Abstract
Primary Retroperitoneal Sarcomas are relatively uncommon tumors with varied manifestations, ill-defined prognostic factors and uncertain management modalities. We undertook this study to review patients who presented with primary or recurrent RPS at our institute during the study period. Between 2008 and 2010, 23 patients were evaluated. Statistical analysis was done using the chi square test or Fisher’s exact test. Recurrence was calculated using Kaplan Meier curves. The log-rank test was used to compare differences in survival or relapse. Among the 23 patients, 13 were males. Majority (52.2 %) presented with abdominal lump located in umbilical and right hypochondrial region. Surgery was done in 20 (87 %) patients for primary RPS and three (13 %) for recurrent RPS at initial presentation. 14 (61 %) received complete tumor resection, six (27 %) incomplete microscopic resection, and three (13 %) incomplete macroscopic resection. In univariate Cox’s proportional hazard model on time (‘timer’) to event (‘relapse’) analysis, all the variables like older age (p = 0.027), male sex (p = 0.012), incomplete resection (p = 0.008), large size (0.047) and high grade (p = 0.047) became significant predictor of early recurrence. However, multivariate analysis showed that only extent of resection, grade and sex were statistically significant predictors. Complete tumor resection of retroperitoneal sarcoma (n = 14) was associated with a significantly lesser recurrence compared with unclear resection (n = 9, p = 0.002). The median time between surgery and first recurrence was 15 months. High-grade sarcomas had a significantly higher recurrence (n = 10; median: 24, 95 % CI) than low-grade sarcomas (n = 13, median: 15; 95 %CI P < 0.01). Furthermore, the survival of male patients were worse than that of females (p = 0.036). Completeness of resection, tumor grade and sex are prognostic factors of retroperitoneal soft tissue sarcomas.
Keywords: Retroperitoneal sarcoama, Radiotherapy, Chemotherapy, Surgery
Introduction
Primary Retroperitoneal Sarcomas (PRS) is a heterogeneous group of malignant neoplasm with very low incidence. It comprises 0. 1 % of all malignancies, 15 % of all sarcomas and approximately 50 % of all retroperitoneal masses [1]. So far, little is known about their biological behaviour and no specific etiological associations have been identified. Several studies have sought to identify factors that predict prognosis after resection of RPS with the goal of identifying patients who may benefit from more aggressive follow-up or investigational adjuvant therapies, but controversies still exist [2]. The management remains a challenge and surgical resection of localized RPS with microscopically negative margins, as the standard of care is usually used [3]. Complete resection is, however, often difficult to carry out because of the frequently large size of the tumor at the time of diagnosis, the deep-seated location and common infiltration of adjacent vital organs [4]. Despite advances in diagnostic modalities, surgical techniques and the adaptation of more aggressive procedures, this disease still has a propensity for local recurrence, even after an apparent complete resection, contributing to the poor outcome [5].
To better understand the complex nature of this group of malignancies, we undertook this study to review the biologic and clinical behavior of retroperitoneal sarcomas. We report our experience of evaluating the principal prognostic factors contributing to tumor recurrence and patient outcome in 23 patients who presented with primary or recurrent RPS at our institute during the 2 years study period.
Materials and Methods
The clinical courses of all patients with loco regional disease (without distant metastasis) at presentation, treated from 2008 to 2010 for soft-tissue sarcomas of the retro peritoneum at our referral-teaching institute were reviewed both prospectively and retrospectively. With a catchment area of approximately 11 million populations (almost ten percent of State’s population), this sampling is comparable to the general eastern Indian Gangetic belt population [6].
Patients were analyzed for survival and potential prognostic factors by patient demographics (sex, age), preoperative symptoms, tumor related data (size, weight, histological type, grade), diagnostic procedures, infiltration of adjacent organs, extent of resection, peri operative complications, mortality, and recurrence after surgery.
