Abstract
Cholesterol is a key molecule in the mammalian physiology of especial particular importance for the reproductive system as it is the common precursor for steroid hormone synthesis. Cholesterol is also a recognized modulator of sperm functions, not only at the level of gametogenesis. Cholesterol homeostasis regulation is crucial for posttesticular sperm maturation, and imbalanced cholesterol levels may particularly affect these posttesticular events. Metabolic lipid disorders (dyslipidemia) affect male fertility but are most of the time studied from the angle of endocrine/testicular consequences. This review will focus on the deleterious effects of a particular dyslipidemia, i.e., hypercholesterolemia, on posttesticular maturation of mammalian spermatozoa.
Keywords: cholesterol, dyslipidemia, epididymis, fertility, posttesticular maturation, spermatozoa
INTRODUCTION
Mammalian spermatozoa are very specialized and polarized cells, with a specific function: fertilizing their female counterpart, the oocyte. The capacity to fertilize an oocyte is acquired during a long-lasting multi-step process that can be defined as “posttesticular maturation.” This maturation, enabling an infertile testicular spermatozoon to become fertile, starts during its epididymal transit1 and ends in the upper female genital tract, in the vicinity of the oocyte, during the capacitation process.2,3 During posttesticular maturation events, the modifications of sperm plasma membrane composition and dynamics are essential for fertility,4,5 and cholesterol plays a crucial role. The importance of epididymal cholesterol homeostasis has been recently reviewed,6 and this epididymal maturation process prepares sperm cells for the next important maturation step, capacitation, which is also highly dependent on the plasma membrane cholesterol content of the male gametes.7,8,9 Even though the central role of cholesterol in male fertility has been known for a long time, few studies have been conducted on the impact of cholesterol metabolism impairment on posttesticular maturation, the emphasis of most being put on the endocrine effects.
Dysregulation of lipid metabolism (dyslipidemia) is increasingly present in occidental societies, with pathophysiological conditions such as obesity, metabolic syndrome and cardiovascular diseases (World Health Organization). Hypercholesterolemia is highly represented among dyslipidemic patients: over 30 million Americans older than 20 years have a high blood cholesterol level (>240 mg dL−1, American Heart Association Statistical Fact sheet 2013). Another study has shown that a high proportion of men at the age for their partners to conceive (45.3% of men aged 30–54 years) were dyslipidemic without taking any treatment.10 The influence of lipid metabolic disorders on fertility has now become a clinical problem11,12 and this review will present the state-of-the-art on the impact hypercholesterolemia can have on male fertility, focusing on posttesticular maturation events and sperm functions.
POSTTESTICULAR MATURATION AND CHOLESTEROL
The posttesticular maturation process particularly concerns membrane composition of gametes and begins in the epididymis. It involves remodeling of membrane proteins (relocalization, addition13,14) and changes in the lipid composition, in particular of phospholipids and sterols. Changes in the sperm cholesterol content during epididymal transit have been studied in several mammalian species,6 and a significant decrease of approximately 50% has been found in the ram,15 rat,16 hamster,17 and mouse.18 The cholesterol loss causes a decrease in the cholesterol/phospholipid ratio, a membrane fluidity indicator, showing that sperm cells increase their membrane fluidity during epididymal transit, which facilitates the subsequent events of capacitation and membrane fusion during fertilization. The distribution of cholesterol in the membrane also appears to be important because the plasma membrane of spermatozoa shows abundant cholesterol-enriched microdomains (lipid rafts), the real organizing centers of cell signaling.19 These lipid rafts modify the local membrane dynamics and are essential for the regionalized transfer of proteins to the male gamete during epididymal maturation. Indeed, a study in bulls showed that the zona pellucida recognition (P25b) protein was specifically transferred to the sperm lipid rafts via lipid rafts found on small lipid vesicles (epididymosomes) emitted by the epididymal epithelium.20 Moreover, three different subtypes of lipid rafts have been previously characterized in mouse sperm cells, each with a different protein profile as determined by proteomic analysis.21 Many of these proteins are acquired by sperm cells during epididymal transit, showing the importance of the lipid composition of gametes for the proper acquisition and localization of proteins during posttesticular maturation.
The next posttesticular maturation step occurs in the female genital tract during the capacitation process. The lipid composition of sperm cells issuing from the epididymis is crucial in most species, as cholesterol efflux from the plasma membrane is the early event triggering capacitation. The consecutive decrease of the cholesterol/phospholipid ratio modifies plasma membrane dynamics,7,22 thereby increasing membrane permeability to bicarbonate and calcium ions,23,24 allowing activation of a soluble adenylyl cyclase (sAC,25) and increase in cAMP production. The Ser/Thr kinase PKA (protein kinase A) is also activated, resulting in the initiation of a signaling cascade.26,27 PKA phosphorylates, among other proteins, SRC kinase, leading to phosphorylation of specific tyrosine residues in sperm proteins.28 Although phosphorylation of tyrosine residues of proteins during capacitation is well described today, the roles of these proteins in the capacitation process remain poorly understood.9 Ultimately, with capacitation gametes acquire a hyperactivated motility,29 the ability to bind to the zona pellucida and to undergo the acrosome reaction.30
The proper lipid composition of gametes is thus essential for fertilization, and transgenic mouse models have shown that dysregulation of cholesterol metabolism may act at different levels of male fertility.
CHOLESTEROL AND INFERTILITY – THE CONTRIBUTION OF TRANSGENIC MOUSE MODELS
Transgenic mouse models have been used to investigate the physiological importance of cholesterol homeostasis regulators (Table 1). In some models, male fertility studies have only been performed for regarding testicular disturbances (Retinoid X Receptor, Rxr−/− mice31 – ATP-binding cassette, Abca1−/− mice32) and will not be developed here. In other cases, an epididymal phenotype was revealed but not in direct relation to cholesterol homeostasis. The ApoER2−/−(Apolipoprotein E receptor 2) mice, for example, suffered from infertility, but no alterations were detected in testicular or epididymal structures and sperm production was normal.33 Even though ApoER2 is a member of the LDL receptor family, highly expressed in the principal cells of epididymal proximal segment,34 no clear phenotype related to cholesterol homeostasis was described, the infertility being due to functional defects of the sperm mitochondria and cell volume dysregulation.
Table 1.
