Abstract
Mycobacterium tuberculosis (M.tb) infection leads to active tuberculosis (TB), a disease that kills one human every 18 seconds. Current therapies available to combat TB include chemotherapy and the preventative vaccine Mycobacterium bovis Bacille Calmette et Guérin (BCG). Increased reporting of drug resistant M.tb strains worldwide indicates that drug development cannot be the primary mechanism for eradication. BCG vaccination has been used globally for protection against childhood and disseminated TB, however, its efficacy at protecting against pulmonary TB in adult and aging populations is highly variable. In this regard, the immune response generated by BCG vaccination is incapable of sterilizing the lung post M.tb infection as indicated by the large proportion of individuals with latent TB infection that have received BCG. Although many new TB vaccine candidates have entered the development pipeline, only a few have moved to human clinical trials; where they showed no efficacy and/or were withdrawn due to safety regulations. These trials highlight our limited understanding of protective immunity against the development of active TB. Here, we discuss current vaccination strategies and their impact on the generation and sustainability of protective immunity against TB.
Keywords: Tuberculosis, Vaccine, Mycobacterium tuberculosis, Mycobacterium bovis bacille Calmette-Guérin (BCG)
Introduction
WHO estimates that by 2020 up to 36 million people will die of TB every year [1]. BCG vaccination has been used globally for over 80 years to protect against TB [2] with variable results. In humans, at best, BCG is 80% effective in preventing TB and, in the majority of the cases protection lasts for only 10–15 years, with the exception of a study in the Native Alaskan Indians community, where protection lasted for over 50 years [3]. Conversely, complete lack of protection has also been reported in communities in India, specifically in Chengalpattu (formerly known as Chingleput) [4], thus highlighting the importance of the human genetic pool and the environment in generating protection. For unknown reasons, the protective efficacy of BCG and its duration vary according to geography and population age [2]. Many new vaccines seek to improve upon BCG using genetic modifications, and although new vaccines are in development there remain several challenges to implementing them worldwide. Here we present and discuss reasons why new vaccine strategies against TB have failed to enhance or replace the current BCG vaccine, discuss the impact of generating a vaccine against the development of active TB vs. targeting M.tb infection, and introduce an element commonly disregarded in vaccine design, the lung environment.
BCG: Route of Vaccination
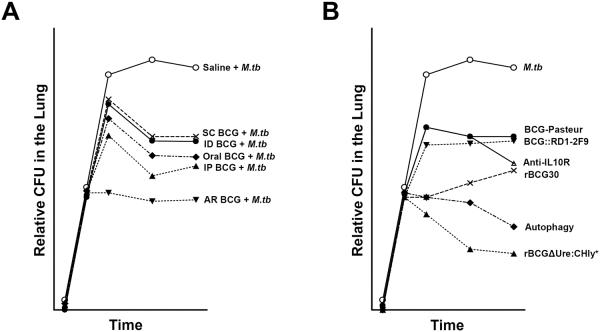
In the following section, we discuss the protective effect conferred by the different routes of BCG vaccination against TB. Figure 1A summarizes results based on our literature search.
Figure 1.
A. Different routes of vaccination with BCG vary in their reduction of M.tb CFU in the lung of vaccinated animals. Shown is a graph compiling the relative protection conferred by distinct routes of vaccination against M.tb using BCG-Pasteur as a reference. The protection by BCG via subcutaneous/intradermal inoculation results in approximately one-log reduction in M.tb CFU in the lung. More immunogenic routes such as oral and intraperitoneal vaccination offer a greater degree of protection mainly due to their ability to stimulate systemic and mucosal immune responses. Immunization via BCG aerosol elicited the greatest degree of protection however this route is associated with lung tissue pathology. B. New vaccines/concepts that have shown greater reduction of M.tb CFU in the lung of vaccinated animals compared to BCG. There is a plethora of vaccines that have been investigated for enhanced protection against M.tb compared to BCG, however here only a few have been summarized. Based on published data, the graph shows the relative number of M.tb CFU in the lung of vaccinated animals challenged with M.tb. Novel strategies, such as the induction of autophagy or blocking of the IL-10R, have been shown to enhance the protective efficacy of the BCG vaccine. Interestingly, rBCG30 was shown to be more protective than BCG∷RD1-2F9, both of which encode highly immunogenic antigens. To date, the rBCG ΔUre:CHly+ vaccine has been shown to be the most protective vaccines in terms of reducing lung CFU.
Oral Immunization
Although this route of immunization is not widely used today, the first dose of BCG administered in 1921 was given orally. Oral BCG remained the chosen route of immunization until 1924 when it was replaced with more immunogenic routes [5]. Several advantages place oral vaccination above other forms of vaccination; it eliminates the need for needles, the requirement for trained clinical staff, and it is more feasible to implement on a large scale [6]. In 1993, Lagranderie et al. were the first to demonstrate that systemic and mucosal immunity could be generated through oral vaccination by producing a recombinant BCG (rBCG) strain expressing the immunogen LacZ, encoding a β-galactosidase from the lac operon [7]. Currently, alternative formulations are being developed to improve oral BCG, mainly focused on lipid-based BCG preparations. Formulas such as lipid microencapsulation of BCG administered via the oral route to mice and Guinea pigs can establish specific systemic cell-mediated immune responses [8–10], and induce long-lived, diverse memory CD4 T cell populations [11], associated with reduced bacterial burden and pathological scores in the lung when compared to unformulated oral vaccines [10;12;13]. However, similar studies in humans failed to generate systemic IFNγ responses to oral BCG vaccination [14]. Hence more studies on oral vaccination are needed before claims about its efficacy can be made, including further exploration of novel delivery systems to enhance its potential.
Cutaneous and Intradermal Immunization
Although BCG was first administered orally, the vaccine later became cutaneously administered due to enhanced induction of delayed type hypersensitivity responses (DTH) to the purified protein derivative (PPD) diagnostic test [15]. Currently, WHO recommends intradermal injection of BCG in the deltoid region [16], although cutaneous injections are performed in some instances. Several studies comparing cutaneous vs. intradermal immunization concluded that both stimulation of a DTH response and production of T helper (Th1) cytokines were more prominent when BCG was intradermally delivered [17–19]. These findings were supported by studies where intradermal vaccination reduced the incidence of childhood TB meningitis [20–22]. Conversely, a randomized trial in South Africa evaluating the efficacy of these two vaccination routes found no significant differences among documented TB cases [23]. These conflicting outcomes were further addressed using animal models, where no immunological differences were found between cutaneous and intradermal BCG delivery [24–26]. Such discrepancies could be attributed to many different factors, including but not limited to the genetics of the subject population, environmental factors, and the origin of the BCG substrain used for vaccination. Referring to the latter, it has been documented that different BCG substrains can induce diverse immune responses and degrees of protection in humans [27;28] and animal models [29;30], although differences were minimal and not significant [31]. Thus, a direct association between BCG substrain immunogenicity and protection cannot be inferred, mainly because correlates of protection against the development of active TB in infected individuals have yet to be identified.
