Abstract
The purpose of the study was to explore the mechanism underlying the enhanced subthalamic nucleus (STN) neural activity during exhausting exercise from the perspective of monoamine neurotransmitters and changes of their corresponding receptors. Rats were randomly divided into microdialysis and immunohistochemistry study groups. For microdialysis study, extracellular fluid of the STN was continuously collected with a microdialysis probe before, during and 90 min after one bout of exhausting exercise. Dopamine (DA) and 5-hydroxytryptamine (5-HT) levels were subsequently detected with high-performance liquid chromatography (HPLC). For immunohistochemistry study, the expression of DRD2 and HT2C receptors in the STN, before, immediately after and 90 min after exhaustion was detected through immunohistochemistry technique. Microdialysis study results showed that the extracellular DA and 5-HT neurotransmitters increased significantly throughout the procedure of exhausting exercise and the recovery period (P<0.05 or P<0.01). Immunohistochemistry study results showed that the expression levels of DRD2 and HT2C in the rat STN immediately after exhausting exercise and at the time point of 90 min after exhaustion were both higher than those of the rest condition, but the difference was not significant (P>0.05). Our results suggest that the increased extracellular DA and 5-HT in the STN might be one important factor leading to the enhanced STN neural activity and the development of fatigue during exhausting exercise. This study may essentially offer useful evidence for better understanding of the mechanism of the central type of exercise-induced fatigue.
Keywords: subthalamic nucleus (STN), central fatigue, monoamine neurotransmitters, microdialysis, immunohistochemistry
INTRODUCTION
It has been demonstrated that, as a complicated symptom, the fatigue phenomenon occurring during exercise can be generally divided into two types: peripheral fatigue, which is caused mainly by peripheral factors, for instance, the depletion of energy in muscle; and central-type fatigue, which is caused mainly by cerebral perturbations [1, 2, 3]. Due to the complexity of the brain as well as the limitations of techniques, previous studies have focused mainly on peripheral fatigue, such as the relationship between fatigue and circulatory, metabolic, muscular, and nutritional factors [4]. Numerous studies have recently verified the effect of the central nervous system (CNS) on fatigue. The results showed that the cerebral metabolic and neurohumoral alterations during strenuous exercise sometimes could lead to deficiencies in the central drive to motoneurons, and thus play important roles in modulating the exercise performance [5, 6]. Therefore, the central type of fatigue has recently received considerable attention.
The basal ganglia (BG) consist of six extensively interconnected nuclei: the caudate nucleus, putamen, globus pallidus, substantia nigra, amygdala (archistriatum) and subthalamic nucleus (STN). Previous findings have provided considerable evidence that the cortex, BG and thalamus are all linked by re-entrant circuits [7]. The STN receives direct excitatory glutamatergic afferent inputs from the frontal cortex and exerts an excitatory drive to the internal globus pallidus (GPi)/substantia nigra pars reticulata (SNr), and then exerts strong inhibitory feedback on the motor cortex through the relay of the thalamus [8]. Thus, changes of STN neural activity play a critical role in modulating cortical activity [9]. In our previous study [10], by using the electrophysiological recording technique, we simultaneously monitored the variance of neuron activity in the STN and motor cortex. The results showed that the decreased motor cortex activity synchronously occurred with the enhanced STN activity when fatigue occurred during exhaustive exercise. Further study found that, when STN activity was suppressed through injecting the glutamate receptor antagonist 6-methyl-2-phenylethynyl-pyridine (MPEP), 10 min before the start of the exhausting exercise, the time from the onset of the exercise to the emergence of fatigue was significantly prolonged [11]. These results strongly implied the close relationship between the enhanced STN activity and the development of fatigue during exercise.
