Abstract
Mammalian fertilization is a complex process that involves different steps of interaction between the male and female gametes. In spite of its relevance, the molecular mechanisms underlying this process still remain to be elucidated. The present review describes the contribution of our laboratory to the understanding of mammalian fertilization using Cysteine-RIch Secretory Proteins (CRISP) as model molecules. Substantial evidence obtained from in vitro assays and knockout models shows that epididymal CRISP1 associates with the sperm surface with two different affinities during maturation, and participates in the regulation of signaling pathways during capacitation as well as in both sperm-zona pellucida interaction and gamete fusion. These observations can be extended to humans as judged by our findings showing that the human homolog of the rodent protein (hCRISP1) is also involved in both stages of fertilization. Evidence supports that other members of the CRISP family secreted in the testis (CRISP2), epididymis (CRISP3-4) or during ejaculation (CRISP3) are also involved in sperm-egg interaction, supporting the existence of a functional redundancy and cooperation between homolog proteins ensuring the success of fertilization. Together, our observations indicate that CRISP proteins accompany spermatozoa along their transit through both the male and female reproductive tracts. We believe these results not only contribute to a better mechanistic understanding of fertilization but also support CRISP proteins as excellent candidates for future research on infertility and contraception.
Keywords: Cysteine-RIch Secretory Proteins, epididymal proteins, fertilization, sperm
INTRODUCTION
Mammalian fertilization is a complex process that involves different steps of interaction between the male and female gametes. Additional complexity comes from the fact that the spermatozoa that leave the testis are not ready to fertilize the oocyte and need to undergo a maturation process as they transit through the epididymis to acquire fully fertilizing competence. A mechanistic understanding of this process will certainly improve the chances of developing new methods for both treatment of infertility and fertility regulation. As a way to contribute to this goal, our laboratory is dedicated to investigating the role of epididymal proteins in the acquisition of the sperm fertilizing ability during maturation, and the potential use of these proteins for contraceptive development. This review describes our contribution to the body of knowledge in the field by studying the role of Cysteine-RIch Secretory Proteins (CRISP) in the different stages of the complex mammalian fertilization process.
CRISP PROTEINS AND EPIDIDYMAL SPERM MATURATION
CRISP1, a glycoprotein of 32 kDa first identified in the rat epididymis,1,2,3 is expressed by the epithelia in an androgen-dependent manner and secreted to the lumen where it associates with the surface of maturing spermatozoa.4,5 To study the mechanisms involved in CRISP1 binding to the cells, mature spermatozoa recovered from the cauda epididymidis were subjected to different extraction procedures. Results revealed that the major amount of CRISP1 can be removed from the cells by exposure to high ionic strength whereas a minor fraction of the protein remains strongly bound to spermatozoa after this treatment and can only be removed by agents that release integral proteins.6 Moreover, whereas the amount of the tightly-attached CRISP1 population was similar in spermatozoa recovered from successive regions of the epididymis, the ionically-bound protein increased from caput to cauda, suggesting that CRISP1 associates strongly with sperm cells as soon as they enter the epididymis and then loosely during epididymal transit.6 Considering that each epididymal segment has its own pattern of gene expression7,8 and that different secretion pathways have been described for this organ (i.e., merocrine and apocrine), it is possible that the two population of CRISP1 are secreted by different pathways in different epididymal regions.
To further characterize the interaction of CRISP1 with spermatozoa, we examined whether the exposure of caput or cauda epididymal spermatozoa to CRISP1 was sufficient to load the protein on the cells. The lack of positive results under all the conditions tested suggested the need of another epididymal fluid component to achieve CRISP1-sperm interaction. Considering the high concentrations of Zn2+ described in the rat epididymis9 and the existence of Zn2+-binding sites in snake CRISP proteins,10 we next investigated the possible role of Zn2+ in the association of CRISP1 with spermatozoa. Western blot results showed an increase in CRISP1 only in extracts from caput spermatozoa that had been incubated with either cauda epididymal fluid or purified CRISP1 in the presence of Zn2+.11 Further experiments supported a direct interaction between the cation and the protein, and their involvement in high molecular weight complexes detected in vitro and in vivo.11 Considering that both the amount of CRISP1 loosely associated with spermatozoa6 and the Zn2+ luminal concentrations12 increase during maturation, our results suggested that Zn2+ might be required for the weak association of CRISP1 with sperm cells. This was later confirmed by the finding that the in vitro-bound protein could be removed from caput cells by the same ionic treatment that releases the loosely attached population from cauda cells. These observations open the possibility that this novel Zn2+-mediated mechanism could be also involved in the binding of other proteins to spermatozoa during epididymal maturation.