Transgenic mice used for genes involved in cholesterol-related biological processes and their associated male fertility phenotypes
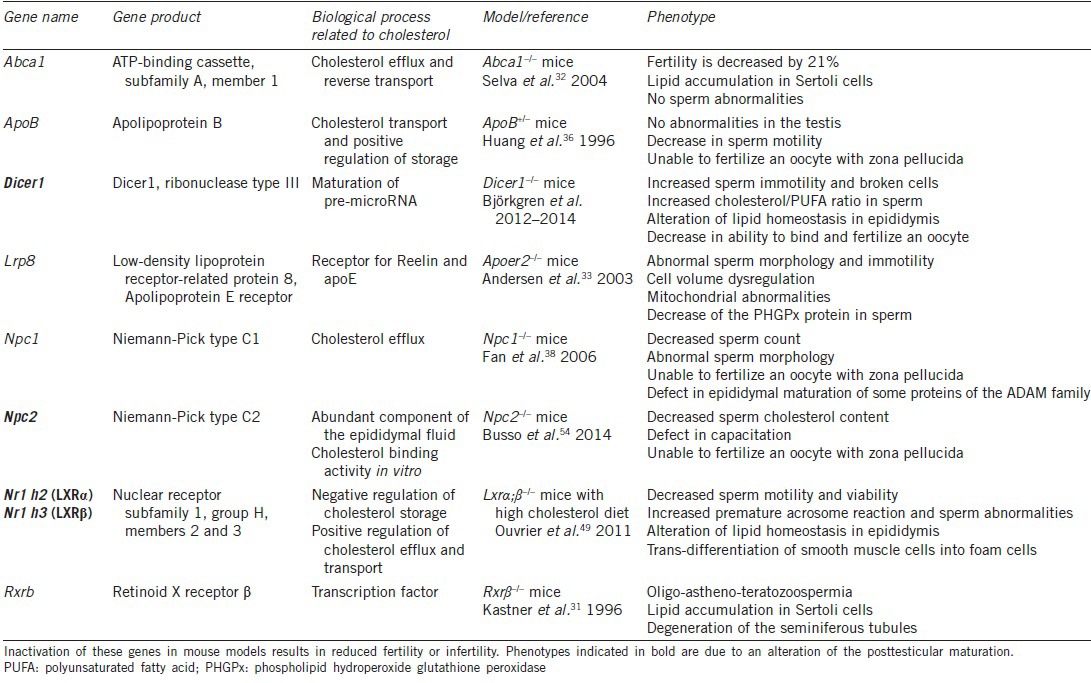
In other models, the authors concluded that the infertility was due to testicular abnormalities while their results also strongly suggested posttesticular maturation disturbances. For example, male mice heterozygous for a null apolipoprotein B allele (Apo B+/−), a structural component of several classes of lipoproteins (chylomicron, VLDL, IDL, LDL), have reduced fertility associated with a decreased cholesterol plasma level.35 The urogenital tract appears normal, but the sperm count is mildly reduced, motility is decreased, and spermatozoa are unable to fertilize an oocyte in the presence of its zona pellucida.36 The authors only evoked the hypothesis of an unknown role of ApoB in spermatogenesis. However, today the importance of epididymal maturation is well-demonstrated for the acquisition of mobility and zona pellucida recognition, and ApoB is also expressed in the epididymis.36 Defects could thus come from posttesticular events. Since this work, the authors have not pursued their investigation on this model.
The Niemann-Pick C1 (NPC1) protein regulates the trafficking of low-density lipoprotein-mediated endocytosed cholesterol.37 Npc1−/− male mice are infertile mostly because of a partial arrest of spermatogenesis.38 Furthermore, spermatozoa show morphological head abnormalities and are unable to fertilize an oocyte in vitro.38 These gametes also have a fault in a disintegrin and metalloprotease (ADAM)family protein maturation, in particular ADAM 3 (cyritestin), known to be essential for fertilization.39 This protein must be cleaved during epididymal transit to be functional40 and sperm cells of Npc1−/− mice show a defect in this cleavage, demonstrating a lack of epididymal maturation, however the authors did not discuss this point.
In these models, the posttesticular sperm defects were often not considered, even though several studies have demonstrated the deleterious impact of dyslipidemia on the posttesticular maturation of gametes and therefore fertility.
The impact of hypercholesterolemia on gamete epididymal maturation has regained some interest since our group has developed a model of diet-induced posttesticular infertility. This model combines knock-out mice for Liver X Receptors (LXRs) with a high cholesterol diet. The transcription factors LXRα (LXRα – Nr1h3) and LXRb (LXRb – Nr1h2) are activated by oxidized forms of cholesterol and have been shown to control the expression of genes involved in cholesterol homeostasis, differentiation, proliferation, inflammation, and reproduction.41,42,43,44,45 Male mice deficient for the two LXR isoforms become totally infertile at 8–9 months, showing a composite phenotype: testicular44 and epididymal (restricted to the proximal caput epididymis46,47,48). Young LXR-deficient male mice (3–4 months) are fertile and do not show any phenotype at all.44,46 However, when young males are fed for 1 month with a high cholesterol diet (HCD, which provokes a large elevation of plasma LDL cholesterol or LDL-C), infertility is triggered and only the epididymal phenotype appears, showing the sensitivity of this organ to a cholesterol overload.49 These animals recapitulate the epididymal phenotype of old mice: accumulation of cholesteryl esters in the proximal caput epididymis, and disruption of the proximal epididymal segments (S1 and S2) with a reduction in the height of the epithelium. These mice present an “epididymosclerotic” phenotype characterized by a global loss of smooth muscle cells accompanied by trans-differentiation of these cells into “foam cells”. These cells appeared to migrate towards and infiltrate the epididymal epithelium, which was corroborated by the presence of the matrix metalloproteinase MMP9. Testes showed no alteration, but the gametes were strongly affected. Motility and viability were decreased, and broken sperm cells were increased, showing a weakness of the head/flagellum junction. Premature acrosomal reactions were also increased and could result from an alteration in the membrane composition. This work clearly showed that, in this model, the epididymis is an early target of lipid-induced infertility. Given the importance of gamete lipid maturation during epididymal transit, we strongly suspect that infertility in this model is caused by an alteration in the membrane lipid composition of spermatozoa. This lipid composition will be crucial especially during capacitation, and this aspect is currently being studied.
