Abstract
The glycan shield on the human immunodeficiency virus 1 (HIV-1) envelope (Env) glycoprotein has drawn attention as a target for HIV-1 vaccine design given that an increasing number of potent and broadly neutralizing antibodies (bNAbs) recognize epitopes entirely or partially comprised of high mannose type N-linked glycans. In an attempt to generate immunogens that target the glycan shield of HIV-1, we previously engineered a triple mutant (TM) strain of Saccharomyces cerevisiae that results in exclusive presentation of high mannose type N-glycans, and identified five TM yeast glycoproteins that support strong binding of 2G12, a bNAb that targets a cluster of high mannose glycans on the gp120 subunit of Env. Here, we further analyzed the antigenicity and immunogenicity of these proteins in inducing anti-HIV responses. Our study demonstrated that the 2G12-reactive TM yeast glycoproteins efficiently bound to recently identified bNAbs including PGT125–130 and PGT135 that recognize high mannose glycan-dependent epitopes. Immunization of rabbits with a single TM yeast glycoprotein (Gp38 or Pst1), when conjugated to a promiscuous T-cell epitope peptide and coadministered with a Toll-like receptor 2 agonist, induced glycan-specific HIV-1 Env cross-reactive antibodies. The immune sera bound to both synthetic mannose oligosaccharides and gp120 proteins from a broad range of HIV-1 strains. The purified antibodies recognized and captured virions that contain both complex- and high mannose-type of N-glycans, and potently neutralized virions from different HIV-1 clades but only when the virions were enforced to retain high mannose N-glycans. This study provides insights into the elicitation of anti-carbohydrate, HIV-1 Env-cross reactive antibodies with a heterologous glycoprotein and may have applications in the design and administration of immunogens that target the viral glycan shield for development of an effective HIV-1 vaccine.
Keywords: AIDS, HIV-1, Carbohydrate, Glycoproteins, Neutralizing antibody, Adjuvant, Vaccine
1. Introduction
An immunogen that can elicit antibodies capable of neutralizing highly diverse HIV-1 strains has yet to be developed. Nevertheless, an increasing number of potent and broadly neutralizing antibodies (bNAbs) isolated from HIV-1 infected patients suggest that developing such an immunogen is feasible. In addition, multiple lines of evidence have demonstrated that the glycan shield on the HIV-1 envelope glycoprotein (Env) may serve as an attractive target for an effective immunogen design. The dense cluster of high mannose glycans on HIV-1 Env provides epitopes for the well characterized bNAbs 2G12 [1–3] and the recently identified PGT 121–123, 125–131 and 135 [4] that offer new clues for carbohydrate-based HIV-1 vaccine development [4–6]. In addition, passive administration of glycan-specific (2G12) or glycan-dependent (PGT121) bNAbs in nonhuman primates is sufficient to protect macaques from SHIV mucosal infection [7–9], suggesting that the glycan shield could be a useful immunogen.
A number of approaches have been made to recapitulate the 2G12 epitope in antigen design in a variety of contexts, including the multivalent display of chemically synthesized oligomannose-containing glycoconjugates [10–15]. Another approach is to develop naturally derived carbohydrate antigens as mimics of the 2G12 epitope, including manipulation of the N-glycan processing pathway in mammalian cells, genetic engineering of yeast strains, and identification of natural lipooligosaccharides in bacteria [16–19]. We have generated a triple mutant (TM) strain of Saccharomyces cerevisiae that expresses strictly the Man8GlcNAc2 form of N-glycans, which is the major form of glycans in the epitope of 2G12 and the PGT bNAbs [1,3,4,20,21]. Immunization of rabbits with whole TM yeast induced antibodies that not only bound specifically to the synthetic glycans containing terminal α1,2-linked mannose residues, but also bound to the high mannose glycans on gp120 from a broad spectrum of HIV-1 and SIV strains [17]. These immune sera efficiently neutralized a genetically diverse panel of HIV-1, but only when the viruses were produced in the presence of the mannosidase inhibitor kifunensine to retain the high mannose type of N-glycans [22,23].
In our earlier studies, we identified five yeast glycoproteins that contain a large number and high density of potential N-linked glycosylation sites (PNGS), like gp120, and support efficient binding to 2G12 [17,24,25]. In this study, we examined their ability to bind glycan-dependent PGT bNAbs and explored the immunization conditions under which glycan-specific HIV-reactive antibodies can be elicited using the yeast glycoproteins in combination with different immunostimulants and/or adjuvants. We found that some of the PGT bNAbs efficiently bound to the 2G12-reactive yeast glycoproteins. Immunization of rabbits with the PGT/2G12-reactive yeast glycoprotein, when conjugated to a promiscuous T-cell epitope peptide and formulated with a Toll-like receptor 2 (TLR2) agonist, induced antibodies that bound to synthetic mannose oligosaccharides as well as gp120 from diverse HIV-1 strains. Furthermore, purified mannose-specific antibodies were able to capture virions that contain both complex- and high mannose-type of N-glycans, and potently neutralize a panel of tier 1 and tier 2 viruses possessing enriched high mannose glycans. Hence, our yeast glycoprotein immunogens represent a promising molecular scaffolding approach to elicit antibodies that cross-react with HIV Env-associated glycans.
2. Materials and methods
2.1. Cloning and protein expression and purification
Genes encoding the yeast glycoproteins Pst1, Gp38, Ecm33, YJL171c and Gas1 were cloned into a modified pYES2/CT yeast expression vector with their endogenous signal sequence and a C-terminal 8×His tag and Strep-II tag [24]. Each of the yeast proteins proved to be inducible with galactose and was secreted into the culture media. Gp38, Ecm33, YJL171c and Gas1were purified using Ni-NTA tag affinity chromatography, while Pst1 was purified using SP-Sepharose C50 ion exchange media due to its high isoelectric point (pH 9.25).
2.2. Immunization of rabbits
Fourteen groups of New Zealand white rabbits, with three rabbits per group, were immunized with TT conjugated or non-conjugated yeast proteins Pst1 or Gp38 with various formulations of adjuvants in two different immunization routes (Table S1). For intravenous (IV) immunization, rabbits were injected in the marginal ear vein twice per week for 12 weeks with 100 µg of antigen, with bleeds taken at weeks 0, 3, 5, 7, 9, 11, and 13. For sub cutaneous (SC) immunizations, rabbits were injected at four sites of the back with a total of 1ml (100 µg) of antigen, with or without 50 µg of Pam3CSK4 (Pam3) or Pam2CSK4 (Pam2) or 50% of Imject Alum (Life Technologies). These rabbits were injected once a week for 6 weeks, and then once every other week until week 17 and bled at weeks 0, 3, 6, 10, 14 and 18.
