Abstract
The thalamo-cortical resting state functional connectivity of 7 sub-thalamic regions were examined in a prospectively recruited population of 77 acute mild TBI (mTBI) patients within the first 10 days (mean 6±3 days) of injury and 35 neurologically intact control subjects using the Oxford thalamic connectivity atlas. Neuropsychological assessments were conducted using the Automated Neuropsychological Assessment Metrics (ANAM). A subset of participants received a magentic resonance spectroscopy (MRS) exam to determine metabolite concentrations in the thalamus and posterior cingulate cortex. Results show that patients performed worse than the control group on various subtests of ANAM and the weighted throughput score, suggesting reduced cognitive performance at this early stage of injury. Both voxel and region of interest based analysis of the resting state fMRI data demonstrated that acute mTBI patients have increased functional connectivity between the various sub-thalamic regions and cortical regions associated with sensory processing and the default mode network (DMN). In addition, a significant reduction in NAA/Cr was observed in the thalamus in the mTBI patients. Furthermore, an increase in Cho/Cr ratio specific to mTBI patients with self-reported sensory symptoms was observed compared to those without self-reported sensory symptoms. These results provide novel insights into the neural mechanisms of the brain state related to internal rumination and arousal, which have implications for new interventions for mTBI patients with persistent symptoms. Furthermore, an understanding of heightened sensitivity to sensory related inputs during early stages of injury may facilitate enhanced prediction of safe return to work.
Keywords: mild traumatic brain injury, resting state functional connectivity, thalamus, H1 magnetic resonance spectroscopy
Introduction
Traumatic Brain Injury (TBI) is one of the most common neurological injuries in the United States, with an estimated 1.7 million Americans experiencing a TBI each year(Faul et al., 2010), with the greater part of these cases (75%) being diagnosed as “mild injuries” (Centers for Disease Control and Prevention, 2003). In contrast to this diagnosis, many of these “mild” patients suffer from a variety of post concussive symptoms in spite of a lack of diagnosed injury based on CT or conventional MR imaging (Iverson et al., 2000). These post concussive symptoms encompass a wide array of symptoms such as cognitive, somatic, sensory, and neurobehavioral symptoms (Halbauer et al., 2009). Physical symptoms are often the most prevalent immediately following injury while cognitive and neurobehavioral symptoms tend to increase with time post injury(Dischinger et al., 2009). This progression of cognitive and neurobehavioral symptoms is likely influenced by a complex interplay between structural and functional damage as well as numerous factors such as such reduced cognitive capacity, somatic symptoms like chronic headaches, altered quality of life due to factors surrounding the incident, post traumatic stress disorder (PTSD) (Hoge et al., 2008), and comorbid disorders including depression and substance abuse. However, the acute physical concussive symptoms may be less influenced by environmental factors and be more directly associated with primary damage to structural and functional connections within various neural networks that support sensory processing. While advances in neuroimaging techniques such as diffusion tensor imaging (DTI), susceptibility weighted imaging (SWI) (Haacke et al., 2004), H1 Magnetic Resonance Spectroscopy (H1-MRS), and resting state functional MRI (fMRI) (Biswal et al., 1995) have had great success in recent years characterizing the subtle damage induced by mTBI, the precise neurobiological basis of these acute symptoms remains elusive to clinicians and researchers.
TBI often involves sudden acceleration-deceleration forces on the brain tissue, which results in a combination of linear, rotational, and angular shearing injuries caused by axonal stretching, which is commonly referred to as diffuse axonal injury (DAI) (Ommaya et al., 2002; Povlishock et al., 1983). Consistent with the diffuse nature of the TBI, DTI has demonstrated wide spread white matter abnormalities suggesting reduced axonal structural integrity and Wallerian degeneration in the corpus callosum (Bazarian et al., 2007; Kumar et al., 2009; Mayer et al., 2010; Warner et al., 2010a), internal capsule (Arfanakis et al., 2002; Bazarian et al., 2007), the superior and inferior longitudinal fasciculus, corona radiata (Messe et al., 2011; Yuh et al., 2013), and cingulum bundles (Mac Donald et al., 2011). This diffuse and heterogenous structural damage likely alters functional communication throughout the neurocircuitry of numerous networks thereby altering regional and global cellular metabolism.
The thalamus is centrally located in the brain and has abundant ascending and descending white matter projections to the multiple cortical regions, and plays an essential role in sensory processing. Therefore, the thalamus acts as the brain’s relay center for the transmission of sensory information. While generally there are no overt lesions within the thalamus following TBI, studies investigating severe TBI populations have consistently reported altered structural properties of the thalamus in the chronic stages of injury including localized atrophy (Warner et al., 2010b), reduced FA (Little et al., 2010), and the association of reduced thalamo-cortical fiber density with unfavorable outcome (Laouchedi et al., 2014). Furthermore, a recent PET study demonstrated increased inflammation in thalamus long after severe TBI regardless of the location of the initial focal injury (Ramlackhansingh et al., 2011). These findings suggest that the thalamus may be especially susceptible to the progressive secondary injury mechanisms induced by TBI. In addition, studies have noted subtle functional damage to the thalamus including reduced thalamic cerebral perfusion in mTBI (Ge et al., 2009) and pediatric mTBI (Bartnik-Olson et al., 2014), reduced BOLD activation of the thalamus during auditory orienting task (Mayer et al., 2009), and altered diffusion parameters in the thalamus of chronic mTBI patients (Grossman et al., 2010; Grossman et al., 2012). These prior findings imply that the thalamus may also act as a centralized location of structural and functional damage from milder forms of trauma, thereby providing a convincing argument for further research investigating the thalamus immediately following mTBI using non-invasive neuroimaging techniques.
The aim of this study is to investigate acute alterations in thalamic resting state functional connectivity and metabolite profiles with the goal of determining the neurobiological mechanism for acute post concussive symptoms. To this end, we predict that in the acute stages following civilian mTBI that there will be functional alterations in the thalamo-cortical connectivity of multiple sub-thalamic regions as measured by resting state functional MRI as well as reduced metabolic integrity of the thalamus as measured by H1-MRS. Furthermore, we anticipate that those patients with greater alterations in functional connectivity and metabolic integrity will have more severe acute post concussive symptoms.
Materials and Methods
Participants
Seventy-seven mTBI patients (44.0±17.0yrs, 59M/18F) were prospectively recruited from the Adam Cowley Shock Trauma Center at the University of Maryland Medical Center as part of a larger protocol using a combination of advanced MR imaging and neuropsychological assessments. Data from a subset of these patients was included in a previous publication (Sours et al., 2014). Thirty-five neurologically intact subjects (37.2±17.3yrs, 19M/16F) served as the control population. All participants were over the age of 18. Control and mTBI participants were excluded for history of neurological or psychiatric disorder, history of stroke, seizures, brain tumors, or previous brain injury requiring hospitalization or medical attention. Patients were classified into the mTBI category if they had an admission Glasgow Coma Scale (GCS) of 13-15 and a mechanism of injury consistent with trauma. In addition, mTBI patients were included based on one of two sets of criteria: (1) positive head CT or (2) loss of consciousness and/or amnesia and evidence of facial trauma consistent with TBI. Based on the inclusion criteria, this study included patients classified as complicated mTBI (positive head CT) and uncomplicated mTBI (negative head CT). Mechanisms of injury included falls, motor vehicle accidents, motor cycle accidents, accidental hits with blunt objects, assaults, bicycle accidents and sports accidents. The average GCS of the participants was 14.9±0.5. Twenty-five out of the 77 mTBI patients (32%) had evidence of injury based on clinical CT and 29 out of the 77 mTBI patients (38%) had evidence of injury based on CT or conventional MR imaging (including T1, T2, FLAIR, and SWI). See Table 1 for group demographics and Supplemental Table 1 for specific information on injury.
Table 1.
