Abstract
End stage renal disease (ESRD) patients on hemodialysis (HD) have an increased oxidative stress, with a high risk of atherosclerosis and other co-morbid conditions. Recent studies have suggested that myeloperoxidase (MPO)—mediated oxidative stress may play a role in the pathogenesis of cardiovascular complications in dialysis patients. Furthermore, dialysis treatment ‘per se’ can aggravate oxidative stress. Hence this study was designed to determine whether HD leads to an alteration in the plasma levels of MPO and malondialdehyde (MDA), a marker of oxidative stress in ESRD patients on maintenance HD. To study the effect of HD, plasma MPO and MDA were determined before and after HD in forty ESRD patients (24 men and 16 women, age between 8 and 71 years, median being 40.5 years) on maintenance HD. Plasma MPO and MDA were assayed by spectrophotometric methods. Haematological and other biochemical parameters were obtained from patients’ case records. Plasma MPO and MDA levels were significantly higher after HD when compared with pre-dialysis levels (p < 0.05). There was no correlation between MPO and MDA (r = 0.184, p = 0.10) and other biochemical parameters (p > 0.05). However, there was a significant correlation between MPO and MDA with haemodialysis vintage (p < 0.05). In univariate regression analysis duration of HD (β = 1.470, p = 0.045, β = 0.388, p = 0.013), was independently associated with MPO and MDA. Although HD is indispensable for survival of patients with ESRD, it is fraught with undesirable side-effects, such as an increase in the plasma MPO and MDA levels. The elevated levels of MPO contribute to the increased oxidative stress as free radicals are produced by the reaction catalyzed by it.
Keywords: ESRD, Hemodialysis, Myeloperoxidase, Oxidative stress, Hemodialysis vintage
Introduction
End stage renal disease (ESRD) patients on hemodialysis (HD) have a high prevalence of cardiovascular disease (CVD), and a significant morbidity and mortality [1]. The classical risk factors only partly explain the high cardiovascular risk in these patients and it is now recognized that non-traditional risk factors play relevant roles among these patients. In this respect, oxidative stress certainly is an emerging issue [2]. While oxidative stress remains as one of the important cardiovascular risk factors in ESRD patients on HD, there is some controversy as to whether regular dialysis has any effect oxidative stress. Moreover, the causes of oxidant stress in these patients are poorly understood.
Myeloperoxidase (MPO), a hemoprotein abundantly stored in primary granulocytes of polymorphonuclear leukocytes (PMNs) and released from degranulated neutrophils, plays an important role in the defense of the organism by catalyzing the production of oxidants such as hypochlorous acid (HOCl) [3]. In addition, it produces hypochlorite (OCl−), that promotes protein oxidation and lipid peroxidation and thereby plays an important role in the pathogenesis of cardiovascular complications [4]. Elevated levels of plasma MPO in patients with chest pain have been shown to predict the early risk of myocardial infarction as well as other major adverse cardiac events [5]. Moreover, plasma MPO activity provides independent prognostic value for the prediction of long-term incident ‘major adverse cardiovascular events’ (MACEs) in a stable, medically managed patient population with coronary artery disease [6].
Because of the many links between elevated MPO levels and atherosclerosis, recent research has focused on a potential role for MPO and its oxidants as predictors of cardiovascular risk in ESRD patients on HD. So far, very few studies [7, 8] have demonstrated that dialysis results in neutrophil activation and release of MPO. Also, MPO has been considered as an important pathophysiological factor during the HD process and may serve as a reliable indicator of oxidative stress [9]. Our earlier study carried out on patients with chronic kidney disease who were not on dialysis, has shown that plasma MPO levels declined progressively and significantly with advancing stages of renal failure [10].
Hence, the present study was designed to determine the effect of HD on plasma MPO and an oxidative stress marker viz, malondialdehyde (MDA). Further this study evaluates the association between MPO, MDA and other biochemical parameters and factors associated with HD.
Materials and Methods
Patients
Forty ESRD patients on maintenance HD at the PSG Super Speciality Hospital, Coimbatore, India, were recruited for this study. The background of each patient such as etiology of ESRD, glycaemic state, BP, and CVD was recorded. Patients suffering from diabetes mellitus, systemic lupus erythematosus, malignancy, acute infections and those on immunosuppressive therapy were excluded from the study.
