Abstract
Reactive oxygen species (ROS) formed in various metabolic reactions cause unlimited damage by attacking and oxidizing the macromolecules. An arsenal of antioxidant substances neutralizes these ROS at various sites of their metabolic cascade, and if disequilibrium exists between the pro and antioxidant system, oxidative stress persists. The present study was undertaken in schizophrenia, to highlight the response and role of some endogenous antioxidants viz. reduced glutathione (GSH), bilirubin, total proteins, albumin and uric acid in scavenging the ROS. The effect of severity of disease, age factor, and substance abuse was also studied. In all, 50 schizophrenics and 50 age and sex-matched controls were enrolled in the present study. Fasting blood samples were drawn for estimating malondialdehyde (MDA), GSH, bilirubin, total proteins, albumin and uric acid in both the groups. The results were statistically analyzed by Z-test and correlated using correlation coefficient (r). The study shows reduction in MDA levels and decline in the level of endogenous antioxidants, but within the normal range. Chronic schizophrenics were at a higher risk of oxidative stress and age and substance abuse seems to worsen the situation.
Keywords: Oxidative stress, Schizophrenia, Antioxidants, Reduced glutathione, Bilirubin, Uric acid
Introduction
Higher eukaryotic aerobic organisms cannot exist without oxygen, yet oxygen is inherently dangerous to their existence [1] as it produces pro-oxidants or reactive oxygen species (ROS) in various metabolic processes [2]. ROS attack macromolecules viz. lipids, proteins/enzymes, carbohydrates and DNA, inducing undesirable oxidation and producing toxic substances that cause membrane damage, protein modification, DNA damage, lipid peroxidation, etc. [3]. This oxidative damage is believed to be involved not only in the process of aging, but accumulating evidence also suggests that it may contribute to pathogenesis of various diseases [4]. The brain tissue contains large amounts of unsaturated fatty acid, catecholamines, increased oxygen consumption, presence of transition metals making the neuronal membrane uniquely vulnerable to free radical mediated damage [5], that may be important in diseases like Alzheimers, Schizophrenia, etc. [6].
Schizophrenia is a psychiatric disorder, with a complex pathophysiology and a broad range of behavioral and biological manifestations [7]. The disease is common to people belonging to all strata of the society who are under constant stress due to socio-economical, environmental and demographic conditions. These conditions may also become etiological factors for the progression of schizophrenia [8]. To better comprehend the relationship between changes in brain membrane lipids and schizophrenia, the protective strategies evolved against free radical toxicity requires to be explored [9]. For combating the offending behavior of ROS, our body is equipped with an arsenal of powerful defense components that include enzymatic, nutrient and endogenous substances known as antioxidants [10]. These are very important for existence and if homeostasis between pro-oxidant and antioxidant is disturbed, it creates oxidative stress [11]. The antioxidants neutralize oxidants by acting cooperatively at different sites of the metabolic cascade of free radicals [12]. Apart from the enzymatic and nutrient antioxidants the biological system has evolved some endogenous defensive substances as additional antioxidative tools. These include glutathione (GSH), bilirubin, total proteins, albumin and uric acid, that as a whole play a homeostatic role against ROS produced during normal cellular metabolism and after active oxidation insults [13].
In our previous studies it was found that nutrient antioxidants [14] as well as enzymatic antioxidant substances [15] are altered in response to oxidative stress in condition of schizophrenia perhaps as a defensive mechanism. Hence, the aim of the present study is to evaluate the response of some endogenous antioxidants in condition of oxidative stress in schizophrenia.
Materials and Methods
The study comprised of 50 schizophrenic patients, screened thoroughly for the disease at Psychiatric Centre, Jaipur. These were compared with another group of 50 normal, healthy, age and sex-matched subjects selected from general population as controls. Personal and clinical history of the subjects was recorded in form of a questionnaire (Table 1). Psychopathological screening of schizophrenic patients was done by positive and negative syndrome scale (PANSS) under the guidance of a trained psychiatrist. Subjects were selected using the following exclusion criterion:
Pregnant and lactating females
Symptoms of any organic illness
History of receiving ECT in last 1 year
Any substance dependence for last 1 month
History or present symptoms of any other stress-induced disorders
Table 1.