Intranasal Immunization
Using different animal models, aerosolized BCG immunization was shown to limit M.tb growth in the lung and enhance the immunogenic control of TB development potentially due to the combination of mucosal and systemic immune activation [32–36]. However, a potential safety concern may be associated with this vaccination route, as more pronounced immunopathologic side effects were observed in the lungs of aerosol vs. intravenously post-vaccinated animals [35]. Thus, by defining bacterial and host components that elicit such inflammatory damage in the lung during intranasal immunization without affecting its protective efficacy, a candidate vaccine could be engineered to induce systemic and mucosal immunity while limiting undesired tissue damage. This could potentially be achieved through limiting the expression/production of some BCG cell wall components such as phosphatidyl-myo-inositol dimannosides, phosphatidyl-myo-inositol, trehalose mono- and di-mycolates, phenolic glycolipid mycoside B and/or other waxes [37]; all abundantly present on the mycobacterial cell wall and known to cause substantial damaging inflammation in tissue [38;39] (Table 1). However, this needs to be carefully balanced, as pathology produced by aerosol vaccination may also be the driving force for the increased protection observed using this route of vaccination. Overcoming intranasal vaccination driven pathologies may allow for the emergence of highly effective tissue specific TB vaccines, where the manipulation of the lung mucosa components (discussed below) may play a significant role in defining the protective immune response to M.tb infection and subsequent development of TB.
Table 1.
Mycobacterial cell wall components and their host interaction outcome.
| Mycobacterial Cell Wall Location | Mycobacterial Cell Wall Component | Host Cell Receptor(s) | Inflammation | Tissue Damage Inducer | Sero-Activity |
|---|---|---|---|---|---|
| Outer Material | α-Glucan | DC-SIGN, CR3? | Anti-inflammatory | ND | ND |
| Peripheral lipid layer | Diacyl- and Triacyl-trehalose (DAT & TAT) | ND | Pro-inflammatory | ND | Yes |
| Higher-order phosphatidyl-myo-inositol mannosides (PIMs) | MR, DC-SIGN | Anti-inflammatory | ND | Yes | |
| Lipooligosaccharides (LOSs) | ND | Pro-inflammatory | ND | Yes | |
| Lipomannan (LM) | TLRs, DC-SIGN | Pro-inflammatory | ND | Yes | |
| Lower-order phosphatidyl-myo-inositol mannosides (PIMs) | CR3, TLRs, DC-SIGN | Pro-inflammatory | ND | Yes | |
| Mannose-capped lipoarabinomannan (ManLAM) | MR, DC-SIGN | Anti-inflammatory | ND | Yes | |
| Phenolic glycolipid (PGL, Mycoside D) | CR3? | Pro-inflammatory | Yes | Yes | |
| Phthiocerol dimycocerosate (PDIM) | Direct insertion into host Membranes | Pro-inflammatory | Yes | ND | |
| Sulfolipid-1 (SL-1) | ND | Pro-inflammatory | Yes | Yes | |
| Trehalose dimycolate (TDM) | Mincle-FcγR TLRs | Pro-inflammatory | Yes | Yes | |
| Trehalose monomycolate (TMM) | ND | Pro-inflammatory | Yes | Yes | |
| Triglycerides | TLRs | Pro-inflammatory | Yes | ND | |
| Cell wall Core | Arabinogalactan (AG) | ND | ND | ND | Yes |
| Mycolic Acids | CD1 (in Ag-presentation | Pro-inflammatory | Yes | Yes | |
| Peptidoglycan (PG)-MDP | Nod2 | Pro-inflammatory | ND | ND |
BCG: Substrain Diversity
The efficacy of different BCG substrains has been thoroughly discussed [29;30], and reviewed [40]; however it is necessary to describe some of their aspects in the context of vaccination studies. BCG vaccination currently covers 80% of the countries where TB is considered endemic. Although one strain of BCG was originally produced, subsequent passages by many laboratories have generated 21 BCG substrains [40–43] (Table 2). Denmark/Copenhagen strain 1331, Russian/Moscow, and Japanese/Tokyo 172 BCG are the predominant substrains currently used for vaccine production, distribution, and administration worldwide [16]. Though unclear, Japanese/Tokyo 172 appears to provide the lowest efficacy at reducing M.tb growth in animal models [40]. Russia/Moscow and Denmark/Copenhagen substrains, although biochemically and genetically different, are equally protective against the development of TB [44;45]. Recently, the presence of different Regions of Difference (RD) with different open reading frames has been suggested as the potential source of variability observed among several BCG substrains in their ability to prevent TB morbidity [41–43;45]. Although genetic and phenotypic analyses have revealed substantial information on the requirements for virulence of M. bovis and the derived BCG strain, they have failed to clarify our understanding of the ability of the original BCG and daughter substrains to stimulate a durable immune response.
Table 2. BCG substrains used for Production, Distribution and Administration.
Frequently used substrains accounting for 90% of BCG vaccine administered worldwide are indicated by (*)
| Origin | BCG Strains |
|---|---|
|
| |
| Europe | • Bulgarian BCG Sophia 222 |
| • Czechoslovakian BCG Prague | |
| • Danish Denmark/Copenhagen strain 1331 (*) | |
| • French Original Bacille Calmete et Guérin strain | |
| • French Pasteur 1173P2 | |
| • Polish BCG Poland | |
| • Romanian BCG Romania 192 | |
| • Russian BCG Moscow (*) | |
| • Swedish BCG Gothenburg | |
| • UK Glaxo strain 1077 | |
|
| |
| Asia | • Chinese BCG Beijing |
| • Chinese BCG Chandan | |
| • Chinese BCG Lanzhou | |
| • Chinese BCG Shanghai | |
| • Japanese BCG Tokyo strain 172 (*) | |
|
| |
| North America | • American BCG Birkhaug |
| • American BCG Phipps | |
| • American BCG Tice | |
| • Canadian BCG Connaught | |
| • Canadian BCG Frappier | |
| • Mexican BCG Mexico | |
|
| |
| South America | • Brazilian BCH Moreau |
An additional concern that should not be overlooked is the way BCG substrain vaccines are selected, prepared, and administered by different laboratories and countries [46]. There remains no consensus or proper regulations in place to control this process. For example, China has developed four BCG substrains; where the protective effects of BCG Shanghai and BCG Beijing are comparable to the Danish BCG Copenhagen-1331 strain (strain from which these were originated); however, BCG Lanzhou is slightly less potent, and BCG Chanchun is completely devoid of protection as measured by the recovery of M.tb colony forming units (CFUs) in the spleen of vaccinated Guinea pigs (both were originally derived from BCG Tokyo-172) [40]. These four BCG substrains are still widely used in China. Moreover, the Russia BCG substrain is predominantly used in countries with high TB burdens [47]. These discrepancies highlight problems associated with lack of standardization. In addition, it underlines that vaccination success may directly depend on the country's social, political, and economic status.