Demographics
| Resting State fMRI | MRS | |||||
|---|---|---|---|---|---|---|
| Control | Acute mTBI | p-value | Control | Acute mTBI | p-value | |
| N | 35 | 77 | NA | 28 | 47 | NA |
| Age | 37.2 ± 17.3 | 44.0 ±17.0 | 0.056 | 38.6 ± 17.5 | 42.7 ± 17.3 | 0.320 |
| Education | 15 ± 2 | 14 ± 3 | 0.018 | 15 ± 2 | 14 ± 3 | 0.053 |
| Gender | 19M/16F | 59M/18F | 0.026# | 17M/11F | 37M/10F | 0.093# |
| Admit GCS | NA | 14.9 ± 0.5 | NA | NA | 14.9 ± 0.3 | NA |
| Days Post Injury | NA | 6 ± 3 | NA | NA | 6 ± 3 | NA |
| Positive Head CT | NA | 25 | NA | NA | 14 | NA |
| Positive Head MR | NA | 29 | NA | NA | 16 | NA |
All 77 mTBI patients participated in the rs-fMRI at the acute stage within 10 days post-injury (mean 6±3 days). Control participants completed one session consisting of rs-fMRI. A subset of these mTBI patients (n=47) and HCs (n=28) received an MRS exam. In addition, a subset of mTBI patients (n=49) and control participants (n=34) completed neurological assessments using the Automated Neuropsychological Assessment Metrics (ANAM), a computerized cognitive assessment.
Neuropsychological Assessment
Level of cognitive functioning was assessed using the Mini Mental State Exam (MMSE) (Folstein MF, Folstein SE, McHugh PR., 1975) and Military Acute Concussion Evaluation (MACE) (McCrea et al., 2000). The level of post concussive symptoms was determined by the scores obtained on the Modified Rivermead Post-Concussion Symptoms Questionnaire (RPQ) during the acute stage. The RPQ asks participants to rate a series of common symptoms following TBI on a Likert scale of 0-4 (King et al., 1995). The total score on the RPQ was determined and used as an overall measure of self-reported symptoms. In addition, the symptoms were divided into four broad classes (cognitive, somatic, sensory, and neurobehavioral symptoms (Halbauer et al., 2009)) and the total score within each class reported. In addition, participants completed the Automated Neuropsychological Assessment Metrics (ANAM). The selected ANAM battery includes seven subtests assessing various aspects of attention, memory, and processing speed (Kane et al., 2007). See Table 2 for list of subtests, abbreviations, and cognitive domains that assesses. For each subtest, a throughput score was calculated which is a combined measure of accuracy and reaction time. Finally, a weighted throughput (WT-TH) (also known as the Index of Cognitive Efficiency) was computed, which is a weighted summary of the throughput scores from each of the seven subtests so that each subtest contributes equally to the WT-TH (Reich et al., 2005). Please see (Sours et al., 2014) for a complete description of the WT-TH.
Table 2.
Automated Neuropsychological Assessment Metrics (ANAM) Subtest Descriptions
| Subtest | Abbreviation | Cognitive Domains |
|---|---|---|
| Simple Reaction Time | SRT | Processing speed |
| Code Substitution | CS | Visual search, sustained attention, working memory, processing speed |
| Procedural Reaction Time | PRT | Processing speed |
| Math | MATH | Computational skills, concentration, working memory |
| Match to Sample | MTS | Spatial processing, visuospatial working memory |
| Code Substitution Delayed | CSD | Sustained attention, working memory, short term memory and learning |
| Simple Reaction Time Repeat | SRT2 | Cognitive fatigue, processing speed |
MR Imaging
MR Image Acquisition
Imaging was performed using a 3T Siemens Tim Trio Scanner (Siemens Medical Solutions; Erlangen, Germany) using a 12-channel receive-only head coil. A high resolution axial T1-weighted-MPRAGE (TE = 3.44 ms, TR = 2250ms, TI = 900ms, flip angle = 9°, resolution = 256 × 256 × 96, FOV = 220 mm, sl. Thick. = 1.5 mm) aligned along the AC-PC line was acquired for anatomic reference. Resting state fMRI was obtained using a T2*-weighted single-shot EPI sequence (TE = 30 ms, TR =2000 ms, FOV = 220 mm, resolution = 64 × 64) with 36 axial AC-PC aligned slices (sl. thick. = 4 mm) over 5 min 42 s that yielded 171 volumes.
MRS was acquired using 3D phase-encoded point-resolved spectroscopy (3D-PRESS) magnetic resonance spectroscopic imaging (MRSI) sequence (TE/TR = 135/1300ms; FOV = 160×160×106mm3; VOI = 106×106×48mm3; acquired resolution = 12×12×8; interpolated resolution = 16×16×8; total acquisition time = 7min 40sec). The VOI was axially prescribed along with AC-PC alignment and centered at the mid-brain at the level of the corpus callosum.
Rs-fMRI Processing
Preprocessing of the imaging data was performed in SPM 8 (http://www.fil.ion.ucl.ac.uk/spm) and included motion correction of the time series, slice timing correction, band pass filtering (.009Hz < f < .08Hz), and registration of all the 171 volumes to the first volume of the time series. The resting state series were spatially normalized to standard space using the Montreal Neurological Institute (MNI) template available in SPM 8. Spatial smoothing was then applied to the resting state data using a 5mm Gaussian kernel. Individual T1-MPRAGE images in MNI space were segmented into white matter (WM), gray matter (GM) and cerebral spinal fluid (CSF). The segmented masks thus created were used to account for time series variance from the non-neuronal contributions of CSF and WM.
The CONN-fMRI Functional Connectivity toolbox v13.h (http://www.nitric.org/projects/conn) was used to process the resting state data (Whitfield-Gabrieli and A. Nieto-Castanon, 2012). The mean Blood Oxygen Level Dependent (BOLD) signal time series from the WM mask, CSF mask, and the 6 motion correction parameters were included in the model as regressors to remove the variance related to non-neuronal contributions and motion.
Seed based resting state functional connectivity analysis was completed using the regions of interest (ROIs) from the 7 sub-thalamic regions described Oxford thalamic connectivity atlas (Behrens et al., 2003) which is based on probabilistic tractography from DTI data. These sub-thalamic ROIs include those regions with projections to primary motor (M1), primary sensory (S1), occipital lobe (OCC), premotor cortex (PM), prefrontal cortex (PFC), posterior parietal cortex (PP) and temporal lobe (TEMP). For each sub-thalamic ROI, voxel-wise group functional connectivity maps were created for the mTBI and HC groups and thresholded at voxel-wise p < 0.001 (uncorrected) and family wise error (FWE) corrected cluster extent threshold p <0.05. In addition, for each sub-thalamic ROI a between groups contrast assessing voxel-wise differences in resting state functional connectivity maps between the mTBI and HC groups was determined and thresholded at voxel-wise p < 0.001 (uncorrected) and cluster extent threshold p <0.05 (FWE corrected).
Based on preliminary voxel-wise results, we determined the resting state functional connectivity between the sub-thalamic regions and specific cortical areas. Two sets of correlation matrices were created using the 7 sub-thalamic ROIs and cortical regions associated with (1) primary sensory areas and (2) the default mode network (DMN). The cortical regions associated with the primary sensory areas include bilateral Broadman’s Areas (BA) 1, 2, 3, 4, 6, 17, 41, 42, 46 and the cortical areas associated with the DMN include 10 mm spherical ROIs in the posterior cingulate cortex (PCC), medial prefrontal cortex (MPFC), left lateral parietal lobe (LLP) and right lateral parietal lobe (RPL). Please see CONN toolbox handbook for specific information regarding these cortical ROIs. In addition to determining pairwise correlations with sub-thalamic ROIs, strength of connectivity with each sub-thalamic ROI was determined. The strength of ROI i was defined as the mean value of ith column of the coherence matrix (Lynall et al., 2010), and represents a measure of the average connectivity of the sub-thalamic ROI with the either the primary sensory ROIs (thalamo-cortical connectivity) or the DMN ROIs (thalamo-DMN connectivity).
MRS Processing
Quantification of MRS data was performed offline using LCModel (Provencher, 2001). Spectral quantification in LCModel was achieved by fitting a model that includes line shape functions, baseline functions, phase correction parameters and chemical shift referencing to the measured in vivo spectra, while utilizing prior information from an in vitro metabolite basis set. In addition, the software generates a Cramer-Rao (CR) bounds value which is indicative of the lower limit of the statistical error realized from the spectral fit. Only metabolite measurements with CR values of below 15% were considered for further analysis in this study. Given the focus on the thalamus, for this study only the voxels that completely overlapped with the thalamus (right and left) were selected for analysis. Metabolite ratios for NAcetylaspartic acid/Creatine (NAA/Cr) and Choline/Creatine (Cho/Cr) were quantified for each voxel and then averaged for each participant. In addition, a single voxel in the posterior cingulate cortex (PCC) was selected and metabolite ratios for the NAA/Cr and Cho/Cr ratios were quantified.