Some anthropometric parameters of control and schizophrenic groups
| Parameters | Control group | Schizophrenic group |
|---|---|---|
| n = 50 (%) | n = 50 (%) | |
| Age (years) | ||
| Mean ± SD | 39.22 + 9.23 | 40.38 + 9.48 |
| ≤40 | 28 (56%) | 25 (50%) |
| >40 | 22 (44%) | 25 (50%) |
| Sex (M/F) | ||
| Male | 35 (70%) | 34 (68%) |
| Female | 15 (30%) | 16 (32%) |
| Diet | ||
| Vegetarian | 38 (76%) | 40 (80%) |
| Non-vegetarian | 12 (24%) | 10 (20%) |
| Phase of illness | ||
| Acute | – | 40 (80%) |
| Chronic | – | 10 (20%) |
| Substance abuse | ||
| Abusers | 18 (36%) | 18 (36%) |
| Non- abusers | 32 (64%) | 32 (64%) |
Subjects under or equal to the age of 40 years were considered as one group and above 40 years of age as another group. Acute and chronic schizophrenics and substance abusers and non-abusers were also counted for in separate groups. Samples of blood from the anticubital vein were drawn and collected in plain vials for serum separation. The samples were centrifuged for 15 min at 2,000 rpm and stored at −20°C until analysis. Endogenous antioxidants were measured using Randox kit methods on the Olympus AU 400 analyser according to the manufacturer’s procedures viz. bilirubin (Diazo kit method), total proteins (Biuret kit method), total proteins (Biuret kit method), albumin (BCG kit method) and uric acid (Uricase-Pod kit method). Reduced GSH was estimated using standard clinical chemistry method [16] and malondialdehyde (MDA) a marker of oxidative stress and end product of lipid peroxidation was estimated in RBC as thiobarbituric acid reactive substance (TBARS) [17]. The data collected was expressed as mean and SD and statistically evaluated by Z-test and Student’s ‘t’ test. Pearson’s correlation coefficient (r) was used to assess the association between related parameters.
Results
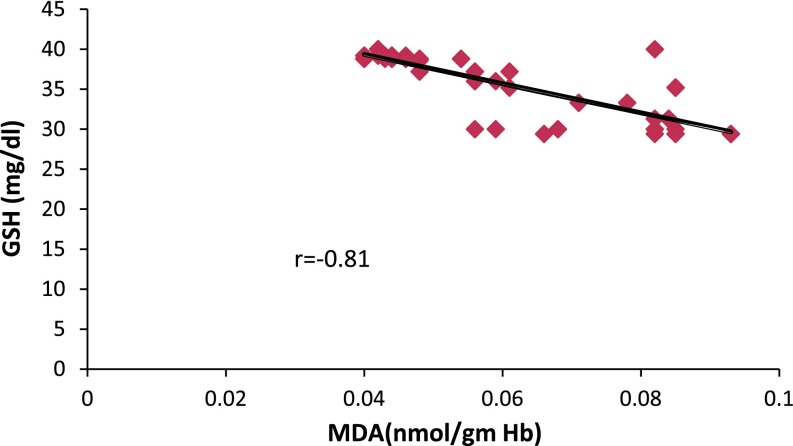
The results are shown in Tables 1, 2, 3, 4, and 5 and Figs. 1, 2, 3. Table 1 shows distribution of subjects according to gender, age, diet, stage of illness and substance abuse. Table 2 shows the level of MDA; the easily extractable product of PUFA oxidation and endogenous antioxidants [18]. The value of MDA in schizophrenics was significantly higher as compared to controls (p = 0.005) indicating pro-oxidant induced damage. When the patients were segregated on the basis of stage of schizophrenia (Table 3), the MDA levels seem to increase in chronic cases as compared to acute (p = 0.005). Also, statistically significant increase in MDA was observed in subjects more than 40 years of age (>40 years) in both control and schizophrenic groups as compared to those less than or equal to 40 years of age (≤40 years) (Table 4). Substance abusers also had higher levels of MDA as compared to non-abusers (Table 5) in both the groups. Glutathione (reduced), a potential antioxidant in