Abstract
Introduction
Plasma D-dimer is a useful clinical test for acute venous thromboembolism (VTE), and concentrations remain higher in VTE patients after treatment than in controls. Yet, evidence is limited on whether higher basal D-dimer concentrations in the general population are associated with greater risk of first VTE.
Objective
To assess the prospective association between D-dimer and incident VTE over a long follow-up.
Methods
We measured plasma D-dimer in 12,097 participants, initially free of VTE, in the Atherosclerosis Risk in Communities Study. Over a median follow-up of 17 years, we identified 521 VTEs. We calculated hazard ratios of VTE using proportional hazards regression.
Results
The age, race, and sex adjusted hazard ratios of VTE across quintiles of D-dimer were 1, 1.5, 1.8, 2.1, and 3.2 (p for trend <0.0001). For the first 10 years of follow-up, the hazard ratio for the highest versus lowest quintile was 3.5, and was 2.9 after 10 years. In both whites and African Americans, VTE risk remained strongly associated with D-dimer after further adjustment for diabetes, body mass index, kidney function, and several thrombophilia genetic markers. D-dimer was associated with both unprovoked and provoked VTE, but more strongly with unprovoked.
Conclusions
A higher basal level of plasma D-dimer in the general population, presumably reflecting a predisposition to thrombosis, is a strong, long-term risk factor for a first VTE.
Keywords: Deep vein thrombosis, D-dimer, Prospective studies, Pulmonary embolism, risk factors
Introduction
The plasma concentration of D-dimer, a fibrin degradation product, is an important clinical marker of acute venous thromboembolism (VTE)—i.e., venous thrombosis (DVT) or pulmonary embolism (PE). Basal D-dimer concentrations vary widely in the general population, and higher levels are strongly associated with increased incidence of atherothrombotic conditions, such as coronary heart disease and stroke [1].
To our knowledge, only one prospective population-based study has linked higher basal plasma D-dimer in the general population to increased risk of future VTE: In a small nested case-control study (n=307 incident VTEs and 616 controls), our Longitudinal Investigation of Thromboembolism Etiology (LITE) found that VTE occurrence during follow-up was four-fold higher in the fifth versus first quintile of baseline plasma D-dimer concentrations [2]. The Leiden Thrombophilia Study reported that a D-dimer above the 70th percentile, measured ≥ 6 months after VTE and compared with controls, was associated with a 2.2 fold increased odds of VTE [3]. Another VTE case-control study in women as well as a prospective study of VTE after hip replacement reported that D-dimer was positively associated with VTE occurrence [4, 5]. Clinical studies have shown that higher D-dimer after VTE recovery is also associated with increased risk of recurrent VTE [6-10].
We recently expanded plasma D-dimer measurement to nearly the entire Atherosclerosis Risk in Communities (ARIC) portion of LITE. Therefore, we now update the prospective associations between D-dimer and incident VTE in ARIC with a larger sample size and longer follow-up than our previous report [2]. The larger sample size allowed us to explore race-specific associations and the possible contribution of several genetic variants to the D-dimer findings.
Methods
Study population
We reported the ARIC study design, methods, and VTE incidence rates in detail elsewhere [11, 12]. In brief, 15,792 men and women aged 45 to 64 years enrolled in the ARIC study in 1987-1989, and had subsequent examinations in 1990-92, 1993-95, 1996-98, and 2011-13, with annual telephone contact between examinations. The institutional review committees at each study center approved the methods and staff obtained informed participant consent.
Plasma D-dimer measurements
ARIC had exhausted most baseline citrate plasma samples previously. Therefore, we measured D-dimer concentrations on fasting citrate plasma collected at ARIC visit 3 (in 1993-95) and stored unthawed at −70°C until analysis in 2014. The Laboratory for Clinical Biochemistry Research at the University of Vermont used an immuno-turbidimetric assay (Liatest D-DI; Diagnostica Stago, Parsippany, NJ) on the Evolution analyzer (Diagnostica Stago, Parsippany, NJ). The analytical coefficient of variation for this assay is 4 - 16%. Blind analysis of 73 pairs of ARIC samples split at the time of blood draw and stored until 2014 yielded an intra-class reliability coefficient of 0.92. The normal reference range is 0.22 - 4.0 μg/mL, with expected normal values <0.4 μg/mL. The laboratory recorded 222 values below the limit of detection (<0.01 μg/mL) and 28 values above the detection limit. For analysis, we assigned these groups respective values of 0.01 and 20 μg/mL (the latter being the maximum in our sample); doing so yielded virtually identical results to dropping them altogether.