Although the regulating effect of STN on development of fatigue during exercise has been demonstrated, the underlying mechanism still remains to be fully understood. Results from our previous study demonstrated that [10] variance of glutamic acid (Glu) and gamma-aminobutyric acid (GABA) levels in STN extracellular fluid, and changes of the expression level of their corresponding receptors [11], mGluR5 and GABA-ARα1 are all closely related to the enhanced STN activity during exhausting exercise. Also, since the STN activity is co-regulated by various afferent projections, besides Glu-ergic and GABA-ergic projections, the dopamine (DA)-ergic projections from the compact part of the substantia nigra (SNc) and the 5-hydroxytryptamine (5-HT)-ergic projections from the dorsal raphe nucleus (DRN) play an important role in modulating STN activity as well [12]. Therefore, testing the dynamic changes of extracellular DA and 5-HT levels in the STN and changes of their corresponding receptor expression levels may substantially facilitate the understanding of the mechanism underlying the enhanced STN neural activity during exhausting exercise. Such a study has not been performed before.
In the present study, by using microdialysis and high performance liquid chromatography-electrochemical detection (HPLC-EC), we observed the dynamic changes of extracellular DA and 5-HT levels in rat STN during exhausting exercise. Changes of the expression levels of their corresponding receptors, the DRD2 and HT2C receptors, before, immediately after and 90 min after exhaustion were detected through immunohistochemistry technique as well. The purpose of this study was to explore the mechanism underlying the enhanced STN neural activity during exhausting exercise. The study may essentially offer useful evidence for better understanding of the mechanism of the central type of fatigue.
MATERIALS AND METHODS
Experimental animals
Male Wistar rats (Beijing Vital River Laboratory Animal Technological Company, Beijing, China), weighing 290±20 g, were used for all experiments. All the rats were randomly divided into four groups: the microdialysis study group, the control immunohistochemistry study group, the immediate exhaustion study group, and the 90 min after exhaustion group. Animals were maintained on a 12 h light/dark cycle and given food and water ad libitum. All the experimental protocol was approved by the local Ethics Committee (Beijing Normal University) and was in compliance with the recommendations of the European Convention on “protection of vertebrate animals used for experimental scientific purposes of 31 May 1986”.
Surgery
Rats of the microdialysis study group were anesthetized with pentobarbital sodium (50 mg · kg-1, Sigma) and placed on a stereotaxic frame. After the hair above the skull had been removed, an incision was made along the midline and a burr hole was drilled, then a cannula (4.15.IC, Microbiotech/se AB, Sweden) with a replaceable inner guide was positioned 1 mm above the left STN. Coordinates relative to the bregma were AP-3.6 mm, L-2.5 mm, and D-6.6 mm [13]. Three holes were drilled around the coordinates designed for the cannula, then screws were secured in each of these holes and fixed with dental cement. Animals were allowed at least 5 days to recover from the surgery. Five days later, rats were subjected to adaptive exercise with progressive loading.
Exhausting exercise protocol
Seven days after the surgery, rats that were able to sustain 30 min of exercise (20 m · min-1) continuously were selected for the next experiments. The microdialysis study was carried out the next day. The progressive loading treadmill exercise protocol was modified from the Bedford treadmill exercise programme, and the loading was classified into 3 stages: stage I: 10 m · min-1, 15 min; stage II: 15 m · min -1, 15 min; stage III: 20 · min-1, till exhaustion (Figure 1) [14].
FIG. 1.
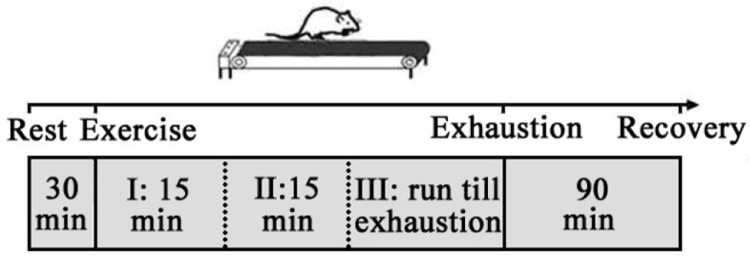
Schematic illustration of exhausting exercise protocol.