An additional element to consider is the human manipulation of the original BCG vaccine strain, when compared to the natural selection of M.tb strains. Recent studies showed that 188 T cell epitopes essential to the human immune response to M.tb infection had been lost, to varying degrees, in all BCG substrains [48]. BCG Tokyo-172 substrain had the highest number of T cell epitopes in relation to M.tb strains; however this BCG substrain has consistently induced poor immunity against TB in animal models and in clinical trials [4;40]. Thus, the number of expressed epitopes is not necessarily a good indicator for vaccine design. In fact, some BCG substrains with fewer epitopes may prevent TB more efficiently compared to substrains with greater number of epitopes [48].
Overall, bacterial genetics may play an important role in determining the ability of BCG substrains to prevent TB morbidity, but this is not the sole factor. Other factors, including global standardization of BCG vaccination, minimizing adverse reactions to BCG vaccination, and the potential variable susceptibility to mycobacterial chemotherapeutics by different BCG substrains are critical to develop an efficient TB vaccine program. In this regard, it is considered costly to standardize the global use of a given BCG vaccine substrain, which is likely one of the reasons why we still use poor-protecting substrains for vaccination programs. In the end, the question of substrain diversity and use lingers, and whether this diversity is harmful or beneficial remains unanswered. Directing the use of a specific BCG substrain for vaccination while keeping in mind M.tb strain diversity and environmental/co-morbidity factors in the targeted region could reveal that a certain BCG substrain may be ideal for one region but not for another. This may also be an approach to bypass the potential interference of other endemic co-infections (i.e. helminthic, HIV, non-tuberculous mycobacteria) and co-morbidities (i.e. smoking, diabetes, aging) that could interfere with the protection induced by BCG substrains; though no evidence has yet been linked between this potential masking effect and the BCG substrain efficacy in protecting against the development of TB [49].
BCG: Novel Approaches
Past and current TB vaccines under development have been extensively discussed in other reviews [50–52], and thus we provide here examples of successes and failures and what we believe are experimentally promising solutions to improve the BCG vaccine. Published results are summarized in Figure 1B. Despite an incomplete understanding of the basic mechanisms of immunity conferred by BCG against the development of TB, researchers have continuously sought to improve it. As a result, many rBCG have been generated throughout the years. One of the pioneering studies tested the protective efficacy of a rBCG vaccine expressing the outer surface protein A (OspA) antigen of Borrelia burgdorferi in mice and found antibody responses against M.tb [53]. Following this initial study, many rBCG vaccines to protect against TB have been developed.
Researchers have aimed to improve BCG by having it expressing single and/or multiple M.tb molecules. One of the first successful attempts to improve BCG was conducted by Horwitz et al. [54]. They constructed a rBCG strain expressing and secreting the M.tb 30-kDa major secretory protein (or Ag85B). rBCG30 vaccinated Guinea pigs challenged with M.tb via aerosol had fewer lung lesions and 0.5 log10 fewer CFUs in the lung when compared to conventional BCG vaccinated animals, while spleen CFU decreased ten-fold [54]. This study highlighted Ag85B antigenicity and linked it to protective immunity against TB. In this regard, rBCG30 can cross-protect against Mycobacterium leprae challenge, which is further enhanced by M.tb 30-kDa Ag85B boosting [55]. In order to address if M.tb secreted antigens are also capable of inducing a protective response, Pym et al. constructed a rBCG strain (BCG:RD1-2F9) containing the complete region of deletion-1 (RD1) locus, which contains 11 genes including the ones encoding the potent, secreted T-cell antigens ESAT-6 (6-kDa early secretory antigenic target) and CFP-10 (10-kDa culture filtrate protein) [56]. Contrary to rBCG30, vaccination of Guinea pigs with rBCG:RD1-2F9 did not significantly reduce M.tb CFUs in the lung when compared to BCG alone; however, dissemination to the spleen was reduced 10% [56]. The failure to reduce M.tb CFUs in lung by rBCG:RD1-2F9 was considered to be tissue specific as this rBCG vaccine was able to reduce dissemination and prolong survival, thus fuelling the idea that a vaccine that inhibits M.tb dissemination from the lungs may have a major positive impact on TB outcome [56]. However, the majority of subsequent studies that are focused on improving TB vaccination emphasize controlling M.tb infection and active TB development in the lung.
Many other rBCG vaccines have been constructed, including rBCG strains expressing a fusion protein of Ag85A-ESAT-6[57;58], the M.tb hspX, perfringolysin O from Clostridium perfringens, human IL-12p70 and Ag85A individually or jointly, Mtb72f (Mtb39 + Mtb32), and many more, all with variable levels of success in reducing M.tb CFUs after challenge. However, similar to BCG, none fully prevent the development of TB [56;59–66]. Nevertheless, a promising development in the TB vaccine field may be the vaccine engineered by Grode et al. based on improving access of mycobacterial antigens to the MHC class I pathway to boost CD8 T-cell responses [67]. Their strain, rBCGΔUre:CHly+ (a BCG strain that secretes listeriolysin (Hly) from Listeria monocytogenes), is highly protective against M.tb challenge via the aerosol route with almost 3 log10 reduction in bacterial load in the lung at day 200 post infection. This enhanced efficacy was attributed to an efficient perforation of the phagocyte phagosomal membrane by Hly [68], promoting antigen translocation into the phagocyte cytoplasm and enhancing cross-priming to both helper and cytotoxic T-cells [69]. The protection afforded by rBCGΔUre:CHly+ supports an important role for CD8 T-cells in reducing M.tb CFUs and potentially establishing protection against the development of active TB. Furthermore, the ability of rBCGΔUre:CHly+ to induce greater numbers of central memory CD4 T-cells was also considered an important step in this process [70]. Due to its increased potential at protecting against TB, rBCGΔUre:CHly+ is now in clinical trials [50–52]. Similarly, autophagy, a catabolic process by which the cell digests cellular components, has been shown to assist DCs in processing extracellular antigens for MHC class I presentation [71]. Jagannath et al. hypothesized that by inducing autophagy, immunity to BCG could be enhanced by increasing MHC class I antigen presentation. Indeed, induction of autophagy in BCG infected DCs which were subsequently transferred to mice reduced M.tb CFU in the lungs by an additional 2 log10 compared to conventional BCG [72]. Thus, in combination with the enhanced protection shown by rBCGΔUre:CHly+, these data indicate that CD8 T-cells may play an underestimated role in reducing the burden of M.tb in the lung and perhaps protection against TB.