Measurement of risk factors
We analyzed risk factors measured at ARIC visit 3, in which D-dimer was measured. We calculated body mass index as measured weight (kg)/height (m)2. We defined diabetes as a fasting blood glucose of 126 mg/dl or higher, non-fasting blood glucose of 200 mg/dl or higher, a physician diagnosis of diabetes, or use of antidiabetic medication in the past 2 weeks. We estimated the glomerular filtration rate (eGFR) from cystatin C at ARIC visit 2 in 1990-92. ARIC also measured several genetic variants (SNPs) associated with VTE: F5 Leiden rs6025, F2 rs1799963, ABO rs8176719 (O vs. non-O groups), FGG rs2066865, F11 rs2036914, and in African Americans, hemoglobin S (rs334) [13-16].
VTE occurrence
Staff contacted ARIC participants annually by phone and asked about all hospitalizations in the previous year. In addition, ARIC conducted surveillance of hospital discharge lists from local hospitals. Staff obtained all International Classification of Diseases (ICD) discharge codes. For ICD codes harboring possible VTE events [12], staff obtained copies of the hospital records. To validate VTE events, two physicians reviewed the records using standardized criteria [12], requiring positive imaging tests for diagnosis of DVT and PE. We restricted DVTs for this analysis to those occurring through 2011 in the lower extremity or vena cava, because upper extremity DVTs were relatively few and almost always the result of venous catheters. The reviewers sub-classified VTEs as provoked (associated with cancer, major trauma, surgery, marked immobility) or unprovoked (all others).
Statistical analysis
Of the 12,887 ARIC participants who attended visit 3, we excluded those without D-dimer measurement (n=340), those with a VTE prior to biomarker assessment (n=293), those taking warfarin (n=119), those who were not white or African American (n=36), and those with no VTE follow-up (n=2). This left a maximum of 12,097 participants for the present analyses of incident VTE. Time at risk was computed from the date of biomarker measurement to the earliest of the following: date of hospital admission for incident VTE, date of death, date of last follow-up contact, or end of follow-up. We used version 9.3 of SAS (SAS Institute, Cary, NC) for analyses.
We analyzed D-dimer as quintiles and as a log-transformed continuous variable. We first described, by D-dimer quintile, participants’ characteristics and frequencies of genetic variants for VTE. The genetic variants were coded as any risk allele versus no risk allele. Our main hypothesis was that D-dimer concentration would be associated positively with VTE incidence. We plotted Kaplan-Meier curves and used Poisson regression to compute incidence rates. We graphically modeled the natural logarithm of D-dimer using restricted cubic splines and then performed Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals of incident VTE by D-dimer quintile. We performed a test for trend in VTE occurrence across D-dimer quintiles in the Cox models using an ordinal variable representing the D-dimer median for each quintile. We confirmed the proportional hazards assumption of the Cox models by testing a D-dimer quintiles by log follow-up time interaction term. Because there was no evidence of a multiplicative D-dimer by race interaction (p>0.05), we pooled whites and African Americans for most analyses. Model 1 analyzed D-dimer with VTE adjusted for age (continuous), sex, and race; Model 2 additionally adjusted for characteristics previously associated with VTE in this cohort: diabetes status (yes or no), body mass index and eGFR (both continuous).
To determine whether D-dimer associations differ according to the presence or absence of each genetic marker of VTE risk, we tested for multiplicative interactions of log-transformed D-dimer and each genetic variant by including cross-product terms in Model 2, by race. Finally, to examine the degree to which the genetic variants for VTE might explain the race-specific D-dimer associations with VTE, we added (in Model 3) the genetic variants individually to Model 2. In the African American analysis, we initially included 10 principal components of ancestry to control for possible population stratification, but results without adjustment were the same.
Results
Among the 12,097 participants initially free of VTE, the proportions of African Americans, women, and diabetic participants and the mean age and BMI were higher across D-dimer quintiles, whereas the mean eGFR was lower across D-dimer quintiles (Table 1). D-dimer was also associated positively with having one or more risk alleles for VTE in F5 Leiden, F2 rs179963, non-O blood group, and hemoglobin S.