The causes of ESRD were hypertension (40%), chronic glomerulonephritis (29%), nephrosclerosis (13%), polycystic kidney disease (8%) and other diseases (10%). All patients were essentially treated with three sessions of routine conventional bicarbonate HD each week (3–4 h/session) using standard polysulfone (PS) membranes (Fresenius F-6, 40 mm thick of 1.32 m2 surface area). The dialysate of standard composition with bicarbonate buffer was used in all patients. The blood flow rate was 200 ml/min and the dialysate flow rate was 500 ml/min. The dose of dialysis was individually adjusted to maintain a Kt/V > 1.2.
The Institutional Human Ethics Committee approved the study protocol and written informed consent was obtained from all patients.
Laboratory Data
Venous blood was collected before and 4 h after the dialysis into vacutainers (Becton–Dickinson, USA) containing fluoride EDTA. The samples were centrifuged for 15 min at 3,000 rpm, plasma was separated and stored at −20°C until analysis. All the other data which included hematological and biochemical parameters were obtained from patients’ dialysis records. The data that was used for this study was rendered anonymous and maintained confidentially.
Estimation of MPO
The activity of plasma MPO was measured spectrophotometrically using o-dianisidine (Sigma-Aldrich) and hydrogen peroxide (H2O2) as described previously [11]. MPO catalyzes the oxidation of o-dianisidine in the presence of H2O2 as oxidizing agent, yielding a brown coloured product according to the following equation.
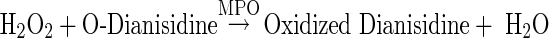 |
The oxidized o-dianisidine that is formed has an absorbance maximum at 470 nm.
One unit (U) of MPO activity was defined as that degrading 1 μmol of H2O2 per minute at 25°C.
Estimation of MDA
Plasma thiobarbituric acid-reactive substances (TBARS) were estimated and expressed as MDA, as described by the method of Wilbur et al. [12].
Statistical Analysis
All the data are presented as mean ± standard deviation (SD). As the data is not distributed normally, the Wilcoxon rank-sum test was used for comparisons of means within the same group. Correlation between variables was calculated using Spearman’s rank-sum test (ρ). A p value <0.05 was considered to be statistically significant. Significant predictors of MPO and MDA were evaluated using simple linear regression analysis. The statistical analysis was performed using the statistical software, MedCalc Version 11.3.3.0.
Results
The base-line and biochemical characteristics of patients studied are shown in Tables 1 and 2. The median duration of HD was 11 months. Among 40 patients, 22 (55%) had a history of cardiovascular events.
Table 1.
Demographic characteristics of hemodialysis patients n = 40, (%)
| 1. Age (years) | 40 ± 15 |
| 2. Gender (male/female) | 24/16 |
| 3. Weight (kg) | 53.0 ± 12.3 |
| 4. Systolic blood pressure (mm Hg) | 151 ± 17 |
| 5. Diastolic blood pressure (mm Hg) | 89 ± 19 |
| 6. History of precious CV events | |
| Myocardial infarction | 03 (7.5) |
| Angina pectoris | 07 (17.5) |
| Ischemic attack | 05 (12.5) |
| Others | 07 (17.5) |
| 7. Aetiology of ESRD | |
| Hypertension | 28 (70.0) |
| Glomerulonephritis | 03 (7.5) |
| Polycystic kidney disease | 05 (12.5) |
| Others | 02 (5.0) |
| Unknown | 02 (5.0) |
| 8. Dialysis dosage (Kt/V) | 1.54 ± 0.48 |
| 9. Hemodialysis vintage (months) | 14.1 ± 9.6 |
Data are expressed as Mean ± SD
Kt/V fractional urea clearance, ESRD end-stage renal disease
Table 2.