Researchers have sought to improve BCG in other ways, by vaccinating with cytokines [73], chemokines [74], lipid mediators [75], nucleotides [76], and antigenic components from M.tb [77]; all with variable levels of success. Other studies have focused on the role of IL-10 as an immunomodulator [78]. The absence of IL-10 enhances the ability of the immune system to clear M.tb infection in the lung and spleen of mice [79]. Recently, Pitt et al. reasoned that by inhibiting IL-10 and simultaneously vaccinating with BCG, a more robust immune response could be mounted against M.tb challenge. Their results showed enhanced cellular activation and 10-fold decrease in bacterial burden in the lung compared to conventional BCG vaccination [80]. This study highlights the importance of understanding the innate immune responses to BCG and the mechanisms that can lead to effective immunity to mycobacteria. As outlined by the many studies above, a basic understanding of the cellular responses generated after BCG vaccination can elucidate bacterial and/or host components required for optimal protection against the development of active TB.
Unfortunately, the failure of the MVA85 vaccine trial was a significant step back for the TB vaccine development field [81]. MVA is an attenuated strain of the vaccinia virus lacking all virulence factors including its ability to replicate within human cells [82]. MVA was turned into MVA85A when it was made to express Ag85A from M.tb. When mice were vaccinated with BCG and boosted with MVA85A, this combination generated higher levels of antigen specific CD4 and CD8 T-cells [83]. In phase I human clinical trials, MVA85A was found to induce high levels of antigen-specific IFNγ secreting T-cells [83] and showed enhanced IFNγ activity in volunteers who were previously vaccinated with BCG (range of 0.5–38 years)[82]. However, despite its success in animal models and early phase human clinical trials, MVA85A (delivered to a BCG vaccinated cohort of infants) was 17.3% effective against the development of TB as detected by microbiological, radiological, and clinical criteria, and had similar efficacy against controlling M.tb infection measured by the QuantiFERON TB Gold In-tube conversion test [81]. No additional protection against the development of TB, on top of that afforded by BCG, was reported in this study [81]. This significant setback highlights the required need for a better understanding of the host immune responses generated by BCG during vaccination, and the need to identify irrefutable correlates of protection against pulmonary and disseminated TB.
Other vaccines designed against TB relate to therapeutic vaccines, useful for TB patients that can no longer be cured by pharmacotherapy (i.e. patients infected with XDR, or XXDR-M.tb strains). These vaccines are mainly based on mycobacterial products (i.e. heat killed whole or fragmented [84] M.tb or its lysates) thought to generate a strong immune response; however, there are concerns about their safety and immunogenicity [85]. To bypass these issues, the concept of using auxotroph attenuated M.tb strains as vaccine candidates appeared more than 20 years ago [86], where recent advances in the development of attenuated M.tb auxotrophs containing mutations that enhance the adaptive immune response are reported [87].
BCG Vaccination and the Lung Environment
Of particular interest to us is the impact of the lung environment in generating and modulating the immune response to M.tb infection. Our laboratory has reported that the human lung mucosa is rich in hydrolytic activities capable of modifying the M.tb cell wall surface exposing previously masked motifs, and releasing M.tb cell wall fragments into the alveolar milieu [88]. These M.tb cell wall alterations had direct consequences on the recognition of M.tb by human macrophages and neutrophils as well as on the ability of these phagocytes to control M.tb intracellular growth [88;89]. As a consequence, immune recognition in the lung may be through modified M.tb bacilli expressing different epitopes on their cell wall surface that could have direct impact on antigen recognition and presentation. Our current findings assessing the bioactivity of the M.tb released fragments by lung mucosa hydrolases suggest that these fragments may be capable of modifying the macrophage phenotype to an alternative state, enhancing its capacity to control infection (unpublished results). Our data suggest a potential role for human lung mucosa components in orchestrating the immune response against M.tb infection. Hence, a way to improve BCG may simply lie in accounting for the lung environment and understanding how it orchestrates M.tb infection and the development of active TB.
Concluding Remarks
Based on all current results and efforts to improve the BCG vaccine, it is first critical to improve our understanding of immunity to mycobacteria, and how this immunity arises, stabilizes and dampens down through the period of post-BCG vaccination. It is also important to contemplate the impact of the route of administration, co-infections with other endemic microbes, as well as the impact of the lung environment in defining how the immune response to M.tb infection is orchestrated. Another concern is the standard laboratory M.tb strain(s) being used to verify the protective role of these rBCG vaccines that present different virulent patterns and pathologies than the strains found in endemic areas, and thus what we may be assessing in the laboratory is a vaccine with the wrong pattern of protection [90]. Moreover, laboratory strains vary between researchers and thus add to the difficulty of comparing vaccine efficacy across studies.
Importantly, most vaccine clinical trials in humans assess vaccine protection by infection transmission or development of TB [91]. In this regard, there is an urgent need to identify correlates of protection that can be extrapolated from the laboratory to the field. Although valuable information can be deduced from animal models of M.tb infection and disease as well as from human field trial records, an important point of concern in the TB field is the disparity between efficacy testing in the laboratory vs. the field. Animal models have revealed great insight into the pathological mechanism employed by M.tb to invade host cells in addition to the immunological responses required for its control [92]. However, these measurements are not always an accurate reflection of TB progression in humans [93]. Studies on animal models center on the idea that reducing CFU in the lung decreases overall morbidity, whereas human clinical trials measure the development of active TB in the vaccinated population as their primary indicator of protection [4;94]. By equating the measurement outcomes in animal models and in humans we can expect to reduce the uncertainty associated with BCG vaccination and its protective efficacy. No current or developed vaccines generate protective immunity sufficient to clear M.tb in animal models. How this protection extends to humans is unclear and leads to some critical questions. Should we expect complete protection from TB disease by BCG in humans, when this cannot be achieved in animal models? Should the measurement of a successful vaccine trial be a delay to active TB disease and prolonged survival? More importantly, should our expectations of protection be held to a higher standard in animal models before returning to human trials? These questions remain to be answered and we may need to reassess and better align the definition of a protective vaccine in humans and animal models.