2.3. Virus stocks
Pseudotyped viruses were produced in HEK293T/17 cells by cotransfection of an HIV env deletion backbone plasmid, pSG3ΔEnv, with plasmids expressing the desired Env protein in the presence or absence of kifunensine (MD Millipore). The tissue culture dose for 50% infectivity (TCID50) was determined for each virus in TZM-bl cells. Viral titer was defined as TCID50/ml. For viral capture assay, viral supernatant was filtered and virions were then pelleted at 30,000 × g (Beckman Coulter F0650) for 3.5 h at 4 °C. Virions were resuspended in D-PBS and the p24 content of the virus was quantified using a p24 ELISA kit (ProSci Inc. Catalog no. PSI-1851).
2.4. Neutralization assay
The susceptibility of pseudovirions bearing different Env proteins to neutralization by rabbit antisera, 2G12 or PGT bNAbs was assayed using TZM-bl cells in 96-well plates as described previously [26].
2.5. Viral capture assay
Capture antibodies including mannose-specific IgG (MS-IgG), 2G12 and a polyclonal anti-influenza H1N1 antibody were diluted in 50mM carbonate buffer (pH 9.5) and coated at 50 µl/well overnight at 4 °C. Wells were washed three times with PBS and blocked with 3% BSA in PBS for 1 h at 37 °C. 50 µl of DPBS containing the equivalent of 10 ng of p24 of pseudotyped virus was then added to each well and incubated for 2.5 h at 37 °C. Following incubation with the virus, the wells were washed six times with PBS to remove unbound virus. The captured virions were lysed by adding 200 µl of 1% Triton X-100 in PBS and incubation for 1 h at RT. The quantity of p24 released from captured virions in each well was measured using a p24 ELISA kit.
3. Results
3.1. Antigenicity of yeast glycoproteins against PGT broad neutralizing antibodies
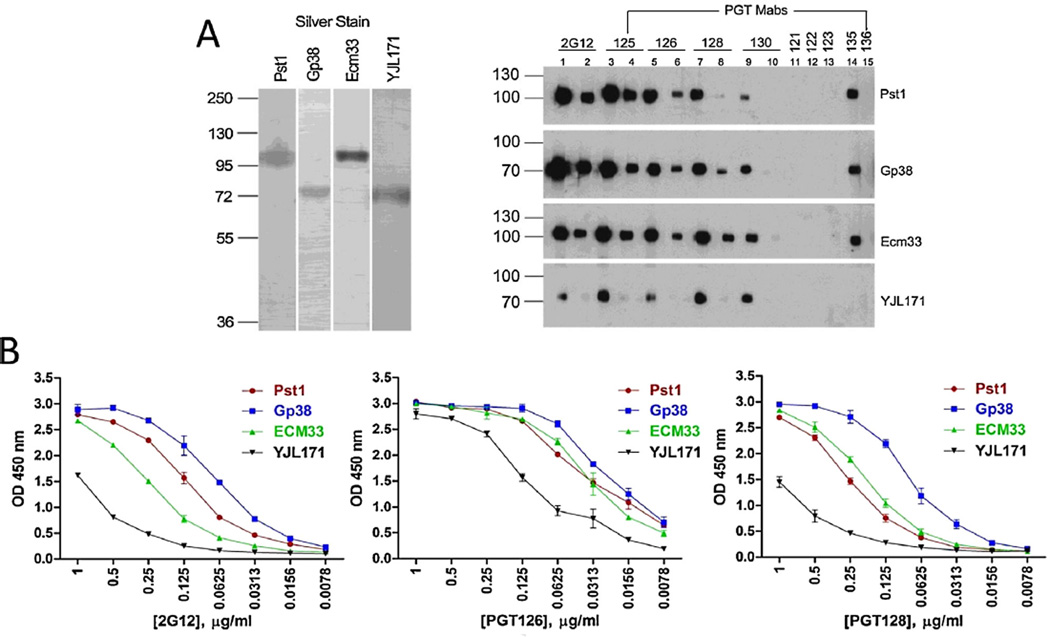
Four out of five previously identified 2G12-reactive glycoproteins were expressed in TM yeast and purified to greater than 95% as determined by SDS-PAGE (Fig. 1A left panel). We assessed the potential of the purified yeast glycoproteins to bind to the newly identified glycan-dependent PGT bNAbs in Western blot and ELISA. PGT 125, 126, 128, 130 and 135 recognized the yeast glycoproteins Pst1, Gp38, Ecm33 and YJL171 (Fig. 1A, right panel), consistent with their specificity for Man8GlcNAc2 and Man9GlcNAc2 forms of N-glycans [4,5,21]. In contrast, PGT 121–123 did not react with the TM yeast glycoproteins, consistent with their preference for binding to more complex glycans [27,28]. The yeast glycoproteins also showed reactivity to PGT bNAbs under native conditions as detected by ELISA (Fig. 1B), with Gp38 being the strongest binder and YJL171 the weakest to PGT128, PGT126 and 2G12.
Fig. 1.
2G12-reactive yeast glycoproteins bind to PGT bNAbs. (A) The indicated yeast glycoproteins were separated on SDS-PAGE gels at 500 ng per lane and stained by silver (left) or blotted with PGT bNAbs and 2G12 (right) at 1 µg/ml (lanes 1, 3, 5, 7, 9 and 11–15) or 0.2 µg/ml (lanes 2, 4, 6, 8 and 10), respectively. (B) The indicated yeast glycoproteins at 300 ng were coated on ELISA plates, and detected with PGT bNAbs and 2G12 in serial dilutions, followed by detection with anti-human IgG-HRP and color development with HRP substrate. The results are representative of two independent experiments in duplicate and the error bars indicate standard deviations.