The association of the tightly-bound population of CRISP1 with spermatozoa is less understood. Interestingly, evidence shows the presence of small membrane vesicles within the epididymal fluid, known as “epididymosomes” and proposed to be involved in the transfer of proteins, lipids, and microRNAs to luminal spermatozoa.13 In terms of cholesterol and sphingomyelin content, these vesicles are similar to lipid rafts, also called detergent-resistant membranes, which are involved in signaling pathways and protein and lipid trafficking.13 Interestingly, proteomic analyses revealed the presence of CRISP1 in rafts from mouse spermatozoa14 as well as in human epididymosomes,15 suggesting the participation of these vesicles in the strong association of CRISP1 with spermatozoa during maturation. At present, efforts are being made in our laboratory to transfer CRISP1 from the epididymosomes onto immature spermatozoa to gain insights into the mechanism involved in CRISP1 association with sperm cells and also as an approach to increase sperm fertilizing ability in vitro.
In summary, our results suggest the existence of two populations of CRISP1, secreted by different segments of the epididymis, that bind to spermatozoa through different mechanisms and which, as described in the next sections, behave differently during capacitation and fertilization.
CRISP PROTEINS AND SPERM CAPACITATION
Different experimental approaches have led us to conclude that the ionically-bound CRISP1 protein is released from spermatozoa during capacitation, suggesting that this population could be acting as a decapacitating factor.5,6 This finding is in agreement with the participation of Zn2+ in the weak binding of CRISP1 to spermatozoa,11 as it is known that loss of this cation from spermatozoa is one of the initial steps in the capacitation process.16 In accordance with our results, Roberts and colleagues17 postulated that a population of CRISP1 interacts in a reversible way with the sperm plasma membrane to modulate capacitation, as judged by the fact that exposure of spermatozoa to the purified protein inhibited capacitation-associated events, such as protein tyrosine phosphorylation and the progesterone-induced acrosome reaction. The loose association of CRISP1 with spermatozoa and its possible decapacitating role are consistent with what it can be expected for an epididymal protein secreted by a merocrine pathway.18
After capacitation, a minor population of CRISP1 remains attached to spermatozoa17,19 and behaves as the tightly-bound CRISP1.6 This protein migrates from the dorsal region of the acrosome to the equatorial segment concomitantly with the occurrence of the acrosome reaction,20 and plays a role in the fertilization process as described in detail below.
CRISP PROTEINS AND FERTILIZATION
The bulk of evidence supports the participation of CRISP1 in the fertilization process.21 Initially, results from our group revealed that CRISP1 participates in an event following the binding of spermatozoa to the oocyte plasma membrane, leading to gamete fusion.22 These studies also showed that CRISP1 binds to complementary sites on the oocyte surface, with CRISP1 and its partner localized in the fusogenic regions of the spermatozoon and oocyte, respectively.20,22,23 Subsequent results revealed that the oocyte-binding ability of CRISP1 resides within the N-terminal domain of the molecule and, more specifically, in a region of only 12 amino acids24 that corresponds to a defining feature of all CRISP family members. In addition to this role in gamete fusion, experiments from our group showed that CRISP1 also participates in the initial step of sperm binding to the zona pellucida (ZP) through its interaction with complementary sites localized in this glycoprotein matrix.25 To further examine the role of CRISP1 in fertilization, we generated a mouse line deficient in CRISP1, which represented the first knockout animal for a CRISP protein.26 Analysis of CRISP1 in spermatozoa by Western blots revealed the existence of two populations of the protein in the mouse gamete (i.e., one that is released during capacitation and one that remains in the capacitated sperm cells) and the lack of both populations in the knockout cells (Figure 1a). In vitro fertilization studies confirmed the previously-proposed roles of the protein in fertilization, as CRISP1-deficient spermatozoa exhibit a clear disadvantage in their ability to interact with both the ZP and the egg plasma membrane compared with controls.26 Interestingly, despite these fertilizing ability deficiencies, normal fertilization rates were observed when CRISP1 knockout spermatozoa were co-incubated with cumulus-intact oocytes,26 suggesting that CRISP1 could be expressed by the oocyte or cumulus cells and compensate for the lack of CRISP1 in the null sperm cells. Consistent with this possibility, CRISP1 exhibits 57% homology with allurin,27 a CRISP protein synthesized by the amphibian oviduct28 and which binds to the vitelline coat surrounding the egg.29 Studies are being conducted in our laboratory to confirm whether CRISP1 is expressed by the cumulus-oocyte complexes and plays an additional role during fertilization.
Figure 1.
