Abstract
Multiple cutaneous leiomyomatosis have been associated with uterine leiomyomatosis and known as Reed’s syndrome or Multiple Cutaneous and Uterine Leiomyomatosis (MCUL). Gastrointestinal Stromal Tumours (GIST) have been reported to be associated with this syndrome only once previously in literature to the best of our knowledge. Here we report a rare case of GIST and multiple uterine leiomyomatosis in a middle aged patient with longstanding cutaneous leiomyomatosis who underwent GIST excision and hysterectomy. A 35 year old female patient with multiple cutaneous leiomyomatosis for the past 20 years was diagnosed to have gastrointestinal stromal tumour and multiple uterine leiomyomatosis for which she underwent laparotomy for GIST excision and hysterectomy. In the report we have elaborated the clinical and pathological observations as well as the anaesthetic management. This case report further substantiates the association of GIST with multiple cutaneous and uterine leiomyomatosis and also reminds us that cutaneous lesions can be clues to the diagnosis of underlying malignancy.
Keywords: Gastrointestinal stromal tumours, Multiple cutaneous and uterine leiomyomatosis, Laparotomy
Introduction
It is well known that multiple cutaneous leiomyomatosis have been associated with uterine leiomyomatosis and known as Reed’s syndrome or Multiple Cutaneous and Uterine Leiomyomatosis (MCUL) [1–5], which is inherited as an autosomal dominant condition with incomplete penetrance [5]. A subset of these patients have been found to have renal cell carcinomas (RCC) and this association has been called Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC) [6–9]. These patients with RCC are known to have mutations in the ‘Fumarate Hydratase’ gene [10]. Apart from RCC, MCUL has also been associated with uterine leiomyosarcomas, macronodular adrenocortical disease, benign ovarian tumours and leydig cell tumours of the testis [11]. It has also recently been associated with gastric leiomyoma and hyperplastic polyposis coli [11]. Gastrointestinal Stromal Tumours (GIST) have been reported to be associated with MCUL only once previously in literature [5] to the best of our knowledge. Here, we introduce a case of middle aged woman with longstanding cutaneous leiomyomatosis presenting with multiple uterine leiomyomas and a gastrointestinal stromal tumour (GIST) who underwent laparotomy for GIST excision and hysterectomy for uterine leiomyomatosis in the same sitting.
Case Report
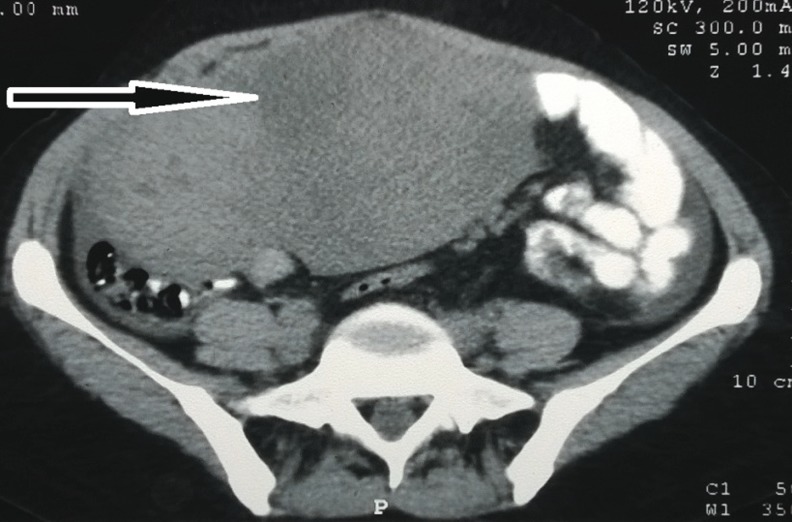
A 35 year old female patient was admitted in All India Institute of Medical Sciences (AIIMS), New Delhi on 1 March, 2014 with complaints of discomfort in lower abdomen with increased intensity on standing as well as after eating food for the past 1 year. It was associated with irregular menstrual cycles. Computerized tomography scan showed large 173 × 135 mm space occupying lesion in abdominopelvic cavity (Fig. 1). An exploratory laparotomy was done in a district hospital in January 2014. They found a large retroperitoneal tumour adhering to the anterior and lateral abdominal wall as well the small intestine and closure of abdomen was done as it was inoperable in a small hospital and referred the patient to AIIMS, a tertiary hospital.