3.2. Induction of gp120 cross-reactive antibodies by intravenous administration of a single yeast glycoprotein
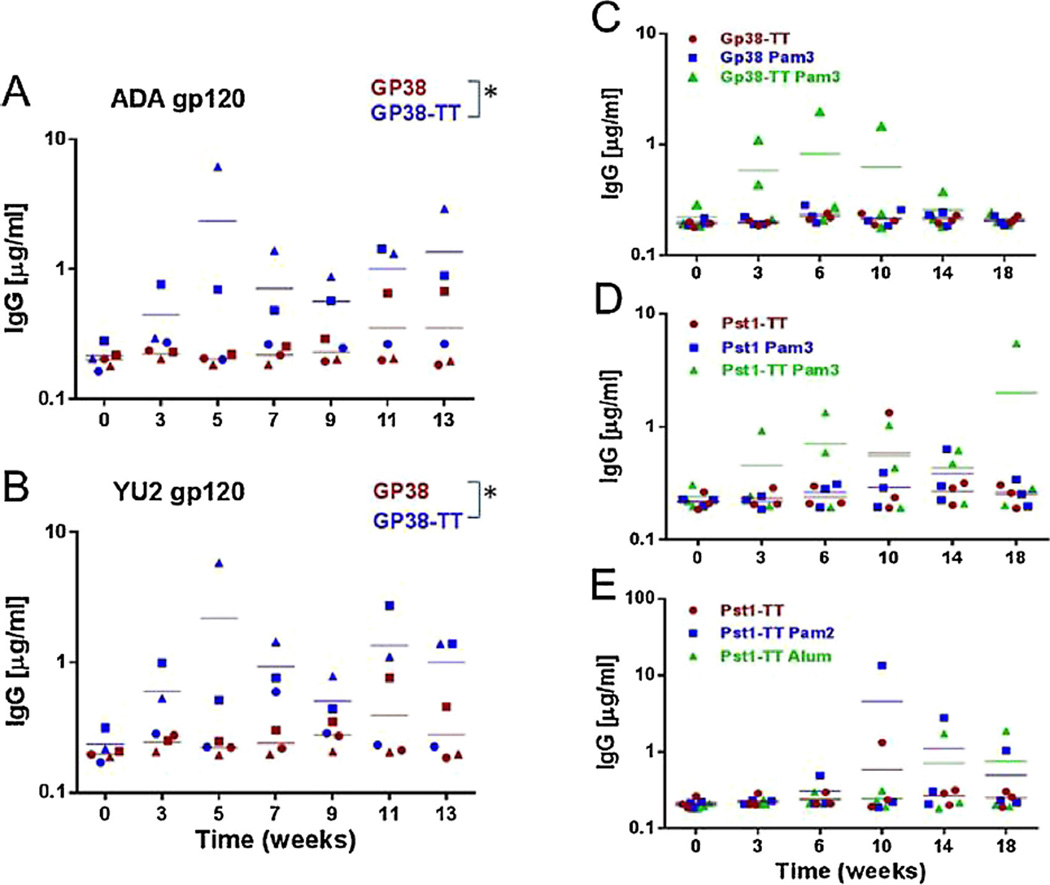
To determine if glycan-specific immune responses could be elicited with a single yeast glycoprotein and to determine the effects of T-cell epitope conjugation on the elicitation of HIV-1 cross-reactive antibodies, we conjugated a promiscuous T-cell epitope from tetanus toxin (TT) [29,30] to the yeast glycoproteins (Fig. S1) and immunized four groups of rabbits via the IV route with Gp38 and Pst1 and their TT-conjugates (groups 1–4 in Table S1). We screened the immune sera for their binding to soluble gp120 proteins from two clade B strains by ELISA. Sera from two out of three rabbits in the Gp38-TT group showed increased antibody responses against both ADA and YU2 gp120 proteins, while the Gp38 group did not display obvious responses until week 11 post-immunization from one out of three animals (Fig. 2A and B). In general, the Gp38-TT group exhibited relatively higher levels of gp120-specific IgG against gp120 proteins as compared to the Gp38 group starting from week 3 post-immunization (Fig. 2A and B), and the differences in the levels of gp120-specific IgG between these two groups were significant (p < 0.05). These results suggest that conjugation of the TT peptide to Gp38 is essential for the elicitation of early glycan-specific HIV-1 cross-reactive immune responses in the context of IV administration. In contrast, immunization with either Pst1 or Pst1-TT failed to elicit significant levels of cross-reactive antibodies to the tested gp120 proteins, even though antibodies to Pst1 itself were readily detected (data not shown).
Fig. 2.
Detection of HIV-1 Env cross-reactive antibodies elicited via intravenous (A–B) or subcutaneous immunization (C–E). (A–B) Rabbit sera at 1:500 dilutions from pre- and post-immunization with Gp38 or Gp38-TT conjugate were tested against the gp120 proteins from strains ADA and YU2 coated on ELISA plate. (C–E) Rabbit sera at 1:500 dilutions from pre- and post-immunization with Gp38-TT or Pst1-TT conjugate were tested against ADA gp120 proteins coated on ELISA plate. Temporal gp120-specific IgG response was determined by ELISA and quantified against a standard curve generated using known amounts of purified normal rabbit IgG. Data were plotted as relative concentration of gp120-specific IgG in each serum. Data represent the average of duplicates from one representative experiment. The solid lines indicate the mean for 3 rabbits in each group. Statistical significance was determined by a two-tailed t-test (* p < 0.05).
3.3. Induction of gp120 cross-reactive antibodies with a single yeast glycoprotein via the subcutaneous route
We also tested subcutaneous (SC) administration of the immunogens to explore the impact of co-administration of the adjuvants Pam3CSK4 (Pam3), Pam2CSK4 (Pam2), and aluminum salt (Alum) on the elicitation of gp120 cross-reactive antibodies. In our previous studies, antibodies against terminal α1,3-linked mannoses were successfully elicited with SC administration of zymosan A [17], a yeast cell wall component that contains α-mannan and β-glucan. Zymosan A is a known ligand for TLR2 [31,32] and may function as an adjuvant to mediate acquired immunity to yeast mannan. Therefore, to enhance humoral immunity against the high mannose structures, we formulated our yeast glycoproteins with a strong TLR2 agonist Pam3, or Pam2 for TLR2/1 or TLR2/6 heterodimerization, respectively [33]. We also tested Alum for its effect on inducing glycan-specific antibodies, as it is licensed for human use in the United States [34,35]. The immunization schedule of ten groups (groups 5–14) with three rabbits per group is shown in Table S1.
Among SC immunization groups, Gp38 alone, Gp38-TT conjugate and Gp38 + Pam3 failed to induce HIV-1 gp120 cross-reactive antibody responses (Fig. 2C and data not shown), despite each eliciting antibodies against the Gp38 immunogen (data not shown). However, co-administration of the TLR2 agonist Pam3 with Gp38-TT induced antibody responses against gp120 after 3 weeks of immunization in two out of three rabbits, suggesting a possible synergistic effect on the elicitation of HIV cross-reactive antibodies (Fig. 2C). A very similar pattern was observed with the Pst1 immunization groups, such that Pst1-TT + Pam3 induced antibody responses against ADA gp120 (Fig. 2D). Additionally, co-inoculation of Pst1-TT with Pam2 could also induce antibody responses against gp120 (Fig. 2E). Finally, formulation of the Pst1-TT conjugate with Alum also elicited a relatively weak response against the gp120 protein after 14 weeks of immunization (Fig. 2E). Taken together, these results suggest that a conjugated T-cell epitope is necessary, but not sufficient, for the induction of glycan-specific HIV cross-reactive immune responses with a single PGT/2G12-reactive yeast glycoprotein via SC administration, and that a TLR2 agonist may have synergistic effects with the T-cell epitope on the induction of such responses although variations between the immunized animals were observed for both immunogens.