blood that neutralizes the oxidants [19] showed an inverse relationship with MDA (Fig. 1), as its levels declined from 41.05 ± 5.89 in controls to 33.40 ± 3.92 mg/dl in schizophrenics (Table 2) which was significant (p = 0.005). The effect of persistent oxidative stress in chronic phase of illness also decreased the level of GSH significantly (p = 0.001) as compared to acute (Table 3). Aging weakens the body physiology which was clearly reflected in the decline (p < 0.001) observed in the levels of GSH in both the groups but more prominent in schizophrenics (Table 4). Substance of abuse metabolize and generate excessive free radicals that create a deficit in GSH levels (Table 5), which was more pronounced in schizophrenics (p < 0.001) due to the generation of free radicals in metabolism of the substance as well as membrane lipid oxidation. In an effort to study the importance of bilirubin as an effective antioxidant, its serum levels were estimated and it was found that there were higher levels in schizophrenics on comparing with controls (Table 2) raising from 0.51 ± 0.08 to 0.68 ± 0.13 mg/dl, but within the normal range (0.6–1.2 mg/dl). And moreover, none of the subjects had bilirubin levels higher than 1.2 mg/dl, the upper limit of normal reference interval for bilirubin in human blood. Stage of illness, aging, and substance abuse did not show any significant change.
Table 2.
Level of MDA and endogenous antioxidants in healthy individuals and Schizophrenic patients
| Parameter | Control group (n = 50) | Schizophrenic group (n = 50) | Z-value (p) | ||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| MDA (nmol/gmHb) | 0.047 ± 0.005 | 0.040–0.062 | 0.061 ± 0.010a | 0.048–0.093 | 7.48 (0.005) |
| GSH (mg/dl) | 41.05 ± 5.89 | 31.30–50.00 | 33.40 ± 3.92a | 26.40–38.00 | 7.65 (0.005) |
| Bilirubin (mg/dl) | 0.51 ± 0.08 | 0.40–0.70 | 0.68 ± 0.13a | 0.52–0.09 | 7.90 (0.005) |
| Total Proteins (gm/dl) | 7.86 ± 0.35 | 7.20–8.60 | 6.80 ± 0.73a | 5.50–7.35 | 9.29 (0.005) |
| Albumin (gm/dl) | 4.67 ± 0.51 | 3.88–5.10 | 4.00 ± 0.52a | 3.20–4.80 | 6.50 (0.005) |
| Uric acid (mg/dl) | 3.94 ± 0.44 | 3.20–4.70 | 3.16 ± 0.48a | 2.50–4.00 | 8.47 (0.005) |
aSignificant change
Table 3.
Level of oxidative stress and endogenous antioxidants in acute and chronic Schizophrenic patients
| Parameter | Acute (n = 40) | Chronic (n = 10) | Z-value (p) |
|---|---|---|---|
| MDA (nmol/gmHb) | 0.058 ± 0.017 | 0.076 ± 0.011a | 5.18 (0.005) |
| GSH (mg/dl) | 36.02 ± 3.78 | 32.03 ± 2.79a | 3.74 (0.005) |
| Bilirubin (mg/dl) | 0.63 ± 0.13 | 0.64 ± 0.14NS | 0.20 (NS) |
| Total proteins (gm/dl) | 7.03 ± 0.66 | 6.22 ± 0.75a | 3.24 (0.005) |
| Albumin (gm/dl) | 4.11 ± 0.51 | 3.75 ± 0.51a | 2.00 (0.025) |
| Uric acid (mg/dl) | 3.40 ± 0.48 | 3.19 ± 0.45NS | 1.30 (NS) |
Values are mean ± SD
NS not significant
aSignificant change
Table 4.