BCG, the only approved vaccine against TB, falls short of protecting against pulmonary disease in humans. New research has focused on shifting the route of immunization from the most commonly used parenteral route to more immunogenic sites within the body. Of particular significance is intranasal immunization, the most protective route against pulmonary TB. However, the caveat associated with this form of immunization lies in the pathology accompanied by delivering live BCG directly into the lung. Thus, exploring the mechanism(s) induced by BCG to cause such pathology could reveal a novel method of immunizing mucosal routes while minimizing tissue damage. Important unconsidered factors in TB vaccine development are the impact of the lung environment on the M.tb cell wall as well as the use of M.tb clinical isolates from TB endemic areas to assess the potential of the candidate vaccine. The prolific generation of rBCG strains has been both promising and unsatisfactory. In this regard, the use of the most protective BCG substrain as the background in the development of all rBCG vaccine candidates has not been standardized.
Other questions remain, why are we only working towards the improvement of a TB vaccine that protects against the development of the disease; and why are we not addressing our efforts in developing a TB vaccine that will prevent M.tb infection and/or assist in eliminating the infection at the very early stages prior to granuloma formation? Are early events (i.e. M.tb-environment contact) dictating the protection and infection outcome? If this is the case, we need to start considering the role of the lung environment in modifying M.tb contact with the host, where this interaction could be dictating the protective balance towards an initial infection that progresses to latency or towards active disease. If infection cannot be circumvented, a vaccine could be designed to maintain M.tb in persistent dormancy, avoiding an uncontrollable reactivation and subsequent active TB.
Despite many setbacks, further research on the immunogenicity of BCG will answer critical questions as to what constitutes immunity to mycobacteria. The answer may be that BCG is not the right vaccine to improve, and thus the use of other Mycobacterium spp as a background to produce an efficient vaccine against TB may need to be considered.
Highlights.
We discuss the impact of the BCG vaccination route in protecting against the development of active TB
We discuss the impact of the variability of using different BCG substrains in protecting against TB
We discuss new vaccine strategies and developments to improve the BCG vaccine, as well as the potential impact of the lung environment in the success of vaccination against M.tb infection and/or the development of active TB
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (NIH/NIAID, grant numbers AI073856 and AI093570 to JBT.; by a Julie Martin Mid-Career award from The American Federation for Aging Research partially supporting JT.; and by The Ohio State University College of Medicine Systems in Integrative Biology Training Program - NIH/National Institute of General Medical Sciences (NIGMS) T32-GM068412 and NIH/NIAID AI093570-S1 partially supporting JIM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest All authors disclose that they do not have a conflict of interest in the presented work.
References
- [1].WHO WHO Tuberculosis Fact Sheet 2015. World Health Organization 15 A.D. Mar 20th; Available from: URL: http://www.who.int/mediacentre/factsheets/fs104/en/
- [2].Fine PEM. Variation in protection by BCG: Implications of and for heterologous immunity. Lancet. 1995;346:1339–45. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- [3].Aronson NE, Santosham M, Comstock GW, et al. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: A 60-year follow-up study. JAMA. 2004 May 5;291(17):2086–91. doi: 10.1001/jama.291.17.2086. [DOI] [PubMed] [Google Scholar]
- [4].Smith KC, Orme IM, Starke JR. Vaccines. Sixth ed Saunders; 2012. Tuberculosis vaccines; pp. 789–811. [Google Scholar]
- [5].Luca S, Mihaescu T. History of BCG Vaccine. Maedica (Buchar) 2013 Mar;8(1):53–8. [PMC free article] [PubMed] [Google Scholar]
- [6].Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol. 2010;8:129. doi: 10.1186/1741-7007-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lagranderie M, Murray A, Gicquel B, Leclerc C, Gheorghiu M. Oral immunization with recombinant BCG induces cellular and humoral immune responses against the foreign antigen. Vaccine. 1993;11:1283–90. doi: 10.1016/0264-410x(93)90096-g. [DOI] [PubMed] [Google Scholar]
- [8].Aldwell FE, Keen DL, Parlane NA, Skinner MA, De Lisle GW, Buddle BM. Oral vaccination with Mycobacterium bovis BCG in a lipid formulation induces resistance to pulmonary tuberculosis in brushtail possums. Vaccine. 2003 Dec 8;22(1):70–6. doi: 10.1016/s0264-410x(03)00539-5. [DOI] [PubMed] [Google Scholar]
- [9].Aldwell FE, Cross ML, Fitzpatrick CE, Lambeth MR, De Lisle GW, Buddle BM. Oral delivery of lipid-encapsulated Mycobacterium bovis BCG extends survival of the bacillus in vivo and induces a long-term protective immune response against tuberculosis. Vaccine. 2006 Mar 15;24(12):2071–8. doi: 10.1016/j.vaccine.2005.11.017. [DOI] [PubMed] [Google Scholar]
- [10].Aldwell FE, Brandt L, Fitzpatrick C, Orme IM. Mice fed lipid-encapsulated Mycobacterium bovis BCG are protected against aerosol challenge with Mycobacterium tuberculosis. Infect Immun. 2005 Mar;73(3):1903–5. doi: 10.1128/IAI.73.3.1903-1905.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ancelet LR, Aldwell FE, Rich FJ, Kirman JR. Oral vaccination with lipid-formulated BCG induces a long-lived, multifunctional CD4(+) T cell memory immune response. PLoS ONE. 2012;7(9):e45888. doi: 10.1371/journal.pone.0045888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vipond J, Cross ML, Lambeth MR, Clark S, Aldwell FE, Williams A. Immunogenicity of orally-delivered lipid-formulated BCG vaccines and protection against Mycobacterium tuberculosis infection. Microbes Infect. 2008 Nov;10(14–15):1577–81. doi: 10.1016/j.micinf.2008.09.004. [DOI] [PubMed] [Google Scholar]
- [13].Clark SO, Kelly DL, Badell E, et al. Oral delivery of BCG Moreau Rio de Janeiro gives equivalent protection against tuberculosis but with reduced pathology compared to parenteral BCG Danish vaccination. Vaccine. 2010 Oct 8;28(43):7109–16. doi: 10.1016/j.vaccine.2010.07.087. [DOI] [PubMed] [Google Scholar]
