload. This review describes the methods used to induce joint injury in each of these non-invasive models, and presents the findings of studies utilizing these models. Altogether, these non-invasive mouse models represent a unique and important spectrum of animal models for studying different aspects of PTOA.
Keywords: Post-traumatic osteoarthritis (PTOA), mouse model, articular cartilage, knee injury
Introduction
Osteoarthritis (OA) currently affects approximately 27 million people in the United States [1], and 630 million people worldwide, and the knee is by far the most commonly affected joint [2]. OA can be classified either as “primary” (or idiopathic), arising from unknown causes and affecting primarily older subjects, or “secondary” (or post-traumatic), arising as a consequence of a joint injury and often affecting much younger subjects. For example, after anterior cruciate ligament (ACL) or meniscus injury, patients are at a much higher risk of developing post-traumatic osteoarthritis (PTOA) within 10–20 years after injury [3, 4]. This risk is even greater following high-energy impact joint injuries involving intra-articular bone fracture [5, 6]. Altogether, approximately 10–12% of symptomatic osteoarthritis cases can be considered post-traumatic [7].
Animal models of OA are essential tools for investigating the development and mechanisms of the disease on a more rapid timeline than human OA. Spontaneous animal models of OA [8–11], in which OA will develop in animals without any “injury” to the joint, are believed to be representative of primary (idiopathic) OA. However, the majority of animal models use a joint injury or other acute insult to initiate joint degeneration, making them more representative of PTOA. Little et al. identified five properties of the “ideal” animal model of OA [12]:
The model should induce consistent reproducible disease that occurs in a suitable time frame to allow reasonably high throughput studies.
The induced disease should be universally progressive in the time frame of the study to allow investigation of early, mid and late pathophysiology and treatment effects.
The animal should be a mammalian species that is tractable, inexpensive, easy to house and manage, large enough to allow multiple analyses/outcome measures, allows genome wide micro-array analysis and proteomic analysis, sequencing etc.
The disease process in the animal recapitulates the human pathology in all tissues of the articulating joint.
The model should be predictive of therapeutic disease modification in humans. Numerous animal models of PTOA have been described, however it is not yet known if any of these models fully exhibit these “ideal” properties.
Mice are particularly useful model organisms and are commonly used to study OA and other musculoskeletal conditions, so extensive normative data on mouse musculoskeletal growth and metabolism are available. The abundance of available genetically modified or inbred mouse strains provide the unique ability to study molecular mechanisms contributing to OA development [13–19]. However, the use of mice for studies of OA also has important limitations [20] including the small size of the joint and the extreme thinness of the articular cartilage, which is only a few cell layers thick. Therapeutic strategies that are shown to be successful on this small scale may not prove as effective in the larger human joint. Additionally, the small joint size and thin cartilage make surgical repair or treatment of joint injuries unfeasible or impractical.
While numerous mouse models of PTOA have been described, no consensus exists on the injury methods used to initiate the development of OA. This limitation is crucially important, since the observed pattern of joint degeneration likely depends on the injury method used. For this reason, injury methods should be utilized in mice that recapitulate the human injury conditions as closely as possible. However, most mouse models use invasive (i.e., surgical) or non-physiologic methods to initiate joint degeneration.
Surgical and Injection Mouse Models of PTOA
Surgical injury methods initiate joint degeneration by disrupting joint structures such as ligaments and menisci that can alter the stability and biomechanics of the joint. Development of mouse models of PTOA based on this concept follows previous studies in larger animals. One of the first surgically-induced animal models of OA was ACL transection (ACLT), or Pond-Nuki model, in dogs in 1973 [21]. The authors noted a histological progression of arthritis similar to naturally occurring arthritis, with gross fibrillation extending deep into the articular cartilage at long time points. This study laid the groundwork for further ACLT studies in dogs [22–26], rats [27–29], rabbits [30–32], cats [33, 34], guinea pigs [35, 36], sheep [37], and mice [38, 39].
Another common method of inducing OA in animal models involves partial or total removal of the medial meniscus [40–44]. Studies have combined meniscectomy and ACLT to model the combination injury seen clinically [45–47]. Destabilization of the medial meniscus (DMM) also initiates OA progression in mice [19, 38, 48–51]; this method has been the most commonly reported for studying OA in mice. DMM produces relatively predictable development of OA, although disease progression may be variable, particularly when the procedure is performed by surgeons with disparate skill or experience. These factors may represent potential drawbacks to the DMM model or any other surgical method. These procedures can be technically difficult to perform, and require specialized equipment and personnel to effectively perform the procedure. Surgical techniques also require opening the joint capsule, which can disrupt the natural environment of the joint, and may therefore come with unintended adaptive and healing processes that are due to the surgery itself, rather than the intended “injury”. This consideration is particularly important when studying early time points following an injurious event, and thus require the use of “sham” surgeries and increased numbers of experimental animals.
Degenerative changes in the knee joint can also be achieved by intra-articular injection of degradative agents into the joint space, including proteolytic enzymes such as papain [52–54] and collagenase [54–57], cytokines such as tumor necrosis factor alpha (TNF-α) [58, 59], transforming growth factor β (TGF-β) [60, 61], and interleukin-1 (IL-1) [56, 62], or chemicals such as monosodium iodoacetate (MIA) [54, 63–65] and colchicine [66]. Different agents will create pathological changes in the joint by different mechanisms, making these approaches appropriate for studies of particular biological mechanisms. However, these methods do not mimic human injury conditions, suggesting that the translatability of information garnered from these models may not be relevant to PTOA.
Non-Invasive Mouse Models of PTOA
Non-invasive models can initiate PTOA using externally applied mechanical loads, but do not break the skin or disrupt the joint capsule. Non-invasive injury models are therefore completely aseptic, and avoid potential confounding effects caused by the trauma of the surgical/invasive injury procedure.
A particular opportunity afforded by non-invasive injury models is investigating early adaptive processes that are initiated at the time of injury, with time scales of hours or days following injury (Fig. 1). This is a key advantage, as the window of opportunity for treatments aimed at slowing or inhibiting PTOA may be only a few days following injury. Non-invasive models more accurately recapitulate the mechanically-induced mechanisms involved in injuries leading to OA in humans, initiating joint degeneration through direct damage to cartilage, bone, or soft tissue structures of the joint. Additionally, non-invasive models may be simpler, quicker experimental procedures that are straightforward to implement and do not require technically difficult surgical or injury techniques.
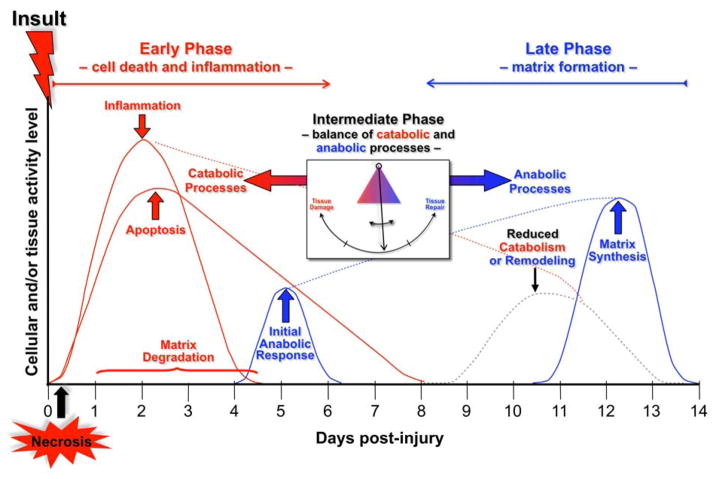
Figure 1.
Conceptual framework of the immediate cellular responses to acute joint trauma. Both catabolic and anabolic processes are involved in the response to the injury, and overlap with one another. Image courtesy of Susanna Chubinskaya. From Anderson et al. [100]. Used with permission.
Several non-invasive mouse models of PTOA have been described within the last few years. Each model initiates joint degeneration using different methods, and is therefore representative of specific conditions leading to the human disease. However, these non-invasive models represent a unique and important spectrum of animal models for studying different aspects of PTOA.