Histologically Grade 1 & 2 was grouped as low and grade 3 & 4 high [7]. The primary RPS was defined as a tumor which was untreated before definitive surgical intervention. We did not separate local vs distant recurrence as the sample size is small and eight of the nine recurrences were local. Surgical resection was classified into complete (R0) or incomplete (R1 and R2). The patients were censored until death or their last follow-up in 2 years.
Statistical analysis was done using the chi square test or Fisher’s exact test for univariate analysis. Recurrence was analyzed by Kaplan and Meier method. The log-rank test was used to compare differences in relapse distributions. There was one post-operative death (3 weeks from operation) but as this is a direct result of the disease burden, this was included in the analysis. Survival endpoints were based on death from disease. Patients with R1 and R2 resection received post-operative radiotherapy /chemotherapy but those having direct morbidity and mortality due to adjuvant treatment were excluded from the analysis as these treatments are still controversial [8–10]. The hazard ratios (HR) and 95 % Confidence Interval (CI) were reported in relapse rate analyses. SPSS software program (SPSS 10) was used. P value less than 0.05 was considered significant.
Results
Twenty- three patients were analyzed,14 in the retrospective and nine in prospective group with follow up of 2 years. The age groups ranged from 12 to 80 years (mean 39.09, S.D 15.34). Patients younger than 10 years were excluded.
All patients with primary RPS were symptomatic (mean 5 months, range 1–12) at presentation. Majority were males (58.3 %) and presented with abdominal swelling (52.2 %). Abdominal discomfort /pain was present in one third but nausea was less common (13 %). Only two patients (8.7 %) had significant weight loss and one presented with sub-acute intestinal obstruction.
On general examination, significant pallor was present in only 13 %. All but one had palpable lump, one third of them tender. Lumps were mostly in the umbilical region or the right hypochondrium, with equal frequency, and in three patients extending into both. Right lumbar was the next most common site. Two had lumps occupying all the three areas. Epigastric region was spared. Consistency varied from firm (75 %) to hard (16.67 %) to soft cystic (8.33 %). Most of the lumps were smooth with ill-defined margins. Majority (65.2 %) had little mobility.
Patient work-up included abdominal ultrasonography and thoraco-abdominal Contrast Enhanced Computed Tomography (CECT) for detailed assessment prior to definitive surgery. Ultrasonography suggested a well-defined solid mass in 17 patients. Associated ascites, lymphadenopathy and liver lesions were seen in five, seven and one patient respectively. CECT mostly supported the USG findings and also picked up mass in the remaining six cases. In addition, it revealed the encasement of superior mesenteric artery, aorta and IVC in one each.
Laparotomy was done in all the cases, 20 (87 %) for primary RPS and three (13 %) for recurrent RPS at initial presentation. Eight (35 %) patients underwent surgery for first recurrence and three patients (13 %) each for the second recurrence and third recurrence. Two patients (8.7 %) presented with recurrent tumors on 1 year follow up and total nine patients presented with recurrence in 24 months follow up, the median time between the primary surgical resection and their first recurrence 15 months (range 6 to 23 months) while that for second recurrence was 20 months. To simplify the study, only the first recurrence from time to surgery was analyzed.
Fourteen patients (61 %) underwent R0, six had R1 and three had R2 resections. Fourteen out of 20 (70 %) patients with primary RPS underwent R0 resections and none of the patients with recurrent RPS (first recurrence, eight patients) could undergo curative resection (P = 0.0019) signifying difficulty in redo surgery to achieve R0 status. Therefore, the first surgery has the best chance of a curative attempt. Among the eight patients with first recurrence, macroscopic tumor free margin were possible in four cases and de-bulking of tumor was done in the remaining four. For analysis, margin status of the first resection was taken.
Wide local excision with excision of contiguous structures (infiltrated grossly) was done in four cases [three of 8 patients with recurrent RPS (37.5 %) and one of the 20 patients with primary RPS (5 %); P = 0.0581; not significant]. Two patients (both with recurrent retroperitoneal tumor) required multi-visceral resection involving right colon with same-sided kidney. Other two underwent a single organ resection (sigmoid colectomy). There was no intra-operative death. However, one patient died on the third week postoperative following Fecal fistula after sigmoid colectomy. Twenty two patients were transfused with average two units of blood during operation.