Cholesterol homeostasis involves different proteins regulating intracellular trafficking. Niemann-Pick C2 (NPC2) is a lysosomal, soluble protein shown to be a cholesterol-binding protein relevant for the efflux of cholesterol from this organelle.50 This protein has been shown to be one of the most abundant proteins in human epididymal fluid51 and seems to participate in cholesterol efflux from spermatozoa during epididymal maturation.52,53 NPC2 deficient mice (Npc2−/−), a model for Niemann-Pick disease type C2 (neurological disease), are also infertile because they do not mate (locomotor dysfunction). However, given the abundance of NPC2 in the epididymis, it was of interest to check whether the spermatozoa of these males were able to fertilize an oocyte. The authors showed that IVF rates were significantly lower with spermatozoa from these mice than with those from the wild type.54 Spermatozoa appeared normal in count, morphology, viability and mobility. After vasectomy and even after a vaso-vasostomy, NPC2 expression in the human epididymis is selectively down-regulated, and the sperm cholesterol content is increased,53 confirming the functional role of NPC2 in cholesterol efflux. In bulls, purified NPC2 is able to reduce the cholesterol content of spermatozoa and to dissociate a large part of P25b molecules from lipid rafts, suggesting that NPC2 is involved in sperm membrane organization.52 Unexpectedly, in the mouse, the lack of NPC2 appears to cause a decrease in sperm plasma cholesterol. Spermatozoa of Npc2−/− animals show reduced capacitation-induced cholesterol efflux, highlighted by a defect in tyrosine phosphorylation. The lower cholesterol content, with increasing membrane fluidity, may alter the distribution of molecules in noncapacitated spermatozoa. Indeed, as mentioned previously, lipid rafts (enriched in cholesterol) allow regionalized acquisition of proteins.21 An impairment of this process, by a reduction in membrane cholesterol content, could ultimately affect the formation of capacitation-related signaling complexes during incubation under capacitation conditions.54 Changes in sperm cholesterol content, either an increase or a decrease in these animals, have a direct impact on capacitation and thus on the ability to fertilize an oocyte.
More recently, a specific model of invalidation of Dicer1 in the caput epididymis was generated (Dicer 1 cKO55) by using a mouse line expressing iCre under the Defensin beta 41 (Defb41) promoter.56 Dicer1 is the RNAse III enzyme required for processing pre-microRNAs into their mature forms. These male mice were infertile, and the epithelial layer of both initial segment (IS) and caput epididymis was disorganized. With age, the Dicer1 cKO IS morphologically resembled that of an undifferentiated prepubertal epididymis. Moreover, the authors showed a decrease in segment-specific gene expression and altered sex steroid receptor ratio.55 Further examination of these Dicer1 cKO mice sperm showed that 33% of sperm cells were broken and that there was an increased spontaneous loss of the acrosome. Eventually, 80% of the spermatozoa were immotile. Such defects could be caused by membrane instability coming from the abnormal lipid composition of these gametes. The authors showed that under capacitating conditions Dicer1 cKO mouse spermatozoa did not lose cholesterol as did wild-type spermatozoa, leading to ineffective capacitation (only a small increase in tyrosine phosphorylation levels). Furthermore, the polyunsaturated fatty acid (PUFA) level was decreased compared with the wild type, consequently increasing the cholesterol/PUFA ratio and, therefore, the membrane rigidity. In the epididymis, expression levels of genes involved in the biosynthesis (Hmgcr, Hsd17b7, and Dhcr24) and transport (Abca1 and Abcg1) of cholesterol were up-regulated.57 This is in accordance with the lack of DICER1, thus strongly reducing the presence of all mature miRNAs from the cells, and ultimately giving rise to up-regulation of target genes. MicroRNAs have previously been shown to be involved in the regulation of lipid metabolism: miR-33 inhibits the expression of Abca1 and Abcg158 in mice and miR-122 inhibits Hmgcr.59 In the Dicer1 cKO, these miRNAs may not be matured, thus explaining the increase in their target gene expression levels, but this hypothesis will need confirmation. This original KO has brought new evidence regarding the importance of cholesterol homeostasis and miRNAs in the epididymis for proper maturation of spermatozoa.
MODELS OF DIET-INDUCED MALE INFERTILITY
Besides transgenic mouse models, hypercholesterolemia can also be diet-induced, mimicking nutritional dyslipidemia. The rabbit is a useful model because hypercholesterolemia can be easily triggered with cholesterol- or fat-enriched diets.60,61 The observed diet effects vary depending on diet composition and duration. The main sperm modifications triggered by hypercholesterolemia are (1) an increase in the cholesterol content and the percentage of morphologically abnormal spermatozoa, (2) a decrease in sperm motility and the progesterone-induced acrosome reaction and (3) a constant sperm count.62,63 The observed higher cholesterol content is an indicator of decreased membrane fluidity, a fact in accordance with an inhibition of capacitation shown by a reduced ability of spermatozoa to reach normal tyrosine phosphorylation levels. The link was established using a cell-permeant synthetic cAMP analog and phosphodiesterase inhibitors, which artificially increase intracellular cAMP levels and lead to a direct activation of PKA, bypassing the early membrane events of capacitation. Under these conditions, phosphotyrosine profiles are restored showing that the capacitation defect is associated with membrane-related events.63
The rat model has been less used for the reason that rats can physiologically manage a cholesterol overload.61 Several studies with high cholesterol64,65 or high fat-fed rats show quite similar results to what has been described in the rabbit:66 decreased sperm motility, viability and sometimes sperm numbers,64,65 and usually the percentage of morphologically abnormal sperm cells is increased.65,66 In all these cases, testicular histology was disrupted, with a decrease in the seminiferous tubule diameter,64 testicular atrophy65 or a decrease in the number of Leydig and Sertoli cells.66 These models are thus not appropriate to study the impact of hypercholesterolemia on epididymal sperm maturation.