Intra-Articular Fracture of the Tibial Plateau
The first non-invasive mouse model of PTOA was described by Furman et al. in 2007 [67]. This model initiates symptoms using intra-articular fracture (IAF) of the proximal tibia, and includes blunt impaction of articular cartilage, fracture of the articular cartilage/subchondral bone layer, fragmentation of the articular surface, residual displacement of the articular surfaces, and exposure of blood and marrow products to the articular surface and synovium. This injury model is representative of higher-energy impact injuries that may be sustained by humans in events such as frontal automobile collisions.
To induce an articular fracture of the tibial plateau, C57BL/6 mice were positioned in a custom cradle with the lower limb at approximately 90° flexion, in a materials testing system (ELF 3200, Bose, Eden Prairie, MN), and a 10 N compressive preload was applied to the proximal tibia using a wedge-shaped indenter mounted to the testing system (Fig. 2) to a target compressive force of 55 N at a rate of 20 N/s. Fractures were characterized by anterior/posterior (A/P) and lateral radiographs. No fixation or surgical intervention was employed so that the natural history and healing of a closed articular fracture could be elucidated. Animals were allowed immediate full weight bearing with unlimited range of motion.
Figure 2.
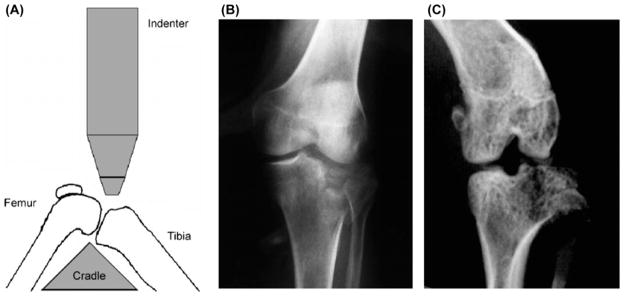
(A) Alignment of the cradle and indenter for creating a closed articular fracture in the mouse knee. (B–C) Radiographs of clinically observed (B) human tibial plateau fracture and (C) experimentally created mouse tibial plateau fracture. From Furman et al. [67]. Used with permission.
The IAF protocol was successful in 87% of the mice (27 of 31). Subsequent studies using this model have reported over 95% success in fracture creation [63–65]. The injuries induced by the protocol varied, with both simple and complex fractures of the tibia. Fractures were commonly located on the lateral side of the plateau and closely resembled those seen clinically (Fig. 2). Micro-computed tomography (μCT) results indicated that trabecular bone volume fraction (BV/TV) in the femoral condyles was significantly reduced in injured limbs at all time points compared to contralateral control limbs. Bone density was significantly lower in injured limbs in the femoral condyles, tibial plateau, and tibial metaphysis. The lateral femoral condyle and tibial plateau exhibited subchondral bone thickening in injured limbs compared to control limbs. Histology showed a progressive loss of Safranin-O staining over the first 8 weeks from the meniscus, and femoral and tibial articular cartilage, indicating a loss of proteoglycan. By 50 weeks, severe loss of articular cartilage and exposure of subchondral bone was present on both the tibia and femur, indicative of terminal OA.
The IAF model provides a fast and fairly reproducible non-invasive injury to the knee joints of mice using a single mechanical load, consistent with human injuries, and the resulting changes in bone and articular cartilage are representative of joint degeneration observed in human OA. This model is representative of articular fracture injuries, therefore this model may not be ideal for studying lower energy non-contact injuries that commonly lead to PTOA (e.g., ACL rupture or meniscus tear). The authors evaluated energy of injury using a liberated surface area analysis and found good correlation between applied energy of fracture and liberated surface area, allowing investigators to create high and low energy articular fracture injuries [63] (Fig. 3). The variability in severity of fracture creates varying degrees of joint inflammation or abnormal loading of cartilage after injury, which affects the rate of joint degeneration on a mouse-to-mouse basis, therefore fracture severity needs to be quantified and included as a co-factor when comparing experimental groups [68]. However, even when fracture severity is quantified and factored into the analysis, the inherent variability in fracture severity must be considered a limitation of this model.
Figure 3.
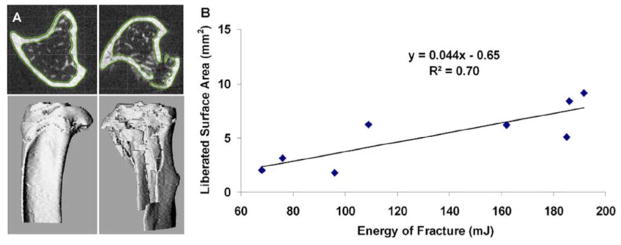
Correlation between liberated surface area and measured energy of fracture for closed articular fracture in the mouse knee. Fracture severity, as measured from the liberated surface area, was well correlated to the energy of fracture, as calculated from the load-displacement data. From Lewis et al. [68]. Used with permission.
Another important advantage of the IAF model is that, as the first non-invasive mouse model of PTOA described, a relatively large body of literature has utilized this model for studies of PTOA development. For example, Ward et al. [13] investigated PTOA development following IAF in MRL/MpJ mice, which have shown unique regenerative abilities following injury. Injured knees of MRL/MpJ mice were protected against changes in bone density, subchondral bone thickness, and cartilage degeneration at 4 and 8 weeks post-injury. The differences in PTOA development between C57BL/6 mice and MRL/MpJ mice after IAF have been further explored [69]. Seifer et al. utilized this mouse model to investigate cartilage oligomeric matrix protein (COMP) in synovial fluid of mice following IAF [70]. Lewis et al. used this model to determine the effect of articular fracture severity on synovial inflammation, bone morphology, liberated fracture area, cartilage pathology, chondrocyte viability, and systemic cytokines and biomarkers levels [68]. Louer et al. utilized IAF in mice to determine whether diet-induced obesity influences the severity of PTOA development [71]. Diekman et al. utilized this animal model to investigate whether delivery of mesenchymal stem cells (MSCs) could affect the development of PTOA by altering inflammation and regeneration after fracture [72]. In two other investigations, intra-articular administration of IL-1 receptor antagonist immediately after fracture limited the development of PTOA in C57BL/6 mice [73, 74]. Altogether, the IAF mouse model is a useful method for studying joint degeneration following high trauma injuries to the knee. The model has been well characterized and accurately recapitulates joint degeneration observed in humans following this type of injury.
Cyclic Tibial Compression of Articular Cartilage
Tibial compression is a commonly used technique for studies of bone adaptation in mice [75–79]. For this loading method, a mouse is subjected to cyclic axial compressive loads applied to the lower leg through the ankle and knee joints (Fig. 4), with loads transmitted through natural joint articulations. This technique has also proven useful for the study of articular cartilage degeneration following mechanical loading. Poulet et al. were the first to exploit this approach to investigate short- and long-term joint degeneration following single or multiple bouts of loading in male CBA mice [80]. In this study, a 9N compressive load was applied every 10 seconds, with 40 cycles for each loading bout, and loading was performed three days per week. Loss of Safranin-O staining and articular cartilage lesions were observed on the lateral femur after two weeks of loading. When an additional three weeks of loading or non-loading was allowed after the initial two weeks of loading, the mean grade of lesion severity increased significantly in the loaded group, but the maximum lesion severity did not change. A single bout of tibial compression loading damaged the articular cartilage, but was not sufficient to create progressive lesions. Early signs of osteophytes were seen on the lateral femur in 57% of mice that were loaded for two weeks, while early signs of osteophytes on the medial and lateral femur were observed in 83% of mice loaded for five weeks. This model has subsequently been used to explore interaction between mechanically-induced cartilage lesions and genetics using the STR/Ort mouse strain (a model of spontaneous OA) to show that increased susceptibility to OA in this mouse strain was unlikely due to greater vulnerability to mechanical trauma [81]. It has also been used to show that cyclic mechanical loading is sufficient to induce subchondral bone thickening, particularly in regions contiguous with articular cartilage lesions in young growing mice [82].
Figure 4.