Median tumors size was 10 cm (cm). Mean weight was 1163 g. Liposarcoma was the most frequent tumor present as in other series but next most common was neurofibroma. Ten out of 23 patients (43.5 %) had high-grade tumors (6/20; with primary RPS and no recurrence vs. 4/8; with recurrent RPS; P = 0.4004, NS). This includes tumors, which changed from low to high grade in recurrence. Two patients died during the 2 years period follow up out of which one was postoperative death due to fecal peritonitis from anastomotic failure. As we had nine recurrences and recurrence is directly related to disease free and overall survival, our analysis of time to event is with reference to recurrence.
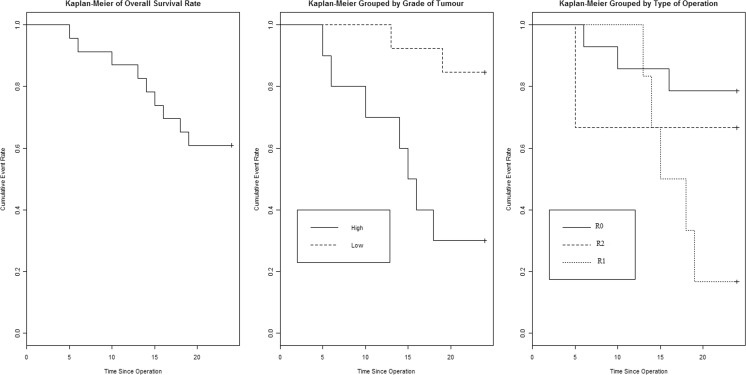
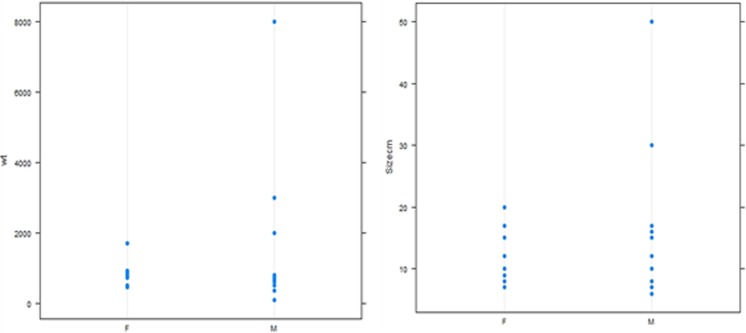
Recurrence Rate Analysis - Kaplan-Meier curves were first constructed to compare relapse rate based on overall patient and disease characteristics. Next, Kaplan-Meier relapse rate analysis was performed with reference to grade of tumors and type of operation,(the two significant factors identified by logistic regression). It showed significant worse prognosis in high-grade tumors (log rank 0.018) and incomplete resection (log rank 0.002) (Fig. 1). (Male sex became non significant when density replaced size and weight). Logistic regression analysis showed significant worse prognosis with male sex, high grade and incomplete surgical resection (Table 1). Interestingly, while analyzing data, we found that tumor size distribution and weight distribution is quite similar in male and female (especially significant as we found male sex was a risk factor for relapse) (Fig. 2).
Fig. 1.