- [14].Vaca M, Moncayo AL, Cosgrove CA, et al. A Single dose of oral BCG Moreau fails to boost systemic IFN-gamma responses to tuberculin in children in the rural tropics: Evidence for a barrier to mucosal immunization. J Trop Med. 2012;2012:132583. doi: 10.1155/2012/132583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bricks LF. Percutaneous or intradermal BCG vaccine? J Pediatr (Rio J) 2004 Mar;80(2):93–8. [PubMed] [Google Scholar]
- [16].Dagg B, Hockley J, Rigsby P, Ho MM. The establishment of sub-strain specific WHO reference reagents for BCG vaccine. Vaccine. 2014 Nov 12;32(48):6390–5. doi: 10.1016/j.vaccine.2014.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Glatthaar E, Kleberg HH. BCG immunization of infants by percutaneous multiple puncture. South African Medical Journal. 1977;52:1173–4. [Google Scholar]
- [18].Jarad NA, Empey DW, Duckworth G. Administration of the BCG vaccination using the multipuncture method in schoolchildren: A comparison with the intradermal method. Thorax. 1999 Sep;54(9):762–4. doi: 10.1136/thx.54.9.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hussey GD, Watkins ML, Goddard EA, et al. Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology. 2002 Mar;105(3):314–24. doi: 10.1046/j.1365-2567.2002.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fine PE. Variation in protection by BCG: Implications of and for heterologous immunity. Lancet. 1995 Nov 18;346(8986):1339–45. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- [21].Awasthi S, Moin S. Effectiveness of BCG vaccination against tuberculosis meningitis. Indian Pediatrics. 1999;36:455–60. [PubMed] [Google Scholar]
- [22].Fine PE. BCG: The challenge continues. Scand J Infect Dis. 2001;33(4):243–5. doi: 10.1080/003655401300077144. [DOI] [PubMed] [Google Scholar]
- [23].Hawkridge A, Hatherill M, Little F, et al. Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: Randomised trial. BMJ. 2008;337:a2052. doi: 10.1136/bmj.a2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Power CA, Wei G, Bretscher PA. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect Immun. 1998 Dec;66(12):5743–50. doi: 10.1128/iai.66.12.5743-5750.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Abolhassani M, Lagranderie M, Chavarot P, Balazuc AM, Marchal G. Mycobacterium bovis BCG induces similar immune responses and protection by rectal and parenteral immunization routes. Infect Immun. 2000 Oct;68(10):5657–62. doi: 10.1128/iai.68.10.5657-5662.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Davids V, Hanekom WA, Mansoor N, et al. The effect of bacille Calmette-Guerin vaccine strain and route of administration on induced immune responses in vaccinated infants. J Infect Dis. 2006 Feb 15;193(4):531–6. doi: 10.1086/499825. [DOI] [PubMed] [Google Scholar]
- [27].Brewer TF, Colditz GA. Relationship between bacille Calmette-Guérin (BCG) strains and the efficacy of BCG vaccine in the prevention of tuberculosis. Clin Infect Dis. 1995;20:126–35. doi: 10.1093/clinids/20.1.126. [DOI] [PubMed] [Google Scholar]
- [28].Davids V, Hanekom W, Gelderbloem SJ, et al. Dose-dependent immune response to Mycobacterium bovis BCG vaccination in neonates. Clin Vaccine Immunol. 2007 Feb;14(2):198–200. doi: 10.1128/CVI.00309-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lagranderie MRR, Balazuc AM, Deriaud E, Leclerc CD, Gheorghiu M. Comparison of immune responses of mice immunized with five different Mycobacterium bovis BCC vaccine strains. Infect Immun. 1996;64:1–9. doi: 10.1128/iai.64.1.1-9.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Castillo-Rodal AI, Castanon-Arreola M, Hernandez-Pando R, Calva JJ, Sada-Diaz E, Lopez-Vidal Y. Mycobacterium bovis BCG substrains confer different levels of protection against Mycobacterium tuberculosis infection in a BALB/c model of progressive pulmonary tuberculosis. Infect Immun. 2006 Mar;74(3):1718–24. doi: 10.1128/IAI.74.3.1718-1724.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. Commonly administered BCG strains including an evolutionarily early strain and evolutionarily late strains of disparate genealogy induce comparable protective immunity against tuberculosis. Vaccine. 2009 Jan 14;27(3):441–5. doi: 10.1016/j.vaccine.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Middlebrook G. Immunological aspects of airborne infection: Reactions to inhaled antigens. Bacteriol Rev. 1961 Sep;25:331–46. doi: 10.1128/br.25.3.331-346.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Barclay WR, Busey WM, Dalgard DW, et al. Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guerin. Am Rev Respir Dis. 1973 Mar;107(3):351–8. doi: 10.1164/arrd.1973.107.3.351. [DOI] [PubMed] [Google Scholar]
- [34].Lefford MJ. Induction and expression of immunity after BCG immunization. Infect Immun. 1977 Dec;18(3):646–53. doi: 10.1128/iai.18.3.646-653.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nuermberger EL, Yoshimatsu T, Tyagi S, Bishai WR, Grosset JH. Paucibacillary tuberculosis in mice after prior aerosol immunization with Mycobacterium bovis BCG. Infect Immun. 2004 Feb;72(2):1065–71. doi: 10.1128/IAI.72.2.1065-1071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen L, Wang J, Zganiacz A, Xing Z. Single intranasal mucosal Mycobacterium bovis BCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis. Infect Immun. 2004 Jan;72(1):238–46. doi: 10.1128/IAI.72.1.238-246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rhoades E, Hsu F, Torrelles JB, Turk J, Chatterjee D, Russell DG. Identification and macrophage-activating activity of glycolipids released from intracellular Mycobacterium bovis BCG. Mol Microbiol. 2003 May;48(4):875–88. doi: 10.1046/j.1365-2958.2003.03473.x. [DOI] [PubMed] [Google Scholar]
- [38].Rhoades ER, Geisel RE, Butcher BA, McDonough S, Russell DG. Cell wall lipids from Mycobacterium bovis BCG are inflammatory when inoculated within a gel matrix: haracterization of a new model of the granulomatous response to mycobacterial components. Tuberculosis (Edinb.) 2005 May;85(3):159–76. doi: 10.1016/j.tube.2004.10.001. [DOI] [PubMed] [Google Scholar]