Statistical Analysis
To determine group differences in demographics independent t-tests were used with the exception of differences in gender which was determined using Fisher’s exact test. Due to the large influence of age on cognitive performance, difference in performance on the ANAM between mTBI patients and controls were determined for each subtest using an Analysis of Covariance (ANCOVA) including age as a covariate. Furthermore, since age likely will have a large influence on imaging measures (both metabolite concentrations and strength of resting state functional connectivity), differences in imaging measures between mTBI patients and controls were determined using an ANCOVA including age as a covariate. Results are presented uncorrected for multiple comparisons.
Results
Participants
Participant demographics are provided in Table 1. For the participants included in the resting state analysis, no differences were noted in age between the mTBI patients and control subjects (p=0.056) but significant differences in education (p=0.018) and gender (p=0.026) were noted with mTBI patients having a higher percentage of males and lower education. For the participants included in the MRS analysis, no differences were noted in age (p=0.320), education (p=0.053), or gender (p=0.093).
Neuropsychological Assessment
For the subset of participants who completed the neuropsychological assessment, we observed a trend in reduced scores on the MMSE (F=3.526, p=0.064) suggesting a subtle reduction in level of awareness (Table 3). MTBI patients had a reduced performance on the overall score of the MACE compared to the control group (F=6.928, p=0.010). Acute mTBI patients performed significantly worse than the control group on multiple subtests of the ANAM include the CSD (F=5.201, p=0.025), PRT (F=4.855, p=0.030), and the overall WT-TH (F=6.623, p=0.012). Trends in reduced performance on the MATH (F=2.937, p=0.090) and MTS (F=3.465, p=0.066) subtests were also observed. No differences in performance were noted for the CS (F=1.943, p=0.167), SRT (F=1.145, p=0.288) or SRT2 (F=0.599, p=0.441) subtests suggesting that various cognitive domains are affected differently by mTBI (Table 3). The results of the RPQ for mTBI patients’ self-reported symptoms are shown in Table 4. Overall, 89% of mTBI patients are experiencing symptoms with the greatest percentage of patients experiencing sensory symptoms (79%), followed by neurobehavioral symptoms (73%), somatic symptoms (70%), and finally cognitive symptoms (55%).
Table 3.
Comparison of Neuropsychological Assessment between patients and control subjects
| Control | Acute mTBI | F | P value | |
|---|---|---|---|---|
| Neuropsychological Assessment | ||||
| n | 34 | 53 | ||
|
Mini-mental state exam
(MMSE) |
29.7±0.7 | 29.2±1.2 | 3.526 | 0.064 |
|
Military Acute Concussion
Evaluation (MACE) |
27.2 ±1.8 | 25.5±2.9 | 6.928 | 0.010* |
| Automated Neuropsychological Assessment Metrics (ANAM) | ||||
| n | 34 | 49 | ||
| CS | 51.5 ±14.9 | 43.9 ± 13.8 | 1.943 | 0.167 |
| CSD | 44.2 ± 19.5 | 33.1 ±13.7 | 5.201 | 0.025* |
| MTS | 33.8 ±11.6 | 27.4 ± 10.9 | 3.465 | 0.066 |
| MATH | 23.5 ± 8.0 | 20.2 ± 7.0 | 2.937 | 0.090 |
| PRT | 100.1 ± 16.8 | 89.0 ±16.8 | 4.855 | 0.030* |
| SRT | 220.0 ± 43.2 | 200.5 ±52.0 | 1.145 | 0.288 |
| SRT2 | 220.4 ± 39.6 | 203.9 ±45.6 | 0.599 | 0.441 |
| WT-TH | 217.7 ± 46.0 | 186.1 ± 40.7 | 6.623 | 0.012* |
Table 4.
Symptoms Rating on the Rivermead Post-Concussive Symptoms Questionnaire (RPQ)
| RPQ (N=56) | Total Score | % Experiencing |
|---|---|---|
| RPQ Total | 16.5 ± 17.4 | 89% |
| RPQ Cognitive | 2.8 ± 3.7 | 55% |
| RPQ Somatic | 5.7 ± 6.3 | 70% |
| RPQ Sensory | 2.7 ± 3.9 | 79% |
| RPQ Neurobehavioral | 4.6 ± 5.0 | 73% |
Rs-FMRI
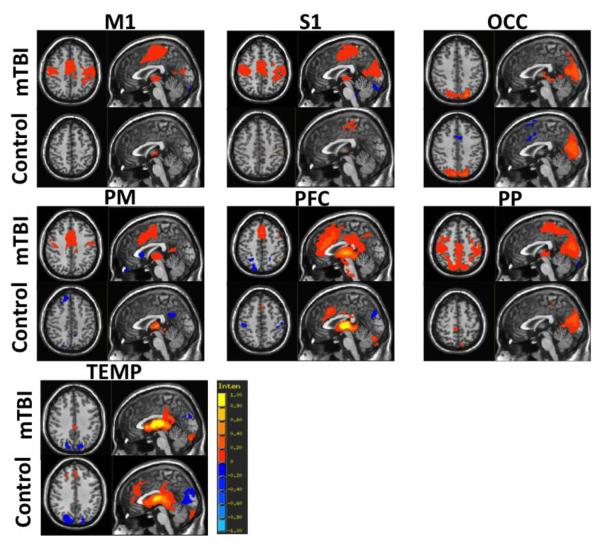
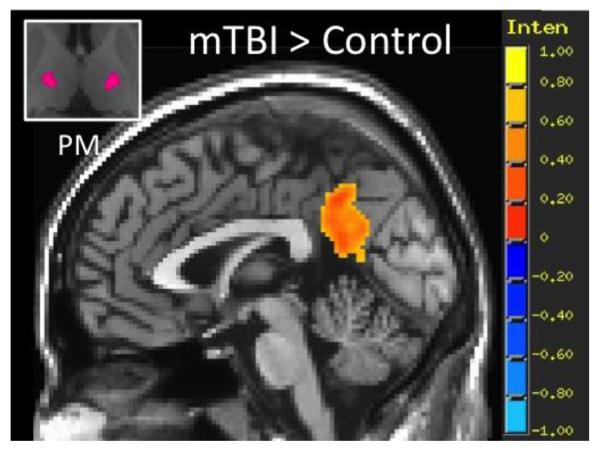
Individual group functional connectivity maps are shown for the acute mTBI and control groups for the 7 sub-thalamic seed regions in Figure 1. Consistent with the thalamic connectivity atlas defined through probabilistic tractography (Behrens et al., 2003), results show the expected thalamo-cortical connectivity for each sub-thalamic ROI. Across the 7 ROIs, mTBI patients demonstrated increased spatial extent of functional connectivity between the thalamus and cortical areas compared to the control group. Group contrasts show that compared to the control group, mTBI patients have statistically increased functional connectivity between the PM sub-thalamic ROI and the posterior cingulate cortex (Figure 2), as well as increased functional connectivity between the PFC sub-thalamic ROI and dorsal anterior cingulate cortex and bilateral medial temporal lobe (Figure 3).
Figure 1.
Results of seed based resting state functional connectivity (rs-FC) maps using the regions of interest (ROIs) from the 7 sub-thalamic regions identified using the Oxford thalamic connectivity Atlas for the control (n=35) and acute mTBI (n=77) groups. Sub-thalamic ROIs include primary motor (M1), primary sensory (S1), occipital lobe (OCC), premotor cortex (PM), prefrontal cortex (PFC), posterior parietal cortex (PP) and temporal lobe (TEMP). Group rs-FC maps are thresholded at voxel-wise p < 0.001 (uncorrected) and cluster extent threshold p <0.05 (FWE corrected).
Figure 2.
Results of seed based resting state functional connectivity (rs-FC) analysis for the premotor (PM) sub-thalamic ROI showing regions of greater rs-FC in the acute mTBI group (n=77) compared to the control group (n=35). Group rs-FC map thresholded at voxel-wise p < 0.001 (uncorrected) and cluster extent threshold p <0.05 (FWE corrected).
Figure 3.