CLINICAL DATA LINKING CHOLESTEROL PLASMA LEVELS AND POSTTESTICULAR MATURATION EVENTS
The first report linking modification of cholesterol metabolism and semen parameters in the human was published in 1993 by Dobs and colleagues. A statistically significant decrease in sperm motility was evident in a subset of 14 male patients included in a Randomized Control Trial (RCT) assessing the efficiency of pravastatin compared with cholestyramine.67 Interestingly, these LDL-lowering drugs act through independent mechanisms. Pravastatin belongs to the statin family of lipid-altering agents. They are competitive inhibitors of Hydroxymethylglutaryl Coenzyme A (HMG-CoA) reductase (HMGR), the rate-limiting enzyme in cholesterol biosynthesis, which irreversibly reduces 3-Hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA) to mevalonate. By occupying a portion of the binding site of HMG-CoA to the enzyme, they block the access of the substrate to the catalytic site of HMGR, which results in a decrease in intracellular cholesterol. The total cholesterol- and low-density lipoprotein-cholesterol (LDL-C)-lowering effect of statins is then explained by their ability to decrease cholesterol production in the liver.68
Cholestyramine belongs to the family of Bile Acid Sequestrants (BAS). They consist of large polymers that are able to bind to bile salts in the intestine, preventing their reabsorption from the gut and increasing their fecal excretion. Increased fecal excretion leads to a subsequent shift in bile salt de novo synthesis from liver cholesterol at the expense of LDL-C synthesis, which results in a lowering of blood LDL-C levels.69 In the initial Dobs et al. study, a decrease in sperm motility was found 6 and 12 months after treatment indicating that this adverse event was the result of a lowering of total cholesterol and LDL-C, and not a specific side effect of a particular treatment. This finding could also not be linked to an alteration of steroid metabolism suggesting that LDL-cholesterol lowering could be acting on posttesticular events, such as epididymal sperm maturation, as epididymides are involved in the acquisition of sperm motility. The authors also noticed a significant decrease in semen volume and sperm count at 6 months, but it returned to baseline after 12 months. This may reflect a transient decrease in testosterone levels since testosterone is essential for normal spermatogenesis and the function of the male accessory glands (epididymides, prostate, and seminal vesicles). However, that testosterone levels and testosterone responses to hCG were not significantly affected during the study, which ruled out this hypothesis.
Interestingly, one blind trial evaluating the impact on testicular function of simvastatin, another molecule of the statin family, found no differences in sperm count or motility after 14 weeks of treatment.70
The long-term effect of pravastatin was further evaluated in eight hypercholesterolemic patients, and no significant changes in values of semen parameters, including motility, were found after 6 months of effective treatment.71 Another RCT comparing the effect of two statins, pravastatin (n = 39), simvastatin (n = 41) and placebo (n = 39) on testicular function and androgen production in hypercholesterolemic patients was published by the same author who first identified a decrease in sperm motility after lowering of LDL-cholesterol.72 This time, no significant changes in semen quality were found after 12 and 24 weeks of treatment and testicular steroid secretion was not altered.
Very recently, a prospective noncontrolled pilot assay aimed at evaluating the impact of a 5-month therapy with atorvastatin on semen parameters of 17 healthy, normo-cholesterolaemic and normozoospermic patients.73 Treatment was significantly associated with a decrease in sperm number, sperm vitality, and seminal concentration of epididymal (neutral alpha 1–4 glucosidase, L-carnitine) and prostatic (acid phosphatase) markers. Sperm morphology was significantly altered in the head, neck, and midpiece regions, and the Multiple Anomalies Index was moderately increased. The percentage of acrosome-reacted spermatozoa was significantly decreased after treatment, but the cholesterol and phospholipid content and ratio in spermatozoa and seminal plasma were unchanged. Surprisingly, sperm motility was significantly but slightly increased at the end of the 5-month treatment. The authors concluded that atorvastatin treatment impaired the fertility potential of the volunteers at the testicular, epididymal and prostatic levels, and that this effect was independent of an alteration of the hypothalamic pituitary testicular axis, as plasma gonadotropin and testosterone levels remained stable during the study. Hypotheses were made on a direct effect of atorvastatin on gonadal, epididymal, and prostatic functions, as some factors identified as determinants of male fertility are classically decreased by statin molecules. This is the case for coenzyme Q10, a provitamin with antioxidant properties synthesized by HMGR. However, a direct effect of total cholesterol- and LDL-C- lowering on epididymides could also be responsible for some of the findings described in the study, especially as total cholesterol and LDL-C were normal before treatment onset. Even though to our knowledge, there are no links between hypocholesterolemia and male infertility in the current scientific literature, this aspect should not be ignored and future studies evaluating the effect of statins on male fertility should include a control group treated with unrelated lipid-lowering drugs.
Interestingly, the same team have previously linked hypercholesterolemia with an increase in seminal carnitine, a biomarker of epididymal function implicated in sperm maturation, metabolism, and motility.74 Neither changes in sperm or seminal plasma lipid content nor an alteration of other male accessory gland function (prostate and seminal vesicle markers) or testosterone levels were found, suggesting that hypercholesterolemia acted solely on carnitine production by the epididymides. Interestingly, there was also no significant difference in basic semen parameter values between hypercholesterolemic (n = 11) and control men (n = 11), although the number of subjects was too low to be clinically relevant.
Taken together, these results suggest that hypercholesterolemia may act on human epididymides by increasing L-carnitine secretion, and that total cholesterol- and LDL-C- lowering could impede sperm motility in previously hypercholesterolemic patients, although available studies have shown contradictory results for the latter finding.
In previously normocholesterolemic and normozoospermic volunteers, total cholesterol- and LDL-C- lowering after 5 months of atorvastatin treatment were associated with a decrease in sperm count, epididymal and prostatic seminal markers, combined with an increase in sperm motility. Even if atorvastatin toxicity through direct or off-target effects could explain these results, a direct action of cholesterol-lowering on epididymides should not be ruled out.
INSIGHTS ON HUMAN FERTILITY OBTAINED FROM TRANSGENIC ANIMAL MODELS
As stated above, in a murine KO model, LDL-C can act on epididymal function through its interaction with ApoER2, a member of the LDL receptor family acting both in endocytosis and in signal transduction through the SRC kinase and Phosphatidyl Inositol-3 kinase pathways. ApoER2 null mice are infertile and present with an alteration of sperm motility and morphology.33 Abnormal morphology consists of tail coiling, bending, angulation, and midpiece deformation involving axonemal structures. These impairments are the results of posttesticular maturation events taking place in the initial portion of the mouse epididymis and involve a decrease in Phospholipid Hydroperoxide Glutathione Peroxidase (PHGPX) protein levels. PHGPH (or GPx4) is a selenoprotein belonging to the family of glutathione peroxidases. In spermatozoa, PHGPX is located in the midpiece and catalyzes the reduction of lipid peroxides, conferring a protective effect against ROS generation by the mitochondrial respiratory chain and subsequent sperm lipid peroxidation. Interestingly, a decrease in sperm PHGPX expression has been found in human males presenting with idiopathic asthenozoospermia.75,76,77 It has also been implicated in the postthaw survival of human spermatozoa.78 However, the alteration of PHGPX expression could not be linked to mutations in the gene, or with single nucleotide polymorphisms of the 5’-untranslated region of the related mRNA,79 and so the mechanism linking ApoE2R and PHGPX expression still remains unclear.