3.4. The immune sera bind gp120s from a broad spectrum of HIV-1 strains
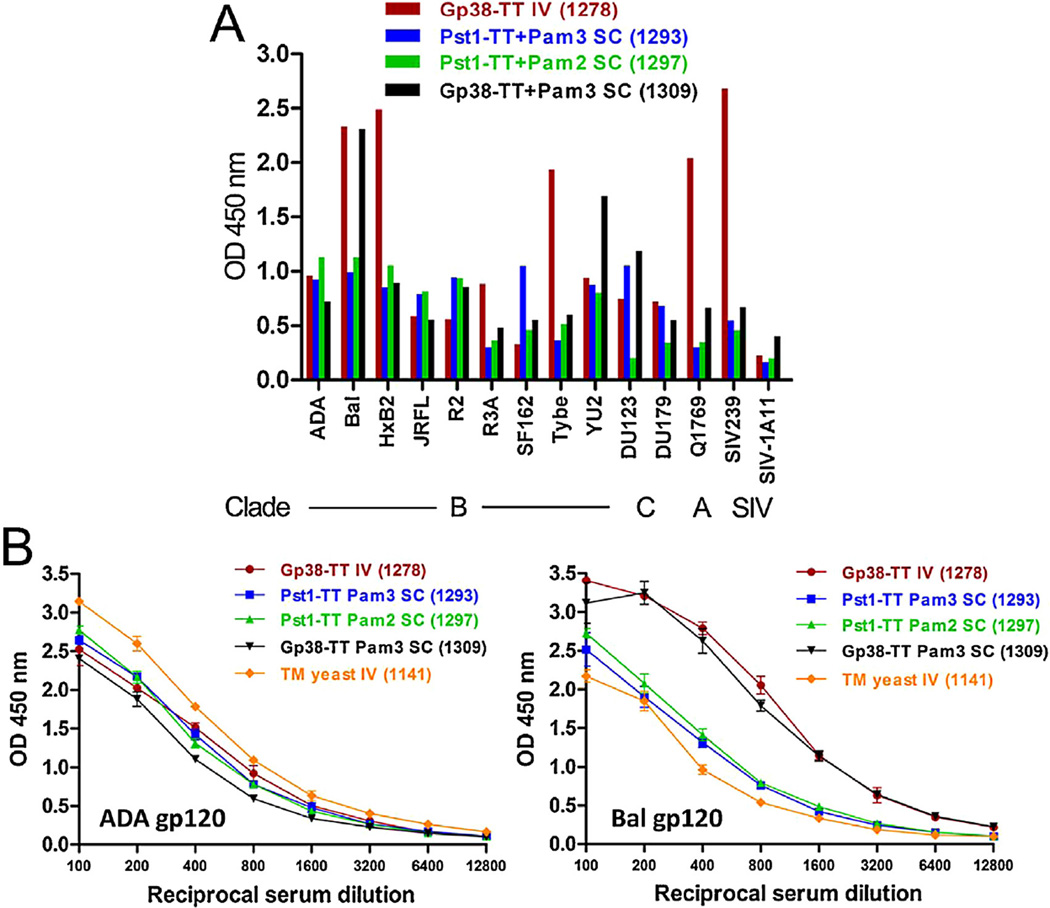
Four immune sera, including three obtained via SC immunization and one through IV immunization, were selected based on their reactivity to the two gp120 proteins tested for further analyses against a larger panel of HIV-1 and SIV gp120s. These immune sera recognized monomeric gp120s from twelve HIV-1 strains including representatives from subtypes A, B, and C and two SIV strains by ELISA (Fig. 3A). Notably, serum from rabbit 1278 (IV administration of Gp38-TT) showed the strongest reactivity (OD > 2.0) to five out of 14 gp120s from HIV-1 and SIV. The serum from rabbit 1309 (SC administration with Gp38-TT + Pam3), exhibited relatively stronger cross-reaction to two gp120s (Bal and YU2 with OD > 1.5–2.0) compared to the rest of gp120s; while the two immune sera from rabbit 1293 (SC administration with Pst1-TT + Pam3) or rabbit 1297 (SC administration with Pst1-TT + Pam2) showed moderate binding to most gp120s (OD < 1.5).
Fig. 3.
Breadth and potency of the immune sera for binding to HIV-1 gp120 proteins. (A) Antisera at 1:500 dilutions from selected rabbit 1278 (week 5), 1293 (week 18), 1297 (week 10) and 1309 (week 6) post immunizations were tested for binding to a panel of Env gp120 proteins (300 ng per well) from clade B, C and A of HIV-1 and SIV by ELISA. (B) A serial of 2-fold dilutions of above antisera were tested against gp120 proteins from ADA and Bal in ELISA. An immune serum (rabbit 1141) was included for comparison. The results are representative of two independent experiments, and the error bars indicate standard deviations.
Next, we examined the binding potency of the four immune sera to HIV-1 gp120s from strains ADA and Bal (Fig. 3B). As a positive control, we included immune serum from rabbit 1141 that was elicited with whole TM yeast and showed the strongest gp120 binding in that study [17]. Remarkably, the gp120-binding profiles of the single yeast protein-elicited sera were similar to that induced with whole TM yeast via IV administration. These results demonstrate that the single PGT/2G12-reactive yeast glycoprotein, Gp38 or Pst1, when conjugated with a TT-peptide and coadministered with a TLR2 agonist, can induce antibodies that cross-react with diverse gp120 proteins with a similar potency and breadth as that induced by whole TM yeast.
