Abstract
Izumo1 is a testis-specific gene product, whose function is essential for sperm-egg fusion. Throughout its lifespan, Izumo1 is posttranslationally modified, being both N-linked glycosylated on its extracellular domain and phosphorylated on the intracellular C-terminal tail. Within the caput regions of the rat epididymis, two phosphorylation events have been documented. However, as sperm pass through the epididymis, this cytoplasmic portion of Izumo1 has been shown to contain up to seven phosphorylation sites. Remarkably, in the rat, in correlation with these events, Izumo1 undergoes sub-cellular re-location, moving from the head/tail regions of the spermatozoa, to a predominantly equatorial segment location once they have reached the caudal end of the epididymis.
Keywords: epididymis, Izumo1, LC-MS, phosphorylation, proteomics
INTRODUCTION
A series of well-orchestrated, highly complex set of events must culminate together in order for a spermatozoon to fertilize an egg. Provided that every component of this network is operational, the final act of fertilization occurs when the spermatozoon engages with the egg plasma membrane and fuses with it.
The molecular identities of proteins involved in sperm-egg fusion are slowly being defined. Initial characterization of the sperm-fusion receptor started by identifying a sperm antigen by 2D-PAGE that cross-reacted with an antibody capable of inhibiting sperm-egg fusion. Following mass spectrometry, this antigen (then unknown) was called “Izumo” (now known as Izumo1) after a Japanese shrine dedicated to marriage.1 Consequent knockout studies have clearly shown that Izumo1 is essential for fertilization. Although male Izumo1−/− mice produce normal sperm cell numbers, with normal motility and morphology, these animals are completely infertile. Further investigation into the reasons for the infertility of male Izumo1−/− mice demonstrated a complete failure of their gametes to fuse with the egg.1 These data provided the definitive evidence that Izumo1 is involved in sperm-egg fusion.
Examination of Izumo1 has demonstrated that it is a Type Ia transmembrane protein and a member of the IgSF (immunoglobulin) family. Izumo1 contains an extracellular domain N-linked glycosylation site.2 As such, it shares characteristics with many viral-cell fusion systems that comprise membrane proteins with adhesion domains and carbohydrate moieties.3 In fact, many facets of Izumo1 appear to be highly conserved across species, suggesting that they play important roles. For example, alignment of Izumo1 across 15 species demonstrates that most of the immunoglobulin molecules (for example the LDC and YRC domains)4 are highly conserved. Furthermore, the extracellular N-linked glycosylation site on Izumo1 was found in all species from positions 198 to 208, suggesting an important functional role. Finally, 10 of the 13 cysteine resides found in human Izumo1 are 100% conserved4 and are likely to be involved in maintaining the three-dimensional structure of the protein.
What makes the protein so interesting, is that by inputing the Izumo1 sequence into the physico-chemical properties program “ProtParam” (http://www.expasy.org/tools/protparam.html) the protein is classified as “unstable.” The reason for this is that the N-terminal portion of Izumo1 contains dipeptides which are typically found in unstable proteins. This is remarkable, given the importance of Izumo1, and suggests that in order not to be proteolytically digested, Izumo1 must somehow be modified. So the question arose, how does an unstable protein prevent itself from becoming proteolytically digested? The answer to this is found on the N-linked glycan moiety. Point mutation of this residue (N204Q) renders it unable to be glycosylated, and results in sub-fertile mice with litter sizes of 8 for wild type but only 4 for the N204Q-mutated Izumo1 mice. Examination of the expression of Izumo1 within these mice clearly showed that the point mutation affected the level of expression of the protein. Thus, the N204Q mice had severely reduced amounts of Izumo1 expression.5 Taken together with the ProtParam data, this suggests that the main mechanism by which the “unstable” Izumo1 becomes stabilized is through N-glycosylation of the conserved asparagine residue. This glycan site must protect the vulnerable proteolytic cleavage sites. Interestingly, data from our laboratory have shown that spermatozoa contain multiple dipeptidases. Since the extracellular domain of Izumo1 sites between the outer acrosomal membrane and plasma membrane, it is plausible to suggest that dipeptidases, such as Angiotensin-converting enzyme may be responsible for the degradation of the protein.