As described above, hypocholesterolemic ApoB+/− mice are infertile with a decrease in sperm count, motility and zona binding, suggesting an alteration of epididymal maturation. The phenotype is partially reversed by using human ApoB as a transgene. Genetic association studies have been subsequently performed in humans. Initial work reported a significant association between a 3-codon deletion polymorphism in the ApoB signal peptide gene and infertility in Slovene Caucasian patients presenting with oligo-astheno-teratozoospermia.80 However, no correlation was found between the polymorphisms and basic semen parameter values, and such an association was not significant when a population of non-Caucasian Indian males was studied for the same deletion.81
In the murine model, Dicer 1 has been implicated in initial segment and caput epididymis homeostasis, and associated with a dramatic decrease in sperm motility, capacitation, and an abnormal phospholipid/cholesterol ratio causing sperm membrane rigidity. This finding could be explained by the absence of mature miRNAs controlling the translation of genes implicated in cholesterol metabolism, such as Abca1, Abcg1, and Hmgcr, as reviewed above. In humans, polymorphism in key enzymes of miRNA pathways, Dicer1 and Drosha, have been associated with semen quality in a Chinese population. But after Bonferroni correction, the association only persisted with oligozoospermia for a polymorphism in Dicer1,82 which does not favor an alteration of posttesticular maturation events. Specific miRNAs profiles have also been described in distinct segments of human epididymides83 and in epididymosomes,84 suggesting an important role of epididymal miRNAs in sperm maturation, as well as a possible uptake of these miRNAs by spermatozoa during epididymal transit. Very recently, it has been demonstrated that a sperm protein, cysteine-rich secretory protein 2 (CRISP2), involved in modulation of sperm motility, acrosome reaction, and gamete fusion, is regulated by miR-27b, a miRNA that has been described as one of 18 seminal plasma miRNAs specifically present in epididymosomes84 and which is differentially expressed in the human epididymal corpus.83 Indeed, a decrease of CRISP2 protein level in spermatozoa from asthenozoospermic patients has been linked to an increase of miR-27b. Furthermore, in a retrospective study, high miR-27b expression or low CRISP2 protein expression was significantly associated with low sperm progressive motility, abnormal morphology, and infertility.85 MiR-27b was earlier described as a regulator of adipocyte differentiation, as expression of miR-27 resulted in blockade of expression of PPARg and C/EBPα, the two master regulators of adipogenesis86 and thus could represent a link between lipid metabolism, sperm epididymal maturation, and male infertility in humans.
CONCLUSION
Human clinical data linking hypercholesterolemia and epididymal function are scarce, and often based on studies evaluating the safety of cholesterol-lowering drugs, as cholesterol is a key player in steroid metabolism. However, some studies showed an association between alterations of total cholesterol and LDL-C levels, and markers of epididymal function, but no modifications of sperm membrane lipid content or capacitation were observed. Furthermore, transgenic animal models have identified several possible molecular targets involved in cholesterol metabolism that are implicated in alteration of posttesticular maturation events. Some, but not all of these molecular targets have already been studied in humans, such as PHGPX, NPC2, and ApoB. The effect of hypercholesterolemia on posttesticular maturation has also been described in animals fed with high-fat diets. The inability to reproduce these results in hypercholesterolemic humans could be due to the impossibility of reproducing such a diet, to the low number of subjects included in these studies or to a lesser sensitivity of the human than mouse epididymis to high cholesterol content. Thus, large comparative and prospective studies assessing basic and extended semen parameter values, as well as capacitation, fertilization biomarkers, and IVF data in hypercholesterolemic compared with control patients are needed to comprehend better the effect of hypercholesterolemia on human male fertility and the implications for future medical intervention.
REFERENCES
- 1.Orgebin-Crist MC. Sperm maturation in rabbit epididymis. Nature. 1967;216:816–8. doi: 10.1038/216816a0. [DOI] [PubMed] [Google Scholar]
- 2.Austin CR. The capacitation of the mammalian sperm. Nature. 1952;170:326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- 3.Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–8. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- 4.Baldi E, Luconi M, Bonaccorsi L, Krausz C, Forti G. Human sperm activation during capacitation and acrosome reaction: role of calcium, protein phosphorylation and lipid remodelling pathways. Front Biosci. 1996;1:d189–205. doi: 10.2741/a125. [DOI] [PubMed] [Google Scholar]
- 5.Martínez P, Morros A. Membrane lipid dynamics during human sperm capacitation. Front Biosci. 1996;1:d103–17. doi: 10.2741/a119. [DOI] [PubMed] [Google Scholar]
- 6.Saez F, Ouvrier A, Drevet JR. Epididymis cholesterol homeostasis and sperm fertilizing ability. Asian J Androl. 2011;13:11–7. doi: 10.1038/aja.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cross NL. Role of cholesterol in sperm capacitation. Biol Reprod. 1998;59:7–11. doi: 10.1095/biolreprod59.1.7. [DOI] [PubMed] [Google Scholar]
- 8.Gadella BM, Tsai PS, Boerke A, Brewis IA. Sperm head membrane reorganisation during capacitation. Int J Dev Biol. 2008;52:473–80. doi: 10.1387/ijdb.082583bg. [DOI] [PubMed] [Google Scholar]
- 9.Visconti PE, Krapf D, de la Vega-Beltrán JL, Acevedo JJ, Darszon A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J Androl. 2011;13:395–405. doi: 10.1038/aja.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrieres J. Prévalence des différentes dyslipidémies en France. Rev Gén Risque Cardiovasc. 2008. [Last accessed on 2015 May 14]. Availalble from: http://www.realites-cardiologiques.com .