Kaplan-Meier curves for survival based on grade of tumour and tumour clearence
Table 1.
logistic regression predicting relapse
| Result from logistic regression with weight and size | Result from logistic regression with density | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude OR (95 %CI) | Adj. OR (95 %CI) | P (Wald’s test) | P (LR-test) | Crude OR (95 %CI) | Adj. OR (95 %CI) | P (Wald’s test) | P (LR-test) | |
| Age (cont. var.) | 1 (0.98,1.02) | 1.13 (0.79,1.61) | 0.492 | 0.432 | 1 (0.98,1.02) | 1.21 (0.85,1.74) | 0.293 | 0.069 |
| Sex: ref.= | 0.036* | 0.761 | ||||||
| F | 1.25 (0.34,4.65) | 0 (0,6792.62) | 0.376 | 1.25 (0.34,4.65) | 0 (0,331.58) | 0.311 | ||
| M | 0.4 (0.13,1.28) | 0 (0,516109.96) | 0.284 | 0.4 (0.13,1.28) | 0 (0,141.35) | 0.249 | ||
| wt (cont. var.) | 1.0001 (0.9996,1.0005) | 1.0013 (0.9993,1.0033) | 0.195 | 0.12 | ||||
| Density = (wt/Size cm) | 1 (0.99,1) | 0.98 (0.93,1.03) | 0.437 | 0.296 | ||||
| Size cm (cont. var.) | 1 (0.96,1.05) | 1.17 (0.92,1.48) | 0.2 | 0.154 | ||||
| Grade of tumour: i vs h | 2.33 (0.6,9.02) | 0 (0,Inf) | 0.997 | 0.018* | 2.33 (0.6,9.02) | 0 (0,Inf) | 0.996 | 0.03* |
| Type of resection ref-R0 | 0.002* | 0.003* | ||||||
| R2 | 0.18 (0.04,0.82) | 0.01 (0,70.02) | 0.292 | 0.18 (0.04,0.82) | 1.67 (0.03,97.59) | 0.805 | ||
| R1 | 0.27 (0.08,0.98) | 99913257729.65 (0,Inf) | 0.996 | 0.27 (0.08,0.98) | 17537648212.92 (0,Inf) | 0.996 | ||
* denots significant
Fig. 2.
Weight and size plotted against sex distribution
The tumors size is an important prognostic criteria and included in TNM Staging (AJCC). In our analysis, size, as measured by greatest tumor length was not significant (p = 0.154) for predicting overall relapse, though it was significant (P = 0.047) in predicting early relapse. When we looked at the weight distribution of the excised mass, a similar variation (a few subjects having data in clustered in one extremes) prompted us to try to combine mass with volume and use density (mass per unit volume). Tumor density itself is also not a significant predictor for relapse, but analysis using tumor density changed male sex from significant to non significant predictor of a poor prognosis (Table 1).
In Cox’s proportional hazard model on time (‘timer’) to event (‘relapse’) analysis -all the variables like higher age, male sex, incomplete resection, large size and weight and high grade become significant predicting early recurrence (Table 2). On replacing wt and size by density and doing the above analysis (Table 3), only age and type of resection remained significant, grade became insignificant.
Table 2.
Cox’s proportional hazard model on time (‘timer’) to event (‘relapse’), grade: h high grade, i low grade
| Crude HR (95 %CI) | Adj. HR (95 %CI) | P (Wald’s test) | P (LR-test) | |
|---|---|---|---|---|
| Age (cont. var.) | 1.06 (1.01,1.12) | 1.17 (0.97,1.42) | 0.096 | 0.027 |
| Sex: M vs F | 0.51 (0.14,1.9) | 0 (0,2.12) | 0.083 | 0.012 |
| Type of resection Ref R0 | 0.008 | |||
| R2 | 1.94 (0.2,18.67) | 0.32 (0.01,15.73) | 0.563 | |
| R1 | 4.82 (1.13,20.46) | 174.4 (0.38,80215.57) | 0.099 | |
| wt (cont. var.) | 1.0005 (1.0001,1.001) | 1.0009 (0.9999,1.0018) | 0.066 | 0.05 |
| Size cm (cont. var.) | 1.1 (1.02,1.17) | 1.11 (0.99,1.25) | 0.076 | 0.047 |
| Grade of tumour: i vs h | 0.14 (0.03,0.67) | 0.09 (0.01,1.49) | 0.092 | 0.047 |
Table 3.