Microdialysis experiments
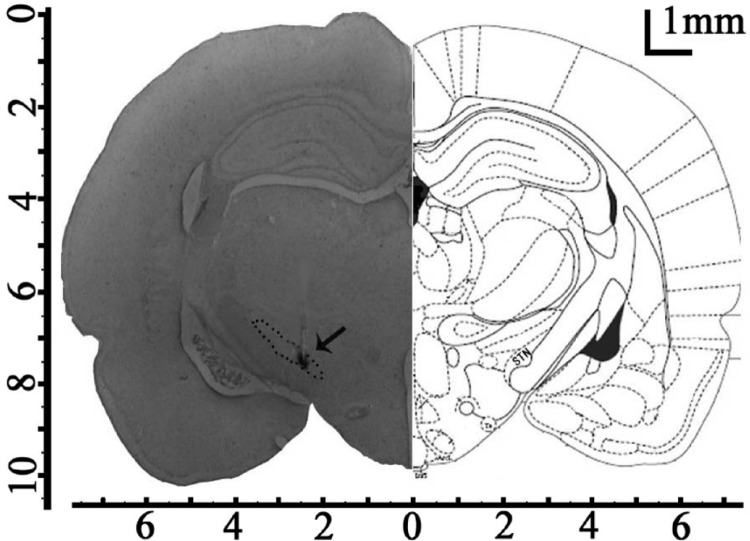
The microdialysis probe (MAB4.15.1, Microbiotech/se AB, Sweden) was implanted through the guide cannula and was perfused with artificial cerebrospinal fluid (aCSF) (126 mM NaCl, 2.4 mM KCl, 1.1 mM CaCl2, 0.85 mM MgCl2, 27.5 mM NaHCO3, 0.5 mM Na2SO4, 0.5 mM KH2PO4, pH 7.0) at a flow rate of 2 µL · min-1 driven by a microinjection pump (MD-0250, Bioanalytical Systems, USA). To limit tissue damage and keeping in mind the small dimensions of the STN, we used a very small microdialysis probe (diameter of 0.2 mm and a membrane length of 1 mm) [15]. After equilibrating for 90 min, the brain microdialysate was collected every 15 min by a refrigerated fraction collector (MAB 85, Microbiotech/se AB, Sweden) before, during and 90 min after exhausting exercise (Figure 2). After microdialysis experiments, Nissl staining was processed, all recording sites in the brain were verified by light microscopy, and data from the wrongly located rats were not used (Figure 3).
FIG. 2.
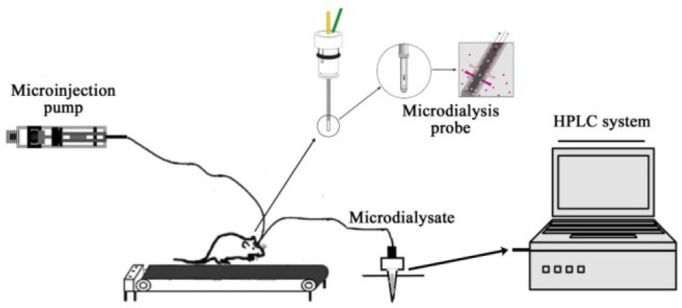
Schematic illustration of experimental setup for continuous collection of microdialysate during exercise and neurochemical detection system.
FIG. 3.
Histological confirmation of microdialysis probe placement. The microdialysis probe was planted in the left STN (arrow) under stereotaxic conditions (AP-3.6 mm; L-2.5 mm; and D-7.6 mm. Scale bar=1 mm.).
Detection of DA and 5-HT in microdialysate
Detection of DA and 5-HT in microdialysate was performed with an ESA Model 5600A CoulArray system (ESA Inc., USA), an ESA Model 582 pump, and an ESA Model 542 refrigerated auto-sampler. Chromatographic separation was achieved by auto-injecting 20 µL of microdialysate onto a Develosil ODS-UG-5 column (150×4.6 mm i.d., Nomura Chemical, Japan) with an ESA guard column (4.0×3.0 mm i.d., Phenomenex, USA). The mobile phase used for compound separation consisted of 75 mM monobasic sodium dihydrogen phosphate, 2.0 mM SDS, 25 mm of EDTA, and 100 µl of triethylamine and 10% acetonitrile (pH 3.0). A flow rate of 0.6 ml · min-1 and analysis time of 45 min were used for all experiments. System control and data acquisition/processing were performed using ESA CoulArray software. Electrochemical detection was designed using two-way electrode potentials (passage I-150 mV, passage IV +450 mV).