Fig. 1.
Computerized tomography scan showing a solid hyperdense- space occupying lesion (diameter of 173 × 135 mm) in abdominopelvic cavity
Biopsy from the pelvic mass showed a spindle cell tumour with mild pleomorphism and occasional mitoses. The tumour cells were immunopositive for CD 117 and negative for Dog1. The features were suggestive of a GIST. On examination a 15 × 10 cm firm mass was palpable involving the right inferior quadrant of abdomen and pelvis.
The patient was evaluated for irregular menses (15–20 days cycle and reduced flow) in the gynecology deptartment, AIIMS and found to have multiple leiomyomas in the uterus, largest of them being 4 × 3 cm in size. A right ovarian cyst and a large cortical simple cyst in the right kidney was also seen on ultrasound. CA-125 levels were raised (172.3 U/ml).
The patient had a history of multiple cutaneous leiomyomatosis for the past 20 years for which she had shown in the dermatology department of AIIMS in 2011. She had multiple hyperpigmented to skin coloured firm papules unilaterally present over the right lower limb extending to the skin of lower abdomen (Fig. 2). A skin biopsy was consistent with cutaneous leiomyoma. The patient had pain in 2–3 lesions for which the dermatologist prescribed her tab. Nifedipine 10 mg OD. The patient’s father also had cutaneous leiomyomatosis on the skin of anterior abdomen. None of her other first degree relatives had any such disease. The patient had completed her family and had two children.
Fig. 2.
Skin lesions, multiple hyperpigmented to skin coloured firm papules unilaterally present over the right lower limb extending to the skin of lower abdomen. A skin biopsy was consistent with cutaneous leiomyoma
An exploratory laparotomy with excision of the GIST with right oophorectomy and hysterectomy was planned.
The patient’s weight was 46 kg and height was 160 cm. She did not have any comorbid illnesses and was ASA (American Society of Anaesthesiologists) grade 1. Her preoperative haemoglobin level was 8.6 g/dl and she had pallor. Other blood investigations were within normal limits. Her airway was normal and Modified Mallampatti Grading was Grade 2. Preoperative blood pressure was 122/77 mmHg and pulse rate was 115/min. The cardiovascular and respiratory system examinations were normal. Adequate blood and blood products were arranged. Epidural was explained in the preoperative period. Informed written consent was taken and patient was kept nil per oral 8 h preoperatively. The patient was premedicated with 150 mg ranitidine and 0.25 mg alprazolam orally in the night before surgery and on the morning of the surgery.
In the operation theatre, pulse oximeter, electrocardiography, end-tidal carbon dioxide, non invasive blood pressure monitors were attached and 18G intravenous cannula was secured in the right hand. An epidural catheter was inserted at L3-L4 interspace by loss of resistance technique. Induction of anaesthesia was done with fentanyl 100 μg, propofol 100 mg followed by atracurium 30 mg. Patient was intubated with 7.5 mm ID cuffed endotracheal tube fixed at 20 cm. Desflurane, 50 % Oxygen and Air were used for maintenance of anaesthesia to maintain a MAC (Minimum alveolar concentration) of 1–1.2. The right Internal Jugular Vein was cannulated using ultrasound guidance under strict aseptic precautions. A 20-gauge cannula was placed into the radial artery for invasive blood pressure (B.P.) monitoring. The patient had episodes of hypotension (B.P. 80/40 mmHg) during the procedure for which ephedrine 6 mg boluses were given intravenously. For analgesia, morphine 6 mg and 1 gm paracetamol was given intravenously. Also, 10 ml of 0.2 % Bupivacaine was given in graded manner by epidural catheter after a negative test dose.