Effect of age on oxidative stress and level of antioxidant substances in controls and Schizophrenics
| Parameter | Control group (n = 50) | Schizophrenic group (n = 50) | ||
|---|---|---|---|---|
| ≤40 years (n = 28) | >40 years (n = 22) | ≤40 year (n = 25) | >40 year (n = 25) | |
| MDA (nmol/gmHb) | 0.044 ± 0.003 | 0.051 ± 0.005* | 0.058 ± 0.007 | 0.076 ± 0.011* |
| GSH (mg/dl) | 44.81 ± 4.31 | 36.26 ± 3.76* | 38.74 ± 0.77 | 32.03 ± 2.74* |
| Bilirubin (mg/dl) | 0.71 ± 0.10 | 0.07 ± 0.13NS | 0.65 ± 0.13 | 0.61 ± 0.13NS |
| Total proteins (gm/dl) | 8.02 ± 0.36 | 7.64 ± 0.25* | 7.53 ± 0.23 | 6.26 ± 0.47 |
| Albumin (gm/dl) | 4.49 ± 0.32 | 3.65 ± 0.26* | 4.48 ± 0.39 | 3.62 ± 0.18* |
| Uric acid (mg/dl) | 3.93 ± 0.41 | 4.00 ± 0.46NS | 3.98 ± 0.39 | 3.05 + 0.38* |
Comparison between age groups <40 years and >40 years of control and schizophrenic groups. Values are mean ± SD
NS not significant
*p < 0.001
Table 5.
Effect of substance abuse on oxidative stress and level of antioxidant substances in controls and Schizophrenics
| Parameter | Control group (n = 50) | Schizophrenic group (n = 50) | ||
|---|---|---|---|---|
| Non users (n = 32) | Users (n = 18) | Non users (n = 32) | Users (n = 18) | |
| MDA (nmol/gmHb) | 0.045 ± 0.004 | 0.051 ± 0.005* | 0.056 ± 0.016 | 0.076 ± 0.014* |
| GSH (mg/dl) | 43.83 ± 5.18 | 36.69 ± 4.43* | 36.37 ± 3.72 | 33.13 ± 3.42* |
| Bilirubin (mg/dl) | 0.53 ± 0.07 | 0.48 ± 0.07** | 0.64 ± 0.13 | 0.61 ± 0.13NS |
| Total Proteins (gm/dl) | 7.80 ± 0.33 | 7.89 ± 0.39NS | 6.48 ± 0.58 | 7.07 ± 0.73* |
| Albumin (gm/dl) | 3.91 ± 0.44 | 4.25 ± 0.51*** | 3.71 ± 0.53 | 4.19 ± 0.31* |
| Uric acid (mg/dl) | 3.86 ± 0.40 | 4.03 ± 0.46NS | 3.43 ± 0.48 | 3.20 ± 0.45* |
Comparison between non-users and users of control and schizophrenic groups. Values are mean ± SD
NS not significant
*p = 0.001; **p < 0.01;***p < 0.02
Fig. 1.
Correlation of oxidative stress and reduced GSH levels in schizophrenics
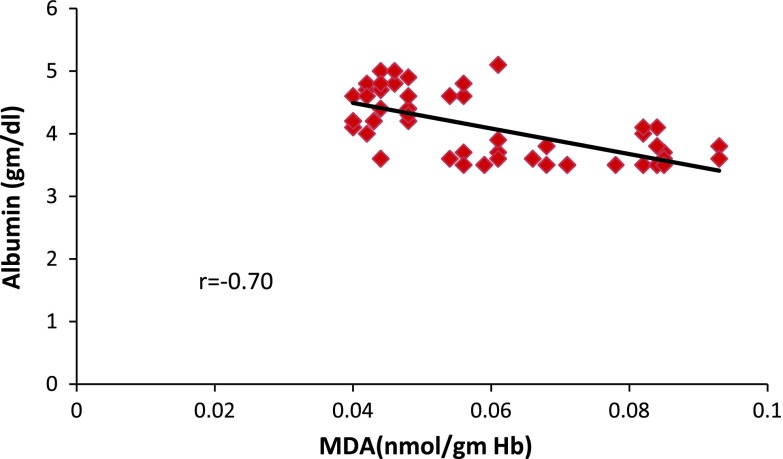
Fig. 2.