- 11.Kasturi SS, Tannir J, Brannigan RE. The metabolic syndrome and male infertility. J Androl. 2008;29:251–9. doi: 10.2164/jandrol.107.003731. [DOI] [PubMed] [Google Scholar]
- 12.Maqdasy S, Baptissart M, Vega A, Baron S, Lobaccaro JM, et al. Cholesterol and male fertility: what about orphans and adopted? Mol Cell Endocrinol. 2013;368:30–46. doi: 10.1016/j.mce.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Aitken RJ, Nixon B, Lin M, Koppers AJ, Lee YH, et al. Proteomic changes in mammalian spermatozoa during epididymal maturation. Asian J Androl. 2007;9:554–64. doi: 10.1111/j.1745-7262.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 14.Dacheux JL, Gatti JL, Dacheux F. Contribution of epididymal secretory proteins for spermatozoa maturation. Microsc Res Tech. 2003;61:7–17. doi: 10.1002/jemt.10312. [DOI] [PubMed] [Google Scholar]
- 15.Parks JE, Hammerstedt RH. Development changes occurring in the lipids of ram epididymal spermatozoa plasma membrane. Biol Reprod. 1985;32:653–68. doi: 10.1095/biolreprod32.3.653. [DOI] [PubMed] [Google Scholar]
- 16.Hall JC, Hadley J, Doman T. Correlation between changes in rat sperm membrane lipids, protein, and the membrane physical state during epididymal maturation. J Androl. 1991;12:76–87. [PubMed] [Google Scholar]
- 17.Awano M, Kawaguchi A, Mohri H. Lipid composition of hamster epididymal spermatozoa. J Reprod Fertil. 1993;99:375–83. doi: 10.1530/jrf.0.0990375. [DOI] [PubMed] [Google Scholar]
- 18.Rejraji H, Sion B, Prensier G, Carreras M, Motta C, et al. Lipid remodeling of murine epididymosomes and spermatozoa during epididymal maturation. Biol Reprod. 2006;74:1104–13. doi: 10.1095/biolreprod.105.049304. [DOI] [PubMed] [Google Scholar]
- 19.Travis AJ, Merdiushev T, Vargas LA, Jones BH, Purdon MA, et al. Expression and localization of caveolin-1, and the presence of membrane rafts, in mouse and Guinea pig spermatozoa. Dev Biol. 2001;240:599–610. doi: 10.1006/dbio.2001.0475. [DOI] [PubMed] [Google Scholar]
- 20.Girouard J, Frenette G, Sullivan R. Compartmentalization of proteins in epididymosomes coordinates the association of epididymal proteins with the different functional structures of bovine spermatozoa. Biol Reprod. 2009;80:965–72. doi: 10.1095/biolreprod.108.073551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asano A, Nelson JL, Zhang S, Travis AJ. Characterization of the proteomes associating with three distinct membrane raft sub-types in murine sperm. Proteomics. 2010;10:3494–505. doi: 10.1002/pmic.201000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Travis AJ, Kopf GS. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J Clin Invest. 2002;110:731–6. doi: 10.1172/JCI16392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MA, Storey BT. Bicarbonate is essential for fertilization of mouse eggs: mouse sperm require it to undergo the acrosome reaction. Biol Reprod. 1986;34:349–56. doi: 10.1095/biolreprod34.2.349. [DOI] [PubMed] [Google Scholar]
- 24.Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, et al. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–37. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- 25.Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, et al. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol. 2006;296:353–62. doi: 10.1016/j.ydbio.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 26.Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, et al. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995;121:1139–50. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- 27.Visconti PE, Ning X, Fornés MW, Alvarez JG, Stein P, et al. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol. 1999;214:429–43. doi: 10.1006/dbio.1999.9428. [DOI] [PubMed] [Google Scholar]
- 28.Baker MA, Hetherington L, Aitken RJ. Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J Cell Sci. 2006;119:3182–92. doi: 10.1242/jcs.03055. [DOI] [PubMed] [Google Scholar]
- 29.Nassar A, Mahony M, Morshedi M, Lin MH, Srisombut C, et al. Modulation of sperm tail protein tyrosine phosphorylation by pentoxifylline and its correlation with hyperactivated motility. Fertil Steril. 1999;71:919–23. doi: 10.1016/s0015-0282(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 30.Naz RK. Involvement of protein tyrosine phosphorylation of human sperm in capacitation/acrosome reaction and zona pellucida binding. Front Biosci. 1996;1:d206–13. doi: 10.2741/a126. [DOI] [PubMed] [Google Scholar]
- 31.Kastner P, Mark M, Leid M, Gansmuller A, Chin W, et al. Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev. 1996;10:80–92. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- 32.Selva DM, Hirsch-Reinshagen V, Burgess B, Zhou S, Chan J, et al. The ATP-binding cassette transporter 1 mediates lipid efflux from Sertoli cells and influences male fertility. J Lipid Res. 2004;45:1040–50. doi: 10.1194/jlr.M400007-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Andersen OM, Yeung CH, Vorum H, Wellner M, Andreassen TK, et al. Essential role of the apolipoprotein E receptor-2 in sperm development. J Biol Chem. 2003;278:23989–95. doi: 10.1074/jbc.M302157200. [DOI] [PubMed] [Google Scholar]
- 34.Stockinger W, Brandes C, Fasching D, Hermann M, Gotthardt M, et al. The reelin receptor ApoER2 recruits JNK-interacting proteins-1 and -2. J Biol Chem. 2000;275:25625–32. doi: 10.1074/jbc.M004119200. [DOI] [PubMed] [Google Scholar]
- 35.Huang LS, Voyiaziakis E, Markenson DF, Sokol KA, Hayek T, et al. apo B gene knockout in mice results in embryonic lethality in homozygotes and neural tube defects, male infertility, and reduced HDL cholesterol ester and apo A-I transport rates in heterozygotes. J Clin Invest. 1995;96:2152–61. doi: 10.1172/JCI118269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang LS, Voyiaziakis E, Chen HL, Rubin EM, Gordon JW. A novel functional role for apolipoprotein B in male infertility in heterozygous apolipoprotein B knockout mice. Proc Natl Acad Sci U S A. 1996;93:10903–7. doi: 10.1073/pnas.93.20.10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–31. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 38.Fan J, Akabane H, Graham SN, Richardson LL, Zhu GZ. Sperm defects in mice lacking a functional Niemann-Pick C1 protein. Mol Reprod Dev. 2006;73:1284–91. doi: 10.1002/mrd.20559. [DOI] [PubMed] [Google Scholar]