GIST 10X12X8 cm adherent to the jejunum, parietal wall and bladder was excised by the General Surgery team (Fig. 3). Hysterectomy and right oophorectomy was done by the Gynecologists (Fig. 4). Maximum allowable blood loss was a mere 216 ml and intraoperative blood loss was 650 ml. Therefore 2 units of PRBCs were transfused intraoperatively. 1.5 L of crystalloids were given. All drugs to manage any hypertensive crisis were kept ready (viz. Sodium Nitroprusside, Labetalol) as GIST has been reported to be associated with undiagnosed paraganglioma (Extra adrenal Pheochromocytoma) with intraoperative hypertensive crisis [12]. Intraoperative Arterial Blood Gas analysis was within normal limits. Intra operative urine output was 300 ml and the duration of procedure was two and half hours. Patient was reversed with neostigmine and glycopyrrolate and extubated. Then the patient was shifted to post anaesthesia care unit. Two mg morphine in 10 ml normal saline was given epidurally every 8 h for 72 h postoperatively for pain relief.
Fig. 3.
a Peroperatory finding: GIST tumor of size10X12X8 cm adherent to the jejunum, parietal wall and bladder b GIST tumor after surgical removal
Fig. 4.
Specimen of uterus showing multiple leiomyomas after right oophorectomy and hysterectomy
Discussion
Gastrointestinal stromal tumours (GIST) are the most common mesenchymal tumours of the gastrointestinal tract [13] and their most common location is the Stomach in 60–70 % of cases [14]. The usual presenting symptoms of GIST are anaemia, weight loss, gastrointestinal bleeding, abdominal pain and mass associated symptoms. [15] Patients may present with acute abdomen, obstruction, perforation, rupture or peritonitis [15]. Some patients can present with hemorrhagic shock with need for emergency surgery [14]. The malignant potential of GISTs is unpredictable [14], as many histologically malignant appearing tumours never metastasize, while rarely benign appearing lesions do. All GIST tumors are believed to have malignant potential, and none of them can be definitively classified as “benign” [16].
The association of GIST with MCUL has only been reported once in literature previously to the best of our knowledge, in a case report by Lamba et al. from Ottawa, Canada in 2005 [5]. They reported a case of cutaneous leiomyomatosis in a 43-year-old woman with history of hysterectomy for uterine leiomyomatosis and partial gastrectomy for excision of GIST.
Hence this is an interesting case of long standing cutaneous leiomyomatosis, with recently diagnosed uterine leiomyomata, and an intestinal GIST in a 35-year-old woman who underwent GIST excision and hysterectomy in the same sitting. This case report further substantiates the possible association of GISTs with MCUL reported by Lamba et al.
Serra and colleages reported a case of gastric leiomyoma and hyperplastic polyposis coli in a 44 year old female with MCUL which carried a new mutation of the fumarate hydratase (FH) gene [11]. Both Leiomyomas and GISTs have a mesenchymal origin, but GISTs are morphologically and biologically different and are derived from the precursors of the interstitial cells of Cajal [17].
This case report brings home the point that cutaneous lesions can be clues to the presence of underlying malignancy and further emphasizes that experts from different disciplines are required for management of this syndrome. Physicians who encounter a case of MCUL should be suspicious of the presence of other tumours that may be present as the complete clinical spectrum of this syndrome is yet to be uncovered. This may lead to early diagnosis and treatment of potentially lethal conditions.
Contributor Information
Ejas P. Bava, Email: ejasaiims@gmail.com
Ankur Sharma, Email: ankuranaesthesia@gmail.com.
Sunil Chumber, Email: sunil_chumber@hotmail.com.
Rahul Kumar Anand, Email: rahulanand00@gmail.com.