Correlation of oxidative stress and albumin levels in schizophrenics
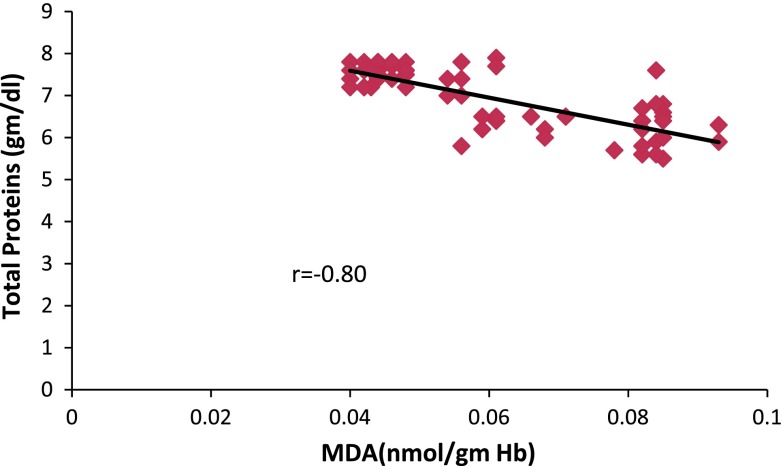
Fig. 3.
Correlation of oxidative stress and Total protein levels in schizophrenics
Albumin levels decreased significantly (p = 0.005) in controls when compared to schizophrenics (Table 2) and had a negative correlation with the MDA level (Fig. 2). This depletion was accompanied by a subsequent fall in serum total protein also showing a negative correlation with MDA levels (Fig. 3). Total protein and albumin levels depleted in chronic patients (Table 3) and also subjects of age group more than 40 years (Table 4). No significant change was observed in control group, but schizophrenics who were substance abusers had significantly (p = 0.001) decreased levels of total proteins and albumin (Table 5).
The role of uric acid and oxygen species in tissue injury is interrelated [20], where uric acid is said to act as an important physiological antioxidant [21]. On investigating the serum levels of uric acid there was found a significant decrease (p = 0.005) in schizophrenics as compared to controls but in the normal range (Table 2). Aging and substance abuse altered the levels of uric acid significantly in schizophrenics (Tables 4, 5) while in chronic stage no significant change was observed. The results reflect altered levels of endogenous antioxidants in response to the increase in MDA levels; an index of oxidative stress.
Discussion
Schizophrenia is associated with a complex pathophysiology and an outcome of radical mediated neurotoxicity. The effectiveness of the antioxidant defense system against the ROS not only depends upon its enzymatic constituents, but non-enzymatic constitutes also contribute to the defensive mechanism [22]. Various biomarkers of oxidative stress are the products of lipid peroxidation [23], of these MDA is measured as TBARS in the erythrocytes. The intensity of peroxidation and antioxidative defense in RBC to a certain extent reflect the state of the cell membrane in different tissues including those of nervous system [24], therefore the increase in MDA necessarily indicates the presence of oxidative stress and associated membrane lipid damage in schizophrenia as it is toxic and disturbs the lipid protein membrane function and neuronal conduction [25]. While measuring the severity of disease by PANSS, in symptologically divided schizophrenics as acute or chronic, it was found that MDA was higher in chronic patients as compared to acute (Table 3) indicating the effect of persisting illness. The process of aging and metabolism of substance of abuse are associated with generation of free radicals which was reflected in the raise in the MDA levels (Table 4, 5). It should be kept in mind that schizophrenics have a high prevalence of smoking [26] and high rates of drug or alcohol abuse [27] as compared to normal population. This fact favors the observations of increased oxidative stress in schizophrenics resulting from drug abuse. There is significant evidence of disturbance of GSH homeostasis that either leads to or results from oxidative stress in neurodegenerative disorders [28].