Laboratory data of hemodialysis patients (n = 40)
| 1. Plasma glucose (mg/dl) | 98.8 ± 13.0 |
| 2. Urea (mg/dl) | 144.8 ± 75.5 |
| 3. Creatinine (mg/dl) | 10.54 ± 3.72 |
| 4. Calcium (mg/dl) | 8.3 ± 3.0 |
| 5. Phosphorus (mg/dl) | 5.9 ± 1.5 |
| 6. Ca × P product (mg2/dl2) | 49.30 ± 12.81 |
| 7. Ferritin (ng/ml) | 554 ± 382 |
| 8. Alkaline phosphatase (U/l) | 122 ± 90 |
| 9. Hemoglobin (g/dl) | 8.08 ± 3.33 |
| 10. Hematocrit (%) | 21.4 ± 6.6 |
| 11. Leukocytes (×1,000/μl) | 7.6 ± 2.4 |
| 12. Neutrophils (×1,000/μl) | 5.5 ± 2.5 |
Data are expressed as Mean ± SD
Effect of Dialysis on Plasma MPO and MDA
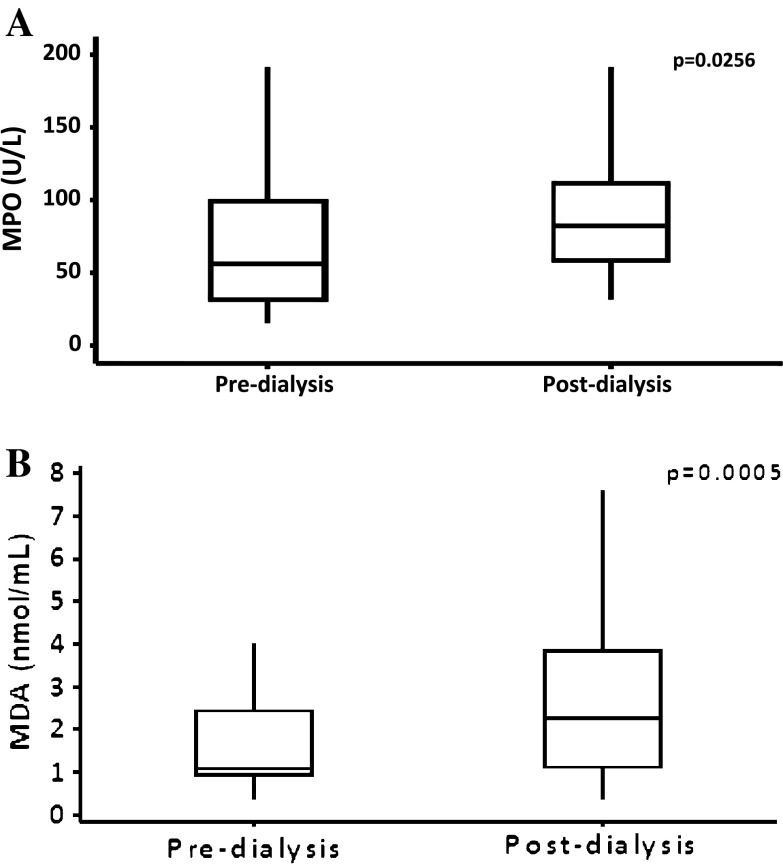
The pre-dialysis MPO levels varied from 15.93 to 191.16 U/l with a median value of 56.86 U/l, whereas post-dialysis, the levels varied from 31.86 to 217.71 U/l, with a median value of 82.31 U/l. The pre-dialysis MDA levels varied from 0.38 to 3.99 n moles/ml with a median value of 1.08 n moles/ml whereas post-dialysis, the levels varied from 0.38 to 7.60 n moles/ml, with a median value of 2.28 n moles/ml (Fig. 1a, b). The plasma concentrations of MPO and MDA were significantly higher in the post-dialysis as compared with the pre-dialysis samples (p < 0.05, p < 0.001 Table 3).
Fig. 1.
Levels of plasma myeloperoxidase (MPO) and malonyldialdehyde (MDA) according to median values in hemodialysis patients
Table 3.