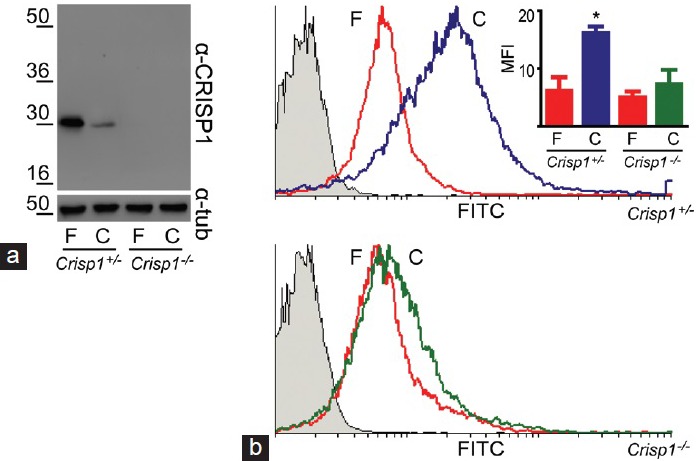
(a) Fate of CRISP1 during mouse sperm capacitation. Total protein extracts from equal amounts of fresh (F) and capacitated (C) epididymal spermatozoa collected from either Crisp1+/− or Crisp1−/− animals were analyzed by SDS-PAGE and Western blots using an anti-CRISP1 antibody (α-CRISP1). β-tubulin was used as control of loading (α-tub). (b) Evaluation of tyrosine phosphorylation in CRISP1-null spermatozoa. Fresh (F) and capacitated (C) epididymal spermatozoa from Crisp1+/− or Crisp1−/− animals were subjected to flow cytometric analysis with an anti-phospho tyrosine antibody. The figure shows representative histograms for each genotype. The gray histograms correspond to sperm cells incubated with normal IgG as primary antibody (negative control). The mean fluorescence intensity (MFI) ± s.e.m. of three experiments is shown as an inset, *P < 0.05.
In addition to these features, CRISP1 knockout spermatozoa do not exhibit the characteristic increase in protein tyrosine phosphorylation associated with sperm capacitation.26 Recent analysis of these spermatozoa by flow cytometry showed that this decrease does not occur as a consequence of the lack of tyrosine phosphorylation in a group of cells but rather to a decrease in phosphorylation in most of the cells (Figure 1b). These observations, together with those reported for the rat,17 support the idea that CRISP1 plays a regulatory role in the intracellular transduction pathways leading to protein tyrosine phosphorylation during capacitation.
In spite of the sperm fertilizing ability deficiencies, CRISP1-null animals exhibited normal fertility rates, suggesting the existence of compensatory mechanisms involving other homolog proteins. In this regard, it is important to note that besides CRISP1, three other CRISP proteins have been reported in mammals (CRISP2-4).30 Whereas CRISP2 and CRISP4 are exclusively found in the testis and epididymis, respectively, CRISP3 exhibits a tissue distribution beyond the reproductive tract.30 Considering results from our group showing that testicular CRISP2 also participates in gamete fusion by binding to the same oocyte-complementary sites as CRISP1,24,31 it is likely that CRISP2 cooperates with CRISP1 to ensure the success of fertilization. Further studies are undergoing in our laboratory to test whether CRISP2 deficiency produces an effect on mouse fertility and/ or fertilization. Another protein that could compensate for the lack of CRISP1 in the knockout model is epididymal CRISP4. Results have shown that mice carrying a deletion in the crisp4 gene are fertile, but exhibit spermatozoa with a reduced ability to bind to the ZP and to undergo the acrosome reaction in response to progesterone.32,33 On the basis of this, CRISP4 could be partially compensating the impaired sperm-ZP binding ability of CRISP1 KO mice, whereas CRISP1 could be doing so in the CRISP4 knockout mice. The generation of mice deficient in more than one CRISP will provide key information on both the existence of cooperation between CRISP homolog members and the relevance of these proteins for animal fertility.