The decline in GSH levels in this study can be explained as a consequence of increased demands on the GSH system in schizophrenia. GSH acts as a substrate for enzymatic antioxidant glutathione peroxidase (GSHPx) and also contributes to the recycling of the ascorbate radicals into ascorbic acid, a major vitamin antioxidant of aqueous medium [29]. The deficit in GSH levels is also evident in chronic condition due to growing oxidative stress. Aging as well as negative lifestyle choices like drug abuse, deplete the levels owing to a heavy cumulative burden of oxidative stress in such conditions [30]. Moreover, aging and decline of GSH levels is considered as a key risk factor in psychiatric illnesses [31].
Bilirubin, the end product of heme-catabolism also participates in antioxidative machinery of the body, by efficiently scavenging peroxyl radicals and playing the role of a chain breaking antioxidant [32]. The elevated serum levels of bilirubin in schizophrenics are due to possible increased vulnerability of red cell membranes in condition of oxidative stress [33]. Also in such a condition the pro-oxidant effect of heme oxygenase, mediated by iron release may outcompete the antioxidant property of bilirubin [34]. Recent studies suggesting that bilirubin synthesis is induced in response to oxidative stress also emphasize that no big difference should be expected [35]. The toxicity created by substances of abuse altered bilirubin levels, reflecting a compensatory mechanism for free radical scavenging, also shown in a prospective study by Breimer et al. [36] who found an inverse relationship between serum bilirubin and smoking. The two factors of aging and substance abuse are a reflection of increasing free radical load, where the level of bilirubin decreased in both these conditions contrary to the overall increase in all other groups. Here, it becomes pertinent to mention that bilirubin is transported as albumin bound complex in plasma [32] and it may be possible that the depleting plasma albumin levels are responsible for similar behavior of bilirubin [37].
It is well known that albumin is an endogenous antioxidant with radical scavenging properties. It is a powerful scavenger of HOCl and also inhibits Cu+ dependent lipid peroxidation by binding copper ions and hydroxyl radical generation [10]. The depletion of albumin and total protein levels in schizophrenic patients suggests its action as a sacrificial molecule while combating ROS. The decrease in their level has also been reported in Taiwanese chronic schizophrenics perhaps due to episodic form of disease [38]. Aging and substance abuse being pro-oxidative processes also take their toll.
The antioxidative property of uric acid is via, the urate radical that is able to react with peroxynitrite intermediates, nitric oxide and peroxyl radical, thus inactivating them [39]. In this process uric acid is destroyed and easily oxidized at a comparable rate [21]. Owing to this property, the levels of serum uric acid deplete in condition of oxidative stress as observed in schizophrenics as compared to controls. Further in accordance with the study of Yao et al. [40] who observed a trend of lower uric acid levels in relapsed patients relative to clinically stable chronic schizophrenics also had depleted levels. Cumulative attack by free radicals, due to substance abuse explains the depletion in uric acid levels [41].
In conclusion the findings of the present study indicate that the levels of endogenous antioxidants viz. GSH, bilirubin, total protein, albumin and uric acid, are disturbed in condition of schizophrenia which is an attempt to neutralize the ROS in body and reduce oxidative stress. These results to some extent support the role of endogenous antioxidants as components of total antioxidant response. Our previous studies have emphasized the role of vitamins [14] and enzymes [15] as antioxidants and further, the role of endogenous substances is emphasized by this study, together they form the total antioxidant defense of the body [42]. Further it opens a new avenue to explore the pattern of dietary antioxidant supplementation that may improve the prognosis of schizophrenia and other neuropsychiatric illnesses.
Contributor Information
Gora Dadheech, Email: gora_dadheech@yahoo.com.
Praveen Sharma, Email: praveensharma55@gmail.com.