Myeloperoxidase and malondialdehyde in hemodialysis patients
| Pre-dialysis | Post-dialysis | p value | |
|---|---|---|---|
| (n = 40) | (n = 40) | ||
| 1. MPO (U/l) | 68.48 ± 45.23 | 92.20 ± 45.03 | 0.0256 |
| 2. MDA (nmol/ml) | 1.65 ± 0.98 | 2.49 ± 1.64 | 0.0005 |
Values are Mean ± SD
Association of MPO and MDA with Other Laboratory Data
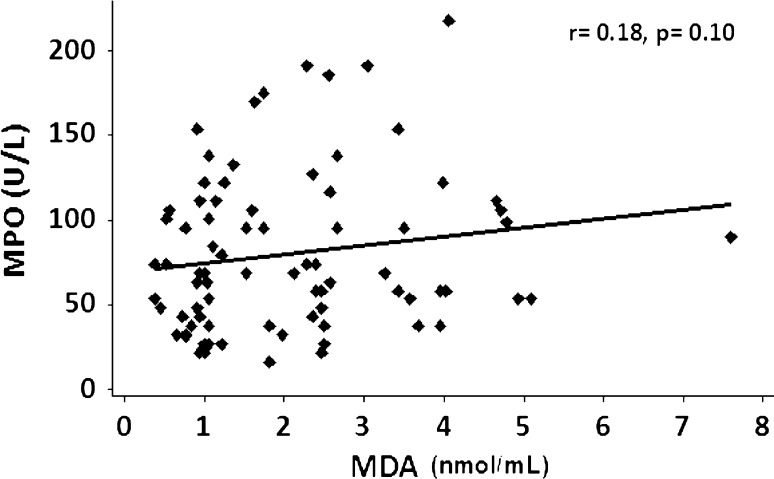
There was a positive correlation between MPO and MDA, but this was not statistically significant (r = +0.18; p = 0.10, Fig. 2). Both MPO (r = +0.322, p = 0.043) and MDA (r = +0.267, p = 0.126) showed a positive correlation with HD duration. Furthermore, a significant negative correlation was observed between MPO and serum creatinine (r = −0.294, p = 0.045) and a positive correlation was observed between MDA and hemoglobin (r = +0.358, p = 0.023). There was no statistically significant correlation of MPO with neutrophils and leucocytes (Table 4).
Fig. 2.
Correlation analysis between myeloperoxidase (MPO) and malonyldialdehyde (MDA) in hemodialysis patients
Table 4.
Correlation between plasma MPO and MDA with clinical parameters in hemodialysis patients
| Variables | MPO | MDA |
|---|---|---|
| 1. Age | 0.194 | 0.082 |
| 2. Hemodialysis vintage (months) | 0.322* | 0.267* |
| 3. Glucose (mg/dl) | −0.201 | −0.048 |
| 4. Urea (mg/dl) | −0.251 | −0.114 |
| 5. Creatinine (mg/dl) | −0.294* | −0.130 |
| 6. Calcium (mg/dl) | 0.111 | 0.162 |
| 7. Phosphorus (mg/dl) | −0.008 | −0.003 |
| 8. Ferritin (ng/ml) | −0.184 | 0.029 |
| 9. ALP (U/l) | −0.080 | 0.004 |
| 10. Hemoglobin (g/dl) | 0.279 | 0.358** |
| 11. Neutrophils (×1,000/μl) | −0.228 | 0.020 |
| 12. Leukocytes (×1,000/μl) | −0.241 | 0.027 |
* p value 0.05–0.01, ** p value 0.01–0.001, *** p value < 0.001
Determination of Significant Predictors for MPO and MDA
To assess the contributors of oxidative stress in these patients we performed univariate linear regression analysis in order to establish the principal determinants for MPO and MDA. Regression model showed that duration of HD (β = 0.388, p = 0.013), was independently associated with increased MPO (Table 5) and MDA (Table 6). Age, neutrophils, leukocytes were not independently associated with MDA. Similarly, there was no significant association between MPO and MDA (β = 0.157, p = 0.165).
Table 5.
Factors contributing to MPO levels
| Independent variable | B | SE | β | t | p |
|---|---|---|---|---|---|
| 1. Age (years) | 0.525 | 0.488 | 0.172 | 10.74 | 0.289 |
| 2. Hemodialysis vintage (months) | 1.470 | 0.728 | 0.311 | 2.019 | 0.045* |
| 3. Neutrophils (×1,000/μl) | −4.030 | 2.959 | −0.216 | 1.362 | 0.181 |
| 4. WBC (×1,000/μl) | −3.938 | 2.834 | −0.220 | −1.389 | 0.173 |
* Pre-dialysis plasma MPO as a dependent variable
Hemodialysis vintage: duration of hemodialysis, B: unstandardized coefficient
SE standard error, β standard coefficient
Table 6.