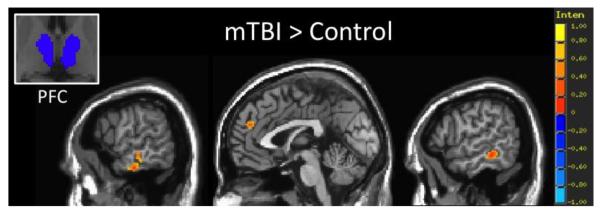
Results of seed based resting state functional connectivity (rs-FC) analysis for the prefrontal cortex (PFC) sub-thalamic ROI showing regions of greater rs-FC in the acute mTBI group (n=77) compared to the control group (n=35). Group rs-FC map thresholded at voxel-wise p < 0.001 (uncorrected) and cluster extent threshold p <0.05 (FWE corrected).
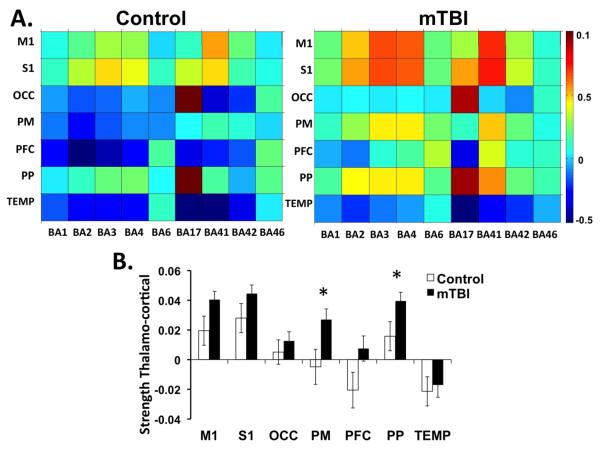
Pair-wise connectivity between sub-thalamic ROIs and cortical areas associated with primary sensory processing are shown in Figure 4A. Overall, mTBI patients have greater thalamo-cortical connectivity compared to the control group across the 7 sub-thalamic ROIs as represented by warmer colors in the connectivity matrix. Upon visual inspection, mTBI patients presented increased connectivity between the M1, PM, and PFC thalamic ROIs and BA1, BA2, BA3, BA4, and BA6 which are cortical areas associated with primary somatosensory and motor processing. In addition, the PFC thalamic ROI showed increased connectivity with BA41 which is associated with auditory processing. After controlling for the influence of age, there is an increased strength of thalamo-cortical connectivity in the PM (F=5.285, p=0.023) and PP (F=5.139, p=0.025) thalamic ROIs in the mTBI group compared to the controls as well as a non-significant trend in increased thalamo-cortical connectivity in the PFC (F=3.564, p=0.062) and M1 (F=3.785, p=0.054) thalamic ROIs (Figure 4B).
Figure 4.
Resting state functional connectivity (rs-FC) between sub-thalamic ROIs and Brodmann’s areas (BA) associated with primary sensory processing. Sub-thalamic ROIs include primary motor (M1), primary sensory (S1), occipital lobe (OCC), premotor cortex (PM), prefrontal cortex (PFC), posterior parietal cortex (PP) and temporal lobe (TEMP) A. Average correlation matrices for control subjects (n=35) and acute mTBI (n=77). The intensity represents the z-transformed corrrelation with warmer colors representing greater functional connectivity between regions. B. Bar graph of the strength of functional connectivity between each thalamic ROI and all BAs defined in the matrices. *p<0.05 based on ANCOVAs controlling for age.
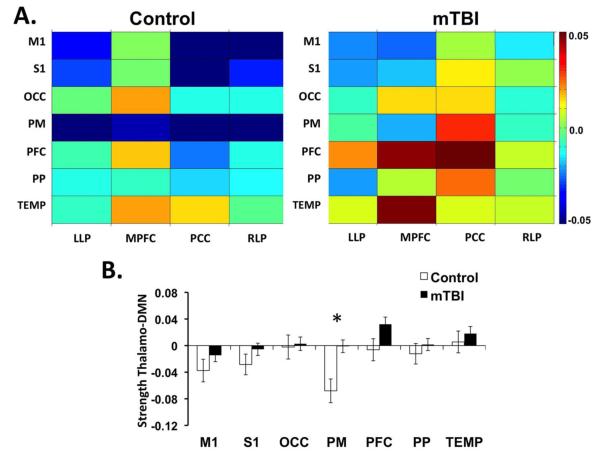
Pair-wise correlation matrices for thalamo-DMN connectivity demonstrate increased connectivity between the 7 sub-thalamic ROIs and the nodes of the DMN for the acute mTBI population compared to the controls (Fig 5A). Upon visual inspection, there is increased connectivity between multiple thalamic ROIs and the PCC as well as increased connectivity between the PM thalamic ROI and the PCC, LLP, and RLP. Quantification of the strength of thalamo-DMN connectivity shows that after controlling for the influence of age, there is increased thalamo-DMN connectivity in the mTBI compared to the controls for the PM subthalamic ROI (F=9.619, p=0.002) (Figure 5B).
Figure 5.
Resting state functional connectivity (rs-FC) between sub-thalamic ROIs and regions associated with the Default Mode Network (DMN). Sub-thalamic ROIs include primary motor (M1), primary sensory (S1), occipital lobe (OCC), premotor cortex (PM), prefrontal cortex (PFC), posterior parietal cortex (PP) and temporal lobe (TEMP). Regions associated with DMN include the posterior cingulate cortex (PCC), medial prefrontal cortex (MPFC), left lateral parietal (LLP), and right lateral parietal (RLP). A. Average correlation matrices for controls (n=35) and acute mTBI (n=77). The intensity represents the z-transformed correlation with warmer colors representing greater functional connectivity between regions. B. Bar graph of the strength of functional connectivity between each thalamic ROI and the four DMN ROIs. *p<0.05 based on ANCOVAs controlling for age.
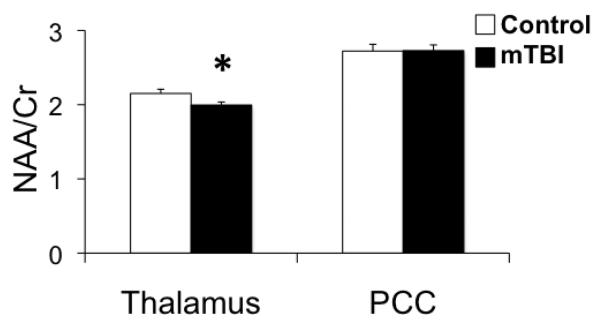
MRS
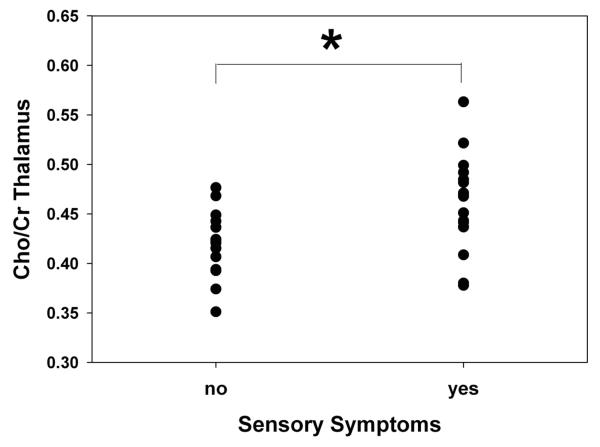
After controlling for the influence of age, results suggest that compared to the control population, the mTBI patients have a reduced NAA/Cr ratio in the thalamus (F=5.887, p=0.018), but that there are no reported group differences in the NAA/Cr ratio in the PCC (F=0.034, p=0.854) (Figure 6). No differences in the Cho/Cr ratio between the mTBI and control groups were noted in the thalamus (F=0.119, p=0.731) or PCC (F=2.183, p=0.144). However, mTBI patients who self-reported sensory symptoms on the RPQ (n=16) had significantly higher Cho/Cr ratio in the thalamus compared to mTBI patients who did not report sensory symptoms (n=18) at the acute time point after controlling for the influence of age (F=8.017, p=0.008) (Figure 7).
Figure 6.
Bar graph of average metabolite concentrations for N-Acetylaspartic acid/Creatine (NAA/Cr) ratio for the control group (n=28) and the mTBI group (n=47) for the thalamus and posterior cingulate cortex (PCC). *p<0.05 based on ANCOVAs controlling for age.
Figure 7.
Graph of average Choline/Creatine ratio (Cho/Cr) for acute mTBI patients with (n=16) and without (n=18) self-reported sensory symptoms. Sensory symptoms were determined by the Rivermead Post Concussion Symptom Questionnaire (RPQ). *p<0.05 based on ANCOVAs controlling for age.