Immunohistochemistry study
Rats for immunohistochemistry study were anesthetized with an overdose of chloral hydrate at rest, immediately after exhaustion or 90 min after exhaustion. Then they were treated with intracardiac perfusion with 4% paraformaldehyde in 0.1 mol · L-1 PBS (pH 7.4). Brains were removed and kept in 4% paraformaldehyde for 12 h, then immersed in 25% sucrose for 3 days at 4°C. Tissues were embedded in paraffin wax using routine histological procedures. Approx. 6 µm thick sections were cut from paraffin blocks and mounted onto polylysine-coated slides. Slides were dried at 60°C for 2 h and then deparaffinized in xylene three times (15 min each time), followed by rehydration in ethanol with declining concentrations from 99% to 70%, then terminated by running tap water. Each section was treated with 3% H2O2 for 20 min to block endogenous peroxidase activity. Sections were then washed three times with PBS, blocked with goat serum albumin for 20 min (37°C), and incubated overnight at 4°C with the rabbit polyclonal anti-DRD2/HT2c antibody (1: 100 and 1: 200 respectively, Bioss, Beijing, China). After washing with PBS, the sections were further incubated with secondary antibody, biotin labelled goat antirabbit IgG (1: 200, Bioss, Beijing, China) for 20 min at room temperature. Sections were then reacted for 20 min using ABC (avidin-biotin peroxidase complex) at room temperature. Labelling was visualized using diaminobenzidine (DAB, Bioss, Beijing, China) for 5 min, and the sections were counterstained with hematoxylin. Cell counting in 3 predefined areas (0.25 mm2) was conducted on ×200 microscopic images using a CK-2 Olympus microscope. Positive cells were counted in three separate fields in three different slices cut at the same level. Three animals per group were analysed.
Data presentation and statistical analyses
DA and 5-HT concentrations in the microdialysate were calculated by comparing peak areas with standard solutions. Values were expressed as percentages of baseline levels to minimize the difference between subject variations. All the data were presented as mean ± SD. The comparison of the DRD2/HT2c positive cells was made with one-way ANOVA. A one-way ANOVA with repeated measures was performed for the analysis of the extracellular DA and 5-HT concentrations. P <0.05 was considered to be statistically significant.
RESULTS
Behavioural studies
The behavioural study showed that rats adjusted themselves well to the treadmill exercise at the beginning. Gradually, the exercise capacity of the rats decreased and they showed difficulty in maintaining the preconditioned speed. Then the rats were given slight sound, light or direct current stimulation till exhaustion.