CRISP PROTEINS IN HUMANS
Rodent CRISP1 exhibits 50% homology with a CRISP protein expressed in the human epididymis known as hCRISP1.34,35 Besides its common tissue origin, hCRISP1 is also a glycoprotein of 30 kDa that associates with the sperm surface during epididymal maturation.34,35 These features, together with results showing the existence of a tightly-bound population of hCRISP1 in spermatozoa,36 led us to explore a possible role of hCRISP1 in human fertilization. For ethical reasons, these studies were carried out with approaches that involve the use of human and rodent gametes. Our results showed that an anti-hCRISP1 antibody significantly inhibits the ability of human spermatozoa to penetrate ZP-free hamster oocytes, and that recombinant hCRISP1 binds to the surface of ZP-free human oocytes, supporting the participation of hCRISP1 in gamete fusion.36 Further experiments were performed in order to investigate whether hCRISP1, as its rodent counterpart, could also mediate the binding of spermatozoa to the ZP. In this case, we used the hemizona (HZ) assay in which matching halves of human ZP from nonliving oocytes are exposed to capacitated human spermatozoa. The results revealed that the presence of anti-hCRISP1 during gamete co-incubation produced a significant inhibition in the number of sperm cells bound per HZ compared with controls.37 As hCRISP1 is capable of binding to human ZP-intact oocytes, human recombinant proteins ZP2, ZP3, and ZP4 expressed in insect cells were co-incubated with hCRISP1 to examine protein–protein interactions by ELISA. The finding that hCRISP1 mostly interacts with ZP3 in a dose-dependent manner supports the involvement of the protein in sperm-ZP binding through its specific interaction with human ZP3.37 There are only three crisp genes in the human genome,30 unlike the rodent's, in which four CRISP proteins have been identified. Moreover, hCRISP1 exhibits a higher sequence homology with rodent CRISP4 (70%) than with rodent CRISP1 (50%), suggesting that hCRISP1 represents the human molecule functionally equivalent to both rodent CRISP1 and CRISP4.30,37
Interesting data have come from epididymal transcriptome studies in men who have undergone vasectomy.38 Results showed that hCRISP1 mRNA is up-regulated in the cauda epididymis of vasectomized men.38 Consistent with this, an increase in hCRISP1 expression has also been observed in the epididymis of azoospermic men.39 Furthermore, after vasectomy reversal, fertility is not recovered in more than 50% of the cases, and hCRISP1 expression remains high as a sequelae in the epididymis.40 In fact, the amount of hCRISP1 in spermatozoa from vaso-vasostomized men is higher than that of healthy donors.40 In addition to these observations, ejaculated spermatozoa from vaso-vasostomized men exhibit higher protein tyrosine phosphorylation,40 a finding consistent with our data showing that mouse sperm cells lacking CRISP1 show lower levels of tyrosine phosphorylation.26 Thus, modifications in the level of hCRISP1 expression may disrupt the signaling cascade underlying capacitation, with potential implications for human fertility, as suggested by the poor recovery of fertility of men with vasectomy reversal. As the levels of soluble hCRISP1 in seminal plasma also vary in cases of obstruction below the epididymis, hCRISP1 has been proposed as a diagnostic marker for the distinction of different types of azoospermia.41
In addition to hCRISP1, the human epididymis expresses CRISP3, a protein which is absent from the rat epididymis and also expressed in the mouse by the accessory glands.42,43 On the basis of this observation, we next investigated the potential involvement of human CRISP3 (hCRISP3) in fertilization. Initial results showed that hCRISP3 was present in ejaculated human spermatozoa as two isoforms of 29 kDa and 31 kDa (depending on its glycosylation state). Whereas the 31 kDa is released during capacitation, the 29 kDa form remains attached to sperm after capacitation and the acrosome reaction (Figure 2a). Subsequent indirect immunofluorescence experiments localized hCRISP3 in the acrosome and tail of capacitated sperm cells42 and in the equatorial segment of calcium ionophore-induced acrosome reacted cells (Figure 2b), suggesting the involvement of hCRISP3 in gamete fusion. Evaluation of this possibility with the hamster oocyte penetration test revealed that the anti-hCRISP3 antibody did not produce a decrease in the percentage of penetrated oocytes, contrary to what was observed for the anti-hCRISP1 and anti-hCRISP2 antibodies (Figure 2c). Although these observations suggest that hCRISP3 does not participate in gamete fusion, the potential involvement of this protein in sperm-ZP interaction remains to be explored.
Figure 2.
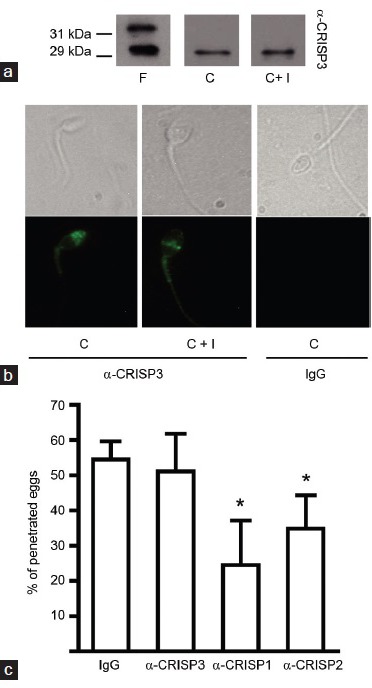
(a) Behavior of CRISP3 during human sperm capacitation and the acrosome reaction. Total protein extracts of fresh (F), capacitated (C) and ionophore-treated (C + I) sperm were analyzed by SDS-PAGE and Western blots with an anti-hCRISP3 antibody (α-CRISP3). (b) Localization of CRISP3 in human spermatozoa. Representative phase contrast (upper panels) and fluorescence (lower panels) microphotographs of human capacitated (C) and ionophore-treated (C + I) spermatozoa subjected to immunofluorescence labeling with α-CRISP3 or normal IgG as primary antibodies. (c) Relevance of CRISP3 for human sperm fusion ability. Capacitated spermatozoa were incubated for 15 min in medium containing either normal IgG, α-CRISP3-, α-CRISP1- or α-CRISP2-antibodies, then co-incubated with ZP-free hamster oocytes for 2.5 h and the percentage of penetrated oocytes was determined. Results represent the mean ± s.e.m. of at least three independent experiments, *P < 0.05 versus IgG.