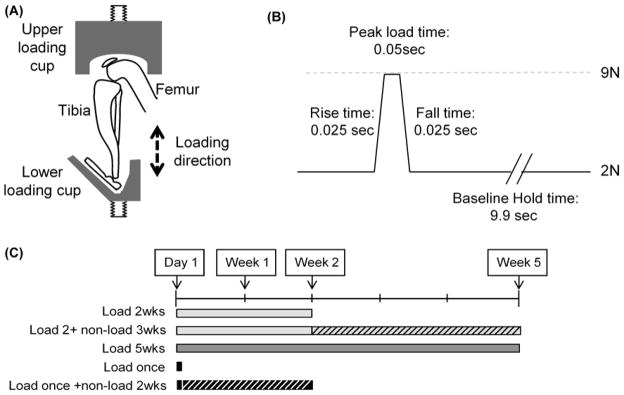
Diagrammatic representation of the cyclic tibial compression loading model. (A) Estimated position of the hind limb and loading direction when placed in the loading apparatus. (B) Diagram of a single cycle of applied load, showing hold and peak load magnitudes, rate of load application, and intervening peak and baseline hold times. (C) Diagrammatic representation of the 5 different loading regimens. From Poulet et al. [80]. Used with permission.
A similar approach was used to examine the effect of compressive load magnitude on the structure and composition of articular cartilage and subchondral bone in the knees of young (10 week-old) and adult (26 week-old) male C57BL/6 mice [83]. In vivo tibial compression loading was performed at defined magnitudes of 4.5 N and 9.0 N in adult mice, and at a peak load of 9.0 N in young mice for 1, 2, and 6 weeks. Loading was applied for 1,200 cycles per day, 4 cycles per second, for 5 days per week. Tibial compression initiated cartilage damage in both young and adult mice, and the severity of cartilage damage was greater with longer duration of loading (Fig. 5). As reported by Poulet et al. [80], cartilage degeneration occurred primarily at the lateral tibial plateau. Trabecular bone volume in the tibial metaphysis increased relative to contralateral controls with loading in young mice, but not in adult mice, whereas trabecular bone volume in the tibial epiphysis decreased with 6 weeks of loading in both young and adult mice. In both age groups, articular cartilage thickness decreased, and subchondral cortical bone thickness increased in the posterior tibial plateau. Mice in both age groups developed periarticular osteophytes at the tibial plateau in response to the 9.0 N peak load, but no osteophytes were observed in mice subjected to 4.5 N loading.
Figure 5.
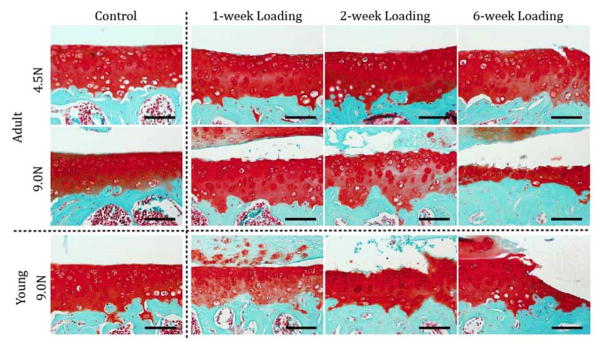
Cartilage matrix changes in the tibia after cyclic tibial compression loading. The left tibiae of young and adult mice were loaded (peak loads of 4.5N and 9.0N in adult mice; 9.0N in young mice) for 1, 2, and 6 weeks. The nonloaded contralateral limb at 6 weeks load duration served as control. Safranin O–fast green staining of the medial articular cartilage reveals that damage to the cartilage matrix occurred following mechanical loading in both young and adult mice, and was exacerbated with longer durations and a higher level of loading in adult mice. Bars = 100 μm. From Ko et al. [83]. Used with permission.
Recently, tibial compression was used to investigate early molecular events following cartilage injury in mice [84]. The right lower leg of 8-week old male C57BL/6 mice was subjected to tibial compression loading at 3, 6, or 9 N peak force for 60 cycles (10 s rest between cycles) in a single loading period, and harvested 5, 9, and 14 days post-loading. The authors reported that loading to 9 N produced a sharp drop in recorded force during the 1st loading cycle, consistent with ACL rupture (Fig. 7). Histology, immunohistochemistry, and ELISA were performed to evaluate chondrocyte viability, cartilage matrix metabolism, synovitis, and serum COMP levels. All loading regimens induced chondrocyte apoptosis, cartilage matrix degradation, and disruption of cartilage collagen fibril arrangement. 6 N loading induced mild synovitis by day 5, while 9 N loading initiated severe synovitis and fibrosis, likely due to joint instability associated with ACL injury. Even with joint loading at low compressive forces, serum COMP was significantly increased in mice. The precise source of COMP is unknown, however evidence of COMP synthesis in the lower zone of mouse cartilage is observable, with more pericellular COMP at higher loading magnitudes over time. Wu et al. recently reported the same COMP response to compression in 3-dimensional cell culture [85]. Similarly, ex vivo studies showed that mechanical loading of cartilage explants increased COMP mRNA expression and synthesis of COMP, suggesting that cartilage tissue can remodel certain aspects of its extracellular matrix in response to an altered mechanical environment [86].
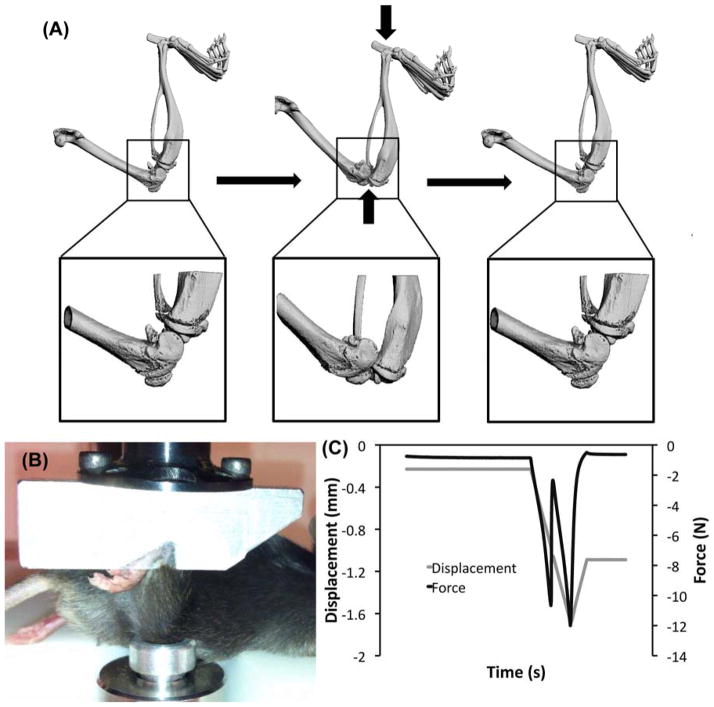
Figure 7.
Tibial compression overload ACL injury (A) Tibial compression loading caused a transient anterior subluxation of the tibia relative to the distal femur. (B) An anesthetized mouse with the right lower leg in the tibial compression loading system. (C) Knee injury during tibial compression loading was identified by a release of compressive force during the loading cycle, with a continued increase in actuator displacement. From Christiansen et al. [88]. Used with permission.
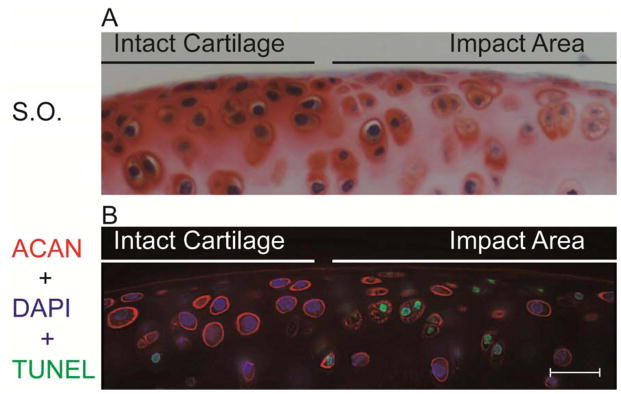
The early molecular and cellular response to joint loading or injury is of particular interest for studies of OA, as these biological responses likely contribute to long-term joint degeneration. Surprisingly, the early response to a single loading session at 3 or 6N magnitudes demonstrated a molecular and cellular response similar to that associated with severe injury [84]. For example, a single loading episode at 3 or 6N magnitude elicited apoptosis of chondrocytes at the site of cartilage compression. Concomitant with cartilage injury and chondrocyte apoptosis, safranin O staining was lost in the surrounding pericellular and interterritorial matrices, and the proteoglycan aggrecan appeared to be internalized into the chondrocyte. The internalization of aggrecan happens only in cells that are undergoing apoptosis (Fig. 6) and may reflect loss of membrane integrity. Longer-term studies suggest that these early events are not repaired, and no evidence of cell proliferation or migration of cells into the injured cartilage is observed (Xin, Rai and Sandell, unpublished data). At these low levels of cartilage injury, inhibition of cell death in cartilage or inhibition of synovial cell proliferation could potentially decrease cartilage damage [87]. Thus, joint loading at sub-injury magnitudes can cause focal damaged patches in the cartilage that have fewer cells and thus less ability to synthesize extracellular matrix and resist mechanical abrasion.