Table 1.
Participant Characteristics [Mean ± SD or %] in Relation to Quintiles of D-dimer, ARIC Visit 3, 1993-1995.
| Quintile of D-dimer (μg/mL) |
|||||
|---|---|---|---|---|---|
| Characteristic | 0.01-0.14 | 0.15-0.22 | 0.23-0.32 | 0.33-0.50 | 0.51-20.0 |
| n | 2,370 | 2,622 | 2,429 | 2,242 | 2,434 |
| Age, y | 58.4 ± 5.4 | 59.3 ± 5.5 | 60.0 ± 5.6 | 60.5 ± 5.8 | 61.5 ± 5.7 |
| BMI, kg/m2 | 27.1 ± 4.7 | 27.9 ± 4.9 | 28.8 ± 5.3 | 29.5 ± 6.1 | 29.1 ± 6.1 |
| eGFR, ml/min/1.73 m2 † | 98.3 ± 14.0 | 97.5 ± 14.7 | 95.8 ± 15.9 | 95.7 ± 16.8 | 92.6 ± 18.6 |
| African American | 11% | 15% | 25% | 33% | 34% |
| Women | 49% | 51% | 58% | 59% | 61% |
| Diabetes | 11% | 14% | 15% | 18% | 18% |
| Whites | |||||
| F5 Leiden rs6025* | 2.7% | 3.8% | 6.1% | 8.4% | 8.1% |
| F2 rs1799963* | 1.6% | 2.5% | 2.8% | 3.3% | 4.6% |
| ABO non-O group | 59% | 58% | 58% | 61% | 63% |
| FGG rs2066865* | 45% | 46% | 46% | 45% | 45% |
| F11 rs2036914* | 79% | 77% | 79% | 77% | 79% |
| African Americans | |||||
| F5 Leiden rs6025* | -- | 0.3% | 0.9% | 1.3% | 1.4% |
| F2 rs1799963* | 0.9% | -- | 0.4% | 0.6% | 1.1% |
| ABO non-O group | 50% | 50% | 51% | 50% | 47% |
| FGG rs2066865* | 50% | 51% | 52% | 54% | 56% |
| F11 rs2036914* | 85% | 86% | 88% | 87% | 87% |
| Hemoglobin S (rs334)* | 2.0% | 3.4% | 4.6% | 7.5% | 9.7% |
Prevalence of any risk allele for VTE.
From 1990-92 visit.
Over a median of 17 years of follow-up, we identified 521 DVTs of the lower extremity or PE. The cumulative incidence of VTE was greater across baseline D-dimer quintiles (Figure 1), with the age, race, and sex adjusted incidence rates of VTE across quintiles being 1.7, 2.4, 3.0, 3.4, and 5.2 per 1,000 person years (Table 2). Thus, the age, race, and sex adjusted hazard ratio of VTE (Model 1) was 3.2 [95% CI 2.3, 4.4] for participants in the highest versus lowest quintile of D-dimer (Table 2). This hazard ratio was 2.7 [95% CI 1.8, 4.2] for men and 4.0 [95% CI 2.4, 6.6] for women. D-dimer was associated with both unprovoked and provoked VTE, but stronger for unprovoked (Table 2). Further adjustment for diabetes, BMI, and eGFR (Model 2) had little impact on hazard ratios (Table 2).
Figure 1.
Survival free of venous thromboembolism (VTE) in relation to quintiles of plasma D-dimer, ARIC, 1993-2011
Table 2.