References
- 1.Davies KJ. Oxidative stress: the paradox of aerobic life. Biochem Soc Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- 2.Leibovitz BE, Siegel BV. Aspects of free radical reactions in biological systems: aging. J Gerontol. 1980;35(1):45–56. doi: 10.1093/geronj/35.1.45. [DOI] [PubMed] [Google Scholar]
- 3.Singh RP, Sharad S, Kapur S. Free radicals and oxidative stress in neurodegenerative diseases: relevance of dietary antioxidants. JIACM. 2004;5(3):218–225. [Google Scholar]
- 4.Gassen M, Youdim MB. Free radical scavengers: chemical concepts and clinical relevance. J Neural Trans Suppl. 1999;56:193–210. doi: 10.1007/978-3-7091-6360-3_13. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa N. Free radicals and neural cell damage. Rivisho Shinkeigaku. 1994;34:1266–1268. [PubMed] [Google Scholar]
- 6.Lohr JB. Oxygen radicals and neuropsychiatric illnesses. Arch Gen Psychiatry. 1991;48:1097–1106. doi: 10.1001/archpsyc.1991.01810360061009. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan SI, Sadock BJ. Schizophrenia. In: Comprehensive textbook of psychiatry. 6th ed. Baltimore: Vol 1, Williams and Wilkins; 1989. p. 699–815.
- 8.Sharma S, Sharma S. Biochemical evaluation of lipid and oxidative stress status in relation to high fat-high antioxidant diets. Ind J Expl Biol. 2001;39(11):1180–1183. [PubMed] [Google Scholar]
- 9.Reddy R, Yao JK. Schizophrenia: role of oxidative stress and essential fatty acids. In: Antioxidants in human health. New York: CAB International; 1999. p. 351–66.
- 10.Halliwell B, Gutteridge JMC. The antioxidants of human extracellular fluids. Arch Biochem Biopsy. 1990;280(1):1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- 11.Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991;91:31S–38S. doi: 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- 12.Marshall WJ, Bangert SK. Free radicals. In: Clinical biochemistry: metabolic and clinical aspects. London: Churchill Livingstone; 1995. p. 765–77.
- 13.Vendemiale G, Grattagliano I, Altomare E. An update on the role of free radicals and antioxidant defense in human disease. Ind J Clin Lab Res. 1999;29:49–55. doi: 10.1007/s005990050063. [DOI] [PubMed] [Google Scholar]
- 14.Dadheech G, Mishra S, Gautam S, Sharma P. Oxidative stress, α-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Ind J Clin Biochem. 2006;21:34–38. doi: 10.1007/BF02912908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dadheech G, Mishra S, Gautam S, Sharma P. Evaluation of antioxidant deficit in schizophrenia. Ind J Psychiatry. 2008;50:16–20. doi: 10.4103/0019-5545.39753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beutler E. Red cell metabolism. In: A manual of biochemical methods. New York: Grune and Stratton; 1971. p. 103–105.
- 17.Stock J, Dormandy TL. The autoxidation of human and red cell lipids induced by hydrogen peroxide. Br J Hematol. 1971;20:95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B. The chemistry of free radicals. Toxicol Ind Health. 1993;9(1-2):1–21. doi: 10.1177/0748233793009001-203. [DOI] [PubMed] [Google Scholar]
- 19.Meister A. Mini review: glutathione–ascorbic acid antioxidant system in animals. J Biol Chem. 1994;269:9397–9400. [PubMed] [Google Scholar]
- 20.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11(32):4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 21.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant and radical causing aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halliwell B. Free radicals, antioxidants and human diseases: curiosity, cause or consequence? Lancet. 1994;344:721–724. doi: 10.1016/S0140-6736(94)92211-X. [DOI] [PubMed] [Google Scholar]
- 23.Stohs SJ. The role of free radicals in toxicity and disease. J Basic Physiol Pharmacol. 1995;6:205–228. doi: 10.1515/JBCPP.1995.6.3-4.205. [DOI] [PubMed] [Google Scholar]