Cox’s proportional hazard model on time (‘timer’) to event (‘relapse’) with density * denotes significant, Grade h high grade, i low grade
| Cox’s proportional hazard model on time (‘timer’) to event (‘relapse’) | ||||
|---|---|---|---|---|
| Crude HR (95 %CI) | Adj. HR (95 %CI) | P (Wald’s test) P (LR-test) | ||
| Age (cont. var.) | 1.06 (1.01,1.12) | 1.13 (1.01,1.28) | 0.041 | 0.005* |
| Sex: M vs F | 0.51 (0.14,1.9) | 0.11 (0,2.71) | 0.176 | 0.081 |
| Dens (cont. var.) | 1.0011 (0.9934,1.0088) | 0.9954 (0.9854,1.0054) | 0.366 | 0.352 |
| Grade of tumour: i vs h | 0.14 (0.03,0.67) | 0.22 (0.03,1.76) | 0.154 | 0.119 |
| Type of resection Ref R0 | 0.019* | |||
| R2 | 1.94 (0.2,18.67) | 2.65 (0.18,39.33) | 0.478 | |
| R1 | 4.82 (1.13,20.46) | 34.27 (0.99,1188.03) | 0.051 | |
| No. of observations = 23 | ||||
Discussion
Retroperitoneal sarcoma continues to pose a challenge with regard to diagnosis, prediction of clinical behavior, and treatment of disease including recurrence. Data from India, especially eastern part of the country is lacking. We present this analysis for a group of patients treated in a standard fashion at one institution.
Similar to other studies, males were more commonly affected but our mean age of presentation was two decades earlier (39.09 years) [5]. The common signs are palpable abdominal mass (40–90 %), vague abdominal or back pain (30–75 %), and increased abdominal girth (10–35 %) [11]. Of our 23 patients, 12 (52.2 %) presented with abdominal mass. Abdominal discomfort /pain (33 %) and nausea (13 %) was less common. CT and USG were helpful in early diagnosis and evaluating local spread of the disease.
We avoided routine pre operative biopsy. There are several instances where biopsy of a suspected sarcoma may be recommended: if the diagnosis (not histology) is truly questioned, if a neoadjuvant therapy protocol is available, or for tissue diagnosis in the face of non resectable metastatic disease [12]. However, not all retroperitoneal sarcomas need to be biopsied before embarking on definitive therapy.
In the report by Heslin [13] the grade of RPS was not a significant predictor of recurrence and survival. But in our and some other studies, patients with high-grade RPS had a significantly higher recurrence and lower survival rate than those with a low-grade tumor [14, 15]. In fact, grade is one of the components of the American Joint Committee on Cancer staging system [16]. It does stimulates new thoughts about pre operative biopsy and neo-adjuvant chemo/radio and new systemic therapies, such as PPAR [gamma] ligands, and loco-regional therapies, such as preoperative intensity modulated radiation therapy, changing the extent of resection or intraoperative radiotherapy in selected high grade tumors [17]. There is also suggestion that RT (pre op or post operative in R0 resection) may most benefit those patients with operable stage I sarcoma irrespective of grade [18]. The role of chemotherapy in handling RPS remains undefined. In 14 trials of adjuvant chemotherapy involving 1568 cases of soft tissue sarcomas at various sites, a modest improvement (10 %) in the recurrence-free survival rate was found [19]. In our study, adjuvant radiotherapy or chemotherapy was used in R1 and R2 resections. This leaves a scope to conduct further studies with aggressive neo-adjuvant and adjuvant therapy.
Additionally, specific tumor histology plays an important role in patient outcome after treatment of retroperitoneal sarcomas [4, 5, 20]. However, in this study we could not assess histological type as a prognostic factor in disease outcome because statistical analysis revealed that histology type and completeness of resection were mutually dependent. (X-squared = 10.0501, df = 4, p-value = 0.03959) This may be due to the fact that more aggressive histology will predict more local spread and more chance of incomplete resection and may be histological type needs to be given equal importance to completeness of resection. However, we have divided histopathology types into liposarcomma, neurofibroma and all other varieties clubbed together and in larger series, this may be inappropriate.