- 39.Shamsadin R, Adham IM, Nayernia K, Heinlein UA, Oberwinkler H, et al. Male mice deficient for germ-cell cyritestin are infertile. Biol Reprod. 1999;61:1445–51. doi: 10.1095/biolreprod61.6.1445. [DOI] [PubMed] [Google Scholar]
- 40.Cho C. Testicular and epididymal ADAMs: expression and function during fertilization. Nat Rev Urol. 2012;9:550–60. doi: 10.1038/nrurol.2012.167. [DOI] [PubMed] [Google Scholar]
- 41.El-Hajjaji FZ, Oumeddour A, Pommier AJ, Ouvrier A, Viennois E, et al. Liver X receptors, lipids and their reproductive secrets in the male. Biochim Biophys Acta. 2011;1812:974–81. doi: 10.1016/j.bbadis.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Jamroz-Wisniewska A, Wójcicka G, Horoszewicz K, Beltowski J. Liver X receptors (LXRs). Part II: non-lipid effects, role in pathology, and therapeutic implications. Postepy Hig Med Dosw (Online) 2007;61:760–85. [PubMed] [Google Scholar]
- 43.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 44.Volle DH, Lobaccaro JM. Role of the nuclear receptors for oxysterols LXRs in steroidogenic tissues: beyond the “foie gras”, the steroids and sex? Mol Cell Endocrinol. 2007;265-266:183–9. doi: 10.1016/j.mce.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 45.Wójcicka G, Jamroz-Wisniewska A, Horoszewicz K, Beltowski J. Liver X receptors (LXRs). Part I: structure, function, regulation of activity, and role in lipid metabolism. Postepy Hig Med Dosw (Online) 2007;61:736–59. [PubMed] [Google Scholar]
- 46.Frenoux JM, Vernet P, Volle DH, Britan A, Saez F, et al. Nuclear oxysterol receptors, LXRs, are involved in the maintenance of mouse caput epididymidis structure and functions. J Mol Endocrinol. 2004;33:361–75. doi: 10.1677/jme.1.01515. [DOI] [PubMed] [Google Scholar]
- 47.Ouvrier A, Cadet R, Vernet P, Laillet B, Chardigny JM, et al. LXR and ABCA1 control cholesterol homeostasis in the proximal mouse epididymis in a cell-specific manner. J Lipid Res. 2009;50:1766–75. doi: 10.1194/jlr.M800657-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saez F, Chabory E, Cadet R, Vernet P, Baron S, et al. Liver X receptors and epididymal epithelium physiology. Asian J Androl. 2007;9:574–82. doi: 10.1111/j.1745-7262.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- 49.Ouvrier A, Alves G, Damon-Soubeyrand C, Marceau G, Cadet R, et al. Dietary cholesterol-induced post-testicular infertility. PLoS One. 2011;6:e26966. doi: 10.1371/journal.pone.0026966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, et al. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci U S A. 2008;105:15287–92. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirchhoff C, Osterhoff C, Young L. Molecular cloning and characterization of HE1, a major secretory protein of the human epididymis. Biol Reprod. 1996;54:847–56. doi: 10.1095/biolreprod54.4.847. [DOI] [PubMed] [Google Scholar]
- 52.Girouard J, Frenette G, Sullivan R. Seminal plasma proteins regulate the association of lipids and proteins within detergent-resistant membrane domains of bovine spermatozoa. Biol Reprod. 2008;78:921–31. doi: 10.1095/biolreprod.107.066514. [DOI] [PubMed] [Google Scholar]
- 53.Légaré C, Thabet M, Gatti JL, Sullivan R. HE1/NPC2 status in human reproductive tract and ejaculated spermatozoa: consequence of vasectomy. Mol Hum Reprod. 2006;12:461–8. doi: 10.1093/molehr/gal050. [DOI] [PubMed] [Google Scholar]
- 54.Busso D, Oñate-Alvarado MJ, Balboa E, Castro J, Lizama C, et al. Spermatozoa from mice deficient in Niemann-Pick disease type C2 (NPC2) protein have defective cholesterol content and reduced in vitro fertilising ability. Reprod Fertil Dev. 2014;26:609–21. doi: 10.1071/RD12059. [DOI] [PubMed] [Google Scholar]
- 55.Björkgren I, Saastamoinen L, Krutskikh A, Huhtaniemi I, Poutanen M, et al. Dicer1 ablation in the mouse epididymis causes dedifferentiation of the epithelium and imbalance in sex steroid signaling. PLoS One. 2012;7:e38457. doi: 10.1371/journal.pone.0038457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jalkanen J, Huhtaniemi I, Poutanen M. Discovery and characterization of new epididymis-specific beta-defensins in mice. Biochim Biophys Acta. 2005;1730:22–30. doi: 10.1016/j.bbaexp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 57.Björkgren I, Gylling H, Turunen H, Huhtaniemi I, Strauss L, et al. Imbalanced lipid homeostasis in the conditional Dicer1 knockout mouse epididymis causes instability of the sperm membrane. FASEB J. 2015;29:433–42. doi: 10.1096/fj.14-259382. [DOI] [PubMed] [Google Scholar]
- 58.Rayner KJ, Suárez Y, Dávalos A, Parathath S, Fitzgerald ML, et al. miR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–3. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Kolodgie FD, Katocs AS, Jr, Largis EE, Wrenn SM, Cornhill JF, et al. Hypercholesterolemia in the rabbit induced by feeding graded amounts of low-level cholesterol. Methodological considerations regarding individual variability in response to dietary cholesterol and development of lesion type. Arterioscler Thromb Vasc Biol. 1996;16:1454–64. doi: 10.1161/01.atv.16.12.1454. [DOI] [PubMed] [Google Scholar]
- 61.Russell JC, Proctor SD. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc Pathol. 2006;15:318–30. doi: 10.1016/j.carpath.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Marchiani S, Vignozzi L, Filippi S, Gurrieri B, Comeglio P, et al. Metabolic syndrome-associated sperm alterations in an experimental rabbit model: relation with metabolic profile, testis and epididymis gene expression and effect of tamoxifen treatment. Mol Cell Endocrinol. 2015;401:12–24. doi: 10.1016/j.mce.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 63.Saez Lancellotti TE, Boarelli PV, Monclus MA, Cabrillana ME, Clementi MA, et al. Hypercholesterolemia impaired sperm functionality in rabbits. PLoS One. 2010;5:e13457. doi: 10.1371/journal.pone.0013457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bataineh HN, Nusier MK. Effect of cholesterol diet on reproductive function in male albino rats. Saudi Med J. 2005;26:398–404. [PubMed] [Google Scholar]