Incidence Rates and Hazard Ratios (HRs) of Venous Thromboembolism in Relation to Quintiles of D-dimer, ARIC, 1993-2011.
| Quintile of D-dimer (μg/mL) |
||||||
|---|---|---|---|---|---|---|
| 0.01-0.14 | 0.15-0.22 | 0.23-0.32 | 0.33-0.50 | 0.51-20.0 | p-trend | |
| Total VTE | ||||||
| N of VTEs | 51 | 84 | 102 | 110 | 174 | |
| Incidence rate (per 103 py)* | 1.7 | 2.4 | 3.0 | 3.4 | 5.2 | |
| [95% CI] | [1.3, 2.2] | [1.9, 3.0] | [2.5, 3.7] | [2.8, 4.1] | [4.4, 6.0] | |
| Model 1 HR* | 1 | 1.5 | 1.8 | 2.1 | 3.2 | <0.0001 |
| [95% CI] | -- | [1.0, 2.1] | [1.3, 2.6] | [1.5, 2.9] | [2.3, 4.4] | |
| Model 2 HR† | 1 | 1.4 | 1.7 | 1.8 | 2.8 | <0.0001 |
| [95% CI] | -- | [1.0, 1.9] | [1.2, 2.4] | [1.3, 2.5] | [2.0, 3.9] | |
| Men | ||||||
| N of VTEs | 32 | 46 | 39 | 46 | 71 | |
| Model 1 HR* | 1 | 1.3 | 1.3 | 1.7 | 2.7 | <0.0001 |
| [95% CI] | -- | [0.8, 2.0] | [0.8, 2.0] | [1.0, 2.6] | [1.8, 4.2] | |
| Women | ||||||
| N of VTEs | 19 | 38 | 63 | 64 | 103 | |
| Model 1 HR* | 1 | 1.7 | 2.6 | 2.8 | 4.0 | <0.0001 |
| [95% CI] | -- | [1.0, 3.0] | [1.6, 4.4] | [1.6, 4.6] | [2.4, 6.6] | |
| Unprovoked VTE | ||||||
| N of VTEs | 13 | 25 | 41 | 45 | 76 | |
| Model 1 HR* | 1 | 1.7 | 3.0 | 3.6 | 6.0 | <0.0001 |
| [95% CI] | -- | [0.9, 3.4] | [1.6, 5.7] | [1.9, 6.7] | [3.3, 11.0] | |
| Provoked VTEs | ||||||
| N of VTEs | 38 | 59 | 61 | 65 | 98 | |
| Model 1 HR* | 1 | 1.4 | 1.4 | 1.6 | 2.3 | <0.0001 |
| [95% CI] | -- | [0.9, 2.0] | [1.0, 2.2] | [1.1, 2.4] | [1.6, 3.4] | |
Adjusted for age, race, and sex, except where sex-stratified,
Adjusted for age, race, sex, diabetes, BMI, and eGFR.
D-dimer was associated positively with incident VTE rates both ≤ 10 years and >10 years of follow-up, although somewhat weaker for later follow-up. For example, the age, race and sex adjusted hazard ratios of total VTE across quintiles (Model 1) were 1, 1.6, 1.8, 2.3, and 3.5 (p-trend < 0.0001) in the first 10 years and 1, 1.4, 1.8, 2.0, and 2.9 (p-trend <0.0001) for >10 years.
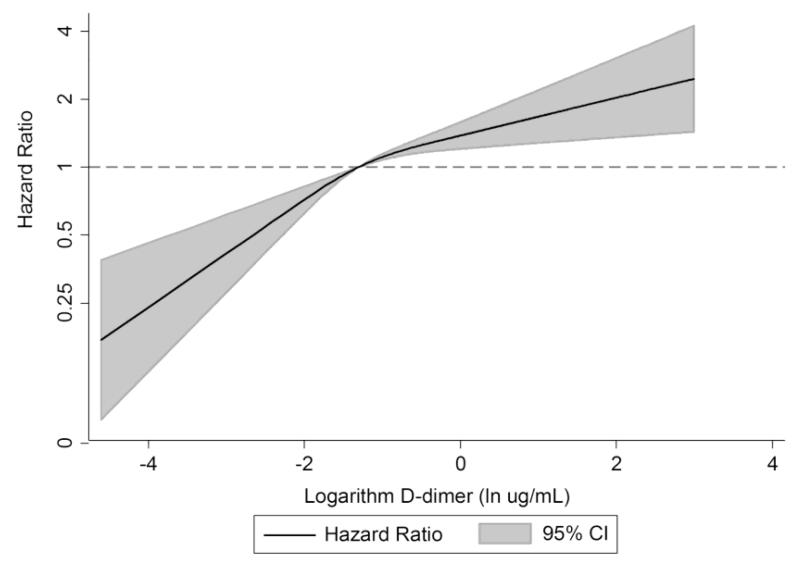
The continuous relation between D-dimer and VTE is shown in Figure 2. Adjusted for age, race, and sex, the hazard of VTE was 33% greater (95% CI 21%, 46%) per standard deviation increment of continuous log D-dimer.