Criteria of exhaustion were as follows: the running posture changed into prostrate-style from stomp-style; remaining stationary in the rear part of the treadmill; and the light/sound/direct current stimulation could not keep it running.
Dynamic changes of extracellular DA and 5-HT concentrations in STN during exhausting exercise
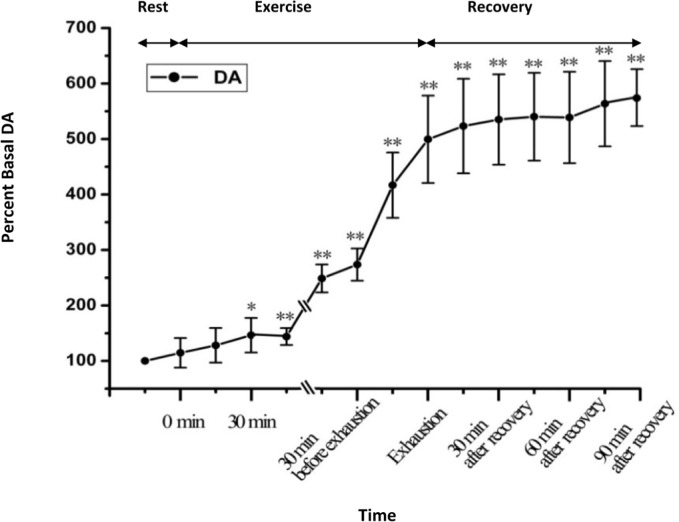
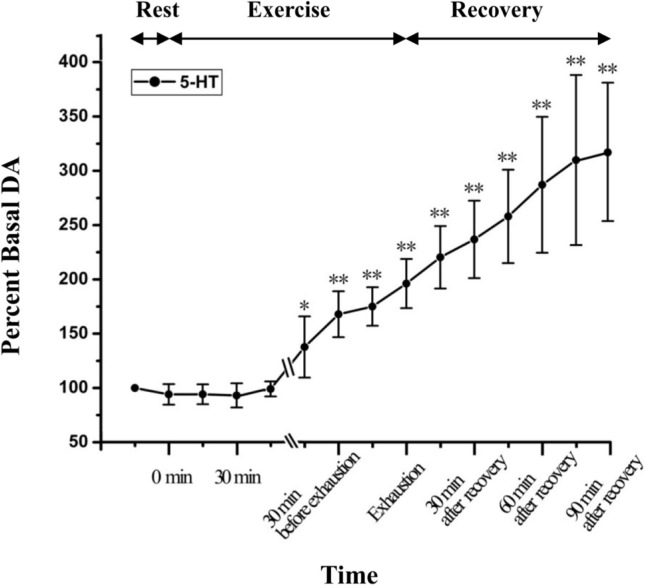
Figure 4 depicts the statistical results of the relative percent change of the microdialysate DA. As shown in Figure 4, the microdialysate DA gradually increased after the onset of exercise. At 30 min after onset of exhausting exercise, the extracellular DA level is significantly higher than that of the rest condition (P<0.05). With the continuation of exercise, the DA level continuously increased; at the time point of exhaustion, extracellular DA reached 499.48±78.74% of the basal levels (P<0.01, n = 6). During recovery, the DA level continuously increased and reached 563.6±76.85% of the basal levels at the time point of 90 min after recovery (P<0.01, n = 6). Figure 5 demonstrates the statistical results of the relative percent change of the microdialysate 5-HT. The results show that dynamic changes of extracellular 5-HT in the STN during exhausting exercise have a similar tendency as changes of DA. Extracellular 5-HT level increased continuously with the continuation of exercise. During recovery, it continuously increased and reached 309.97±78.32% of the basal levels at the time point of 90 min after recovery (P<0.01, n = 6).
FIG. 4.
Dynamic changes of DA levels in STN extracellular fluid with the development of fatigue (n=6). *P <0.05, ** P < 0.01 vs rest condition.
FIG. 5.
Dynamic changes of 5-HT levels in STN extracellular fluid with the development of fatigue (n=6). *P <0.05, ** P < 0.01 vs rest condition.
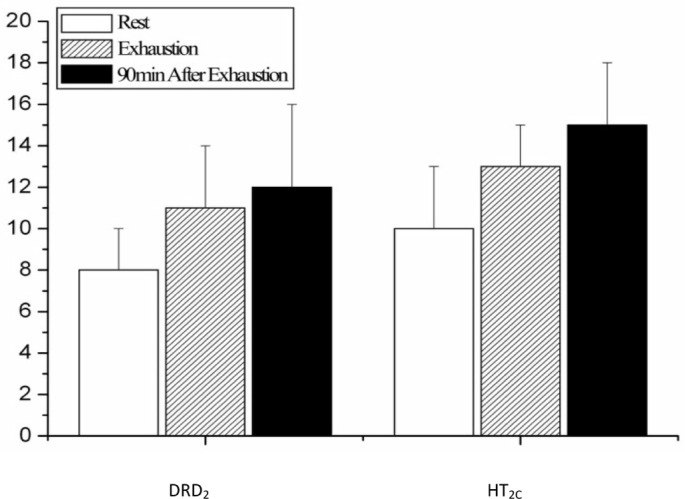
Changes of DRD2 and HT2c expression levels before and after exhausting exercise
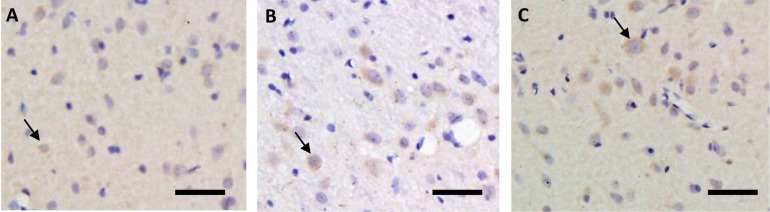
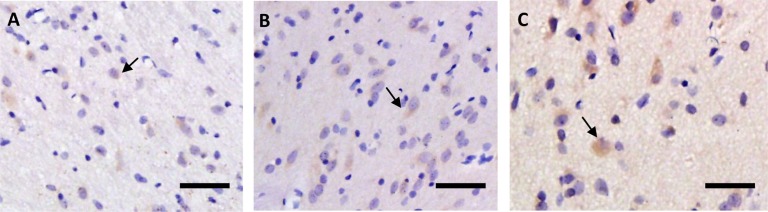
As shown in figures 6 and 8, the expression levels of DRD2 in the rat STN immediately after exhausting exercise and at the time point of 90 min after exhaustion were both higher than those of the rest condition, but the difference is not significant (P>0.05). The change of expression level of HT2C showed the same tendency as DRD2, as shown in figures 7 and 8.