Discussion
The thalamus has an essential role in sensory processing often acting as a relay center for the transmission of sensory information and it has been implicated as damaged in both mild and severe TBI. Therefore, this study sought to simultaneously investigate changes in thalamic functional connectivity as well as altered metabolite concentrations in acute mTBI patients. This study reports three main findings in an acute civilian mTBI population: 1) increased functional connectivity between the thalamus and cortical regions associated with primary sensory processing; 2) increased functional connectivity between the thalamus and the DMN; and 3) altered thalamic metabolic profiles in mTBI patients as well as altered metabolic concentrations specific to mTBI patients with acute sensory symptoms.
Implications of increased thalamo-cortical connectivity
MTBI is a diffuse injury that induces subtle functional alterations in a widespread set of cortical regions resulting in a wide array of post concussive symptoms including cognitive, neurobehavioral, somatic, and sensory impairments. While a constellation of symptoms are present immediately following injury in the mTBI population studied in this analysis, the largest percentage of mTBI patients self-reported sensory complaints with 79% of patients reporting sensory symptoms in this study (Table 4). Since the severity of somatic and sensory symptoms generally diminishes with time (Dischinger et al., 2009), this suggests that there is an ability to compensate for the acute functional alterations present in the neural communication of sensory systems.
To our knowledge, this study is the first to report an acute increase in functional connectivity specifically between various thalamus ROIs and cortical regions associated with primary sensory processing (Figure 4). The occurrence of increased thalamo-cortical functional connectivity in mTBI patients is consistent with current literature in patient populations at similar stages (Iraji et al., 2014), as well as later stages of injury (Sours et al., 2014; Tang et al., 2011). However, alternative analysis of the data from Tang et al. (2011), yielded somewhat contradictory results of both increased and reduced functional connectivity between certain thalamic sub-regions and cortical regions (Zhou et al., 2013). This implies that the specific analysis method selected has a substantial impact on reported results and findings must always be interpreted in the context of the particular methodology.
Specifically in this analysis, mTBI patients demonstrate increased functional connectivity between multiple thalamic sub-regions and primary somatosensory, motor, auditory, and premotor cortices (Figure 3 and 4). The thalamus has numerous projections that are sent to the cortex through the internal capsule, a white matter bundle that has been implicated by DTI as showing reduced structural integrity following severe TBI (Squarcina et al., 2012), civilian mTBI, (Stokum et al., 2014) and military mTBI (Morey et al., 2013). Furthermore, Yeh and colleagues found associations between increased post concussive and PTSD symptomology and reduced FA in the fronto-thalamic-cerebellar circuitry in a cohort of military TBI ranging from the acute to chronic stages of injury (Yeh et al., 2014). These findings further insinuate that there is an association between damaged thalamo-cortical projections and increased symptomology. Yet, the reported functional results suggest a hyper-connectivity of functional communication between the thalamus and cortical areas processing sensory information. These apparently contradictory findings may perhaps be explained by specific damage to GABAergic inhibitory interneurons in the thalamus that would effectively reduce the inhibitory control over these thalamo-cortical communications(Galarreta and S. Hestrin, 2001). For instance, an increased glutamate/GABA ratio in the PCC has been shown to be positively associated with increased functional connectivity within the DMN (Kapogiannis et al., 2013), suggesting that a reduction in GABAergic interneurons may contribute to the noted hyper-connectivity between the thalamus and cortex. Although further experimental confirmation is necessary in a human population, recent work in animal models of TBI using lateral fluid-percussion injury (FPI) provides evidence for this notion of reduced inhibitory tone within the thalamus. For example, groups have shown both a reduction in parvalbumin positive GABAergic interneurons (Huusko and A. Pitkanen, 2014) and a down regulation of GABAA and GABAB receptor subunit mRNAs in the thalamus following experimental TBI (Drexel et al., 2015). Since 79% of mTBI patients in this sample have reported sensory symptoms in the acute stage, this may imply that a lack of inhibitory control within the thalamus might contribute to the high prevalence of sensory symptoms such as sensitivity to noise and light. Further support for this interpretation comes from the study by Zhou et al, who examined functional coherence between a single thalamic ROI and Broadman’s areas 1-52. Their findings of an increased coherence with the frequency range of 0.01Hz-0.08Hz between the thalamus and cortical areas that was positively associated with increased severity of self-reported post concussive symptoms (Zhou et al., 2013). While this hyper-connectivity between the thalamus and cortical regions appears to contribute to post concussive symptoms, future work should explore the subtle intricacies of these associations.
Alterations in resting state neural communication patterns in the acute stage of mTBI raise difficult questions in regards to the safe time for civilian mTBI patients to return to work or contact sports and in the case of the military servicemen the safe time to return to the frontlines of a combat zone. The capability to process and integrate information from multiple sensory modalities as well as the ability to inhibit extraneous sensory stimuli is crucial for the safe return to every day life for these individuals. Moreover, this high prevalence of sensory symptoms suggest that the brain is still in a state of recovery and should be allowed time to recover. The existence of an objective measure of functional recovery will ultimately reduce the likelihood of secondary impact syndrome (SIS) when a second minor head injury, occurring in close proximity to an initial mild head injury, results in exacerbated and often fatal consequences (Bey and B. Ostick, 2009). Therefore, the ability to non-invasively measure this disrupted sensory processing in vivo can provide crucial information to clinicians, coaches, and military personnel to allow for them to make informed decisions regarding the best course of treatment for these patients.
Implications of increased thalamo-DMN connectivity
Voxel wise results showing increased functional connectivity between the PFC thalamus ROI and the posterior cingulate cortex (Figure 2) directed the course of this analysis in a novel way, resulting in preliminary evidence for an increased functional connectivity noted between the DMN and thalamus in acute mTBI (Figure 5). The role of the DMN is multifaceted and includes internally generated thoughts (Callard et al., 2012; Gusnard et al., 2001) and mind wandering (Mason et al., 2007). Furthermore, the functional connectivity of the DMN has been extensively investigated by our group and others following military (Robinson et al., 2014) and civilian mTBI (Iraji et al., 2014; Johnson et al., 2012; Mayer et al., 2011; Sours et al., 2013; Zhou et al., 2012). The general consensus across studies points toward reduced functional connectivity within the DMN that is likely due to axonal damage to white matter pathways connecting various nodes of this network. However, evidence also indicates that there is an increased functional connectivity between the DMN, the salience network (SN), and the task positive network (TPN) (Mayer et al., 2011; Sours et al., 2013). As the Default Mode Interference Hypothesis proposes, the complex interactions between the DMN, SN, and TPN are essential for the balance of internally versus externally directed attention (Sonuga-Barke and F. X. Castellanos, 2007). Intriguingly, recent work has implicated the thalamus as a crucial component responsible for the modulatory interactions between these three networks (Di and B. B. Biswal, 2014). Taken in this context, the findings outlined in this study showing increased thalamo-DMN connectivity could extend this concept to suggest that the functionally detrimental hyper-communication of the DMN with neural networks involved in cognitive processes can be extended to include the thalamic neurocircuitry.
Thalamo-DMN connectivity has been shown to be altered in numerous patient populations including reduced connectivity in patients with disordered consciousness (He et al., 2014), but increased connectivity in individuals with depression (Greicius et al., 2007) and PTSD (Yin et al., 2011). Furthermore chronic pain patients, greater connectivity between the thalamus and DMN was found to be associated with increased pain rumination (Kucyi et al., 2014). In addition, a recent study in healthy population found an intriguing association between the functional connectivity between the DMN and thalamus and mindfulness, with individuals with a weaker connectivity being more mindful and aware of the present (Wang et al., 2014). It could be argued that it is common among mTBI and PTSD patients to relive and dwell in the past traumatic event resulting in an inability to be mindfully aware of the present. This finding may contribute to the increased likelihood of PTSD in military personnel who have experienced a mTBI (Hoge et al., 2008). The unique finding of increased functional connectivity between the thalamus and DMN in acute mTBI provides novel evidence for the neural correlates of this internal rumination on the past events, possibly leading to new interventions for mTBI suffering from PTSD.