References
- 1.Kloepfer HW, Krafchuk J, Derbes V, et al. Hereditary multiple leiomyoma of the skin. Am J Hum Genet. 1958;10(1):48–52. [PMC free article] [PubMed] [Google Scholar]
- 2.Mezzadra G, et al. Multiple hereditary cutaneous leiomyoma. Study of a systemic case in a male subject related to a family with cutaneous leiomyomatosis and uterine fibromyomatosis. Minerva Dermatol. 1965;40(10):388–393. [PubMed] [Google Scholar]
- 3.Reed WB, Walker R, Horowitz R, et al. Cutaneous leiomyomata with uterine leiomyomata. Acta Derm Venereol. 1973;53(5):409–416. [PubMed] [Google Scholar]
- 4.Mandal RK, Koley S, Banerjee S, et al. Familial leiomyomatosiscutis affecting nine family members in two successive generations including four cases of Reed’s syndrome. Indian J Dermatol Venereol Leprol. 2013;79(1):83–87. doi: 10.4103/0378-6323.104674. [DOI] [PubMed] [Google Scholar]
- 5.Lamba M, Verma S, Prokopetz R, et al. Multiple cutaneous and uterine leiomyomas associated with gastric GIST. J Cutan Med Surg. 2005;9(6):332–335. doi: 10.1007/s10227-005-0114-3. [DOI] [PubMed] [Google Scholar]
- 6.Emer JJ, Solomon S, Mercer SE. Reed’s syndrome: a case of multiple cutaneous and uterine leiomyomas. J Clin Aesthet Dermatol. 2011;4(12):37–42. [PMC free article] [PubMed] [Google Scholar]
- 7.Braun SA, Hanneken S, Reifenberger J, et al. Hereditary cutaneous leiomyomatosis. Hautarzt. 2012;63(4):276–278. doi: 10.1007/s00105-012-2357-4. [DOI] [PubMed] [Google Scholar]
- 8.Michelon MA, Layton CJ, Jessup CJ, et al. Cutaneous clues to renal cell carcinoma: hereditary leiomyomatosis and renal cell carcinoma. J Drugs Dermatol. 2013;12(5):578–579. [PubMed] [Google Scholar]
- 9.Henley ND, Tokarz VA, et al. Multiple cutaneous and uterine leiomyomatosis in a 36-year-old female, and discussion of hereditary leiomyomatosis and renal cell carcinoma. Int J Dermatol. 2012;51(10):1213–1216. doi: 10.1111/j.1365-4632.2011.05456.x. [DOI] [PubMed] [Google Scholar]
- 10.Tomlinson IP, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30(4):406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 11.Serra D, Amaro P, Gonçalo M, et al. Gastric leiomyoma and hyperplastic polyposis coli in a patient with multiple cutaneous and uterine leiomyomatosis. J Cutan Med Surg. 2012;16(3):208–211. doi: 10.1177/120347541201600315. [DOI] [PubMed] [Google Scholar]
- 12.Shinn HK, Jung JK, Park JK, et al. Hypertensive crisis during wide excision of gastrointestinal stromal cell tumor (GIST): undiagnosed paraganglioma - a case report. Korean J Anesthesiol. 2012;62(3):289–292. doi: 10.4097/kjae.2012.62.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinomura Y, Kinoshita K, Tsutsui S, Hirota S. Pathophysiology, diagnosis, and treatment ofgastrointestinal stromal tumors. J Gastroenterol. 2005;40:775–780. doi: 10.1007/s00535-005-1674-0. [DOI] [PubMed] [Google Scholar]
- 14.MajdoubHassani KI, Zahid FZ, Ousadden A, et al. Gastrointestinal stromal tumors and shock. J Emerg Trauma Shock. 2009;2(3):199–202. doi: 10.4103/0974-2700.55344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorour MA, Kassem MI, Ghazal A-H, et al. Gastrointestinal stromal tumors (GIST) related emergencies. Int J Surg. 2014;12(4):269–280. doi: 10.1016/j.ijsu.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Raut C, Dematteo R. Evidence-guided surgical management of GIST: beyond a simple case of benign and malignant. Ann Surg Oncol. 2008;15(5):1542. doi: 10.1245/s10434-008-9817-1. [DOI] [Google Scholar]
- 17.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]