FIG. 6.
Immunohistochemical staining of the DRD2 in rat STN before (A), immediately after (B) and 90 min after (C) one bout of exhausting exercise. Scale bar, 50 µm.
FIG. 8.
Quantification of DRD2 and HT2C positive neurons in rat STN before, immediately after and 90 min after one bout of exhausting exercise.
FIG. 7.
Immunohistochemical staining of HT2C in rat STN before (A), immediately after (B) and 90 min after (C) one bout of exhausting exercise. Scale bar, 50 µm.
DISCUSSION
Numerous studies have demonstrated that the basal ganglia is closely relevant to the control of voluntary movements, in which three motor information processing pathways connecting the motor cortex and basal ganglia are involved: the ‘direct’ pathway (cortico-striato-GPi/SNr); the ‘indirect’ pathway (cortico-striato-globus pallidus externus (GPe)-STN-GPi/SNr) [7]; and the ‘hyper-direct’ pathway (cortico-STN-GPi/SNr). These three pathways converge onto the GPi/SNr, and finally return back to the motor cortex through the relay of the thalamus [8]. Since they use different neurotransmitters, the activation of different pathway has different effects on the excitability of the motor cortex. The activation of the ‘direct’ pathway may exert a excitatory effect on the motor cortex, while the activation of the ‘indirect’ and ‘hyper-direct’ pathway may exert an inhibitory one. For the ‘indirect’ and ‘hyper-direct’ pathway both involve the STN. Changes of STN activity play a critical role in modulating cortical activity [9]. In the past few years, numerous pathological studies have suggested the close relationship between enhanced STN activity and motor dysfunctions [16, 17]. In 2012, evidence from our previous work for the first time demonstrated the correlation between enhanced STN activity and the development of fatigue during exercise [10]. Although the modulating effect of enhanced STN activity on development of fatigue during exercise has been demonstrated, the possible mechanism whereby the STN activity becomes enhanced still remains elusive.
Previous studies have verified that DA-ergic projections from the SNc and 5-HT-ergic projections from the DRN play an important role in modulating STN activity [12]. Therefore, in the present study, we tested the dynamic changes of extracellular DA and 5-HT levels in the STN during exhausting exercise. The results showed that, with the continuation of exercise, the DA and 5-HT levels gradually increased. Throughout the exercise procedure, the DA and 5-HT levels showed a continuous increasing tendency and reached 563.6±76.85% and 309.97±78.32% of the basal levels respectively at the time point of 90 min after recovery.
Numerous studies suggest that DA can exerted an excitatory influence on STN neurons in parkinsonian and control rats [18, 20, 20]. Flores et al. found that 5-HT increased the spontaneous firing rate in 84% of STN neurons in a rat brain slice [21]. Since DA and 5-HT are both excitatory neurotransmitters in the STN, the increased extracellular DA and 5-HT levels might be of great importance to the enhanced STN activity. This result is consistent with previous pathological studies. Arfani [14] reported that the increased extracellular STN DA level induced by long-term L-3,4-dihydroxyphenylalanine (L-DOPA) injection might result in dyskinesia and athetosis. Eberle-Wang demonstrated that injection of a 5-HT-like agonist in the subthalamic nucleus elicits orofacial dyskinesia in the rat [22]. In the present study, the increased extracellular DA and 5-HT in STN may enhance the STN activity through activation of their receptors and further participated in the development of fatigue.