Implications of altered thalamic metabolite concentrations
MRS analyses revealed decreased NAA/Cre values measured in the thalamus for the mTBI cohort when compared to control subjects (Figure 6). Measured NAA levels are generally believed to be a robust marker of neuronal integrity and neuronal density following trauma; hence, noted decreases in NAA or NAA/Cre levels are presumably indicative of either reversible neuronal dysfunction or irreversible neuronal depletion. Such decreases in NAA levels following mTBI have been well documented in previous studies. (Cohen et al., 2007; Govindaraju et al., 2004; Henry et al., 2010; Henry et al., 2011; Holshouser et al., 2006; Kirov et al., 2013; Vagnozzi et al., 2008; Vagnozzi et al., 2010). More specifically, our findings replicate previous mTBI studies that have also observed reduced NAA levels in the thalamus in mTBI patients examined 0-7 years post-injury (Kirov et al., 2007).
Similar to our group’s previous findings showing no acute alterations in the Cho/Cr levels in the thalamus when considering the mTBI group as a whole (George et al., 2014), no changes in Cho/Cr levels were reported in the acute mTBI population in this current study. However, the current study revealed increased thalamic Cho/Cre levels in patients experiencing sensory symptoms when compared to patients who did not report sensory symptoms (Figure 7). Alterations in measured Cho levels is believed to be a marker of cell membrane turnover, myelin breakdown (Davie et al., 1993; Yeo et al., 2006), and glial cell proliferation in more severe progressions of TBI sequealae (Friedman et al., 1998; Garnett et al., 2000; Marino et al., 2007). While changes in Cho levels is not a popular finding in mTBI literature, the observed increase in measured Cho/Cre levels in symptomatic patients when compared to asymptomatic patients does agree with previous studies investigating specific symptoms such as headache following mTBI. Sarmento and associates found increased Cho/Cre in patients who experienced post-traumatic headaches when compared to healthy volunteers (Sarmento et al., 2009). This suggests that increases in Cho may be tied to symptomatic and more severe occurrences of head injury. While we found metabolic differences between mTBI patients with and without sensory symptoms, our analysis failed to find any significant associations between neuroimaging measures and neuropsychological performance. Overall, the reduced NAA/Cr and increased Cho/Cr reported following mTBI are likely the results of changes in the metabolic state within the thalamus. This may be due to the alteration in the balance of excitatory sensory inputs and inhibitory GABAergic transmission in the thalamus as suggested by the thalamo-cortical hyper-connectivity as measured by the resting state fMRI.
While in healthy populations Cre levels are considered to remain stable, Cre levels have been shown to deviate from normative values in other cerebral pathologies including Parkinson disease, multiple sclerosis, (Hattingen et al., 2009; Inglese et al., 2003) and TBI (Friedman et al., 1998; Gasparovic et al., 2009). Unfortunately, water only spectra were not available to obtain absolute spectroscopic quantification in this study. However, in a previous study conducted by our group, no statistical differences were found between Cre levels of the mTBI group and healthy subjects for either the thalamus or centrum semiovale across the first six months following injury (George et al., 2014), suggesting that in milder populations there may be a lack significant cell death or astroglial proliferation that occurs with more severe trauma. Therefore, we opted to investigate the ratio of Cho and NAA to Cre. Future studies investigating metabolic changes following TBI would benefit from the analysis of absolute concentrations of metabolites to remove any confounds from the possibility of changing Cr levels.
Limitations
While this study makes new contributions to the current literature regarding alterations in thalamic integrity and functional communication in the period immediately following mTBI, the results must be taken in the context of the limitations of this study. While sensory complaints are most common in the acute stage following injury, for a small percentage of patients, these specific symptoms fail to diminish over time. Therefore, a limitation of the current study is that it only assesses alterations in the acute stages of injury. Furthermore, while alterations in both thalamo-cortical connectivity and reductions in cognitive performance were noted within the mTBI population, our analysis failed to find significant associations between functional measures and cognition. Future studies investigating longitudinal changes in the thalamo-cortical circuitry are needed to further distinguish the abnormal progression thalamo-cortical communication associated with persistent symptoms and neurocognitive deficits.
An additional limitation of this current study is the great variety of injury mechanisms included in our mTBI population. For example, it has been shown that victims of assault report greater emotional and psychosocial symptoms compared to those who were injured as a results of sports related injuries (Mathias et al., 2014). This suggests that the mechanism of injury likely influences the length and extent of recovery following mTBI. However, given the vast heterogeneity of mTBI injuries regardless of injury mechanism, we opted to include multiple civilian injury mechanisms within this analysis. With that consideration, studies investigating specific functional and structural damage associated with distinct injury mechanisms are needed in order to effectively tease apart the unique characteristics of each mechanism of injury. In addition, it should be noted that while we screened and excluded both control participants and mTBI patients who had previously been hospitalized due to a head injury, there is a significant possibility that individuals in either population may have experienced an unreported or undiagnosed concussion. This is due to the fact that until recent years there was a lack of knowledge and awareness of the impact of mild head injuries; however, assuming that individuals in each group have an equal likelihood of unreported concussions and considering that this study investigates the acute effects of mTBI, we believe that the results reported are not confounded by this possibility.
In addition, multiple studies have provided evidence for altered cerebral perfusion of the thalamus following mTBI (Ge et al., 2009; Grossman et al., 2013) likely due to various secondary injury mechanisms including changes in resting metabolism, neurovascular coupling, and heightened inflammatory responses such as reactive microgliosis ((Mayer et al., 2013; Pop and J. Badaut, 2011; Zhuo et al., 2012)). Therefore, the findings in regard to alterations in BOLD functional connectivity measures must be taken in the context of possible contamination of resting state measures with altered regional perfusion. Furthermore, while we parcellated the thalamus into sub-thalamic regions based on a previously defined atlas, the native spatial resolution of the resting state data may have limited the accuracy of this parcellation. Finally, while preliminary results point towards increased Cho/Cr ratio unique to mTBI with sensory symptoms is novel, the limited number of patients in this study that received both MRS and neuropsychological assessments limits the applicability of these findings. Replication of this finding in a larger data set across multiple stages of injury is needed to further validate this finding and determine the association between altered metabolic profiles and persistent sensory symptoms.
Conclusion
This study demonstrates for the first time specific increases in thalamic functional connectivity with primary sensory cortices and the DMN in the context of altered metabolic integrity of the thalamus in a population of acute mTBI patients. These results provide novel insights into neural mechanisms of acute sensitivity to sensory inputs providing an avenue towards enhanced prediction of the safe return to work for civilian and military populations. In addition, these results provide intriguing evidence of altered brain states related to internal rumination and arousal which may ultimately lead to novel interventions for the subset of mTBI patients who suffer from PTSD and related neurobehavioral sequelae.
Supplementary Material
Acknowledgements
The authors would like to thank Joshua Betz, Jacqueline Janowich, Teodora Stoica and Joseph Rosenberg for their help with patient recruitment and George Makris for his help with acquiring and processing the data. Support for this work was in part provided by the Department of Defense (W81XWH-08-1-0725 & W81XWH-12-1-0098 to RPG).
Footnotes
Author Disclosure Section
None of the authors have any relevant disclosures. No competing financial interests exist.
References
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- Bartnik-Olson BL, Holshouser B, Wang H, Grube M, Tong K, Wong V, Ashwal S. Impaired neurovascular unit function contributes to persistent symptoms after concussion: a pilot study. J Neurotrauma. 2014;31:1497–1506. doi: 10.1089/neu.2013.3213. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bey T, Ostick B. Second impact syndrome. West J Emerg Med. 2009;10:6–10. [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Callard F, Smallwood J, Margulies DS. Default Positions: How Neuroscience’s Historical Legacy has Hampered Investigation of the Resting Mind. Front Psychol. 2012;3:321. doi: 10.3389/fpsyg.2012.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Report to congress on mild traumatic brain injury in the united states: steps to prevent a serious public health problem. 2003.