Figure 2.
Age, race, sex-adjusted hazard ratio of venous thromboembolism in relation to logarithm-transformed D-dimer*, ARIC, 1993-2011
* Analyzed using restricted cubic splines with knots at the fifth, fiftieth, and ninety-fifth percentiles of the log D-dimer distribution.
Race-specific associations of D-dimer with VTE were similar by genotype (p for multiplicative interaction ≥0.05, testing all of the individual SNPs depicted in Table 1; data shown for factor V Leiden and non-O type blood type in Table 3). That is, there was no evidence on the multiplicative scale that D-dimer was more strongly associated with VTE in the presence or absence of risk alleles for any of the VTE SNPs. Likewise, the SNPs were not more strongly associated with VTE at higher versus lower D-dimer levels.
Table 3.
Hazard Ratios (HRs) and 95% CI of Venous Thromboembolism (VTE) in Relation to Dichotomized D-dimer and Thrombophilia SNPs, ARIC, 1993-2011.
| Factor V Leiden, No |
Factor V Leiden, Yes |
|||
|---|---|---|---|---|
| Low D-dimer* | High D-dimer* | Low D-dimer* | High D-dimer* | |
|
|
||||
| HR† of VTE |
1 | 1.8 | 2.0 | 4.3 |
| [95% CI] | -- | [1.5, 2.1] | [1.2, 3.5] | [2.9, 6.2] |
| n VTEs | 212 | 245 | 14 | 30 |
| O-Blood Type |
Non-O Blood Type |
|||
|---|---|---|---|---|
| Low D-dimer* | High D-dimer* | Low D-dimer* | High D-dimer* | |
|
|
|
|||
| HR† of VTE |
1 | 1.7 | 1.4 | 2.6 |
| [95% CI] | -- | [1.3, 2.4] | [1.1, 1.9] | [2.6, 3.4] |
| n VTEs | 79 | 93 | 146 | 175 |
Low D-dimer is lowest three quintiles (≤ 0.32 μg/mL) and high D-dimer is highest two quintiles (>0.32 μg/mL).
HR adjusted for sex, race, and age.
Finally, we tested, in whites and African Americans separately, the degree to which the genetic variants for VTE may explain the D-dimer associations with VTE (Table 4). For whites, the 3.0-fold gradient in VTE incidence across D-dimer quintiles fell to 2.9-fold with adjustment for the five main variants. For African Americans, the gradient in VTE remained 2.3-fold with or without adjustment for the five variants plus the hemoglobin S variant (Table 4).
Table 4.
Race-Specific Hazard Ratios (HRs) of Total Venous Thromboembolism in Relation to Quintiles of D-dimer, ARIC, 1993-2011.
| Quintile of D-dimer (μg/mL) |
||||||
|---|---|---|---|---|---|---|
| 0.01-0.14 | 0.15-0.22 | 0.23-0.32 | 0.33-0.50 | 0.51-20.0 | p-trend | |
| Whites | ||||||
| N of VTEs | 42 | 67 | 71 | 61 | 101 | |
| Model 2 HR* | 1 | 1.4 | 1.8 | 1.7 | 3.0 | <0.0001 |
| [95% CI] | -- | [0.9, 2.0] | [1.2, 2.6] | [1.2, 2.6] | [2.1, 4.3] | |
| Model 3 HR† | 1 | 1.2 | 1.8 | 1.6 | 2.9 | <0.0001 |
| [95% CI] | -- | [0.8, 1.9] | [1.2, 2.7] | [1.1, 2.5] | [1.9, 4.3] | |
| African Americans | ||||||
| N of VTEs | 9 | 17 | 31 | 49 | 73 | |
| Model 2 HR* | 1 | 1.3 | 1.3 | 1.7 | 2.3 | 0.002 |
| [95% CI] | -- | [0.6, 2.9] | [0.6, 2.7] | [0.8, 3.4] | [1.1, 4.7] | |
| Model 3 HR‡ | 1 | 1.2 | 1.1 | 1.4 | 2.1 | 0.008 |
| [95% CI] | -- | [0.5, 3.0] | [0.5, 2.6] | [0.6, 3.1] | [0.9, 4.7] | |
Adjusted for age, sex, diabetes, BMI, and eGFR.