Besides neurotransmitters, change of the receptor expression level during or after exercise might be another key factor influencing STN activity. Previous studies have shown that the DRD2 and HT2C receptors are the most dominant receptors modulating DA and 5-HT mediated neuronal activity in the STN [23, 24]. Therefore, in the present study, we further explored the changes of DRD2 and 5-HT2C expression levels. The results showed that the expression levels of DRD2 and 5-HT2C in the rat STN immediately after exhausting exercise and at the time point of 90 min after exhaustion were both higher than those of the rest condition, but the difference was not significant (P>0.05). As far as we know, no study has examined the changes of DRD2 and HT2C expression levels in the STN during and after exhausting exercise so far. However, in pathological studies, numerous studies have demonstrated that altered DA and 5-HT receptor expression levels in the STN contribute to the pathophysiology of several neuropsychiatric disorders, such as schizophrenia, depression, and Parkinson's disease. For instance, Zhang once reported that [25] an altered balance between 5-HT2 subtype receptors in the STN may influence signal transduction pathways and thus result in movement disorders. In the present study, changes of DRD2 and HT2C expression levels in the STN were not significant. We consider that this might be mainly due to the following reasons: First, the duration of the exhausting exercise was very short, as complexes of protein and changes of receptors did not appear. Second, exercise fatigue is a general physiological phenomenon; therefore, unlike the pathological state, the changes of receptor expression levels are less intense.
CONCLUSIONS
In summary, the present study showed that the extracellular DA and 5-HT neurotransmitters increased significantly throughout the procedure of exhausting exercise and the recovery period, while the changes of their corresponding receptors was not significant. We propose that the increased extracellular DA and 5-HT in the STN might be one important factor leading to the enhanced STN neural activity and the development of fatigue during exhausting exercise. This study may essentially offer useful evidence for better understanding of the mechanism of the central type of exercise-induced fatigue.
Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 31171138) and the Natural Science Foundation of Beijing (Grant No. 5142012).
Conflict of interests
The authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Amann M. Central and peripheral fatigue: interaction during cycling exercise in humans. Med Sci Sport Exer. 2011;43:2039–2045. doi: 10.1249/MSS.0b013e31821f59ab. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhuri A, Behan PO. Fatigue and basal ganglia. J Neurol Sci. 2000;179:34–42. doi: 10.1016/s0022-510x(00)00411-1. [DOI] [PubMed] [Google Scholar]
- 3.Decorte N, Lafaix PA, Millet GY, Wuyam B, Verges S. Central and peripheral fatigue kinetics during exhaustive constant-load cycling. Scand J Med Sci Spor. 2012;22:381–391. doi: 10.1111/j.1600-0838.2010.01167.x. [DOI] [PubMed] [Google Scholar]
- 4.Nybo L, Secher NH. Cerebral perturbations provoked by prolonged exercise. Prog Neurobiol. 2004;72:223–261. doi: 10.1016/j.pneurobio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sport Exer. 1997;29:45–57. doi: 10.1097/00005768-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Nybo L, Rasmussen P. Inadequate cerebral oxygen delivery and central fatigue during strenuous exercise. Exerc Sport Sci Rev. 2007;35:110–118. doi: 10.1097/jes.0b013e3180a031ec. [DOI] [PubMed] [Google Scholar]
- 7.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 8.Plenz D, Kital ST. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999;400:677–682. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- 9.Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang DL, Liu XL, Qiao DC. Modulatory effect of subthalamic nucleus on the development of fatigue during exhausting exercise: An in vivo electrophysiological and microdialysis study in rats. J Sport Sci Med. 2012;11:286–293. [PMC free article] [PubMed] [Google Scholar]