- Cohen BA, Inglese M, Rusinek H, Babb JS, Grossman RI, Gonen O. Proton MR spectroscopy and MRI-volumetry in mild traumatic brain injury. AJNR Am J Neuroradiol. 2007;28:907–913. [PMC free article] [PubMed] [Google Scholar]
- Davie CA, Hawkins CP, Barker GJ, Brennan A, Tofts PS, Miller DH, McDonald WI. Detection of myelin breakdown products by proton magnetic resonance spectroscopy. Lancet. 1993;341:630–631. doi: 10.1016/0140-6736(93)90390-3. [DOI] [PubMed] [Google Scholar]
- Di X, Biswal BB. Modulatory interactions between the default mode network and task positive networks in resting-state. PeerJ. 2014;2:e367. doi: 10.7717/peerj.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dischinger PC, Ryb GE, Kufera JA, Auman KM. Early predictors of postconcussive syndrome in a population of trauma patients with mild traumatic brain injury. J Trauma. 2009;66:289–96. doi: 10.1097/TA.0b013e3181961da2. discussion 296-7. [DOI] [PubMed] [Google Scholar]
- Drexel M, Puhakka N, Kirchmair E, Hortnagl H, Pitkanen A, Sperk G. Expression of GABA receptor subunits in the hippocampus and thalamus after experimental traumatic brain injury. Neuropharmacology. 2015;88:122–133. doi: 10.1016/j.neuropharm.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald M, Coronado V. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Center of Disease Control. 2010 [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Brooks WM, Jung RE, Hart BL, Yeo RA. Proton MR spectroscopic findings correspond to neuropsychological function in traumatic brain injury. AJNR Am J Neuroradiol. 1998;19:1879–1885. [PMC free article] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Electrical synapses between GABA-releasing interneurons. Nat Rev Neurosci. 2001;2:425–433. doi: 10.1038/35077566. [DOI] [PubMed] [Google Scholar]
- Garnett MR, Blamire AM, Corkill RG, Cadoux-Hudson TA, Rajagopalan B, Styles P. Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. Brain. 2000;123(Pt 10):2046–2054. doi: 10.1093/brain/123.10.2046. [DOI] [PubMed] [Google Scholar]
- Gasparovic C, Yeo R, Mannell M, Ling J, Elgie R, Phillips J, Doezema D, Mayer AR. Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: an 1H-magnetic resonance spectroscopy study. J Neurotrauma. 2009;26:1635–1643. doi: 10.1089/neu.2009.0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Patel MB, Chen Q, Grossman EJ, Zhang K, Miles L, Babb JS, Reaume J, Grossman RI. Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labelling MR imaging at 3T. Brain Inj. 2009;23:666–674. doi: 10.1080/02699050903014899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EO, Roys S, Sours C, Rosenberg J, Zhuo J, Shanmuganathan K, Gullapalli RP. Longitudinal and prognostic evaluation of mild traumatic brain injury: A 1H-magnetic resonance spectroscopy study. J Neurotrauma. 2014;31:1018–1028. doi: 10.1089/neu.2013.3224. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Gauger GE, Manley GT, Ebel A, Meeker M, Maudsley AA. Volumetric proton spectroscopic imaging of mild traumatic brain injury. AJNR Am J Neuroradiol. 2004;25:730–737. [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman EJ, Ge Y, Jensen JH, Babb JS, Miles L, Reaume J, Silver JM, Grossman RI, Inglese M. Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. J Neurotrauma. 2012;29:2318–2327. doi: 10.1089/neu.2011.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman EJ, Inglese M, Bammer R. Mild traumatic brain injury: is diffusion imaging ready for primetime in forensic medicine? Top Magn Reson Imaging. 2010;21:379–386. doi: 10.1097/RMR.0b013e31823e65b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman EJ, Jensen JH, Babb JS, Chen Q, Tabesh A, Fieremans E, Xia D, Inglese M, Grossman RI. Cognitive impairment in mild traumatic brain injury: a longitudinal diffusional kurtosis and perfusion imaging study. AJNR Am J Neuroradiol. 2013;34:951–7. S1–3. doi: 10.3174/ajnr.A3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52:612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- Halbauer JD, Ashford JW, Zeitzer JM, Adamson MM, Lew HL, Yesavage JA. Neuropsychiatric diagnosis and management of chronic sequelae of war-related mild to moderate traumatic brain injury. J Rehabil Res Dev. 2009;46:757–796. doi: 10.1682/jrrd.2008.08.0119. [DOI] [PubMed] [Google Scholar]
- Hattingen E, Magerkurth J, Pilatus U, Mozer A, Seifried C, Steinmetz H, Zanella F, Hilker R. Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain. 2009;132:3285–3297. doi: 10.1093/brain/awp293. [DOI] [PubMed] [Google Scholar]
- He JH, Cui Y, Song M, Yang Y, Dang YY, Jiang TZ, Xu RX. Decreased functional connectivity between the mediodorsal thalamus and default mode network in patients with disorders of consciousness. Acta Neurol Scand. 2014 doi: 10.1111/ane.12299. [DOI] [PubMed] [Google Scholar]
- Henry LC, Tremblay S, Boulanger Y, Ellemberg D, Lassonde M. Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. J Neurotrauma. 2010;27:65–76. doi: 10.1089/neu.2009.0962. [DOI] [PubMed] [Google Scholar]
- Henry LC, Tremblay S, Leclerc S, Khiat A, Boulanger Y, Ellemberg D, Lassonde M. Metabolic changes in concussed American football players during the acute and chronic post-injury phases. BMC Neurol. 2011;11:105-2377–11-105. doi: 10.1186/1471-2377-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Holshouser BA, Tong KA, Ashwal S, Oyoyo U, Ghamsary M, Saunders D, Shutter L. Prospective longitudinal proton magnetic resonance spectroscopic imaging in adult traumatic brain injury. J Magn Reson Imaging. 2006;24:33–40. doi: 10.1002/jmri.20607. [DOI] [PubMed] [Google Scholar]
- Huusko N, Pitkanen A. Parvalbumin immunoreactivity and expression of GABAA receptor subunits in the thalamus after experimental TBI. Neuroscience. 2014;267:30–45. doi: 10.1016/j.neuroscience.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Inglese M, Li BS, Rusinek H, Babb JS, Grossman RI, Gonen O. Diffusely elevated cerebral choline and creatine in relapsing-remitting multiple sclerosis. Magn Reson Med. 2003;50:190–195. doi: 10.1002/mrm.10481. [DOI] [PubMed] [Google Scholar]
- Iraji A, Benson RR, Welch RD, O’Neil BJ, Woodard JL, Ayaz SI, Kulek A, Mika V, Medado P, Soltanian-Zadeh H, Liu T, Haacke EM, Kou Z. Resting State Functional Connectivity in Mild Traumatic Brain Injury at the Acute Stage: Independent Component and Seed Based Analyses. J Neurotrauma. 2014 doi: 10.1089/neu.2014.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson GL, Lovell MR, Smith S, Franzen MD. Prevalence of abnormal CT-scans following mild head injury. Brain Inj. 2000;14:1057–1061. doi: 10.1080/02699050050203559. [DOI] [PubMed] [Google Scholar]
- Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, Slobounov S. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage. 2012;59:511–518. doi: 10.1016/j.neuroimage.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane RL, Roebuck-Spencer T, Short P, Kabat M, Wilken J. Identifying and monitoring cognitive deficits in clinical populations using Automated Neuropsychological Assessment Metrics (ANAM) tests. Arch Clin Neuropsychol. 2007;22(Suppl 1):S115–26. doi: 10.1016/j.acn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Reiter DA, Willette AA, Mattson MP. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 2013;64:112–119. doi: 10.1016/j.neuroimage.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242:587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- Kirov I, Fleysher L, Babb JS, Silver JM, Grossman RI, Gonen O. Characterizing ‘mild’ in traumatic brain injury with proton MR spectroscopy in the thalamus: Initial findings. Brain Inj. 2007;21:1147–1154. doi: 10.1080/02699050701630383. [DOI] [PubMed] [Google Scholar]
- Kirov II, Tal A, Babb JS, Lui YW, Grossman RI, Gonen O. Diffuse axonal injury in mild traumatic brain injury: a 3D multivoxel proton MR spectroscopy study. J Neurol. 2013;260:242–252. doi: 10.1007/s00415-012-6626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci. 2014;34:3969–3975. doi: 10.1523/JNEUROSCI.5055-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Husain M, Gupta RK, Hasan KM, Haris M, Agarwal AK, Pandey CM, Narayana PA. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J Neurotrauma. 2009;26:481–495. doi: 10.1089/neu.2008.0461. [DOI] [PubMed] [Google Scholar]
- Laouchedi M, Galanaud D, Delmaire C, Fernandez-Vidal S, Messe A, Mesmoudi S, Oulebsir Boumghar F, Pelegrini-Issac M, Puybasset L, Benali H, Perlbarg V. Deafferentation in thalamic and pontine areas in severe traumatic brain injury. J Neuroradiol. 2014 doi: 10.1016/j.neurad.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Little DM, Kraus MF, Joseph J, Geary EK, Susmaras T, Zhou XJ, Pliskin N, Gorelick PB. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74:558–564. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Snyder AZ, Raichle ME, Witherow JR, Fang R, Flaherty SF, Brody DL. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S, Zei E, Battaglini M, Vittori C, Buscalferri A, Bramanti P, Federico A, De Stefano N. Acute metabolic brain changes following traumatic brain injury and their relevance to clinical severity and outcome. J Neurol Neurosurg Psychiatry. 2007;78:501–507. doi: 10.1136/jnnp.2006.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias JL, Harman-Smith Y, Bowden SC, Rosenfeld JV, Bigler ED. Contribution of psychological trauma to outcomes after traumatic brain injury: assaults versus sporting injuries. J Neurotrauma. 2014;31:658–669. doi: 10.1089/neu.2013.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Toulouse T, Klimaj S, Ling J, Pena A, Bellgowan P. Investigating the Properties of the Hemodynamic Response Function Following Mild Traumatic Brain Injury. J Neurotrauma. 2013 doi: 10.1089/neu.2013.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D, Reichard R, Yeo RA. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74:643–650. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Elgie R, Gasparovic C, Phillips JP, Doezema D, Yeo RA. Auditory orienting and inhibition of return in mild traumatic brain injury: a FMRI study. Hum Brain Mapp. 2009;30:4152–4166. doi: 10.1002/hbm.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp. 2011;32:1825–1835. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea M, Kelly J, Randolph C. Standardized Assessment of Concussion (SAC): Manual for Adminstration, Scoring, and Interpretation, Comprehensive Neuropsychological Services. 2nd Eddition 2000.