3.5. Elicited antibodies recognize and capture HIV-1 virions
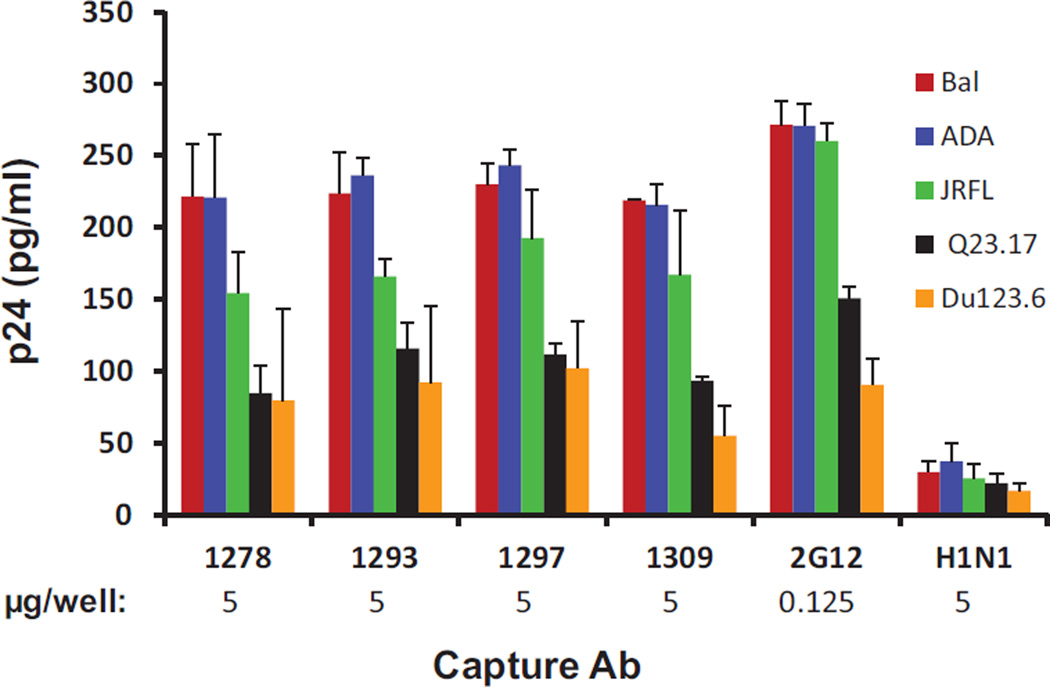
To determine if the immune sera could recognize virion-associated glycans, we performed a viral capture assay by using mannose-specific IgGs (MS-IgG) that were immunoaffinity-purified from the immune sera using heterologous PGT/2G12-reactive yeast glycoproteins (for instance using Gp38 to purify Pst1 antisera and vice-versa) or 2G12 as the capture antibodies. Results in Fig. 4 show that MS-IgGs from the four antisera were able to capture the pseudoviruses from clades A, B and C. As expected, 2G12 captured virions more efficiently than any of the antibodies tested (Fig. 4). Approximately 50-fold higher concentration of MS-IgG compared to 2G12 was required for capturing an equivalent quantity of viral particles (Fig. 4), suggesting that MS-IgG may contain a moderate level of virion binding antibodies and/or has lower affinity than 2G12. Like 2G12, MS-IgG displayed a relatively higher capture capacity to virions from clade B (ADA, Bal and JRFL) as compared with those from clades A (Q23.17) and C (Du123.6) (Fig. 4). Collectively, these results indicate that the antibodies elicited with single PGT/2G12-reactive yeast glycoprotein are able to bind monomeric gp120 and virions from diverse HIV-1 strains.
Fig. 4.
Purified antibodies capture virions. The capture antibodies (5 µg per well for MS-IgG and H1N1; 0.125 µg per well for 2G12) were immobilized on ELISA plate, and equivalent quantities of the pseudotyped viruses bearing the ADA, Bal, JRFL, Q23.17 and Du123.6 envelope were added to the wells. The captured virus was lysed and quantified by p24 ELISA. The results are average from two independent experiments, and the error bars indicate standard deviations.
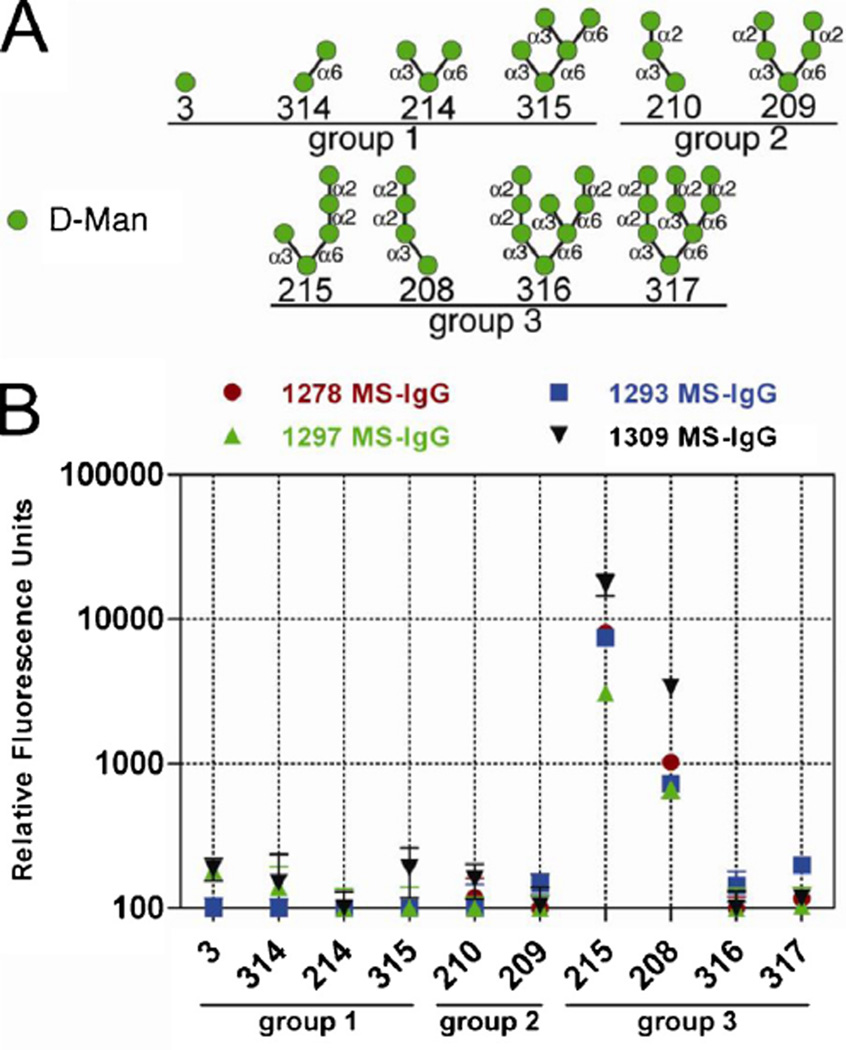
3.6. Single yeast protein-elicited antibodies bind specifically to mannose glycans with Manα1,2-Manα1,2-Man trisaccharide structure
To dissect the binding specificities of single yeast protein-elicited antibodies to oligosaccharides, a glycan binding profile analysis was performed. Synthetic mannose-containing glycans were divided into three groups, with group 1 glycans containing no terminal Manα1,2 structures, group 2 glycans containing terminal Manα1,2-Man disaccharides, and group 3 containing terminal Manα1,2-Manα1,2-Man trisaccharides (Fig. 5A). Purified antibodies from the four pre-immune sera did not recognize any of the glycans (data not shown). By contrast, the antibodies from the four post-immune sera exhibited efficient binding to glycans 215 and 208 in group 3, but not to glycans 316 and 317 (Fig. 5B). The presence of the terminal Manα1,2-Manα1,2-Man trisaccharide structure but not the additional bulky branches present in glycans 316 and 317 could possibly allow for tighter packing of glycans 215 and 208 on the array, and may thus account for the higher reactivity. In summary, the yeast glycoprotein-induced antibodies recognize terminal Manα1,2-Manα1,2-Man trisaccharides, similar to 2G12 [1,3,20].