CRISP PROTEINS AND CONTRACEPTION
On the basis of the participation of CRISP proteins in different stages of the fertilization process, our laboratory explored their potential use for male contraceptive development. As a first approach, male and female rats were immunized with CRISP1 and both the immune response and animal fertility were evaluated. Results revealed that immunization with either native or recombinant rat CRISP1 produced specific antibodies against the protein in over 90% of the animals, as well as a significant and reversible inhibition of fertility in both sexes.44,45,46 These observations support the idea that epididymal proteins are good candidates for posttesticular contraception, as they are specifically involved in the acquisition of sperm fertilizing ability and not in key processes such as spermatogenesis or hormone production. In spite of the disadvantage of using testicular proteins, immunization experiments were also carried out with recombinant testicular CRISP2. The lack of immune response and the normal fertility of immunized males and females were consistent with the intracellular localization of CRISP2.47 In view of the results obtained with rodent CRISP1, we next explored the potential contraceptive use of epididymal hCRISP1 in a nonhuman primate model. Immunization of male and female monkeys with recombinant hCRISP1 elicited a specific and reversible immune response.48 Furthermore, results revealed the presence of specific antibodies against the protein bound to ejaculated spermatozoa from hCRISP1- but not control-immunized animals, confirming the entry of the antibodies into the male reproductive tract and their ability to bind to sperm cells.48 These results together with the finding that anti-hCRISP1 antibodies are capable of inhibiting the ability of spermatozoa both to fuse with the oocyte36 and to bind to the ZP37 support hCRISP1 as an epididymal contraceptive target.
PERSPECTIVES
Evidence obtained by using different cellular, molecular and genetic approaches supports different roles for CRISP proteins in sperm function in rodents as well as in humans. According to our observations and those reported by other groups, these molecules accompany sperm along their transit through both the male and female reproductive tracts. Functional studies of the different CRISP members revealed overlapping roles in the fertilization process (Figure 3), supporting the existence of a functional redundancy and cooperation between these homolog protein members, ensuring the success of fertilization. We believe these results will contribute to a better understanding of the mechanisms involved in the acquisition of sperm fertilizing ability during epididymal maturation, as well as to the development of new methods for diagnosis and treatment of human infertility and fertility regulation.
Figure 3.
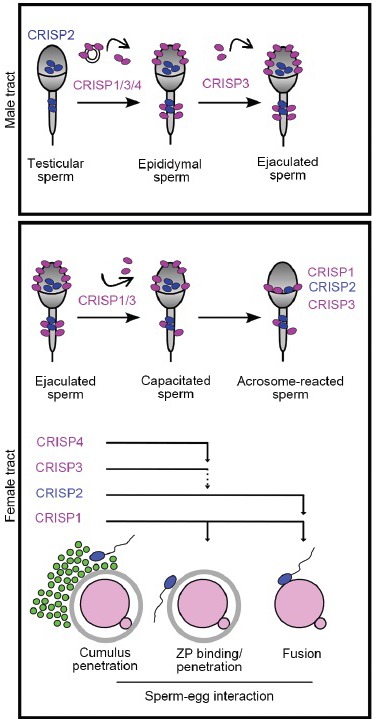
CRISP proteins in spermatozoa during their transit along the male and female reproductive tracts. Spermatozoa leaving the testis carry CRISP2 inside the head and tail. During epididymal transit, CRISP1, CRISP3, and CRISP4, soluble or associated with epididymosomes, bind loosely or strongly to the sperm surface. CRISP3 can also bind to the cells during ejaculation. In the female tract, the loosely associated proteins are partially released from sperm cells during capacitation whereas the intracellular or tightly-bound population remain in the cells and relocalize to the equatorial segment of acrosome-reacted spermatozoa. Each sperm CRISP protein participates in more than one step of fertilization and cooperates with other CRISP homologs in each fertilization step.