IZUMO1 BINDING DOMAINS AND PARTNERS
In spermatozoa
Recently, binding partners of Izumo1 have been identified both within spermatozoa and within the oocyte. Specifically, Izumo2, Izumo3, and Izumo4 have been shown to have significant homology to the N-terminal domain of Izumo1.6 EST expression analysis suggested that like their counterpart, Izumo1, Izumo2, and Izumo3 were only expressed in the testis. In order to determine if these proteins formed a complex, spermatozoa were solubilized in perfluoro-octanoic acid and run in SDS-PAGE under mildly denaturing conditions. Immunoblot analysis showed that three complexes were formed of different molecular weight.6 Since all three complexes were multiples of 60 kDa, it is possible that Izumo1 forms homo-tetramers, trimers, and dimers.6
In the oocyte
The domains responsible for Izumo1 binding to the egg plasma membrane have been mapped.2 By using a proteolytic strategy, combined with three antibodies that cross-reacted with different domains of the protein, Inoue and colleagues have demonstrated that residues 5–113 on the N-terminal are critical for binding to the egg.2 Yet the key question has been what is it that Izumo1 is binding to in the egg? Initial investigations have tried to establish a link between Izumo1 and CD9. A monoclonal antibody raised against CD9 has been shown to be capable of inhibiting sperm-egg fusion, suggesting that this tetraspanin is the egg receptor for Izumo1.7 Indeed, CD9−/− females are healthy but are sub-fertile, owing to an inability of the spermatozoa to fuse with their eggs.8 Despite these data, what is surprising is that Izumo1 does not appear to bind directly to CD9, since by incubating the essential Izumo1 proteins (residues 5–113) with mouse eggs deficient in CD9, Izumo1 still could bind to them.2 Hence, it was clear that another Izumo1-receptor on the egg remained to be characterized.
Our understanding of exactly what it is that Izumo1 is binding to within the egg has recently been advanced by a series of elegant, low-affinity binding studies. Herein, a GPI-linked cell surface folate receptor, now renamed “Juno” (after the Roman goddess of fertility and marriage) was discovered to be the Izumo1-binding protein on the egg. Indeed, the Juno knockout mice are infertile with a failure of the spermatozoa to fuse with the egg.9
SUB-CELLULAR CHANGES TO IZUMO1 AND JUNO DURING FERTILIZATION
Several changes to both Izumo1 and Juno occur leading up to and following binding of these proteins. In the case of Izumo1, the initial location of this protein is within the acrosomal cap of spermatozoa, in both the inner and outer acrosomal membranes.10 However, upon undergoing the acrosome reaction, the protein migrates to the plasma membrane and covers the entire sperm head when the outer acrosomal and plasma membranes fuse to form pores and allow exocytosis of the acrosomal contents. Once Izumo1 spreads out over the entire head, it then has the ability to migrate toward the equatorial segment, whereupon it can engage in sperm-egg interactions with Juno.10 Following docking of spermatozoa with the oocyte, there is an immediate shedding of Juno from the oocyte plasma membrane, which must be considered to be the first step in preventing polyspermia.9,11
Changes to Izumo1 during epididymal maturation
Interestingly, changes to Izumo1's sub-cellular location also occur well before its movement within the acrosomal and plasma membranes. Indeed, we have shown that Izumo1 undergoes major changes during sperm transit through the epididymis. In work undertaken over many years, we have identified and quantified phosphorylation changes that occur during epididymal sperm maturation.12,13,14,15,16 Intriguingly, within this dataset, we found that Izumo1 was highly phospho-regulated (Figure 1). Within immature spermatozoa (taken from the proximal caput region of the epididymis), Izumo1 is phosphorylated on two residues (S339 and S346). However, mature spermatozoa taken from the cauda epididymidis demonstrate seven sites of phosphorylation (S346, S352, S356, S366, T372, S374, and S375) on Izumo117 (Figure 1).
Figure 1.
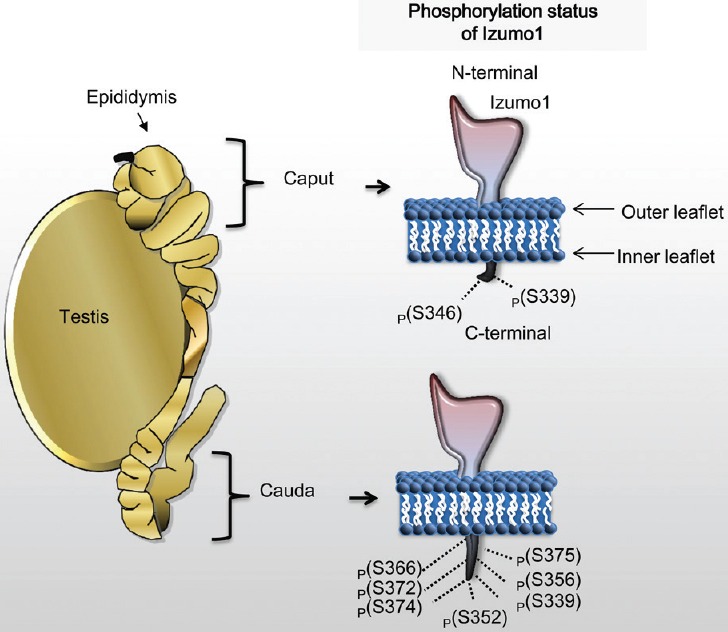
Phosphorylation of Izumo1 during epididymal transit of spermatozoa. Within the caput region, Izumo1 is phosphorylated on two amino acid residues. After spermatozoa have transited to the cauda epididymidis, Izumo1 has seven phosphorylated residues.