Fig. 5.
Purified antibodies preferentially bind to Manα1,2-Manα1,2-Man trisaccharide structure. (A) Schematic representation of the synthetic mannose-containing carbohydrate structures present on the consortium for functional glycomics printed glycan array, version 5.1. (B) Mannose-specific IgG from each animal was tested at 10 µg/ml for binding to the various mannose-containing synthetic carbohydrates. Fluorescent antibodies were used to detect antibody binding, which is measured in relative light units.
3.7. Purified mannose-specific antibodies broadly and potently neutralize HIV-1 strains that express solely high-mannose N-glycans
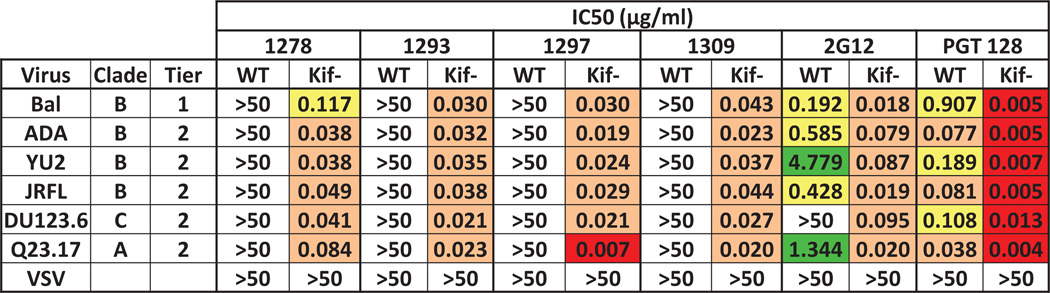
MS-IgG from the four rabbits (1278, 1293, 1297, and 1309) were tested for their neutralizing activities against a panel of 6 pseudoviruses, including tier 1 and tier 2 viruses from HIV-1 clades A, B and C in a TZM-bl cell-based neutralization assay. We found that the four MS-IgGs were unable to neutralize native virions, while control antibodies 2G12 and PGT128 exhibited strong neutralization activity (Fig. 6). However, when virions were produced in the presence of kifunensine, the MS-IgG showed consistently strong neutralization activity. This is consistent with our viral glycan profile study in which the kifunensine-treated virus harbored only Endo H-sensitive high mannose type N-glycans (Fig. S2, lane 4), while the non-treated virus displayed a relatively heterogeneous glycan profile (Fig. S2, lane 3). In addition, both 2G12 and PGT128 neutralized the kifunensine-treated virus more potently as compared to the non-treated virus. The profound effect of kifunensine-treatment on neutralization enhancement of MS-IgG was observed with both tier 1 and tier 2 viruses regardless their subtypes, while the negative-control virus bearing vesicular stomatitis virus glycoprotein (VSV-G) was not neutralized by any of the MS-IgG (Fig. 6), although it was also produced in the presence of kifunensine. The prebleed sera for these rabbits did not show neutralization to the kifunensine-treated virus (IC50 > 50 µg/ml).
Fig. 6.
Purified antibodies potently neutralize the kifunensine-treated viruses. Purified MS-IgG or T-IgG from protein A-purified prebleed was tested for neutralization of pseudoviruses produced in the presence or absence of 25 µg/ml kifunensine, with 2G12 and PGT128 as controls. Neutralizing activity is reported as 50% inhibition concentration (IC50, µg/ml). Boxes are color coded as follows: white, IC50 >50 µg/ml; green, 50 µg/ml > IC50 > 1 µg/ml; yellow, 1 µg/ml > IC50 > 0.1 µg/ml; orange, 0.1 µg/ml > IC50 > 0.01 µg/ml; and red, IC50 < 0.01 µg/ml. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The increasing number of potent and broadly neutralizing antibodies that recognize carbohydrate-dependent epitopes on HIV-1 Env [36–39] suggests that the viral glycan shield is an attractive target for HIV-1 vaccine immunogen design. However, elicitation of glycan-dependent antibodies is highly challenging as carbohydrates are in general poorly immunogenic. Numerous published studies with HIV-1 Env-based immunogens including gp120 monomers or gp140 trimers have failed to induce glycan-directed antibodies in animals [40–44]. Moreover, molecular scaffolds usually result in antibodies that bind mainly to the scaffolds, but rarely recognize HIV-1 Env glycans [10–19]. Our TM yeast is fundamentally different from other immunogens that have been developed in attempts to target the glycan shied of HIV, as TM yeast not only supports efficient 2G12 binding, but elicits antibodies that bind to gp120 glycans. This is a significant advance, although the resulting sera did not neutralize HIV-1 unless if virus was produced under conditions where N-linked glycans remain strictly in the high-mannose form. The recent identification of additional bNAbs that target high mannose glycans on gp120 provided new tools that could be used to identify specific TM yeast glycoproteins that most efficiently support binding of the newer generation of carbohydrate-dependent bNAbs.
Our binding studies indicated that Gp38 and Pst1, among the yeast TM proteins tested, mimicked the epitopes recognized by 2G12 and a number of the PGT bNAbs most efficiently. We then sought to determine if either could elicit antibody responses to HIV-1 that were superior to those obtained with intact TM yeast. However, since carbohydrates generally elicit weak antibody responses [45–48], we therefore included a conjugated T cell epitope as well as various adjuvants in our immunization study. In general, we found that coupling a T helper cell epitope peptide from tetanus toxin to TM yeast proteins resulted in the elicitation of stronger HIV-1 cross-reactive antibody responses, though significant variations in the levels of the responses between animals were observed. Interestingly, all animals mounted strong humoral responses to the immunogens themselves—the variability was observed when assessing responses to high mannose glycans on the immunogens that could cross-react with HIV-1 gp120. The addition of adjuvants also had a positive effect on the elicitation of antibodies that could cross-react with glycans on gp120, though again significant variability between animals was observed, and in some cases the use of both a T helper cell epitope and a TLR2 agonist was beneficial, suggesting a possible synergistic effect under subcutaneous immunization conditions. While our initial results are promising many additional approaches can be contemplated to not only boost the humoral immune response, but also to achieve greater consistency between immunized animals.