REFERENCES
- 1.Cameo MS, Blaquier J. Androgen-controlled specific proteins in rat epididymis. J Endocrinol. 1976;69:317–24. doi: 10.1677/joe.0.0690047. [DOI] [PubMed] [Google Scholar]
- 2.Garberi JC, Kohane AC, Cameo MS, Blaquier JA. Isolation and characterization of specific rat epididymal proteins. Mol Cell Endocrinol. 1979;13:73–82. doi: 10.1016/0303-7207(79)90077-7. [DOI] [PubMed] [Google Scholar]
- 3.Garberi JC, Fontana JD, Blaquier JA. Carbohydrate composition of specific rat epididymal protein. Int J Androl. 1982;5:619–26. doi: 10.1111/j.1365-2605.1982.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 4.Kohane AC, Cameo MS, Piñeiro L, Garberi JC, Blaquier JA. Distribution and site of production of specific proteins in the rat epididymis. Biol Reprod. 1980;23:181–7. doi: 10.1095/biolreprod23.1.181. [DOI] [PubMed] [Google Scholar]
- 5.Kohane AC, González Echeverría FM, Piñeiro L, Blaquier JA. Interaction of proteins of epididymal origin with spermatozoa. Biol Reprod. 1980;23:737–42. doi: 10.1095/biolreprod23.4.737. [DOI] [PubMed] [Google Scholar]
- 6.Cohen DJ, Rochwerger L, Ellerman DA, Morgenfeld MM, Busso D, et al. Relationship between the association of rat epididymal protein “DE” with spermatozoa and the behavior and function of the protein. Mol Reprod Dev. 2000;56:180–8. doi: 10.1002/(SICI)1098-2795(200006)56:2<180::AID-MRD9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Johnston DS, Jelinsky SA, Bang HJ, DiCandeloro P, Wilson E, et al. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol Reprod. 2005;73:404–13. doi: 10.1095/biolreprod.105.039719. [DOI] [PubMed] [Google Scholar]
- 8.Thimon V, Koukoui O, Calvo E, Sullivan R. Region-specific gene expression profiling along the human epididymis. Mol Hum Reprod. 2007;13:691–704. doi: 10.1093/molehr/gam051. [DOI] [PubMed] [Google Scholar]
- 9.Mawson CA, Fischer MI. Zinc content of the genital organs of the rat. Nature. 1951;167:859. doi: 10.1038/167859a0. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki N, Yamazaki Y, Brown RL, Fujimoto Z, Morita T, et al. Structures of pseudechetoxin and pseudecin, two snake-venom cysteine-rich secretory proteins that target cyclic nucleotide-gated ion channels: implications for movement of the C-terminal cysteine-rich domain. Acta Crystallogr D Biol Crystallogr. 2008;64(Pt 10):1034–42. doi: 10.1107/S0907444908023512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maldera JA, Vasen G, Ernesto JI, Weigel-Muñoz M, Cohen DJ, et al. Evidence for the involvement of zinc in the association of CRISP1 with rat sperm during epididymal maturation. Biol Reprod. 2011;85:503–10. doi: 10.1095/biolreprod.111.091439. [DOI] [PubMed] [Google Scholar]
- 12.Stoltenberg M, Ernst E, Andreasen A, Danscher G. Histochemical localization of zinc ions in the epididymis of the rat. Histochem J. 1996;28:173–85. doi: 10.1007/BF02331441. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan R, Saez F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction. 2013;146:R21–35. doi: 10.1530/REP-13-0058. [DOI] [PubMed] [Google Scholar]
- 14.Sleight SB, Miranda PV, Plaskett NW, Maier B, Lysiak J, et al. Isolation and proteomic analysis of mouse sperm detergent-resistant membrane fractions: evidence for dissociation of lipid rafts during capacitation. Biol Reprod. 2005;73:721–9. doi: 10.1095/biolreprod.105.041533. [DOI] [PubMed] [Google Scholar]
- 15.Thimon V, Frenette G, Saez F, Thabet M, Sullivan R. Protein composition of human epididymosomes collected during surgical vasectomy reversal: a proteomic and genomic approach. Hum Reprod. 2008;23:1698–707. doi: 10.1093/humrep/den181. [DOI] [PubMed] [Google Scholar]
- 16.Andrews JC, Nolan JP, Hammerstedt RH, Bavister BD. Role of zinc during hamster sperm capacitation. Biol Reprod. 1994;51:1238–47. doi: 10.1095/biolreprod51.6.1238. [DOI] [PubMed] [Google Scholar]
- 17.Roberts KP, Wamstad JA, Ensrud KM, Hamilton DW. Inhibition of capacitation-associated tyrosine phosphorylation signaling in rat sperm by epididymal protein Crisp-1. Biol Reprod. 2003;69:572–81. doi: 10.1095/biolreprod.102.013771. [DOI] [PubMed] [Google Scholar]