- [39].Torrelles JB. Broadening our view about the role of Mycobacterium tuberculosis cell envelope components during infection: A battle for survival. In: Cardona PJ, editor. Understanding Tuberculosis - Analyzing the Origin of Mycobacterium tuberculosis Pathogenicity. Rijeka, Croatia, Intech; 2012. pp. 1–46. [Google Scholar]
- [40].Ritz N, Hanekom WA, Robins-Browne R, Britton WJ, Curtis N. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol Rev. 2008 Aug;32(5):821–41. doi: 10.1111/j.1574-6976.2008.00118.x. [DOI] [PubMed] [Google Scholar]
- [41].Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M-bovis. J Bacteriol. 1996;178:1274–82. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Behr MA, Wilson MA, Gill WP, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–3. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- [43].Brosch R, Gordon SV, Garnier T, et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci U S A. 2007 Mar 27;104(13):5596–601. doi: 10.1073/pnas.0700869104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Minnikin DE, Parlett JH, Magnusson M, Ridell M, Lind A. Mycolic acid patterns of representatives of Mycobacterium bovis BCG. J Gen Microbiol. 1984 Oct;130(10):2733–6. doi: 10.1099/00221287-130-10-2733. [DOI] [PubMed] [Google Scholar]
- [45].Behr MA, Small PM. A historical and molecular phylogeny of BCG strains. Vaccine. 1999 Feb 26;17(7–8):915–22. doi: 10.1016/s0264-410x(98)00277-1. [DOI] [PubMed] [Google Scholar]
- [46].Dietrich G, Viret JF, Hess J. Mycobacterium bovis BCG-based vaccines against tuberculosis: Novel developments. Vaccine. 2003 Jan 30;21(7–8):667–70. doi: 10.1016/s0264-410x(02)00577-7. [DOI] [PubMed] [Google Scholar]
- [47].Ritz N, Curtis N. Mapping the global use of different BCG vaccine strains. Tuberculosis (Edinb.) 2009 Jul;89(4):248–51. doi: 10.1016/j.tube.2009.03.002. [DOI] [PubMed] [Google Scholar]
- [48].Zhang W, Zhang Y, Zheng H, et al. Genome sequencing and analysis of BCG vaccine strains. PLoS ONE. 2013;8(8):e71243. doi: 10.1371/journal.pone.0071243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mangtani P, Abubakar I, Ariti C, et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin Infect Dis. 2014 Feb;58(4):470–80. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- [50].Orme IM. Vaccine development for tuberculosis: Current progress. Drugs. 2013 Jul;73(10):1015–24. doi: 10.1007/s40265-013-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kaufmann SH, Lange C, Rao M, et al. Progress in tuberculosis vaccine development and host-directed therapies - a state of the art review. Lancet Respir Med. 2014 Apr;2(4):301–20. doi: 10.1016/S2213-2600(14)70033-5. [DOI] [PubMed] [Google Scholar]
- [52].da Costa C, Walker B, Bonavia A. Tuberculosis vaccines - state of the art, and novel approaches to vaccine development. Int J Infect Dis. 2015 Mar;32:5–12. doi: 10.1016/j.ijid.2014.11.026. [DOI] [PubMed] [Google Scholar]
- [53].Stover CK, de la Cruz VF, Fuerst TR, et al. New use of BCG for recombinant vaccines. Nature. 1991;351(6326):456–60. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- [54].Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. Recombinant bacillus Calmette-Guérin (BCG) vaccines expressing the Mycobacteirum tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci USA. 2000;97(25):13853–8. doi: 10.1073/pnas.250480397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gillis TP, Tullius MV, Horwitz MA. rBCG30-induced immunity and cross-protection against Mycobacterium leprae challenge are enhanced by boosting with the Mycobacterium tuberculosis 30-kilodalton antigen 85B. Infect Immun. 2014 Sep;82(9):3900–9. doi: 10.1128/IAI.01499-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pym AS, Brodin P, Majlessi L, et al. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med. 2003 May;9(5):533–9. doi: 10.1038/nm859. [DOI] [PubMed] [Google Scholar]
- [57].Deng YH, He HY, Zhang BS. Evaluation of protective efficacy conferred by a recombinant Mycobacterium bovis BCG expressing a fusion protein of Ag85A ESAT-6. Journal of Microbiology, Immunology and Infection. 2014;47(1):48–56. doi: 10.1016/j.jmii.2012.11.005. [DOI] [PubMed] [Google Scholar]
- [58].Wang C, Fu R, Chen Z, et al. Immunogenicity and protective efficacy of a novel recombinant BCG strain overexpressing antigens Ag85A and Ag85B. Clin Dev Immunol. 2012;2012:563838. doi: 10.1155/2012/563838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bastos RG, Borsuk S, Seixas FK, Dellagostin OA. Recombinant Mycobacterium bovis BCG. Vaccine. 2009 Nov 5;27(47):6495–503. doi: 10.1016/j.vaccine.2009.08.044. [DOI] [PubMed] [Google Scholar]
- [60].Dhar N, Rao V, Tyagi AK. Immunogenicity of recombinant BCG vaccine strains overexpressing components of the antigen 85 complex of Mycobacterium tuberculosis. Med Microbiol Immunol. 2004 Feb;193(1):19–25. doi: 10.1007/s00430-002-0170-x. [DOI] [PubMed] [Google Scholar]
- [61].Badell E, Nicolle F, Clark S, et al. Protection against tuberculosis induced by oral prime with Mycobacterium bovis BCG and intranasal subunit boost based on the vaccine candidate Ag85B-ESAT-6 does not correlate with circulating IFN-gamma producing T-cells. Vaccine. 2009 Jan 1;27(1):28–37. doi: 10.1016/j.vaccine.2008.10.034. [DOI] [PubMed] [Google Scholar]
- [62].Qie YQ, Wang JL, Zhu BD, et al. Evaluation of a new recombinant BCG which contains mycobacterial antigen ag85B-mpt64(190-198)-mtb8.4 in C57/BL6 mice. Scand J Immunol. 2008 Feb;67(2):133–9. doi: 10.1111/j.1365-3083.2007.02048.x. [DOI] [PubMed] [Google Scholar]
- [63].Tang C, Yamada H, Shibata K, et al. Efficacy of recombinant bacille Calmette-Guerinvaccine secreting interleukin-15/antigen 85B fusion protein in providing protection against Mycobacterium tuberculosis. J Infect Dis. 2008 May 1;197(9):1263–74. doi: 10.1086/586902. [DOI] [PubMed] [Google Scholar]
- [64].Sun R, Skeiky YA, Izzo A, et al. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine. 2009 Jul 16;27(33):4412–23. doi: 10.1016/j.vaccine.2009.05.048. [DOI] [PubMed] [Google Scholar]
- [65].Rao M, Vogelzang A, Kaiser P, Schuerer S, Kaufmann SH, Gengenbacher M. The tuberculosis vaccine candidate Bacillus Calmette-Guerin DeltaureC∷hly coexpressing human interleukin-7 or -18 enhances antigen-specific T cell responses in mice. PLoS ONE. 2013;8(11):e78966. doi: 10.1371/journal.pone.0078966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kita Y, Tanaka T, Yoshida S, et al. Novel recombinant BCG and DNA-vaccination against tuberculosis in a cynomolgus monkey model. Vaccine. 2005 Mar 18;23(17–18):2132–5. doi: 10.1016/j.vaccine.2005.01.057. [DOI] [PubMed] [Google Scholar]