Figure 6.
Reduction in pericellular aggrecan (ACAN) thickness and in the intensity of extracellular distribution around the cells in the impact area in mouse knee joint cartilage injured by 3N compressive loading. (A) Loss of Safranin O (S.O.) staining in the impact area. (B) Representative images from TUNEL assay combined with immunofluorescence staining for aggrecan. Note the inferior aggrecan encapsulation and thickness around apoptotic chondrocytes (nuclei stained green) in the injured area, compared to clear pericellular aggrecan in TUNEL-negative cells (nuclei stained blue). Bar = 20 μm. From Wu et al. [84]. Used with permission.
The cyclic tibial compression model provides several unique benefits for the study of OA development. The adjustability of this model is a major benefit. By loading at a higher compressive force or for longer duration, the severity of joint degeneration can be controlled. However, regardless of the loading protocol used, the subsequent joint degeneration is typically mild (in the absence of ligament failure), even when loading at high force for several weeks, therefore severe OA may not be easily achievable. This model is highly reproducible between individual mice (and theoretically between research groups; see “Additional Considerations” below), and produces predictable structural changes in articular cartilage and subchondral bone. Importantly, the joint degeneration observed with this model (for sub-injury forces) does not appear to be a secondary consequence of joint destabilization or altered joint biomechanics, but is ascribable to direct overload of articular cartilage. In fact, acute degeneration can be observed within a few days at the sites of contact between the femur and tibia (Fig. 6) [84]. A limitation of this approach is that multiple days of anesthesia and loading are required in order to initiate cartilage degeneration. Despite the recent findings of Poulet et al. [82], it is also unclear if bone changes induced with this model occur by a similar mechanism to PTOA, or if they are an anabolic response to cyclic mechanical loading. In fact, the same loading protocol that can be used to produce articular cartilage degeneration and epiphyseal bone changes [80, 83, 84] can also be used for studies of anabolic bone adaptation of metaphyseal trabecular bone in mice [75–77]. Altogether, the cyclic loading model is an extremely useful non-invasive model of OA in mice because it is adjustable (i.e., severity of initiated OA can be controlled), and can initiate OA features without disrupting joint function or altering the biomechanical stability of the joint in a measurable fashion (if the peak force is below the ACL-rupture threshold). From this perspective, this model may be more representative of OA driven by chronic mechanical overuse rather than acute injury.
ACL Rupture via Tibial Compression Overload
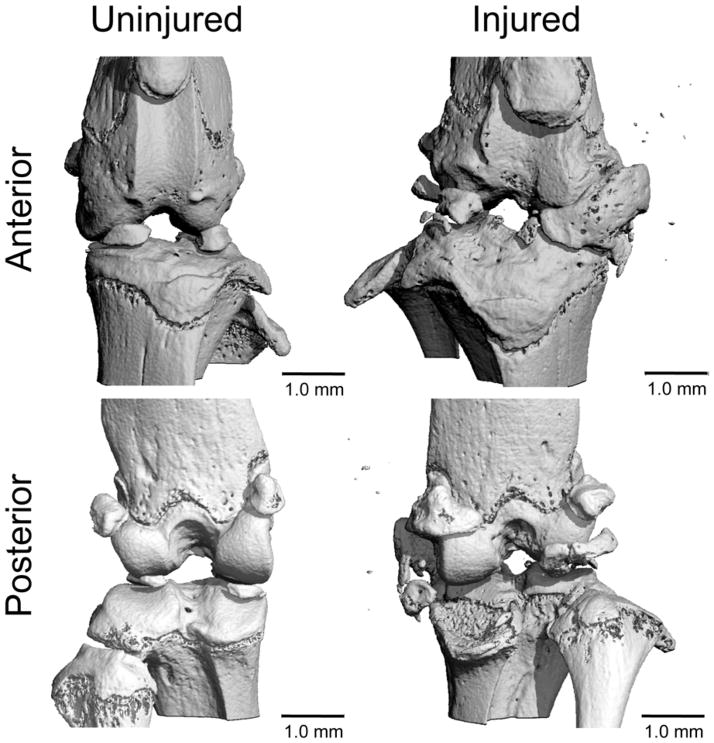
A similar tibial compression loading method, first described by Christiansen et al. [88], has been used for studies of PTOA by non-invasively creating an acute knee injury in mice by rupturing the ACL. This method is analogous to previous studies that have used external mechanical loading to non-invasively injure the ACL of rabbits [89]. A single compressive load was applied to the lower leg of C57BL/6 mice (10 weeks old at injury) using a tibial compression setup similar to those described above, to a target force of 12 N with a loading rate of 1 mm/s. Injury was noted by a release in compressive force and an audible “click” (Fig. 7). Mice were sacrificed at 1, 3, 7, 14, 28, or 56 days post-injury. MicroCT analysis revealed a dramatic loss of trabecular bone at the femoral and tibial epiphysis and tibial metaphysis by 7 days post-injury compared to contralateral knees. This bone volume deficit persisted for at least 56 days post-injury. Considerable mineralized osteophyte volume was also observed in the injured limb compared to the contralateral control by 28 and 56 days after injury. Histological results at 56 days post-injury indicated degenerative changes in the articular cartilage of injured knees, including loss of Safranin-O staining, fissuring in the cartilage surface, and chondrocyte cell death in the superficial zone.
A subsequent study using this model investigated biomechanical and structural changes in the joint following ACL rupture either with avulsion fracture or midsubstance tear [90]. This was achieved by increasing the loading rate during knee injury to decrease the likelihood of avulsion fracture [91, 92]. Joint degeneration following low-speed injury (1 mm/s; ACL rupture with avulsion) or high-speed injury (500 mm/s; ACL rupture via mid-substance tear) was quantified at 10 days, 12 and 16 weeks to determine structural changes in subchondral bone and epiphyseal trabecular bone, osteophyte formation, articular cartilage degeneration, and biomechanical stability of injured vs. uninjured knees. Knee injury with both injury modes caused considerable trabecular bone loss by 10 days post-injury, with avulsion initiating a greater amount of bone loss than midsubstance tear. Immediately after injury, both injury modes resulted in greater than twofold increases in anterior-posterior (AP) joint laxity relative to control knees. However, by 12 and 16 weeks post-injury, AP laxity was restored to uninjured control values, possibly due to knee stabilization by osteophytes. By 12 and 16 weeks post-injury both high-speed and low-speed injury resulted in severe joint degeneration and osteophyte formation (Fig. 8). A primary site of degeneration was the posterior aspect of the medial tibia, similar to previously studies using surgical ACL transection in mice [38].
Figure 8.
MicroCT images of injured and uninjured mouse knees 12 weeks after non-invasive ACL injury. Substantial osteophyte formation and joint degeneration were observed in all injured knees. In particular, osteophytes were observed on the anteriomedial aspect of the distal femur, the posteromedial aspect of the proximal tibia, and the medial meniscus. From Lockwood et al. [90]. Used with permission.
A recent study by Onur et al. [93] subjected 3-month old FVB mice to cyclical axial loads of 12 N for 240 cycles or until the ACL ruptured. One and eight weeks after this procedure, knees were evaluated histologically for OA. Consistent with Wu et al. [84], the ACL-ruptured group exhibited significantly greater joint degeneration than either control (non-loaded) joints or joints that were cyclically loaded without ACL injury at both 1 and 8 weeks. Additionally, only ACL-ruptured knees consistently showed synovitis after 1 week and osteophyte formation after 8 weeks. The authors concluded that ACL rupture consistently creates a severe osteoarthritis phenotype, while a single bout of tibial compression loading alone did not consistently create an OA phenotype in this mouse strain.