- 18.Cooper TG. Epididymal research: more warp than weft? Asian J Androl. 2015;17:1–5. doi: 10.4103/1008-682X.146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cameo MS, Gonzalez Echeverria MF, Blaquier JA, Burgos MH. Immunochemical localization of epididymal protein DE on rat spermatozoa: its fate after induced acrosome reaction. Gamete Res. 1986;15:247–58. [Google Scholar]
- 20.Rochwerger L, Cuasnicú PS. Redistribution of a rat sperm epididymal glycoprotein after in vivo and in vitro capacitation. Mol Reprod Dev. 1992;31:34–41. doi: 10.1002/mrd.1080310107. [DOI] [PubMed] [Google Scholar]
- 21.Cohen DJ, Maldera JA, Vasen G, Ernesto JI, Muñoz MW, et al. Epididymal protein CRISP1 plays different roles during the fertilization process. J Androl. 2011;32:672–8. doi: 10.2164/jandrol.110.012922. [DOI] [PubMed] [Google Scholar]
- 22.Rochwerger L, Cohen DJ, Cuasnicú PS. Mammalian sperm-egg fusion: the rat egg has complementary sites for a sperm protein that mediates gamete fusion. Dev Biol. 1992;153:83–90. doi: 10.1016/0012-1606(92)90093-v. [DOI] [PubMed] [Google Scholar]
- 23.Cohen DJ, Munuce MJ, Cuasnicú PS. Mammalian sperm-egg fusion: the development of rat oolemma fusibility during oogenesis involves the appearance of binding sites for sperm protein “DE”. Biol Reprod. 1996;55:200–6. doi: 10.1095/biolreprod55.1.200. [DOI] [PubMed] [Google Scholar]
- 24.Ellerman DA, Cohen DJ, Da Ros VG, Morgenfeld MM, Busso D, et al. Sperm protein “DE” mediates gamete fusion through an evolutionarily conserved site of the CRISP family. Dev Biol. 2006;297:228–37. doi: 10.1016/j.ydbio.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Busso D, Cohen DJ, Maldera JA, Dematteis A, Cuasnicu PS. A novel function for CRISP1 in rodent fertilization: involvement in sperm-zona pellucida interaction. Biol Reprod. 2007;77:848–54. doi: 10.1095/biolreprod.107.061788. [DOI] [PubMed] [Google Scholar]
- 26.Da Ros VG, Maldera JA, Willis WD, Cohen DJ, Goulding EH, et al. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1) Dev Biol. 2008;320:12–8. doi: 10.1016/j.ydbio.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burnett LA, Anderson DM, Rawls A, Bieber AL, Chandler DE. Mouse sperm exhibit chemotaxis to allurin, a truncated member of the cysteine-rich secretory protein family. Dev Biol. 2011;360:318–28. doi: 10.1016/j.ydbio.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 28.Olson JH, Xiang X, Ziegert T, Kittelson A, Rawls A, et al. Allurin, a 21-kDa sperm chemoattractant from Xenopus egg jelly, is related to mammalian sperm-binding proteins. Proc Natl Acad Sci U S A. 2001;98:11205–10. doi: 10.1073/pnas.211316798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang X, Kittelson A, Olson J, Bieber A, Chandler D. Allurin, a 21 kD sperm chemoattractant, is rapidly released from the outermost jelly layer of the Xenopus egg by diffusion and medium convection. Mol Reprod Dev. 2005;70:344–60. doi: 10.1002/mrd.20201. [DOI] [PubMed] [Google Scholar]
- 30.Gibbs GM, Roelants K, O’Bryan MK. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins – roles in reproduction, cancer, and immune defense. Endocr Rev. 2008;29:865–97. doi: 10.1210/er.2008-0032. [DOI] [PubMed] [Google Scholar]
- 31.Busso D, Goldweic NM, Hayashi M, Kasahara M, Cuasnicú PS. Evidence for the involvement of testicular protein CRISP2 in mouse sperm-egg fusion. Biol Reprod. 2007;76:701–8. doi: 10.1095/biolreprod.106.056770. [DOI] [PubMed] [Google Scholar]
- 32.Gibbs GM, Orta G, Reddy T, Koppers AJ, Martínez-López P, et al. Cysteine-rich secretory protein 4 is an inhibitor of transient receptor potential M8 with a role in establishing sperm function. Proc Natl Acad Sci U S A. 2011;108:7034–9. doi: 10.1073/pnas.1015935108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turunen HT, Sipilä P, Krutskikh A, Toivanen J, Mankonen H, et al. Loss of cysteine-rich secretory protein 4 (Crisp4) leads to deficiency in sperm-zona pellucida interaction in mice. Biol Reprod. 2012;86:1–8. doi: 10.1095/biolreprod.111.092403. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi M, Fujimoto S, Takano H, Ushiki T, Abe K, et al. Characterization of a human glycoprotein with a potential role in sperm-egg fusion: cDNA cloning, immunohistochemical localization, and chromosomal assignment of the gene (AEGL1) Genomics. 1996;32:367–74. doi: 10.1006/geno.1996.0131. [DOI] [PubMed] [Google Scholar]