- [67].Winau F, Weber S, Sad S, et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006 Jan;24(1):105–17. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- [68].Beauregard KE, Lee KD, Collier RJ, Swanson JA. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J Exp Med. 1997 Oct 6;186(7):1159–63. doi: 10.1084/jem.186.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Grode L, Seiler P, Baumann S, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest. 2005 Sep;115(9):2472–9. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Vogelzang A, Perdomo C, Zedler U, et al. Central memory CD4+ T cells are responsible for the recombinant Bacillus Calmette-Guerin DeltaureC∷hly vaccine's superior orotection against tuberculosis. J Infect Dis. 2014 Jun 18; doi: 10.1093/infdis/jiu347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Li Y, Wang LX, Yang G, Hao F, Urba WJ, Hu HM. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 2008 Sep 1;68(17):6889–95. doi: 10.1158/0008-5472.CAN-08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009 Mar;15(3):267–76. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- [73].Freidag BL, Melton GB, Collins F, et al. CpG oligodeoxynucleotides and interleukin-12 improve the efficacy of Mycobacterium bovis BCG vaccination in mice challenged with M. tuberculosis. Infect Immun. 2000 May;68(5):2948–53. doi: 10.1128/iai.68.5.2948-2953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ryan AA, Spratt JM, Britton WJ, Triccas JA. Secretion of functional monocyte chemotactic protein 3 by recombinant Mycobacterium bovis BCG attenuates vaccine virulence and maintains protective efficacy against M. tuberculosis infection. Infect Immun. 2007 Jan;75(1):523–6. doi: 10.1128/IAI.00897-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wedlock DN, Denis M, Painter GF, et al. Enhanced protection against bovine tuberculosis after coadministration of Mycobacterium bovis BCG with a mycobacterial protein vaccine-adjuvant combination but not after coadministration of adjuvant alone. Clin Vaccine Immunol. 2008 May;15(5):765–72. doi: 10.1128/CVI.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cervantes-Villagrana AR, Hernandez-Pando R, Biragyn A, et al. Prime-boost BCG vaccination with DNA vaccines based in beta-defensin-2 and mycobacterial antigens ESAT6 or Ag85B improve protection in a tuberculosis experimental model. Vaccine. 2013 Jan 11;31(4):676–84. doi: 10.1016/j.vaccine.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cardona PJ, Amat I. Origin and development of RUTI, a new therapeutic vaccine against Mycobacterium tuberculosis infection. Arch Bronconeumol. 2006 Jan;42(1):25–32. doi: 10.1016/s1579-2129(06)60110-9. [DOI] [PubMed] [Google Scholar]
- [78].Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- [79].Redford PS, Boonstra A, Read S, et al. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur J Immunol. 2010 Aug;40(8):2200–10. doi: 10.1002/eji.201040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Pitt JM, Stavropoulos E, Redford PS, et al. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol. 2012 Oct 15;189(8):4079–87. doi: 10.4049/jimmunol.1201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tameris MD, Hatherill M, Landry BS, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: A randomised, placebo-controlled phase 2b trial. Lancet. 2013 Mar 23;381(9871):1021–8. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].McShane H, Pathan AA, Sander CR, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004 Nov;10(11):1240–4. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- [83].Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol. 2003 Aug 1;171(3):1602–9. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- [84].Nell AS, D'lom E, Bouic P, et al. Safety, tolerability, and immunogenicity of the novel antituberculous vaccine RUTI: Randomized, placebo-controlled phase II clinical trial in patients with latent tuberculosis infection. PLoS ONE. 2014;9(2):e89612. doi: 10.1371/journal.pone.0089612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Groschel MI, Prabowo SA, Cardona PJ, Stanford JL, Van der Werf TS. Therapeutic vaccines for tuberculosis - a systematic review. Vaccine. 2014 May 30;32(26):3162–8. doi: 10.1016/j.vaccine.2014.03.047. [DOI] [PubMed] [Google Scholar]
- [86].Guleria I, Teitelbaum R, McAdam RA, Kalpana G, Jacobs WR, Jr, Bloom BR. Auxotrophic vaccines for tuberculosis. Nature Med. 1996;2:334–7. doi: 10.1038/nm0396-334. [DOI] [PubMed] [Google Scholar]
- [87].Lee S, Jeon BY, Bardarov S, Chen M, Morris SL, Jacobs WR., Jr Protection elicited by two glutamine auxotrophs of Mycobacterium tuberculosis and in vivo growth phenotypes of the four unique glutamine synthetase mutants in a murine model. Infect Immun. 2006 Nov;74(11):6491–5. doi: 10.1128/IAI.00531-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Arcos J, Sasindran SJ, Fujiwara N, Turner J, Schlesinger LS, Torrelles JB. Human lung hydrolases delineate Mycobacterium tuberculosis-macrophage interactions and the capacity to control infection. J Immunol. 2011 May 20;187(1):372–81. doi: 10.4049/jimmunol.1100823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Arcos J, Diangelo L, Scordo J, et al. Lung mucosa lining fluid modifies Mycobacterium tuberculosis to reprogram human neutrophil killing mechanisms. J Infect Dis. 2015 doi: 10.1093/infdis/jiv146. DOI: 110.1093/infdis/jiv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Jeon BY, Derrick SC, Lim J, et al. Mycobacterium bovis BCG immunization induces protective immunity against nine different Mycobacterium tuberculosis strains in mice. Infect Immun. 2008 Nov;76(11):5173–80. doi: 10.1128/IAI.00019-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Frick M. The Tuberculosis Vaccines Pipeline: A New Path to the Same Destination? 2015 HIV-HCV-TB 2015 Pipeline Report - TAG ibase. http://i-base.info/htb/28472.
- [92].Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- [93].Dharmadhikari AS, Nardell EA. What animal models teach humans about tuberculosis. Am J Respir Cell Mol Biol. 2008 Nov;39(5):503–8. doi: 10.1165/rcmb.2008-0154TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994 Mar 2;271(9):698–702. [PubMed] [Google Scholar]