The ability to completely resect a retroperitoneal sarcoma remains the most important predictor of recurrence and overall survival [21, 22]. This study supports the findings both in univariate and multivariate analysis and KM curve visualization (Fig. 1). In our patients, the incompleteness of resection were usually due to the involvement of the large vessels and base of the mesentery. Of note, patients with microscopically positive margin appeared to have worse survival than patients with gross residual tumor which is in contrary to common concept, but on closer look, it becomes apparent that there is initial sharper drop in the R2 (PI) group as normally expected and then it plateaus probably because very limited number in that group confounding the graph (Fig. 1). In R2 cases, recurrence was taken according to “RECIST” guideline for progressive disease, i.e., any distant recurrence or 20 % increase in the sum of the longest diameter of target lesions in local imaging. As margin status is highly significant in all the analysis, it does stimulate us to think about intra-operative frozen section as a guide to the extent of resection specially when technically it is possible to convert R1 to R0 resection. Also, as histology and grade both are important, the problem with consistently grading these tumors even by experienced pathologists encourage use of molecular genetics combined with morphology.
As with most malignancies and similar to other studies, we also found that the first operation is the best chance for cure [12]. Multivisceral resections facilitate complete tumor removal and reduce the chances of local recurrence [23]. Some authors also recommend vascular resection and prosthetic vascular replacement or the use of temporary bypass for reconstruction of infra-renal aorta and vena cava after a resection in selected patients [24, 25]. Aggressive vascular resection to achieve R0 resection may be tried in future studies in our set up as well to improve outcome.
The overall survival (OS) of 91 % and recurrence-free survival is 63 % at 2 years in our study. Complete resection (R0) rate was 61 % which is comparable to other studies [1, 26, 27], although few authors have reported higher R0 rates [28, 29]. Factors predictive of local recurrence, calculated using logistic regression with tumor weight and size, included incomplete tumor resection (p = 0.002), high histologic grade (p = 0.018) and male sex (p = 0.036). In contrast logistic regression with tumor density (combining volume and weight) excluded male sex as a poor prognostic factor. In most studies, either tumor size or tumor density did not determine prognosis [2, 5]. Analysis using Cox’s proportional hazard model for time to relapse (early relapse) showed that factors like old age, male sex, high tumor grade, incomplete tumor resection and large tumor size, all significantly shortened the time of first recurrence. Analysis using tumor density instead of weight and size showed that only type of resection and age. was statistically significant. Grade, a very important factor in prognosis, became less significant (P = 0.119). This data shows that we may need to consider density as factor while analyzing prognostic indicators. Larger studies are necessary to reach a conclusion.
Two-year follow-up was chosen a priority, as a reasonable cutoff point because of concerns of greater loss to follow-up and compromised data quality associated with longer study duration. We have shown the treatment (surgery) effects on overall relapse or progression-free survival rate.
Conclusions
In this study retroperitoneal Sarcoma, tumor size, histologic grade, incomplete resection, increasing age and male sex are strongly associated with recurrence. Complete (R0) resection appeared most significant.
Our mean age of presentation was two decades earlier than in other studies. Also statistical analysis revealed that histology type and completeness of resection were mutually dependent. This may be due to the fact that more aggressive histology will predict more local spread and more chance of incomplete resection . We proposed that may be histological type needs to be given equal importance to completeness of resection.
In most studies, either tumor size or tumor density did not determine prognosis. However in our study tumor size and weight did predict local recurrence.
Tumor density appears to have reduced the effect of sex as a prognostic factor but density itself is not important in either univariate or multivariate analysis.
Analysis using tumor density instead of weight and size showed that only type of resection and age was statistically significant. Grade, a very important factor in prognosis, became less significant . male sex and large tumor size also became less significant, This data shows that we may need to consider density as factor while analyzing prognostic indicators.