While individual yeast proteins elicited antibodies that bind to high mannose structures on gp120, they failed to neutralize the virus unless the virions were forced to retain only high-mannose type N-glycans by kifunensine treatment. This discrepancy between virion binding and neutralization could be due to several factors. First, the antibodies induced by the yeast glycoproteins may recognize high mannose structures that are different from those bound by bNAbs. Second, the induced antibodies captured virions about 2 orders of magnitude less efficiently than 2G12, and this could be the result of lower binding affinity. Third, it is possible that only a small subset of the induced antibodies in the polyclonal antibody pool is able to efficiently bind to the glycan epitopes on intact virions, which is supported by the fact that virions produced in the presence of kifunensine were universally sensitive to neutralization by the immune sera. A similar phenomenon has been seen in some patient-derived sera, in which enriching for high-mannose glycans with kifunensine-treatment led to higher neutralization sensitivity of the virus [49]. It is possible that homogeneous presentation of Man9GlcNAc2 may increase the numbers of high mannose clusters and thus form diverse conformational epitopes that are targeted by the polyclonal antibodies with diverse specificities. In addition, the low intrinsic affinity could be amplified by the avidity effect when the virus presents only high-mannose type N-glycans. Thus, the lack of neutralization of non-treated virions may be attributed to both the specificity and avidity of the elicited polyclonal antibodies.
In summary, we have advanced our previous success with the TM yeast-based genetic scaffold by employing and evaluating the single yeast glycoproteins as an HIV-1 vaccine immunogen to target the viral glycan shield. First, we have identified specific yeast glycoproteins as efficient PGT bNAb binders that can be selected for more detailed studies, such as further manipulation and optimization of the glycan clusters on these proteins to best mimic PGT/2G12 bNAb epitopes. Second, we have extended our immunization strategies from IV delivery of whole TM yeast cells to SC administration of single yeast glycoproteins. Third, we have shown that the antibodies elicited with single yeast glycoproteins are able to capture virions but lack neutralization activity unless if virions harbor exclusively high mannose N-linked glycans. Collectively, this pilot study highlights the potential of these yeast glycoproteins as functional genetic scaffolds to recapitulate the PGT/2G12 bNAb epitopes, at least partially. It also confirms the feasibility to elicit HIV-1 Env cross-reactive antibodies with heterologous glycoproteins by targeting the HIV-1 glycan shield. Further improvement in immunization strategies, such as including additional adjuvants to elicit high titer, sustainable and consistent HIV-1 cross-reactive antibodies, optimizing immunization schedules to promote affinity maturation, and employing prime-boost regimens to focus the immune responses on the glycan-specific or glycan-dependent neutralizing epitopes, will need to be explored to elicit neutralizing antibodies to native virions.
Supplementary Material
Acknowledgements
This work was supported by The International AIDS Vaccine Initiative Innovation Fund Grant and NIH grant R43AI112484 (to Yu Geng).
Footnotes
Author contribution
Study design: Y.G., H.Z. and R.J.L; data acquisition and analysis: H.Z., H.F., R.J.L., B.L. and Y.G.; providing key reagents: F-H.L. and R.W.D.; manuscript writing: H.Z., Y.G., R.J.L. and R.W.D.
Conflict of interest statement
Yu Geng, Founder and CEO of ProSci Incorporated, acknowledges a potential conflict of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2015.08.012
References
- 1.Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, et al. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1 → 2 mannose residues on the outer face of gp120. J Virol. 2002;76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 3.Calarese DA, Lee HK, Huang CY, Best MD, Astronomo RD, Stanfield RL, et al. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc Natl Acad Sci USA. 2005;102:13372–13377. doi: 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong L, Lee JH, Doores KJ, Murin CD, Julien JP, McBride R, et al. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat Struct Mol Biol. 2013;20:796–803. doi: 10.1038/nsmb.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doores KJ, Kong L, Krumm SA, Le KM, Sok D, Laserson U, et al. Two classes of broadly neutralizing antibodies within a single lineage directed to the high-mannose patch of HIV envelope. J Virol. 2015;89:1105–1118. doi: 10.1128/JVI.02905-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 8.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci USA. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni J, Song H, Wang Y, Stamatos NM, Wang LX. Toward a carbohydrate-based HIV-1 vaccine: synthesis and immunological studies of oligomannose-containing glycoconjugates. Bioconjug Chem. 2006;17:493–500. doi: 10.1021/bc0502816. [DOI] [PubMed] [Google Scholar]
- 11.Wang LX. Toward oligosaccharide- and glycopeptide-based HIV vaccines. Curr Opin Drug Discovery Dev. 2006;9:194–206. [PubMed] [Google Scholar]
- 12.Astronomo RD, Lee HK, Scanlan CN, Pantophlet R, Huang CY, Wilson IA, et al. A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120. J Virol. 2008;82:6359–6368. doi: 10.1128/JVI.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joyce JG, Krauss IJ, Song HC, Opalka DW, Grimm KM, Nahas DD, et al. An oligosaccharide-based HIV-1 2G12 mimotope vaccine induces carbohydrate-specific antibodies that fail to neutralize HIV-1 virions. Proc Natl Acad Sci USA. 2008;105:15684–15689. doi: 10.1073/pnas.0807837105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang SK, Liang PH, Astronomo RD, Hsu TL, Hsieh SL, Burton DR, et al. Targeting the carbohydrates on HIV-1: interaction of oligomannose dendrons with human monoclonal antibody 2G12 and DC-SIGN. Proc Natl Acad Sci USA. 2008;105:3690–3695. doi: 10.1073/pnas.0712326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Astronomo RD, Kaltgrad E, Udit AK, Wang SK, Doores KJ, Huang CY, et al. Defining criteria for oligomannose immunogens for HIV using icosahedral virus capsid scaffolds. Chem Biol. 2010;17:357–370. doi: 10.1016/j.chembiol.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scanlan CN, Ritchie GE, Baruah K, Crispin M, Harvey DJ, Singer BB, et al. Inhibition of mammalian glycan biosynthesis produces non-self antigens for a broadly neutralising, HIV-1 specific antibody. J Mol Biol. 2007;372:16–22. doi: 10.1016/j.jmb.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Luallen RJ, Lin J, Fu H, Cai KK, Agrawal C, Mboudjeka I, et al. An engineered Saccharomyces cerevisiae strain binds the broadly neutralizing human immunodeficiency virus type 1 antibody 2G12 and elicits mannose-specific gp120-binding antibodies. J Virol. 