- Messe A, Caplain S, Paradot G, Garrigue D, Mineo JF, Soto Ares G, Ducreux D, Vignaud F, Rozec G, Desal H, Pelegrini-Issac M, Montreuil M, Benali H, Lehericy S. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum Brain Mapp. 2011;32:999–1011. doi: 10.1002/hbm.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Haswell CC, Selgrade ES, Massoglia D, Liu C, Weiner J, Marx CE, MIRECC Work Group. Cernak I, McCarthy G. Effects of chronic mild traumatic brain injury on white matter integrity in Iraq and Afghanistan war veterans. Hum Brain Mapp. 2013;34:2986–2999. doi: 10.1002/hbm.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ommaya AK, Goldsmith W, Thibault L. Biomechanics and neuropathology of adult and paediatric head injury. Br J Neurosurg. 2002;16:220–242. doi: 10.1080/02688690220148824. [DOI] [PubMed] [Google Scholar]
- Pop V, Badaut J. A Neurovascular Perspective for Long-Term Changes After Brain Trauma. Transl Stroke Res. 2011;2:533–545. doi: 10.1007/s12975-011-0126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock JT, Becker DP, Cheng CL, Vaughan GW. Axonal change in minor head injury. J Neuropathol Exp Neurol. 1983;42:225–242. doi: 10.1097/00005072-198305000-00002. [DOI] [PubMed] [Google Scholar]
- Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- Reich S, Short P, Kane R, Weiner W, Shulman L, Anderson K. Validation of the ANAM Test Battery in Parkinson’s Disease. Defense Technical Information Center; Ft. Belvoir: 2005. [Google Scholar]
- Robinson ME, Lindemer ER, Fonda JR, Milberg WP, McGlinchey RE, Salat DH. Close-range blast exposure is associated with altered functional connectivity in Veterans independent of concussion symptoms at time of exposure. Hum Brain Mapp. 2014 doi: 10.1002/hbm.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmento E, Moreira P, Brito C, Souza J, Jevoux C, Bigal M. Proton spectroscopy in patients with post-traumatic headache attributed to mild head injury. Headache. 2009;49:1345–1352. doi: 10.1111/j.1526-4610.2009.01494.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Sours C, Rosenberg J, Kane R, Roys S, Zhuo J, Shanmuganathan K, Gullapalli RP. Associations between interhemispheric functional connectivity and the Automated Neuropsychological Assessment Metrics (ANAM) in civilian mild TBI. Brain Imaging Behav. 2014 doi: 10.1007/s11682-014-9295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sours C, Zhuo J, Janowich J, Aarabi B, Shanmuganathan K, Gullapalli RP. Default mode network interference in mild traumatic brain injury - A pilot resting state study. Brain Res. 2013;1537:201–215. doi: 10.1016/j.brainres.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarcina L, Bertoldo A, Ham TE, Heckemann R, Sharp DJ. A robust method for investigating thalamic white matter tracts after traumatic brain injury. Neuroimage. 2012;63:779–788. doi: 10.1016/j.neuroimage.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokum J, Sours C, Zhuo J, Shanmuganathan K, Gullapalli R. A longitudinal evaluation of diffusion kurtosis imaging in patients with mild traumatic brain injury. Brain injury : [BI] 2014 doi: 10.3109/02699052.2014.947628. In press. [DOI] [PubMed] [Google Scholar]
- Tang L, Ge Y, Sodickson DK, Miles L, Zhou Y, Reaume J, Grossman RI. Thalamic resting-state functional networks: disruption in patients with mild traumatic brain injury. Radiology. 2011;260:831–840. doi: 10.1148/radiol.11110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Cristofori L, Alessandrini F, Floris R, Isgro E, Ria A, Marziali S, Zoccatelli G, Tavazzi B, Del Bolgia F, Sorge R, Broglio SP, McIntosh TK, Lazzarino G. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133:3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Tavazzi B, Floris R, Ludovici A, Marziali S, Tarascio G, Amorini AM, Di Pietro V, Delfini R, Lazzarino G. Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes--part III. Neurosurgery. 2008;62:1286–95. doi: 10.1227/01.neu.0000333300.34189.74. discussion 1295-6. [DOI] [PubMed] [Google Scholar]
- Wang X, Xu M, Song Y, Li X, Zhen Z, Yang Z, Liu J. The network property of the thalamus in the default mode network is correlated with trait mindfulness. Neuroscience. 2014;278:291–301. doi: 10.1016/j.neuroscience.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Warner MA, Marquez de la Plata C, Spence J, Wang JY, Harper C, Moore C, Devous M, Diaz-Arrastia R. Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. J Neurotrauma. 2010a;27:2121–2130. doi: 10.1089/neu.2010.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner MA, Youn TS, Davis T, Chandra A, Marquez de la Plata C, Moore C, Harper C, Madden CJ, Spence J, McColl R, Devous M, King RD, Diaz-Arrastia R. Regionally selective atrophy after traumatic axonal injury. Arch Neurol. 2010b;67:1336–1344. doi: 10.1001/archneurol.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Yeh PH, Wang B, Oakes TR, French LM, Pan H, Graner J, Liu W, Riedy G. Postconcussional disorder and PTSD symptoms of military-related traumatic brain injury associated with compromised neurocircuitry. Hum Brain Mapp. 2014;35:2652–2673. doi: 10.1002/hbm.22358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo RA, Phillips JP, Jung RE, Brown AJ, Campbell RC, Brooks WM. Magnetic resonance spectroscopy detects brain injury and predicts cognitive functioning in children with brain injuries. J Neurotrauma. 2006;23:1427–1435. doi: 10.1089/neu.2006.23.1427. [DOI] [PubMed] [Google Scholar]
- Yin Y, Jin C, Hu X, Duan L, Li Z, Song M, Chen H, Feng B, Jiang T, Jin H, Wong C, Gong Q, Li L. Altered resting-state functional connectivity of thalamus in earthquake-induced posttraumatic stress disorder: a functional magnetic resonance imaging study. Brain Res. 2011;1411:98–107. doi: 10.1016/j.brainres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Yuh EL, Mukherjee P, Lingsma HF, Yue JK, Ferguson AR, Gordon WA, Valadka AB, Schnyer DM, Okonkwo DO, Maas AI, Manley GT, TRACK-TBI Investigators Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol. 2013;73:224–235. doi: 10.1002/ana.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Milham MP, Lui YW, Miles L, Reaume J, Sodickson DK, Grossman RI, Ge Y. Default-Mode Network Disruption in Mild Traumatic Brain Injury. Radiology. 2012;265:882–892. doi: 10.1148/radiol.12120748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Lui YW, Zuo XN, Milham MP, Reaume J, Grossman RI, Ge Y. Characterization of thalamo-cortical association using amplitude and connectivity of functional MRI in mild traumatic brain injury. J Magn Reson Imaging. 2013 doi: 10.1002/jmri.24310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, Gullapalli RP. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage. 2012;59:467–477. doi: 10.1016/j.neuroimage.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.