Surgical treatment of retroperitoneal sarcoma should consist of an aggressive approach to achieve a complete surgical resection as there is sharp drop in recurrence free survival between R0 and R1. We identified areas where modification of surgical techniques like “Aggressive vascular resection” may improve outcome.
Acknowledgments
This study has been approved by the appropriate ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study. Details that might disclose the identity of the subjects under study has omitted.
References
- 1.Storm FK, Mahvi D. Diagnosis and management of retroperitoneal soft-tissue sarcoma. Ann Surg. 1991;214:2–10. doi: 10.1097/00000658-199107000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan H, Raut CP, Thornton K, Herman JM, Ahuja N, Schulick RD, et al. Predictors of survival after resection of retroperitoneal sarcoma: a population-based analysis and critical appraisal of the AJCC staging system. Ann Surg. 2009;250(6):970–976. doi: 10.1097/SLA.0b013e3181b25183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Qahtani M, Asiri A. Retroperitoneal sarcomas - a retrospective study. Biomed Res. 2009;20(1):1–6. [Google Scholar]
- 4.Chiappa A, Zbar AP, Bifffi R, Bertani E, Biella F, Viale G, et al. Effect of resection and outcome in patients with retroperitoneal sarcoma. ANZ J Surg. 2006;76:462–466. doi: 10.1111/j.1445-2197.2006.03753.x. [DOI] [PubMed] [Google Scholar]
- 5.Hassan I, Park SZ, Donohue JH, Nagorney DM, Kay PA, Nasciemento AG, et al. Operative management of primary retroperitoneal sarcomas: a reappraisal of an institutional experience. Ann Surg. 2004;239(2):244–250. doi: 10.1097/01.sla.0000108670.31446.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.http://censusindia.gov.in/2011-prov-results/prov_data_products_wb.html. At a Glance Provisional Population Totals at a Glance Figure: 2011 - West Bengal
- 7.Skubitz KM, D’Adamo DR. Sarcoma. Mayo Clin Proc. 2007;82(11):1409–32. doi: 10.4065/82.11.1409. [DOI] [PubMed] [Google Scholar]
- 8.The ESMO / European Sarcoma Network Working Group Clinical practice guidelines.Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:vii92–vii99. doi: 10.1093/annonc/mds310. [DOI] [PubMed] [Google Scholar]
- 9.Krikelis D, Judson I. Role of chemotherapy in the management of soft tissue sarcomas. Expert Rev Anticancer Ther. 2010;10:249–260. doi: 10.1586/era.09.176. [DOI] [PubMed] [Google Scholar]
- 10.Feng M, Murphy J, Griffith KA, et al. Long-term outcomes after radiotherapy for retroperitoneal and deep truncal sarcoma. Int J Radiat Oncol Biol Phys. 2007;69:103–110. doi: 10.1016/j.ijrobp.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 11.Ziran BH, Makley JT, Carter JR. Primary retroperitoneal sarcomas: common symptoms, common diagnoses, uncommon disease. Clin Orthop Relat Res. 1996;331:277–282. doi: 10.1097/00003086-199610000-00039. [DOI] [PubMed] [Google Scholar]
- 12.Woodall C, Scoggins R. Retroperitoneal and visceral sarcomas: issues for the general surgeon. Am Surg. 2007;73(6):631–35. [PubMed] [Google Scholar]
- 13.Heslin MJ, Lewis JJ, Nadler E, Newman E, Woodruff JM, Casper ES, et al. Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol. 1997;15:2832–2839. doi: 10.1200/JCO.1997.15.8.2832. [DOI] [PubMed] [Google Scholar]
- 14.Neuhaus SJ, Barry P, Clark MA, Hayes AJ, Fisher C, Thomas JM. Surgical management of primary and recurrent retroperitoneal liposarcoma. Br J Surg. 2005;92:246–252. doi: 10.1002/bjs.4802. [DOI] [PubMed] [Google Scholar]