- 11.Dalei W, Xiaoli L, Decai Q. Effect of exhausting exercise on the mGluR5/GABA-ARα1 express in rat STN and the modulatory effect of MPEP intervention on the motor cortex excitability. Chin J Sports Med. 2010;29:542–546. [Google Scholar]
- 12.Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM. The subthalamic nucleus in the context of movement disorders. Brain. 2004;127:4–20. doi: 10.1093/brain/awh029. [DOI] [PubMed] [Google Scholar]
- 13.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. San Diego: Academic press; 1997. [DOI] [PubMed] [Google Scholar]
- 14.El Arfani A, Bentea E, Aourz N, Ampe B, De Deurwaerdère P, Van Eeckhaut A, et al. NMDA receptor antagonism potentiates the L-DOPA-induced extracellular dopamine release in the subthalamic nucleus of hemi-parkinson rats. Neuropharmacology. 2014;85:198–205. doi: 10.1016/j.neuropharm.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Ampe B, Massie A, D'Haens J, Ebinger G, Michotte Y, Sarre S. NMDA-mediated release of glutamate and GABA in the subthalamic nucleus is mediated by dopamine: an in vivo microdialysis study in rats. J Neurochem. 2007;103:1063–1074. doi: 10.1111/j.1471-4159.2007.04847.x. [DOI] [PubMed] [Google Scholar]
- 16.Strutt AM, Simpson R, Jankovic J, York MK. Changes in cognitive-emotional and physiological symptoms of depression following STN-DBS for the treatment of Parkinson's disease. Eur J Neurol. 2012;19:121–127. doi: 10.1111/j.1468-1331.2011.03447.x. [DOI] [PubMed] [Google Scholar]
- 17.Swann N, Poizner H, Houser M, Gould S, Greenhouse I, Cai W, et al. Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson's disease. J Neurosci. 2011;31:5721–5729. doi: 10.1523/JNEUROSCI.6135-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell GA, Eckardt MJ, Weight FF. Dopaminergic mechanisms in subthalamic nucleus of rat: analysis using horseradish peroxidase and microiontophoresis. Brain Res. 1985;333:261–270. doi: 10.1016/0006-8993(85)91580-x. [DOI] [PubMed] [Google Scholar]
- 19.Mintz I, Hammond C, Feger J. Excitatory effect of iontophoretically applied dopamine on identified neurons of the rat subthalamic nucleus. Brain Res. 1986;375:172–175. doi: 10.1016/0006-8993(86)90971-6. [DOI] [PubMed] [Google Scholar]
- 20.Ni ZG, Bouali-Benazzouz R, Gao DM, Benabid AL, Benazzouz A. Intrasubthalamic injection of 6-hydroxydopamine induces changes in the firing rate and pattern of subthalamic nucleus neurons in the rat. Synapse. 2001;40:145–153. doi: 10.1002/syn.1036. [DOI] [PubMed] [Google Scholar]
- 21.Flores G, Rosales MG, Hernández S, Sierra A, Aceves J. 5-Hydroxytryptamine increases spontaneous activity of subthalamic neurons in the rat. Neurosci Lett. 1995;192:17–20. doi: 10.1016/0304-3940(95)11597-p. [DOI] [PubMed] [Google Scholar]
- 22.Eberle-Wang K, Lucki I, Chesselet MF. A role for the subthalamic nucleus in 5-HT2C-induced oral dyskinesia. Neuroscience. 1996;72:117–128. doi: 10.1016/0306-4522(95)00548-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang QJ, Liu X, Liu J, Wang S, Ali U, Wu ZH, et al. Subthalamic neurons show increased firing to 5-HT2C receptor activation in 6-hydroxydopamine-lesioned rats. Brain Res. 2009;1256:180–189. doi: 10.1016/j.brainres.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 24.Zhu ZT, Shen KZ, Johnson SW. Pharmacological identification of inward current evoked by dopamine in rat subthalamic neurons in vitro. Neuropharmacology. 2002;42:772–781. doi: 10.1016/s0028-3908(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Andren PE, Svenningsson P. Changes on 5-HT2 receptor mRNAs in striatum and subthalamic nucleus in Parkinson's disease model. Physiol Behav. 2007;92:29–33. doi: 10.1016/j.physbeh.2007.05.033. [DOI] [PubMed] [Google Scholar]