- 65.Shalaby MA, el-Zorba HY, Kamel GM. Effect of alpha-tocopherol and simvastatin on male fertility in hypercholesterolemic rats. Pharmacol Res. 2004;50:137–42. doi: 10.1016/j.phrs.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 66.Mortazavi M, Salehi I, Alizadeh Z, Vahabian M, Roushandeh AM. Protective Effects of Antioxidants on Sperm Parameters and Seminiferous Tubules Epithelium in High Fat-fed Rats. J Reprod Infertil. 2014;15:22–8. [PMC free article] [PubMed] [Google Scholar]
- 67.Dobs AS, Sarma PS, Schteingart D. Long-term endocrine function in hypercholesterolemic patients treated with pravastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Metabolism. 1993;42:1146–52. doi: 10.1016/0026-0495(93)90272-p. [DOI] [PubMed] [Google Scholar]
- 68.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160–4. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 69.Out C, Groen AK, Brufau G. Bile acid sequestrants: more than simple resins. Curr Opin Lipidol. 2012;23:43–55. doi: 10.1097/MOL.0b013e32834f0ef3. [DOI] [PubMed] [Google Scholar]
- 70.Purvis K, Tollefsrud A, Rui H, Haug E, Norseth J, et al. Short-term effects of treatment with simvastatin on testicular function in patients with heterozygous familial hypercholesterolaemia. Eur J Clin Pharmacol. 1992;42:61–4. doi: 10.1007/BF00314921. [DOI] [PubMed] [Google Scholar]
- 71.Bernini GP, Brogi G, Argenio GF, Moretti A, Salvetti A. Effects of long-term pravastatin treatment on spermatogenesis and on adrenal and testicular steroidogenesis in male hypercholesterolemic patients. J Endocrinol Invest. 1998;21:310–7. doi: 10.1007/BF03350334. [DOI] [PubMed] [Google Scholar]
- 72.Dobs AS, Miller S, Neri G, Weiss S, Tate AC, et al. Effects of simvastatin and pravastatin on gonadal function in male hypercholesterolemic patients. Metabolism. 2000;49:115–21. doi: 10.1016/s0026-0495(00)90938-7. [DOI] [PubMed] [Google Scholar]
- 73.Pons-Rejraji H, Brugnon F, Sion B, Maqdasy S, Gouby G, et al. Evaluation of atorvastatin efficacy and toxicity on spermatozoa, accessory glands and gonadal hormones of healthy men: a pilot prospective clinical trial. Reprod Biol Endocrinol. 2014;12:65. doi: 10.1186/1477-7827-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grizard G, Sion B, Jouanel P, Benoit P, Boucher D. Cholesterol, phospholipids and markers of the function of the accessory sex glands in the semen of men with hypercholesterolaemia. Int J Androl. 1995;18:151–6. doi: 10.1111/j.1365-2605.1995.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 75.Diaconu M, Tangat Y, Böhm D, Kühn H, Michelmann HW, et al. Failure of phospholipid hydroperoxide glutathione peroxidase expression in oligoasthenozoospermia and mutations in the PHGPx gene. Andrologia. 2006;38:152–7. doi: 10.1111/j.1439-0272.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- 76.Imai H, Suzuki K, Ishizaka K, Ichinose S, Oshima H, et al. Failure of the expression of phospholipid hydroperoxide glutathione peroxidase in the spermatozoa of human infertile males. Biol Reprod. 2001;64:674–83. doi: 10.1095/biolreprod64.2.674. [DOI] [PubMed] [Google Scholar]
- 77.Shen S, Wang J, Liang J, He D. Comparative proteomic study between human normal motility sperm and idiopathic asthenozoospermia. World J Urol. 2013;31:1395–401. doi: 10.1007/s00345-013-1023-5. [DOI] [PubMed] [Google Scholar]
- 78.Meseguer M, Garrido N, Simón C, Pellicer A, Remohí J. Concentration of glutathione and expression of glutathione peroxidases 1 and 4 in fresh sperm provide a forecast of the outcome of cryopreservation of human spermatozoa. J Androl. 2004;25:773–80. doi: 10.1002/j.1939-4640.2004.tb02855.x. [DOI] [PubMed] [Google Scholar]
- 79.Liu SY, Zhang CJ, Si XM, Yao YF, Shi L, et al. Association between single nucleotide polymorphisms of 5’-untranslated region of GPx4 gene and male infertility. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2011;28:270–4. doi: 10.3760/cma.j.issn.1003-9406.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Peterlin B, Zorn B, Volk M, Kunej T. Association between the apolipoprotein B signal peptide gene insertion/deletion polymorphism and male infertility. Mol Hum Reprod. 2006;12:777–9. doi: 10.1093/molehr/gal088. [DOI] [PubMed] [Google Scholar]
- 81.Khattri A, Pandey RK, Gupta NJ, Chakravarty B, Deenadayal M, et al. APOB gene signal peptide deletion polymorphism is not associated with infertility in Indian men. J Androl. 2009;30:734–8. doi: 10.2164/jandrol.109.007898. [DOI] [PubMed] [Google Scholar]
- 82.Qin Y, Xia Y, Wu W, Han X, Lu C, et al. Genetic variants in microRNA biogenesis pathway genes are associated with semen quality in a Han-Chinese population. Reprod Biomed Online. 2012;24:454–61. doi: 10.1016/j.rbmo.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Belleannée C, Calvo E, Thimon V, Cyr DG, Légaré C, et al. Role of microRNAs in controlling gene expression in different segments of the human epididymis. PLoS One. 2012;7:e34996. doi: 10.1371/journal.pone.0034996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Belleannée C, Légaré C, Calvo E, Thimon V, Sullivan R. microRNA signature is altered in both human epididymis and seminal microvesicles following vasectomy. Hum Reprod. 2013;28:1455–67. doi: 10.1093/humrep/det088. [DOI] [PubMed] [Google Scholar]
- 85.Zhou JH, Zhou QZ, Lyu XM, Zhu T, Chen ZJ, et al. The expression of cysteine-rich secretory protein 2 (CRISP2) and its specific regulator miR-27b in the spermatozoa of patients with asthenozoospermia. Biol Reprod. 2015;92:28. doi: 10.1095/biolreprod.114.124487. [DOI] [PubMed] [Google Scholar]
- 86.Lin Q, Gao Z, Alarcon RM, Ye J, Yun Z. A role of miR-27 in the regulation of adipogenesis. FEBS J. 2009;276:2348–58. doi: 10.1111/j.1742-4658.2009.06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