Factors contributing to MDA levels
| Independent variable | B | SE | β | t | p |
|---|---|---|---|---|---|
| 1. Age (years) | 0.004 | 0.011 | 0.058 | 0.359 | 0.722 |
| 2. Hemodialysis vintage (months) | 0.040 | 0.015 | 0.388 | 2.596 | 0.013* |
| 3. Neutrophils (×1,000/μl) | 0.032 | 0.065 | 0.078 | 0.484 | 0.631 |
| 4. WBC (×1,000/μl) | 0.039 | 0.063 | 0.100 | 0.618 | 0.540 |
| 5. MPO (U/l) | 0.005 | 0.003 | 0.157 | 1.402 | 0.165 |
* Pre-dialysis plasma MDA as a dependent variable
Hemodialysis vintage: duration of hemodialysis, B: unstandardized coefficient
SE standard error, β standard coefficient
Discussion
The major finding of this study was that a single session of dialysis resulted in significantly higher levels of plasma MPO and MDA in ESRD patients. Another novel observation in the present study is that, the duration of dialysis is the most significant contributor to higher MPO and MDA levels. Other factors such as age, total WBC counts as well as neutrophil counts did not have any impact on both MPO as well as MDA levels.
The results of the present study are in agreement with that of others [9, 13] who have also shown a significant rise in MPO after a single dialysis session. MPO is abundantly stored in the primary granulocytes of PMNs and is secreted by activated neutrophils [3]. The increased MPO levels are said to be the result of the degranulation of PMNs, consequent to the shear stress that they undergo when blood is forced through the dialyzer membrane [14]. MPO is an enzyme that catalyzes the reaction between the chloride ion and hydrogen peroxide to form a powerful oxidant, sodium hypochlorite as the product. Since each session of dialysis leads to the release of this enzyme, the cumulative effect would be even more deleterious. This could account for the association between both MPO and MDA with the dialysis vintage.
Recently Krieter et al. [13] have observed that contact of blood with the dialysis membrane was not the only source of the higher MPO levels and that there are unrecognized factors which were responsible for its up-regulation during HD. The type of anticoagulant used and the nature of the dialysis membrane might also have an impact on the generation of MPO. Barowski et al. [15] demonstrated that MPO was an abundant constituent of the vascular vessel wall and could be extensively mobilized into circulating blood by exogenous heparin. Furthermore, in the study conducted by Gritters et al. [16], serum MPO levels almost double with the first passage of blood through the high flux poly-sulfone dialyzer. Notably the effect was observed only when either un-fractionated heparin or low molecular weight heparin was used for temporary HD and disappeared afterwards with tri-sodium citrate anticoagulation. In the present study since un-fractionated heparin was used as the anti-coagulant, this could also be responsible for the release of MPO from the vascular vessel wall, which then manifests as raised plasma MPO.
An increase in oxidative stress is recognized as an important metabolic accompaniment to ESRD [17]. Our results are in agreement with the study of Nguyen-Khoa et al. [18], who suggested that the presence of inflammation and the duration of dialysis are the most important determinants of oxidative stress in HD patients. This implicates a significant effect of dialysis procedure in the elevation of oxidative stress.
Recent research has focused on mediators of oxidative stress in dialysis. It has been suggested that massive liberation of MPO from activated neutrophils triggered by the HD procedure leads to generation of oxidants which oxidize plasma proteins resulting in the formation of advanced oxidation protein products (AOPP), which are associated with coronary and carotid artery disease in ESRD patients [19]. Capeillere-Blandin et al. [20] demonstrated that plasma AOPPs concentration correlated with the neutrophil MPO activity in HD patients. Further, Hawkins et al. [21] also reported that plasma proteins are the major targets for HOCl, a product of MPO and showed that the reaction of fresh diluted plasma with HOCl gives rise to protein-derived nitrogen-centered radicals in a time and HOCl concentration-dependent manner. Identical radicals have been detected with isolated HOCl-treated plasma proteins. Thus, it has been suggested that AOPPs act as mediators of oxidative process in HD patients. It is not possible to draw a definite conclusion on this with our results, as we have not measured the AOPP levels. However, a weak correlation between MPO and MDA found in this study suggests that increased MPO would have contributed, at least in part, to the increased peroxidation of lipids resulting in increased MDA following HD.
In conclusion, even a single session of HD results in a significant increase in plasma MPO and MDA levels. These increases have a direct association with the duration of dialysis or the dialysis vintage. Raised levels of MPO and MDA serve to reflect the greater oxidative stress and consequently the increased cardio-vascular risk in ESRD patients undergoing HD. However, the present study failed to demonstrate a significant association between MPO with MDA, for which further evaluation in a larger patient population is needed.
Acknowledgments
The authors thank the Indian Council of Medical Research (ICMR), New Delhi, for their financial support.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
All the authors contributed in research design, data collection, analysis of data and drafting article.