Adjusted for age, sex, diabetes, BMI, eGFR, and presence of ≥1 risk allele for five SNPs adjusted individually (F5 Leiden rs6025, F2 rs1799963, ABO non-O group rs8176719, FGG rs2066865, F11 rs2036914).
Adjusted for age, sex, diabetes, BMI, eGFR, and presence of ≥1 risk allele for six SNPs adjusted individually (F5 Leiden rs6025, F2 rs1799963, ABO non-O group rs8176719, FGG rs2066865, F11 rs2036914, hemoglobin S rs334).
Discussion
This large population-based prospective study documented that a higher basal plasma concentration of D-dimer was associated moderately strongly with greater risk of VTE over a median of 17 years of follow-up. The association proved stronger for unprovoked (or spontaneous) VTE than for VTE provoked by triggers such as cancer, major trauma, or surgery. The association also was somewhat stronger for the first 10 years of follow-up, but remained significant even after 10 years, suggesting that elevated D-dimer is a marker of increased risk of VTE over the long-term. Multiplicative interaction testing showed that key SNPs did not modify the association of D-dimer with VTE. However, adjustment for the genetic variants slightly weakened the D-dimer associations with VTE. Notably, the D-dimer levels associated with increased risk of VTE were well below clinical D-dimer cutpoints for acute VTE diagnosis.
Our previous publication from LITE included D-dimer measured in ARIC by a research assay on samples from 1987-89 and 169 incident VTEs occurring from 1987 through 1998 [2]. In contrast, the present ARIC study included a D-dimer measured using a commercial assay on samples from 1993-95 and 521 incident VTEs from 1993 through 2011. The VTEs mostly did not overlap, and the hazard ratios here were quite similar to those reported previously for ARIC [2]. Case-control and clinical epidemiological studies also have corroborated strong, positive associations between D-dimer and VTE occurrence [3-10].
The most likely explanation for higher basal D-dimer concentrations being associated with long-term VTE risk in the general population is that elevated D-dimer is a marker of genetic and environmental contributors to thrombosis. Indeed, this study and others [17-19] found D-dimer associated with environmental risk factors for VTE (i.e., BMI, diabetes, and eGFR), ethnicity (higher in African Americans than whites) and several genetic variants. Most genetic variants associated with D-dimer are located in hemostatic factor genes (e.g., F5, F3, FGA, and FGG) [18]. We confirmed that D-dimer was higher in participants with risk alleles for F5 Leiden, F2, and in African Americans, FGG and hemoglobin S, but not with ABO or F11. Genome-wide association studies have consistently reported the SNPs we studied to be associated with VTE [13-16]. These variants explained part of the D-dimer association with VTE. Yet, even after adjustment for age, sex, genetic variants, and environmental factors, D-dimer remained independently associated with VTE. In addition, the waning of the association between D-dimer and VTE with longer follow-up suggests that D-dimer is not reflecting only genetic risk of VTE.
Some potential limitations of our study warrant consideration. Firstly, we measured D-dimer on plasma samples that had been stored for approximately 20 years at −70°C. Yet, previous evidence suggests D-dimer is stable in samples frozen for up to 6 years [20]. Any sample deterioration, if uniform across the D-dimer distribution, should not have biased our results. However, it is possible that sample deterioration had a non-uniform distribution. Secondly, D-dimer concentrations may have fluctuated during the long follow-up, and such fluctuations would tend to weaken the observed association with VTE. Thirdly, we identified VTEs via participant recall of hospitalizations and via local hospital surveillance. The few hospitalizations missed should be random with respect to D-dimer, and thus not significantly bias results. We also would have missed sudden fatal VTEs or outpatient-treated VTEs. However, ARIC pilot data suggest the vast majority of patients with first VTEs in ARIC during 1999 through 2011 were hospitalized.