2008;82:6447–6457. doi: 10.1128/JVI.00412-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunlop DC, Bonomelli C, Mansab F, Vasiljevic S, Doores KJ, Wormald MR, et al. Polysaccharide mimicry of the epitope of the broadly neutralizing anti-HIV antibody, 2G12, induces enhanced antibody responses to self oligomannose glycans. Glycobiology. 2010;20:812–823. doi: 10.1093/glycob/cwq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark BE, Auyeung K, Fregolino E, Parrilli M, Lanzetta R, De Castro C, et al. A bacterial lipooligosaccharide that naturally mimics the epitope of the HIV-neutralizing antibody 2G12 as a template for vaccine design. Chem Biol. 2012;19:254–263. doi: 10.1016/j.chembiol.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Sanders RW, Venturi M, Schiffner L, Kalyanaraman R, Katinger H, Lloyd KO, et al. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J Virol. 2002;76:7293–7305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334:1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elbein AD. Glycosidase inhibitors: inhibitors of N-linked oligosaccharide processing. FASEB J. 1991;5:3055–3063. doi: 10.1096/fasebj.5.15.1743438. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal-Gamse C, Luallen RJ, Liu B, Fu H, Lee FH, Geng Y, et al. Yeast-elicited cross-reactive antibodies to HIV Env glycans efficiently neutralize virions expressing exclusively high-mannose N-linked glycans. J Virol. 2011;85:470–480. doi: 10.1128/JVI.01349-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luallen RJ, Fu H, Agrawal-Gamse C, Mboudjeka I, Huang W, Lee FH, et al. A yeast glycoprotein shows high-affinity binding to the broadly neutralizing human immunodeficiency virus antibody 2G12 and inhibits gp120 interactions with 2G12 and DC-SIGN. J Virol. 2009;83:4861–4870. doi: 10.1128/JVI.02537-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang LX, Costantino P, Geng Y, Zhang H. Anti-carbohydrate HIV vaccine design. In: Pantophlet R, editor. HIV glycans in infection and immunity. New York, NY: Springer; 2014. pp. 143–176. [Google Scholar]
- 26.Zhang H, Rola M, West JT, Tully DC, Kubis P, He J, et al. Functional properties of the HIV-1 subtype C envelope glycoprotein associated with mother-to-child transmission. Virology. 2010;400:164–174. doi: 10.1016/j.virol.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci USA. 2012;109:E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Julien JP, Sok D, Khayat R, Lee JH, Doores KJ, Walker LM, et al. Broadly neutralizing antibody PGT121 allosterically modulates CD4 binding via recognition of the HIV-1 gp120 V3 base and multiple surrounding glycans. PLoS Pathog. 2013;9:e1003342. doi: 10.1371/journal.ppat.1003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahlborg N, Ling IT, Holder AA, Riley EM. Linkage of exogenous T-cell epitopes to the 19-kilodalton region of plasmodium yoelii merozoite surface protein 1 (MSP1(19)) can enhance protective immunity against malaria and modulate the immunoglobulin subclass response to MSP1(19) Infect Immun. 2000;68:2102–2109. doi: 10.1128/iai.68.4.2102-2109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theisen DM, Bouche FB, El Kasmi KC, von der Ahe I, Ammerlaan W, Demotz S, et al. Differential antigenicity of recombinant polyepitope-antigens based on loop- and helix-forming B and T cell epitopes. J Immunol Methods. 2000;242:145–157. doi: 10.1016/s0022-1759(00)00197-6. [DOI] [PubMed] [Google Scholar]
- 31.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi O, Akira S. Toll-like receptors; their physiological role and signal transduction system. Int Immunopharmacol. 2001;1:625–635. doi: 10.1016/s1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 33.Gambhir V, Yildiz C, Mulder R, Siddiqui S, Guzzo C, Szewczuk M, et al. The TLR2 agonists lipoteichoic acid and Pam3CSK4 induce greater pro-inflammatory responses than inactivated Mycobacterium butyricum. Cell Immunol. 2012;280:101–107. doi: 10.1016/j.cellimm.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noe SM, Green MA, HogenEsch H, Hem SL. Mechanism of immunopotentiation by aluminum-containing adjuvants elucidated by the relationship between antigen retention at the inoculation site and the immune response. Vaccine. 2010;28:3588–3594. doi: 10.1016/j.vaccine.2010.02.085. [DOI] [PubMed] [Google Scholar]
- 36.Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. Antibodies in HIV-1 vaccine development and therapy. Science. 2013;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blattner C, Lee JH, Sliepen K, Derking R, Falkowska E, de la Pena AT, et al. Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers. Immunity. 2014;40:669–680. doi: 10.1016/j.immuni.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sok D, Doores KJ, Briney B, Le KM, Saye-Francisco KL, Ramos A, et al. Promiscuous glycan site recognition by antibodies to the high-mannose patch of gp120 broadens neutralization of HIV. Sci Transl Med. 2014;6:236–263. doi: 10.1126/scitranslmed.3008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West AP, Jr, Scharf L, Scheid JF, Klein F, Bjorkman PJ, Nussenzweig MC. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell. 2014;156:633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wrin T, Nunberg JH. HIV-1MN recombinant gp120 vaccine serum, which fails to neutralize primary isolates of HIV-1, does not antagonize neutralization by antibodies from infected individuals. Aids. 1994;8:1622–1623. doi: 10.1097/00002030-199411000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 42.Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis. 2005;191:666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 43.Selvarajah S, Puffer B, Pantophlet R, Law M, Doms RW, Burton DR. Comparing antigenicity and immunogenicity of engineered gp120. J Virol. 2005;79:12148–12163. doi: 10.1128/JVI.79.19.12148-12163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Svehla K, Mathy NL, Voss G, Mascola JR, Wyatt R. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J Virol. 2006;80:1414–1426. doi: 10.1128/JVI.80.3.1414-1426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pashov A, Garimalla S, Monzavi-Karbassi B, Kieber-Emmons T. Carbohydrate targets in HIV vaccine research: lessons from failures. Immunotherapy. 2009;1:777–794. doi: 10.2217/imt.09.44. [DOI] [PubMed] [Google Scholar]
- 46.Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337:183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hevey R, Ling CC. Recent advances in developing synthetic carbohydrate-based vaccines for cancer immunotherapies. Future Med Chem. 2012;4:545–584. doi: 10.4155/fmc.11.193. [DOI] [PubMed] [Google Scholar]
- 48.Wang LX. Synthetic carbohydrate antigens for HIV vaccine design. Curr Opin Chem Biol. 2013;17:997–1005. doi: 10.1016/j.cbpa.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavine CL, Lao S, Montefiori DC, Haynes BF, Sodroski JG, Yang X, et al. High-mannose glycan-dependent epitopes are frequently targeted in broad neutralizing antibody responses during human immunodeficiency virus type 1 infection. J Virol. 2012;86:2153–2164. doi: 10.1128/JVI.06201-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.