References
- 1.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 2.Himmelfarb J. Hemodialysis complications. Am J Kidney Dis. 2005;45:1122–1131. doi: 10.1053/j.ajkd.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Arnhold J. Properties, functions, and secretion of human myeloperoxidase. Biochemistry. 2003;69:4–9. doi: 10.1023/b:biry.0000016344.59411.ee. [DOI] [PubMed] [Google Scholar]
- 4.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 5.Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 6.Tang WH, Wu Y, Nicholls SJ, Hazen SL. Plasma myeloperoxidase predicts incident cardiovascular risks in stable patients undergoing medical management for coronary artery disease. Clin Chem. 2011;57:1–7. doi: 10.1373/clinchem.2010.152827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bansal V, Cunanan J, Hoppensteadt D, Wahi R, Fareed J. Up regulation of myeloperoxidase in end stage renal disease and its modulation by hemodialysis. J Thromb Haemost. 2007;52:S609. [Google Scholar]
- 8.Kalantar-Zadeh K, Brennan ML, Hazen SL. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2006;48:59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 9.Wu CC, Chen JS, Wu WM, et al. Myeloperoxidase serves as a marker of oxidative stress during single haemodialysis session using two different biocompatible dialysis membranes. Nephrol Dial Transplant. 2005;20:1134–1139. doi: 10.1093/ndt/gfh764. [DOI] [PubMed] [Google Scholar]
- 10.Madhusudhana Rao A, Anand U, Anand CV. Myeloperoxidase in chronic kidney disease. Indian J Clin Biochem. 2011;26:28–31. doi: 10.1007/s12291-010-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger AJ, Yang JJ, Roy TA, Robbins DJ, Mackerer CR. An automated myeloperoxidase assay. Clin Chem. 1990;36:158. [PubMed] [Google Scholar]
- 12.Wilbur KM, Bernheim F, Shapiro OW. The TBARS reagent as a test for the oxidation of unsaturated fatty acids by various agents. Arch Biochem Biophys. 1943;24:305–313. [PubMed] [Google Scholar]
- 13.Krieter DH, Lemke HD, Wanner C. Myeloperoxidase serves as a marker of oxidative stress during single haemodialysis session using two different biocompatible membranes. Nephrol Dial Transplant. 2006;21:546. doi: 10.1093/ndt/gfi222. [DOI] [PubMed] [Google Scholar]
- 14.Rutgers A, Heeringa P, Kooman JP, et al. Peripheral blood myeloperoxidase activity increases during hemodialysis. Kidney Int. 2003;64:760. doi: 10.1046/j.1523-1755.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- 15.Borawski J. Myeloperoxidase as a marker of hemodialysis biocompatibility and oxidative stress: the underestimated modifying effects of heparin. Am J Kidney Dis. 2006;47:37–41. doi: 10.1053/j.ajkd.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Gritters M, Grooteman MPC, Schoorl M, et al. Citrate anticoagulation abolishes degranulation of polymorphonuclear cells and platelets and reduces oxidative stress during haemodialysis. Nephrol Dial Transplant. 2006;21:153–159. doi: 10.1093/ndt/gfi069. [DOI] [PubMed] [Google Scholar]
- 17.Himmelfarb J, Stenvinkel P, Ikizler TA, et al. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen-Khoa T, Massy ZA, De Bandt JP, et al. Oxidative stress and haemodialysis: role of inflammation and duration of dialysis treatment. Nephrol Dial Transplant. 2001;16:335–340. doi: 10.1093/ndt/16.2.335. [DOI] [PubMed] [Google Scholar]
- 19.Drueke T, Witko-Sarsat V, Massy Z, et al. Iron therapy, advanced oxidation protein products and carotid artery intima-media thickness in end-stage renal disease. Circulation. 2002;106:2212–2217. doi: 10.1161/01.CIR.0000035250.66458.67. [DOI] [PubMed] [Google Scholar]
- 20.Capeillere BC, Gausson V, Nguyen AT, Descamps LB, Drueke T, Witko SV. Respective role of uraemic toxins and myeloperoxidase in the uraemic state. Nephrol Dial Transplant. 2006;21:1555–1563. doi: 10.1093/ndt/gfl007. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins CL, Davies MJ. Hypochlorite-induced oxidation of proteins in plasma: formation of chloramines and nitrogen-centered radicals and their role in protein fragmentation. Biochem J. 1999;340:539–548. doi: 10.1042/0264-6021:3400539. [DOI] [PMC free article] [PubMed] [Google Scholar]