- 24.Vilkov GA, Kiroi RI, Stepnina EG, Smimova OV, Kovalenko VA, Trapezontseva RA. Lipid peroxidation and microviscosity of erythrocyte membranes in patients with schizophrenia. Zn Nevropatol Psikhiatr. 1991;91:45–47. [PubMed] [Google Scholar]
- 25.Drewa G, Bala G, Gzerwionka Szaflarska M, Szaflarska-Szazepanik S. Oxyradicals and other oxidants: their generation, specificity and reactivity in biological systems. Med Sci Monit. 1996;2(5):681–687. [Google Scholar]
- 26.Hughes JR, Hatsukami DK, Mitchell JE, et al. Prevalence of smoking among psychiatric patients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- 27.Fowler IL, Carr VJ, Carter NT, Lewin TJ. Patterns of current and lifetime substance use in schizophrenia. Schizophr Bull. 1998;24(3):443–455. doi: 10.1093/oxfordjournals.schbul.a033339. [DOI] [PubMed] [Google Scholar]
- 28.Schultz JB, Lindenan J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267(16):4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 29.Grima G, Benz B, Parpura V, Cuenod M, Do KQ. Dopamine-induced stress in neurons with glutathione deficiency implication for schizophrenia. Schizophr Res. 2003;62(3):213–224. doi: 10.1016/S0920-9964(02)00405-X. [DOI] [PubMed] [Google Scholar]
- 30.Kidd PM. Natural antioxidants and first line of defense. In: Kidd PM, Huber W, editors. Living with the AIDS virus: a strategy for long-term survival. California: PMK Biomedical-Nutritional Consulting; 1991. p. 115–142.
- 31.Sohal RS, Weindruch R. Oxidative stress, caloric restriction and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stocker R, Glazer AN, Ames BN. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci USA. 1987;84(16):5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller N, Schiller P, Ackenheil M. Coincidence of schizophrenia and hyperbilirubinemia. Pharmacopsychiatry. 1991;24(6):225–228. doi: 10.1055/s-2007-1014472. [DOI] [PubMed] [Google Scholar]
- 34.Dani C, Martelli E, Bertini G, Pezzati M, Filippi L, Rossetti M, Rizzuti G, Rubattelli FF. Plasma bilirubin level and oxidative stress in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2003;88(2):F. 119–F. 123. doi: 10.1136/fn.88.2.F119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hidalgo FJ, Zamora R, Dillard CJ, Tappel AL. Can serum bilirubin be an index of invivo oxidative stress? Med Hypotheses. 1990;33(3):207–211. doi: 10.1016/0306-9877(90)90178-H. [DOI] [PubMed] [Google Scholar]
- 36.Breimer LH, Wannamethee G, Ebrahim S, Shaper AG. Serum bilirubin and risk of ischaemic heart disease in middle aged British men. Clin Chem. 1995;41(10):1504–1508. [PubMed] [Google Scholar]
- 37.Frei B, Stocker R, Ames BN. Antioxidant defense and lipid peroxidation in human blood plasma. Proc Natl Acad Sci USA. 1988;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang TL. Decreased serum albumin levels in Taiwanese patients with schizophrenia. Psychiatry Clin Neurosci. 2002;56(6):627–630. doi: 10.1046/j.1440-1819.2002.01066.x. [DOI] [PubMed] [Google Scholar]
- 39.Rodionov RN. Urate as an endogenous antioxidant. Free Radic Biol Med. 2003; 77(222):1–11.
- 40.Yao JK, Reddy R, Van Kammen DP. Reduced status of plasma total antioxidant capacity in schizophrenia. Schizophr Res. 1998;32(1):1–8. doi: 10.1016/S0920-9964(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 41.Yao JK, Reddy R, Van Kammen DP. Reduced level of plasma antioxidant uric acid in schizophrenia. Psychiatry Res. 1988;80(1):29–39. doi: 10.1016/S0165-1781(98)00051-1. [DOI] [PubMed] [Google Scholar]
- 42.Uma DP, Devipriya D, Chinnaswamy P. Age and gender related changes in total antioxidant response and oxidative stress in patients with schizophrenia. J Clin Diagn Res. 2008;14(2):627–633. [Google Scholar]