Given the fact that in the rat, Izumo1 is highly phosphorylated, we wanted to understand the physiological importance of this event. Immunocytochemical analysis of Izumo1 in immature caput epididymidal spermatozoa (two phosphorylation events) demonstrated midpiece/flagellar staining in these cells. This was in stark contrast to Izumo1 in mature spermatozoa from the caudal region (seven phosphorylation events; Figure 1), which demonstrated a specific midpiece and inner acrosomal/equatorial membrane head staining, indicating that Izumo1 undergoes translocation during rat epididymal transit.17
Thus, apart from the acrosome reaction, our studies have shown that Izumo1 also undergoes translocation during rat epididymal migration, which is highly correlated with its phosphorylation status.
Izumo1 and its role in idiopathic male infertility
What is particularly important to us is the role of Izumo1 in explaining idiopathic male infertility. Globally, more than 80 million people suffer from infertility,18 with approximately one in every seven couples in Australia being so affected.19 In at least half of these cases, a defect in one or more aspects of sperm function appears to be the cause,19 and a major reason for couples to be referred for assisted reproductive technologies (ART).20 One of the most difficult cases to handle is “idiopathic” (unexplained) male infertility, which are thought to characterize 24%21 of all cases referred to ART. Idiopathic male infertility is particularly difficult to diagnose because the classical clinical criteria used, including sperm counts, morphology, and motility, always lie within the “normal” limits. As such, when an idiopathic, male-infertile couple presents to an IVF clinic, the burden of the diagnostic tests falls disproportionately on the female, even when the male gametes are at fault. Hence, if we could achieve a precise diagnosis of male infertility from the onset, this would avoid the unnecessary hormonal, pelvic and laparoscopic examinations in women.
Izumo1, or the regulation of this key protein, represents an intriguing causal possibility for cases of idiopathic male infertility. Clearly, without the protein, fertilization will not proceed. Furthermore, as demonstrated by the knockout mice, sperm cell numbers, motility, and morphology would lie within “normal” limits. However, investigations on 13 men with oligozoospermia or asthenozoospermia and 12 infertile patients with fertilization failure, did not identify any defects on Izumo1 protein by immunocytochemistry.22 This suggests that to date, Izumo1 has never been shown to be involved in human male infertility.
In our own studies, we have performed immunoblots on spermatozoa from men who are attending an IVF clinic. Interestingly, in some men, we could show that although Izumo1 was present within their gametes, the protein was either not phosphorylated, or its phosphorylation status was reduced compared with that of known fertile counterparts. Thus, it may well be possible that despite Izumo1's presence within the gametes of these infertile males, regulation of the protein may affect its fertilization capacity. Intriguingly, spermatozoa from mice that were deficient in the testis-specific kinase 6 (TSSK) failed to fuse with the egg,23 suggesting that TSSK6 is responsible (in part) for Izumo1 phosphorylation.23 However, preliminary data by our group have shown in mice that all of the phosphorylation sites within Izumo1 are still present. Thus, this interaction of TSSK6 may be indirect.
Despite these exciting data, and links between the lack of Izumo1 regulation and infertility, it should be remembered that the C-terminal section of Izumo1 is highly variable among species. Although in the human this region contains a 56-amino acid stretch immediately adjacent to the transmembrane domain, in the pig, only a single amino acid is present within the same region. This large variability raises questions over how each species regulates the protein. Thus, further research is still needed to uncover the exact mechanism of Izumo1 regulation. The discovery of the Izumo1 binding partner Juno raises the possibility that Izumo1 relocalization is essential for bringing Izumo1 and Juno into close enough proximity for adhesion to occur. Understanding this process may very well lead to the discovery of more potential drug targets for male contraceptive development.