The ACL rupture model applies loads with similar methods to those used for the cyclic tibial compression model, however the early biological response is likely very different. Once the tibial compression forces are high enough to cause rupture of the ACL, the cartilage is no longer loaded in the same way as it is in the cyclic compression model. In fact, once the ACL is ruptured, the position of the tibia and femur change in relationship to each other and thus the position of joint contact changes and is reflected in two or more zones of apoptosis [84]. This joint destabilization causes considerably more synovial cell proliferation than sub-injury cyclic compression, which sets the stage for the formation of cartilaginous nodules that will eventually mineralize and become visible on microCT. The rapid and considerable synovial response following ACL rupture may reflect several contributing factors, including soft tissue injury, destabilization of the joint, increased concentrations of inflammatory cytokines, and hemarthrosis.
The tibial compression overload/ACL rupture model has several unique advantages for studies of OA development. This model closely replicates ACL injury in humans, as ACL rupture is induced with a single mechanical overload, with subsequent OA symptoms developing due to a known etiology. This method produces a highly reproducible injury, and a predictable pattern of joint degeneration. However, this model is not easily adjusted like the cyclic tibial compression model. Joint degeneration can likely be accelerated by applying additional compressive loads following ACL injury [84, 93], but producing joint degeneration of a lesser severity is difficult. Another consideration is that the observed joint degeneration is likely due in a large part to the joint instability initiated by this injury. The severe osteophyte formation observed at 8–16 weeks may also be part of the compensatory response to this joint instability [94–96], although osteophyte formation is also present with sub-injury cyclic loading in which no instability is created [80, 83, 84]. Another limitation of this model is the severity of OA symptoms at long time points after ACL injury. The instability created by the failure of the ACL results in the distal femur translating posteriorly relative to the tibial plateau, creating a new articulating surface on the posterior aspect of the proximal tibia. Over time, this new articulation will erode articular cartilage from both the posterior tibial surface and the femoral condyles, particularly at the medial aspect of the joint, with degeneration often extending into the subchondral bone. This severe degeneration can be clearly observed by 12 and 16 weeks post-injury [90], and often as early as 8 weeks post-injury [97]. For this reason, the tibial compression overload model may be more useful for studies of acute processes initiated by ACL rupture on a scale of hours or days following injury, and may not be ideal for long-term studies investigating the onset of OA symptoms on a scale of weeks or months.
Additional Considerations
The selection of an appropriate mouse model involves not only the proposed research question, but also factors such as the logistics of performing the appropriate injury. For example, the non-invasive methods described in this review typically require specialized loading equipment with custom-made attachments. Much like surgical expertise and training, these specialized systems may not be readily available in all laboratories. If a suitable system is available, preliminary studies are still required to confirm the ability to reliably create the desired injury. Subtle differences in equipment and animal position may alter the site of maximal loading and the load-to-failure of the ACL, and thus possibly the pathology observed. These considerations are particularly important when attempting to create or avoid ACL rupture (as in the cyclic loading model) during tibial compression. Some tibial compression systems induce ACL rupture at relatively low forces (8–10 N), while others apply multiple loads of relatively high magnitude compression (11–12 N) without causing ACL injury. Contributing factors may include hip angle, which can create tension in the quadriceps during hip extension (pulling the tibia anteriorly, making ACL rupture more likely) or tension in the hamstrings during flexion (pulling the tibia posteriorly, making ACL rupture less likely). The angle of extension of the ankle, along with any rotation of the lower limb also plays a role in the likelihood of inducing an ACL rupture during tibial compression. Even among groups that apply tibial compression without ACL rupture, subtle system-specific differences may exist in the loading of the joint that affect the tissue changes reported [98]. Because of these subtle differences, each team of investigators should carefully and rigorously characterize the methods used to initiate joint degeneration. The specific mechanical loading applied to the cartilage or the injury methods used may be subtley different than published results from other groups, and differences in OA development may reflect this.
The specific strain, sex, or age of mice used for studies of OA may also have a significant effect on outcomes. The majority of studies describing injury methods have used young, male, “wild-type” mice. Using mice of a different age, sex, or genetic strain will affect the ability to induce an injury, or the adaptation of the joint following the injury [13, 81]. Indeed, studies examining such interplay have already revealed mechanisms contributing to susceptibility [81] or resistance [13] to OA following non-invasive injury. Further studies may reveal the roles of joint size and shape, the inherent mechanical properties of bone, ligaments, or other musculoskeletal tissues, or the biological response to injury in OA development. Each animal phenotype should be carefully characterized, and appropriate controls should be utilized to ensure the fidelity of results obtained with these models.
Careful consideration must also be given to appropriate controls for studies of OA that utilize non-invasive injury methods. Many studies compare the injured joint to the contralateral joint as an internal control. However, systemic effects of inflammation and bone turnover exist following an injury [88, 97, 99], which may confound the results obtained when only the contralateral limb is used as a control. Therefore, each investigative team should independently perform a comparison of sham vs. contralateral controls as part of the characterization of an animal model; when appropriate, uninjured (sham) controls should be utilized. This experimental design allows a true comparison of injured vs. uninjured effects, and is necessary for investigators to determine contralateral or systemic effects of injury.
Conclusion
Non-invasive mouse models of post-traumatic osteoarthritis are a significant step forward for the study of OA mechanisms and therapies. Non-invasive models aseptically initiate joint degeneration, and are more representative of human injury conditions than surgical or invasive injury models because they use externally applied mechanical loading to directly damage bone, cartilage, or soft tissues of the joint. Non-invasive models are particularly useful for studying injury-induced biological processes at early time points following injury, which may be difficult with surgical models due to complications of the invasive injury methods. Non-invasive models are also typically faster and easier to perform than invasive or surgical injury methods, which often require special tools or training to consistently produce the desired injury. Currently, a small number of non-invasive mouse models have been described for studies of PTOA. These models vary in the methods and severity of injury, and are each translatable to specific human injuries and conditions that may lead to PTOA. These non-invasive models represent a unique and important spectrum of animal models for studying different aspects of PTOA, and may lead to novel insights into the underlying mechanisms of joint disease, although considerable work remains before results from these models will be truly translatable to human disease and treatment.
Acknowledgments
The authors would like to thank Christopher Little for providing helpful comments during drafting of this review. We would like to thank Susanna Chubinskaya and Donald Anderson for graciously allowing us to use Figure 1 for this review. We would like to acknowledge the contributions of the various members of our laboratories who have contributed to these studies over the last few years. Research described in this review was supported in part by the Department of Defense, the Arthritis Foundation, Arthritis Research UK, and National Institutes of Health grants AR48182, AR50245, AG46927, AR062603, AR063348, AR063757, and AR064034.
Footnotes
Author Contributions
All authors were fully involved in the preparation of this manuscript and approved the final version.
Competing Interest Statement
The authors have no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Blaine A. Christiansen, Email: bchristiansen@ucdavis.edu.
Farshid Guilak, Email: guilak@duke.edu.
Kevin A. Lockwood, Email: lockwoodemail@gmail.com.
Steven A. Olson, Email: steven.olson@duke.edu.
Andrew A. Pitsillides, Email: apitsillides@rvc.ac.uk.
Linda J. Sandell, Email: sandelll@wudosis.wustl.edu.
Matthew J. Silva, Email: silvam@wudosis.wustl.edu.
Marjolein C. H. van der Meulen, Email: mcv3@cornell.edu.
Dominik R. Haudenschild, Email: drhaudenschild@ucdavis.edu.