- 15.Ferrario T, Karakousis CP. Retroperitoneal sarcomas: grade and survival. Arch Surg. 2003;138:248–251. doi: 10.1001/archsurg.138.3.248. [DOI] [PubMed] [Google Scholar]
- 16.Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging manual. 6. New York: Springer; 2002. [Google Scholar]
- 17.Alektiar KM, Hu K, Anderson L, Brennan MF, Harrison LB. High-dose-rate intraoperative radiation therapy (HDR-IORT) for retroperitoneal sarcomas. Int J Radiat Oncol Biol Phys. 2000;47:157–163. doi: 10.1016/S0360-3016(99)00546-5. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Z, McDade TP, Simons JP, Ng SC, Lambert LA, Whalen GF, et al. Surgery and radiotherapy for retroperitoneal and abdominal sarcoma, both necessary and sufficient. Arch Surg. 2010;145(5):426–431. doi: 10.1001/archsurg.2010.70. [DOI] [PubMed] [Google Scholar]
- 19.Sarcoma Meta-analysis Collaboration Adjuvant chemo- therapy for localized respectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647–1654. doi: 10.1016/S0140-6736(97)08165-8. [DOI] [PubMed] [Google Scholar]
- 20.Shibata D, Lewis JJ, Leung DH, Brennan MF. Is there a role for incomplete resection in the management of retroperitoneal Iiposarcomas? J Am Coll Surg. 2001;193:373–9. doi: 10.1016/S1072-7515(01)01024-9. [DOI] [PubMed] [Google Scholar]
- 21.Singer S, Corson JM, Demetri GD, et al. Prognostic factors predictive of survival for truncal and retroperitoneal soft-tissue sarcoma. Ann Surg. 1995;221:185–195. doi: 10.1097/00000658-199502000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis J, Leung D, Woodruff J, et al. Retroperitoneal soft-tissue sarcoma. Ann Surg. 1998;228:355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Yin L, Peng C, Cai Y, Li Y, Zhao R, Zhou H. Li H Prognostic factors of retroperitoneal soft tissue sarcomas: analysis of 132 cases. Chin Med J. 2007;120(12):1047–1050. [PubMed] [Google Scholar]
- 24.Fueglistaler P, Gurke L, Stierli P, Obeid T, Koella C, Oertli D, et al. Major vascular resection and prosthetic replacement for retroperitoneal tumors. World J Surg. 2006;30:1344–1349. doi: 10.1007/s00268-005-0555-2. [DOI] [PubMed] [Google Scholar]
- 25.Chervenkoff V, Kirilova K, Tschirkov AL. Venous reconstruction of the inferior vena cava bifurcation for retroperitoneal rhabdomyosarcoma in a child. Case report. J Cardiovasc Surg. 2005;46:47–49. [PubMed] [Google Scholar]
- 26.Kinsella TJ, Sindelar WF, Lack E, et al. Preliminary results of a randomized study of adjuvant radiation therapy in resectable adult retroperitoneal soft tissue sarcomas. J Clin Oncol. 1988;6:18–25. doi: 10.1200/JCO.1988.6.1.18. [DOI] [PubMed] [Google Scholar]
- 27.McBride SM, Raut PC, Lapidus M, Devlin MP, Marcus KJ, Bertagnolli M, et al. Locoregional recurrence after preoperative radiation therapy for retroperitoneal sarcoma. Ann Surg Oncol. 2013 doi: 10.1245/s10434-013-2868-y. [DOI] [PubMed] [Google Scholar]
- 28.Karakousis CP, Kontzoglou K, Driscoll DL. Resectability of retroperitoneal sarcomas: a matter of surgical technique? Eur J Surg Oncol. 1995;21:617–622. doi: 10.1016/S0748-7983(95)95305-1. [DOI] [PubMed] [Google Scholar]
- 29.Storm FK, Eilber FR, Mirra J, Morton DL. Retroperitoneal sarcomas: a reappraisal of treatment. J Surg Oncol. 1981;17:1–7. doi: 10.1002/jso.2930170102. [DOI] [PubMed] [Google Scholar]