- 35.Krätzschmar J, Haendler B, Eberspaecher U, Roosterman D, Donner P, et al. The human cysteine-rich secretory protein (CRISP) family. Primary structure and tissue distribution of CRISP-1, CRISP-2 and CRISP-3. Eur J Biochem. 1996;236:827–36. doi: 10.1111/j.1432-1033.1996.t01-1-00827.x. [DOI] [PubMed] [Google Scholar]
- 36.Cohen DJ, Ellerman DA, Busso D, Morgenfeld MM, Piazza AD, et al. Evidence that human epididymal protein ARP plays a role in gamete fusion through complementary sites on the surface of the human egg. Biol Reprod. 2001;65:1000–5. doi: 10.1095/biolreprod65.4.1000. [DOI] [PubMed] [Google Scholar]
- 37.Maldera JA, Weigel Muñoz M, Chirinos M, Busso D, Raffo FG, et al. Human fertilization: epididymal hCRISP1 mediates sperm-zona pellucida binding through its interaction with ZP3. Mol Hum Reprod. 2014;20:341–9. doi: 10.1093/molehr/gat092. [DOI] [PubMed] [Google Scholar]
- 38.Thimon V, Calvo E, Koukoui O, Légaré C, Sullivan R. Effects of vasectomy on gene expression profiling along the human epididymis. Biol Reprod. 2008;79:262–73. doi: 10.1095/biolreprod.107.066449. [DOI] [PubMed] [Google Scholar]
- 39.Dubé E, Hermo L, Chan PT, Cyr DG. Alterations in gene expression in the caput epididymides of nonobstructive azoospermic men. Biol Reprod. 2008;78:342–51. doi: 10.1095/biolreprod.107.062760. [DOI] [PubMed] [Google Scholar]
- 40.Légaré C, Boudreau L, Thimon V, Thabet M, Sullivan R. Vasectomy affects cysteine-rich secretory protein expression along the human epididymis and its association with ejaculated spermatozoa following vasectomy surgical reversal. J Androl. 2010;31:573–83. doi: 10.2164/jandrol.109.009860. [DOI] [PubMed] [Google Scholar]
- 41.Légaré C, Cloutier F, Makosso-Kallyth S, Laflamme N, Jarvi K, et al. Cysteine-rich secretory protein 1 in seminal plasma: potential biomarker for the distinction between obstructive and nonobstructive azoospermia. Fertil Steril. 2013;100:1253–60. doi: 10.1016/j.fertnstert.2013.07.1984. [DOI] [PubMed] [Google Scholar]
- 42.Udby L, Bjartell A, Malm J, Egesten A, Lundwall A, et al. Characterization and localization of cysteine-rich secretory protein 3 (CRISP-3) in the human male reproductive tract. J Androl. 2005;26:333–42. doi: 10.2164/jandrol.04132. [DOI] [PubMed] [Google Scholar]
- 43.Reddy T, Gibbs GM, Merriner DJ, Kerr JB, O’Bryan MK. Cysteine-rich secretory proteins are not exclusively expressed in the male reproductive tract. Dev Dyn. 2008;237:3313–23. doi: 10.1002/dvdy.21738. [DOI] [PubMed] [Google Scholar]
- 44.Cuasnicú PS, Conesa D, Rochwerger L. Potential contraceptive use of an epididymal protein that participates in fertilization. In: Alexander NJ, Griffin D, Spieler JM, Waites GM, editors. Gamete Interaction. Prospects for Immunocontraception. New York: Wiley-Liss; 1990. pp. 143–53. [Google Scholar]
- 45.Pérez Martinez S, Conesa D, Cuasnicú PS. Potential contraceptive use of epididymal proteins: evidence for the participation of specific antibodies against rat epididymal protein DE in male and female fertility inhibition. J Reprod Immunol. 1995;29:31–45. doi: 10.1016/0165-0378(95)00927-d. [DOI] [PubMed] [Google Scholar]
- 46.Ellerman DA, Busso D, Maldera JA, Cuasnicú PS. Immunocontraceptive properties of recombinant sperm protein DE: implications for the development of novel contraceptives. Fertil Steril. 2008;89:199–205. doi: 10.1016/j.fertnstert.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 47.Muñoz MW, Ernesto JI, Bluguermann C, Busso D, Battistone MA, et al. Evaluation of testicular sperm CRISP2 as a potential target for contraception. J Androl. 2012;33:1360–70. doi: 10.2164/jandrol.112.016725. [DOI] [PubMed] [Google Scholar]
- 48.Ellerman DA, Cohen DJ, Weigel Muñoz M, Da Ros VG, Ernesto JI, et al. Immunologic behavior of human cysteine-rich secretory protein 1 (hCRISP1) in primates: prospects for immunocontraception. Fertil Steril. 2010;93:2551–6. doi: 10.1016/j.fertnstert.2010.01.075. [DOI] [PubMed] [Google Scholar]