References
- 1.Arthritis Foundation. Learn About Osteoarthritis. Retrieved April 15, 2014, from Arthritis Foundation Web site: https://www.arthritis.org/conditions-treatments/disease-center/osteoarthritis/
- 2.Felson DT. Epidemiology of hip and knee osteoarthritis. Epidemiol Rev. 1988;10:1–28. doi: 10.1093/oxfordjournals.epirev.a036019. [DOI] [PubMed] [Google Scholar]
- 3.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 4.Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37(7):1434–43. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 5.Dirschl DR, Marsh JL, Buckwalter JA, Gelberman R, Olson SA, Brown TD, et al. Articular fractures. J Am Acad Orthop Surg. 2004;12(6):416–23. doi: 10.5435/00124635-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Marsh JL, Buckwalter J, Gelberman R, Dirschl D, Olson S, Brown T, et al. Articular fractures: does an anatomic reduction really change the result? J Bone Joint Surg Am. 2002;84-A(7):1259–71. [PubMed] [Google Scholar]
- 7.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739–44. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 8.Chateauvert J, Pritzker KP, Kessler MJ, Grynpas MD. Spontaneous osteoarthritis in rhesus macaques. I. Chemical and biochemical studies. J Rheumatol. 1989;16(8):1098–104. [PubMed] [Google Scholar]
- 9.Chateauvert JM, Grynpas MD, Kessler MJ, Pritzker KP. Spontaneous osteoarthritis in rhesus macaques. II. Characterization of disease and morphometric studies. J Rheumatol. 1990;17(1):73–83. [PubMed] [Google Scholar]
- 10.Das-Gupta EP, Lyons TJ, Hoyland JA, Lawton DM, Freemont AJ. New histological observations in spontaneously developing osteoarthritis in the STR/ORT mouse questioning its acceptability as a model of human osteoarthritis. Int J Exp Pathol. 1993;74(6):627–34. [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez PA, Glasson SS, Trubetskoy OV, Haimes HB. Spontaneous osteoarthritis in Dunkin Hartley guinea pigs: histologic, radiologic, and biochemical changes. Lab Anim Sci. 1997;47(6):598–601. [PubMed] [Google Scholar]
- 12.Little CB, Smith MM. Animal models of osteoarthritis. Current Rheumatology Reviews. 2008;4:175–82. [Google Scholar]
- 13.Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum. 2008;58(3):744–53. doi: 10.1002/art.23288. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald J, Rich C, Burkhardt D, Allen J, Herzka AS, Little CB. Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthritis Cartilage. 2008;16(11):1319–26. doi: 10.1016/j.joca.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Chen Q, Lanske B, Fleming BC, Terek R, Wei X, et al. Disrupting the Indian hedgehog signaling pathway in vivo attenuates surgically induced osteoarthritis progression in Col2a1-CreERT2; Ihhfl/fl mice. Arthritis Res Ther. 2014;16(1):R11. doi: 10.1186/ar4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roudier M, Li X, Niu QT, Pacheco E, Pretorius JK, Graham K, et al. Sclerostin is expressed in articular cartilage but loss or inhibition does not affect cartilage remodeling during aging or following mechanical injury. Arthritis Rheum. 2013;65(3):721–31. doi: 10.1002/art.37802. [DOI] [PubMed] [Google Scholar]
- 17.Lodewyckx L, Luyten FP, Lories RJ. Genetic deletion of low-density lipoprotein receptor-related protein 5 increases cartilage degradation in instability-induced osteoarthritis. Rheumatology (Oxford) 2012;51(11):1973–8. doi: 10.1093/rheumatology/kes178. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto S, Rai MF, Janiszak KL, Cheverud JM, Sandell LJ. Cartilage and bone changes during development of post-traumatic osteoarthritis in selected LGXSM recombinant inbred mice. Osteoarthritis Cartilage. 2012;20(6):562–71. doi: 10.1016/j.joca.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malfait AM, Ritchie J, Gil AS, Austin JS, Hartke J, Qin W, et al. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthritis Cartilage. 2010;18(4):572–80. doi: 10.1016/j.joca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010;16(1):105–15. doi: 10.1089/ten.teb.2009.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pond MJ, Nuki G. Experimentally-induced osteoarthritis in the dog. Ann Rheum Dis. 1973;32(4):387–388. doi: 10.1136/ard.32.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandt KD, Braunstein EM, Visco DM, O’Connor B, Heck D, Albrecht M. Anterior (cranial) cruciate ligament transection in the dog: a bona fide model of osteoarthritis, not merely of cartilage injury and repair. J Rheumatol. 1991;18(3):436–46. [PubMed] [Google Scholar]
- 23.Marshall KW, Chan AD. Arthroscopic anterior cruciate ligament transection induces canine osteoarthritis. J Rheumatol. 1996;23(2):338–43. [PubMed] [Google Scholar]
- 24.Guilak F, Ratcliffe A, Lane N, Rosenwasser MP, Mow VC. Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. J Orthop Res. 1994;12(4):474–84. doi: 10.1002/jor.1100120404. [DOI] [PubMed] [Google Scholar]
- 25.Palmoski MJ, Brandt KD. Immobilization of the knee prevents osteoarthritis after anterior cruciate ligament transection. Arthritis Rheum. 1982;25(10):1201–8. doi: 10.1002/art.1780251009. [DOI] [PubMed] [Google Scholar]
- 26.Palmoski MJ, Brandt KD. Proteoglycan aggregation in injured articular cartilage. A comparison of healing lacerated cartilage with osteoarthritic cartilage. J Rheumatol. 1982;9(2):189–97. [PubMed] [Google Scholar]
- 27.Hayami T, Pickarski M, Wesolowski GA, McLane J, Bone A, Destefano J, et al. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50(4):1193–206. doi: 10.1002/art.20124. [DOI] [PubMed] [Google Scholar]
- 28.Chou MC, Tsai PH, Huang GS, Lee HS, Lee CH, Lin MH, et al. Correlation between the MR T2 value at 4.7 T and relative water content in articular cartilage in experimental osteoarthritis induced by ACL transection. Osteoarthritis Cartilage. 2009;17(4):441–7. doi: 10.1016/j.joca.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Williams JM, Felten DL, Peterson RG, O’Connor BL. Effects of surgically induced instability on rat knee articular cartilage. J Anat. 1982;134(Pt 1):103–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Batiste DL, Kirkley A, Laverty S, Thain LM, Spouge AR, Holdsworth DW. Ex vivo characterization of articular cartilage and bone lesions in a rabbit ACL transection model of osteoarthritis using MRI and micro-CT. Osteoarthritis Cartilage. 2004;12(12):986–96. doi: 10.1016/j.joca.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Tiraloche G, Girard C, Chouinard L, Sampalis J, Moquin L, Ionescu M, et al. Effect of oral glucosamine on cartilage degradation in a rabbit model of osteoarthritis. Arthritis Rheum. 2005;52(4):1118–28. doi: 10.1002/art.20951. [DOI] [PubMed] [Google Scholar]
- 32.Messner K, Gillquist J, Bjornsson S, Lohmander LS. Proteoglycan fragments in rabbit joint fluid correlated to arthrosis stage. Acta Orthop Scand. 1993;64(3):312–6. doi: 10.3109/17453679308993633. [DOI] [PubMed] [Google Scholar]
- 33.Boyd SK, Muller R, Leonard T, Herzog W. Long-term periarticular bone adaptation in a feline knee injury model for post-traumatic experimental osteoarthritis. Osteoarthritis Cartilage. 2005;13(3):235–42. doi: 10.1016/j.joca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Herzog W, Adams ME, Matyas JR, Brooks JG. Hindlimb loading, morphology and biochemistry of articular cartilage in the ACL-deficient cat knee. Osteoarthritis Cartilage. 1993;1(4):243–51. doi: 10.1016/s1063-4584(05)80330-9. [DOI] [PubMed] [Google Scholar]
- 35.Teeple E, Elsaid KA, Fleming BC, Jay GD, Aslani K, Crisco JJ, et al. Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orthop Res. 2008;26(2):231–7. doi: 10.1002/jor.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jimenez PA, Harlan PM, Chavarria AE, Haimes HB. Induction of osteoarthritis in guinea pigs by transection of the anterior cruciate ligament: radiographic and histopathological changes. Inflamm Res. 1995;44(Suppl 2):S129–30. doi: 10.1007/BF01778296. [DOI] [PubMed] [Google Scholar]