In conclusion, a higher basal level of D-dimer in the general population is a strong risk factor for VTE over at least two decades in African Americans, as well as whites. We clearly need a better understanding of why some ostensibly healthy people have elevated basal D-dimer levels, as well as further clarification of genetic and preventable environmental and lifestyle contributors to thrombosis. In this regard, “maintenance of ideal cardiovascular health” is associated with lower D-dimer [19] and reduced VTE incidence [21, 22], and may be one strategy to reduce the large public health burden of VTE. Use of statins also can lower D-dimer [23] but whether they reduce VTE remains controversial [24].
Highlights.
We measured D-dimer in 12,097 participants free of venous thromboembolism (VTE).
Over a median of 17 years, 521 developed VTEs.
VTE risk was strongly, independently, and positively associated with D-dimer.
This was true in both whites and African Americans.
Acknowledgements
The authors thank the staff and participants of the ARIC Study for their important contributions, and Elaine Cornell for supervising D-dimer measurements.
Sources of Funding
The National Heart, Lung, and Blood Institute (NHLBI) supported the D-dimer measurements via U01 HL096902, LITE via R01-HL0597367, and ARIC via contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C.
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- DVT
venous thrombosis
- eGFR
estimate glomerular filtration rate
- HR
hazard ratio
- LITE
Longitudinal Investigation of Thromboembolism Etiology
- PE
pulmonary embolism
- SNP
single nucleotide polymorphisms
- VTE
venous thromboembolism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None.
Addendum
All authors contributed to critical revision of the manuscript and approved the final version. In addition, A. R. Folsom contributed to concept and design, classified VTE cases, and drafted the manuscript; A. Alonso helped obtain funding; K. M. George analyzed the data; N. S. Roetker analyzed the data; W. Tang contributed to concept and design; and M. Cushman contributed to concept and design, and classified VTE cases.
References
- [1].Willeit P, Thompson A, Aspelund T, Rumley A, Eiriksdottir G, Lowe G, et al. Hemostatic factors and risk of coronary heart disease in general populations: New prospective study and updated meta-analyses. PLoS One. 2013;8(2):e55175. doi: 10.1371/journal.pone.0055175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cushman M, Folsom AR, Wang L, Aleksic N, Rosamond WD, Tracy RP, et al. Fibrin fragment D-dimer and the risk of future venous thrombosis. Blood. 2003;101(4):1243–1248. doi: 10.1182/blood-2002-05-1416. [DOI] [PubMed] [Google Scholar]
- [3].Andreescu AC, Cushman M, Rosendaal FR. D-dimer as a risk factor for deep vein thrombosis: the Leiden Thrombophilia Study. Thromb Haemost. 2002;87(1):47–51. [PubMed] [Google Scholar]
- [4].Lowe G, Woodward M, Vessey M, Rumley A, Gough P, Daly E. Thrombotic variables and risk of idiopathic venous thromboembolism in women aged 45-64 years. Relationships to hormone replacement therapy. Thromb Haemost. 2000;83(4):530–535. [PubMed] [Google Scholar]
- [5].Lowe GD, Haverkate F, Thompson SG, Turner RM, Bertina RM, Turpie AG, et al. Prediction of deep vein thrombosis after elective hip replacement surgery by preoperative clinical and haemostatic variables: the ECAT DVT Study. European Concerted Action on Thrombosis. Thromb Haemost. 1999;81(6):879–886. [PubMed] [Google Scholar]
- [6].Baglin T, Palmer CR, Luddington R, Baglin C. Unprovoked recurrent venous thrombosis: prediction by D-dimer and clinical risk factors. J Thromb Haemost. 2008;6(4):577–582. doi: 10.1111/j.1538-7836.2008.02889.x. [DOI] [PubMed] [Google Scholar]
- [7].Shrivastava S, Ridker PM, Glynn RJ, Goldhaber SZ, Moll S, Bounameaux H, et al. D-dimer, factor VIII coagulant activity, low-intensity warfarin and the risk of recurrent venous thromboembolism. J Thromb Haemost. 2006;4(6):1208–1214. doi: 10.1111/j.1538-7836.2006.01935.x. [DOI] [PubMed] [Google Scholar]
- [8].Cosmi B, Legnani C, Cini M, Guazzaloca G, Palareti G. D-dimer levels in combination with residual venous obstruction and the risk of recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Haemost. 2005;94(5):969–974. doi: 10.1160/TH05-02-0095. [DOI] [PubMed] [Google Scholar]