REFERENCES
- 1.Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–8. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- 2.Inoue N, Hamada D, Kamikubo H, Hirata K, Kataoka M, et al. Molecular dissection of IZUMO1, a sperm protein essential for sperm-egg fusion. Development. 2013;140:3221–9. doi: 10.1242/dev.094854. [DOI] [PubMed] [Google Scholar]
- 3.Dimitrov DS. Virus entry: molecular mechanisms and biomedical applications. Nat Rev Microbiol. 2004;2:109–22. doi: 10.1038/nrmicro817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xing WJ, Han BD, Wu Q, Zhao L, Bao XH, et al. Molecular cloning and characterization of Izumo1 gene from sheep and cashmere goat reveal alternative splicing. Mol Biol Rep. 2011;38:1995–2006. doi: 10.1007/s11033-010-0322-9. [DOI] [PubMed] [Google Scholar]
- 5.Inoue N, Ikawa M, Okabe M. Putative sperm fusion protein IZUMO and the role of N-glycosylation. Biochem Biophys Res Commun. 2008;377:910–4. doi: 10.1016/j.bbrc.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 6.Ellerman DA, Pei J, Gupta S, Snell WJ, Myles D, et al. Izumo is part of a multiprotein family whose members form large complexes on mammalian sperm. Mol Reprod Dev. 2009;76:1188–99. doi: 10.1002/mrd.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okabe M, Yagasaki M, Oda H, Matzno S, Kohama Y, et al. Effect of a monoclonal anti-mouse sperm antibody (OBF13) on the interaction of mouse sperm with zona-free mouse and hamster eggs. J Reprod Immunol. 1988;13:211–9. doi: 10.1016/0165-0378(88)90002-2. [DOI] [PubMed] [Google Scholar]
- 8.Miyado K, Yamada G, Yamada S, Hasuwa H, Nakamura Y, et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science. 2000;287:321–4. doi: 10.1126/science.287.5451.321. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508:483–7. doi: 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satouh Y, Inoue N, Ikawa M, Okabe M. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J Cell Sci. 2012;125:4985–90. doi: 10.1242/jcs.100867. [DOI] [PubMed] [Google Scholar]
- 11.Bianchi E, Wright GJ. Izumo meets Juno: preventing polyspermy in fertilization. Cell Cycle. 2014;13:2019–20. doi: 10.4161/cc.29461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker MA, Hetherington L, Aitken RJ. Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J Cell Sci. 2006;119:3182–92. doi: 10.1242/jcs.03055. [DOI] [PubMed] [Google Scholar]
- 13.Baker MA, Hetherington L, Curry B, Aitken RJ. Phosphorylation and consequent stimulation of the tyrosine kinase c-Abl by PKA in mouse spermatozoa; its implications during capacitation. Dev Biol. 2009;333:57–66. doi: 10.1016/j.ydbio.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Baker MA, Smith ND, Hetherington L, Pelzing M, Condina MR, et al. Use of titanium dioxide to find phosphopeptide and total protein changes during epididymal sperm maturation. J Proteome Res. 2011;10:1004–17. doi: 10.1021/pr1007224. [DOI] [PubMed] [Google Scholar]
- 15.Baker MA, Smith ND, Hetherington L, Taubman K, Graham ME, et al. Label-free quantitation of phosphopeptide changes during rat sperm capacitation. J Proteome Res. 2010;9:718–29. doi: 10.1021/pr900513d. [DOI] [PubMed] [Google Scholar]
- 16.Baker MA, Witherdin R, Hetherington L, Cunningham-Smith K, Aitken RJ. Identification of post-translational modifications that occur during sperm maturation using difference in 2D-Gel electrophoresis. Proteomics Suppl. 2005;5:1003–12. doi: 10.1002/pmic.200401100. [DOI] [PubMed] [Google Scholar]
- 17.Baker MA, Hetherington L, Weinberg A, Naumovski N, Velkov T, et al. Analysis of phosphopeptide changes as spermatozoa acquire functional competence in the epididymis demonstrates changes in the post-translational modification of Izumo1. J Proteome Res. 2012;11:5252–64. doi: 10.1021/pr300468m. [DOI] [PubMed] [Google Scholar]
- 18.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLachlan RI, de Kretser DM. Male infertility: the case for continued research. Med J Aust. 2001;174:116–7. doi: 10.5694/j.1326-5377.2001.tb143180.x. [DOI] [PubMed] [Google Scholar]
- 20.Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, et al. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed) 1985;291:1693–7. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller CH. The andrology laboratory in an Assisted Reproductive Technologies program. Quality assurance and laboratory methodology. J Androl. 1992;13:349–60. [PubMed] [Google Scholar]
- 22.Hayasaka S, Terada Y, Inoue N, Okabe M, Yaegashi N, et al. Positive expression of the immunoglobulin superfamily protein IZUMO on human sperm of severely infertile male patients. Fertil Steril. 2007;88:214–6. doi: 10.1016/j.fertnstert.2006.11.086. [DOI] [PubMed] [Google Scholar]
- 23.Sosnik J, Miranda PV, Spiridonov NA, Yoon SY, Fissore RA, et al. Tssk6 is required for Izumo relocalization and gamete fusion in the mouse. J Cell Sci. 2009;122:2741–9. doi: 10.1242/jcs.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]