- 37.Funakoshi Y, Hariu M, Tapper JE, Marchuk LL, Shrive NG, Kanaya F, et al. Periarticular ligament changes following ACL/MCL transection in an ovine stifle joint model of osteoarthritis. J Orthop Res. 2007;25(8):997–1006. doi: 10.1002/jor.20370. [DOI] [PubMed] [Google Scholar]
- 38.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9):1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005;13(7):632–41. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro F, Glimcher MJ. Induction of osteoarthrosis in the rabbit knee joint. Clin Orthop Relat Res. 1980;(147):287–95. [PubMed] [Google Scholar]
- 41.Meacock SC, Bodmer JL, Billingham ME. Experimental osteoarthritis in guinea-pigs. J Exp Pathol (Oxford) 1990;71(2):279–93. [PMC free article] [PubMed] [Google Scholar]
- 42.Armstrong SJ, Read RA, Ghosh P, Wilson DM. Moderate exercise exacerbates the osteoarthritic lesions produced in cartilage by meniscectomy: a morphological study. Osteoarthritis Cartilage. 1993;1(2):89–96. doi: 10.1016/s1063-4584(05)80023-8. [DOI] [PubMed] [Google Scholar]
- 43.Pastoureau P, Leduc S, Chomel A, De Ceuninck F. Quantitative assessment of articular cartilage and subchondral bone histology in the meniscectomized guinea pig model of osteoarthritis. Osteoarthritis Cartilage. 2003;11(6):412–23. doi: 10.1016/s1063-4584(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 44.Wancket LM, Baragi V, Bove S, Kilgore K, Korytko PJ, Guzman RE. Anatomical localization of cartilage degradation markers in a surgically induced rat osteoarthritis model. Toxicol Pathol. 2005;33(4):484–9. doi: 10.1080/01926230590965364. [DOI] [PubMed] [Google Scholar]
- 45.Karahan S, Kincaid SA, Kammermann JR, Wright JC. Evaluation of the rat stifle joint after transection of the cranial cruciate ligament and partial medial meniscectomy. Comp Med. 2001;51(6):504–12. [PubMed] [Google Scholar]
- 46.Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2005;13(7):632–41. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Jones MD, Tran CW, Li G, Maksymowych WP, Zernicke RF, Doschak MR. In vivo microfocal computed tomography and micro-magnetic resonance imaging evaluation of antiresorptive and antiinflammatory drugs as preventive treatments of osteoarthritis in the rat. Arthritis Rheum. 2010;62(9):2726–35. doi: 10.1002/art.27595. [DOI] [PubMed] [Google Scholar]
- 48.Ma HL, Blanchet TJ, Peluso D, Hopkins B, Morris EA, Glasson SS. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthritis Cartilage. 2007;15(6):695–700. doi: 10.1016/j.joca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16(6):687–93. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 50.Moodie JP, Stok KS, Muller R, Vincent TL, Shefelbine SJ. Multimodal imaging demonstrates concomitant changes in bone and cartilage after destabilisation of the medial meniscus and increased joint laxity. Osteoarthritis Cartilage. 2011;19(2):163–70. doi: 10.1016/j.joca.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Li J, Anemaet W, Diaz MA, Buchanan S, Tortorella M, Malfait AM, et al. Knockout of ADAMTS5 does not eliminate cartilage aggrecanase activity but abrogates joint fibrosis and promotes cartilage aggrecan deposition in murine osteoarthritis models. J Orthop Res. 2011;29(4):516–22. doi: 10.1002/jor.21215. [DOI] [PubMed] [Google Scholar]
- 52.Marcelon G, Cros J, Guiraud R. Activity of anti-inflammatory drugs on an experimental model of osteoarthritis. Agents Actions. 1976;6(1–3):191–4. doi: 10.1007/BF01972207. [DOI] [PubMed] [Google Scholar]
- 53.Inoue S, Glimcher MJ. The reaction of cartilage and osteophyte formation after the intraarticular injection of papain. Nihon Seikeigeka Gakkai Zasshi. 1982;56(5):415–30. [PubMed] [Google Scholar]
- 54.van der Kraan PM, Vitters EL, van de Putte LB, van den Berg WB. Development of osteoarthritic lesions in mice by “metabolic” and “mechanical” alterations in the knee joints. Am J Pathol. 1989;135(6):1001–14. [PMC free article] [PubMed] [Google Scholar]
- 55.van der Kraan PM, Vitters EL, van Beuningen HM, van de Putte LB, van den Berg WB. Degenerative knee joint lesions in mice after a single intra-articular collagenase injection. A new model of osteoarthritis. J Exp Pathol (Oxford) 1990;71(1):19–31. [PMC free article] [PubMed] [Google Scholar]
- 56.Borella L, Eng CP, DiJoseph J, Wells C, Ward J, Caccese R, et al. Rapid induction of early osteoarthritic-like lesions in the rabbit knee by continuous intra-articular infusion of mammalian collagenase or interleukin-1. Agents Actions. 1991;34(1–2):220–2. doi: 10.1007/BF01993285. [DOI] [PubMed] [Google Scholar]
- 57.van Osch GJ, van der Kraan PM, Vitters EL, Blankevoort L, van den Berg WB. Induction of osteoarthritis by intra-articular injection of collagenase in mice. Strain and sex related differences. Osteoarthritis Cartilage. 1993;1(3):171–7. doi: 10.1016/s1063-4584(05)80088-3. [DOI] [PubMed] [Google Scholar]
- 58.Hui W, Rowan AD, Richards CD, Cawston TE. Oncostatin M in combination with tumor necrosis factor alpha induces cartilage damage and matrix metalloproteinase expression in vitro and in vivo. Arthritis Rheum. 2003;48(12):3404–18. doi: 10.1002/art.11333. [DOI] [PubMed] [Google Scholar]
- 59.Malfait AM, Tortorella M, Thompson J, Hills R, Meyer DM, Jaffee BD, et al. Intra-articular injection of tumor necrosis factor-alpha in the rat: an acute and reversible in vivo model of cartilage proteoglycan degradation. Osteoarthritis Cartilage. 2009;17(5):627–35. doi: 10.1016/j.joca.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 60.van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest. 1994;71(2):279–90. [PubMed] [Google Scholar]
- 61.van Beuningen HM, Glansbeek HL, van der Kraan PM, van den Berg WB. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-beta injections. Osteoarthritis Cartilage. 2000;8(1):25–33. doi: 10.1053/joca.1999.0267. [DOI] [PubMed] [Google Scholar]
- 62.van Beuningen HM, Arntz OJ, van den Berg WB. In vivo effects of interleukin-1 on articular cartilage. Prolongation of proteoglycan metabolic disturbances in old mice. Arthritis Rheum. 1991;34(5):606–15. doi: 10.1002/art.1780340513. [DOI] [PubMed] [Google Scholar]
- 63.Dunham J, Hoedt-Schmidt S, Kalbhen DA. Prolonged effect of iodoacetate on articular cartilage and its modification by an anti-rheumatic drug. Int J Exp Pathol. 1993;74(3):283–9. [PMC free article] [PubMed] [Google Scholar]
- 64.van Osch GJ, van der Kraan PM, van den Berg WB. Site-specific cartilage changes in murine degenerative knee joint disease induced by iodoacetate and collagenase. J Orthop Res. 1994;12(2):168–75. doi: 10.1002/jor.1100120204. [DOI] [PubMed] [Google Scholar]
- 65.Guingamp C, Gegout-Pottie P, Philippe L, Terlain B, Netter P, Gillet P. Mono-iodoacetate-induced experimental osteoarthritis: a dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 1997;40(9):1670–9. doi: 10.1002/art.1780400917. [DOI] [PubMed] [Google Scholar]
- 66.Frankl U, Pogrund H, Yosipovitch Z. The effect of intra-articular colchicine on the knee joint of the rat. Clin Orthop Relat Res. 1983;(178):270–5. [PubMed] [Google Scholar]
- 67.Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res. 2007;25(5):578–92. doi: 10.1002/jor.20331. [DOI] [PubMed] [Google Scholar]