- [9].Cosmi B, Legnani C, Cini M, Favaretto E, Palareti G. D-dimer and factor VIII are independent risk factors for recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Res. 2008;122(5):610–617. doi: 10.1016/j.thromres.2007.12.024. [DOI] [PubMed] [Google Scholar]
- [10].Palareti G, Legnani C, Cosmi B, Valdré L, Lunghi B, Bernardi F, et al. Predictive value of D-dimer test for recurrent venous thromboembolism after anticoagulation withdrawal in subjects with a previous idiopathic event and in carriers of congenital thrombophilia. Circulation. 2003;108(3):313–318. doi: 10.1161/01.CIR.0000079162.69615.0F. [DOI] [PubMed] [Google Scholar]
- [11].The ARIC investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- [12].Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the Longitudinal Investigation of Thromboembolism Etiology. Am J Med. 2004;117(1):19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- [13].Trégouët DA, Heath S, Saut N, Biron-Andreani C, Schved JF, Pernod G, et al. Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: results from a GWAS approach. Blood. 2009;113(21):5298–5303. doi: 10.1182/blood-2008-11-190389. [DOI] [PubMed] [Google Scholar]
- [14].Germain M, Saut N, Greliche N, Dina C, Lambert JC, Perret C, et al. Genetics of venous thrombosis: insights from a new genome wide association study. PLoS One. 2011;6(9):e25581. doi: 10.1371/journal.pone.0025581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Heit JA, Armasu SM, Asmann YW, Cunningham JM, Matsumoto ME, Petterson TM, et al. A genome-wide association study of venous thromboembolism identifies risk variants in chromosomes 1q24.2 and 9q. J Thromb Haemost. 2012;10(8):1521–1531. doi: 10.1111/j.1538-7836.2012.04810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tang W, Teichert M, Chasman DI, Heit JA, Morange PE, Li G, et al. A genome-wide association study for venous thromboembolism: the extended Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium. Genet Epidemiol. 2013;37(5):512–521. doi: 10.1002/gepi.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lutsey PL, Cushman M, Steffen LM, Green D, Barr RG, Herrington D, et al. Plasma hemostatic factors and endothelial markers in four racial/ethnic groups: the MESA Study. J Thromb Haemost. 2006;4(12):2629–2635. doi: 10.1111/j.1538-7836.2006.02237.x. [DOI] [PubMed] [Google Scholar]
- [18].Smith NL, Huffman JE, Strachan DP, Huang J, Dehghan A, Trompet S, et al. Genetic predictors of fibrin D-dimer levels in healthy adults. Circulation. 2011;123(17):1864–1872. doi: 10.1161/CIRCULATIONAHA.110.009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, et al. Ideal cardiovascular health: Associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014;130(19):1676–1683. doi: 10.1161/CIRCULATIONAHA.114.009273. [DOI] [PubMed] [Google Scholar]
- [20].Lewis MR, Callas PW, Jenny NS, Tracy RP. Longitudinal stability of coagulation, fibrinolysis, and inflammation factors in stored plasma samples. Thromb Haemost. 2011;86(6):1495–1500. [PubMed] [Google Scholar]
- [21].Olson NC, Cushman M, Judd SE, McClure LA, Lakoski SG, Folsom AR, et al. American Heart Association’s Life’s Simple 7 and risk of venous thromboembolism: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. J Am Heart Assoc. 2015;4(3):e001494. doi: 10.1161/JAHA.114.001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Folsom AR, Olson NC, Lutsey PL, Roetker NS, Cushman M. American Heart Association’s Life’s Simple 7 and incidence of venous thromboembolism [Correspondence] Am J Hematol. 2015;90(5):E92. doi: 10.1002/ajh.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Adams NB, Lutsey PL, Folsom AR, Herrington DH, Sibley CT, Zakai NA, et al. Statin therapy and levels of hemostatic factors in a healthy population: the Multi-Ethnic Study of Atherosclerosis. J Thromb Haemost. 2013;11(6):1078–1084. doi: 10.1111/jth.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li L, Zhang P, Tian JH, Yang K. Statins for primary prevention of venous thromboembolism. Cochrane Database Syst Rev. 2014;12:CD008203. doi: 10.1002/14651858.CD008203.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]