- 68.Lewis JS, Hembree WC, Furman BD, Tippets L, Cattel D, Huebner JL, et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthritis Cartilage. 2011;19(7):864–73. doi: 10.1016/j.joca.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewis JS, Jr, Furman BD, Zeitler E, Huebner JL, Kraus VB, Guilak F, et al. Genetic and cellular evidence of decreased inflammation associated with reduced incidence of posttraumatic arthritis in MRL/MpJ mice. Arthritis Rheum. 2013;65(3):660–70. doi: 10.1002/art.37796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seifer DR, Furman BD, Guilak F, Olson SA, Brooks SC, 3rd, Kraus VB. Novel synovial fluid recovery method allows for quantification of a marker of arthritis in mice. Osteoarthritis Cartilage. 2008;16(12):1532–8. doi: 10.1016/j.joca.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Louer CR, Furman BD, Huebner JL, Kraus VB, Olson SA, Guilak F. Diet-induced obesity significantly increases the severity of posttraumatic arthritis in mice. Arthritis Rheum. 2012;64(10):3220–30. doi: 10.1002/art.34533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diekman BO, Wu CL, Louer CR, Furman BD, Huebner JL, Kraus VB, et al. Intra-articular delivery of purified mesenchymal stem cells from C57BL/6 or MRL/MpJ superhealer mice prevents posttraumatic arthritis. Cell Transplant. 2013;22(8):1395–408. doi: 10.3727/096368912X653264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kimmerling KA, Furman BD, Mangiapani DS, Moverman MA, Sinclair SM, Huebner JL, et al. Sustained intra-articular delivery of IL-1Ra from a thermally-responsive elastin-like polypeptide as a therapy for post- traumatic arthritis. Eur Cells Mater. 2015 doi: 10.22203/ecm.v029a10. p. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Furman BD, Mangiapani DS, Zeitler E, Bailey KN, Horne PH, Huebner JL, et al. Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res Ther. 2014;16(3):R134. doi: 10.1186/ar4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Souza RL, Matsuura M, Eckstein F, Rawlinson SC, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37(6):810–8. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 76.Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared to young-adult mice following 1 week of axial tibial compression. J Bone Miner Res. 2010 doi: 10.1002/jbmr.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lynch ME, Main RP, Xu Q, Walsh DJ, Schaffler MB, Wright TM, et al. Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. J Appl Physiol. 2010;109(3):685–91. doi: 10.1152/japplphysiol.00210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zaman G, Jessop HL, Muzylak M, De Souza RL, Pitsillides AA, Price JS, et al. Osteocytes use estrogen receptor alpha to respond to strain but their ERalpha content is regulated by estrogen. J Bone Miner Res. 2006;21(8):1297–306. doi: 10.1359/jbmr.060504. [DOI] [PubMed] [Google Scholar]
- 79.De Souza RL, Pitsillides AA, Lanyon LE, Skerry TM, Chenu C. Sympathetic nervous system does not mediate the load-induced cortical new bone formation. J Bone Miner Res. 2005;20(12):2159–68. doi: 10.1359/JBMR.050812. [DOI] [PubMed] [Google Scholar]
- 80.Poulet B, Hamilton RW, Shefelbine S, Pitsillides AA. Characterising a novel and adjustable non-invasive murine knee joint loading model. Arthritis Rheum. 2011;63(1):137–47. doi: 10.1002/art.27765. [DOI] [PubMed] [Google Scholar]
- 81.Poulet B, Westerhof TA, Hamilton RW, Shefelbine SJ, Pitsillides AA. Spontaneous osteoarthritis in Str/ort mice is unlikely due to greater vulnerability to mechanical trauma. Osteoarthritis Cartilage. 2013;21(5):756–63. doi: 10.1016/j.joca.2013.02.652. [DOI] [PubMed] [Google Scholar]
- 82.Poulet B, de Souza R, Kent AV, Saxon L, Barker O, Wilson A, et al. Intermittent applied mechanical loading induces subchondral bone thickening that may be intensified locally by contiguous articular cartilage lesions. Osteoarthritis Cartilage. 2015 doi: 10.1016/j.joca.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ko FC, Dragomir C, Plumb DA, Goldring SR, Wright TM, Goldring MB, et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 2013;65(6):1569–78. doi: 10.1002/art.37906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu P, Holguin N, Silva MJ, Fu M, Liao W, Sandell LJ. Early response of mouse joint tissues to noninvasive knee injury suggests treatment targets. Arthritis Rheumatol. 2014 doi: 10.1002/art.38375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu P, DeLassus E, Patra D, Liao W, Sandell LJ. Effects of serum and compressive loading on the cartilage matrix synthesis and spatiotemporal deposition around chondrocytes in 3D culture. Tissue Eng Part A. 2013;19(9–10):1199–208. doi: 10.1089/ten.tea.2012.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong M, Siegrist M, Cao X. Cyclic compression of articular cartilage explants is associated with progressive consolidation and altered expression pattern of extracellular matrix proteins. Matrix Biol. 1999;18(4):391–9. doi: 10.1016/s0945-053x(99)00029-3. [DOI] [PubMed] [Google Scholar]
- 87.Carames B, Taniguchi N, Seino D, Blanco FJ, D’Lima D, Lotz M. Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection. Arthritis Rheum. 2012;64(4):1182–92. doi: 10.1002/art.33444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JH, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2012;20(7):773–82. doi: 10.1016/j.joca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 89.Killian ML, Isaac DI, Haut RC, Dejardin LM, Leetun D, Donahue TL. Traumatic anterior cruciate ligament tear and its implications on meniscal degradation: a preliminary novel lapine osteoarthritis model. J Surg Res. 2010;164(2):234–41. doi: 10.1016/j.jss.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 90.Lockwood KA, Chu BT, Anderson MJ, Haudenschild DR, Christiansen BA. Comparison of loading rate-dependent injury modes in a murine model of post-traumatic osteoarthritis. J Orthop Res. 2013;32(1):79–88. doi: 10.1002/jor.22480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crowninshield RD, Pope MH. The strength and failure characteristics of rat medial collateral ligaments. J Trauma. 1976;16(2):99–105. doi: 10.1097/00005373-197602000-00004. [DOI] [PubMed] [Google Scholar]
- 92.Noyes FR, DeLucas JL, Torvik PJ. Biomechanics of anterior cruciate ligament failure: an analysis of strain-rate sensitivity and mechanisms of failure in primates. J Bone Joint Surg Am. 1974;56(2):236–53. [PubMed] [Google Scholar]
- 93.Onur TS, Wu R, Chu S, Chang W, Kim HT, Dang AB. Joint instability and cartilage compression in a mouse model of posttraumatic osteoarthritis. J Orthop Res. 2014;32(2):318–23. doi: 10.1002/jor.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Osch GJ, Blankevoort L, van der Kraan PM, Janssen B, Hekman E, Huiskes R, et al. Laxity characteristics of normal and pathological murine knee joints in vitro. J Orthop Res. 1995;13(5):783–91. doi: 10.1002/jor.1100130519. [DOI] [PubMed] [Google Scholar]
- 95.Pottenger LA, Phillips FM, Draganich LF. The effect of marginal osteophytes on reduction of varus-valgus instability in osteoarthritic knees. Arthritis Rheum. 1990;33(6):853–8. doi: 10.1002/art.1780330612. [DOI] [PubMed] [Google Scholar]
- 96.Suzuki T, Motojima S, Saito S, Ishii T, Ryu K, Ryu J, et al. Osteoarthritis of the patella, lateral femoral condyle and posterior medial femoral condyle correlate with range of motion. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2584–9. doi: 10.1007/s00167-013-2508-x. [DOI] [PubMed] [Google Scholar]
- 97.Satkunananthan PB, Anderson MJ, De Jesus NM, Haudenschild DR, Ripplinger CM, Christiansen BA. In vivo fluorescence reflectance imaging of protease activity in a mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2014;22(10):1461–9. doi: 10.1016/j.joca.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Holguin N, Brodt MD, Sanchez ME, Kotiya AA, Silva MJ. Adaptation of tibial structure and strength to axial compression depends on loading history in both C57BL/6 and BALB/c mice. Calcif Tissue Int. 2013;93(3):211–21. doi: 10.1007/s00223-013-9744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Christiansen B, Emami A, Fyhrie D, Satkunananthan PB, Hardisty M. Trabecular bone loss at a distant skeletal site following non-invasive knee injury in mice. J Biomech Eng. 2014 doi: 10.1115/1.4028824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802–9. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]