Abstract
Schwann cells (SCs) are the principal glia of the peripheral nervous system. The end point of SC development is the formation of myelinating and nonmyelinating cells which ensheath large and small diameter axons, respectively. They play an important role in axon regeneration after injury, including cavernous nerve injury that leads to erectile dysfunction (ED). Despite improvement in radical prostatectomy surgical techniques, many patients still suffer from ED postoperatively as surgical trauma causes traction injuries and local inflammatory changes in the neuronal microenvironment of the autonomic fibers innervating the penis resulting in pathophysiological alterations in the end organ. The aim of this review is to summarize contemporary evidence regarding: (1) the origin and development of SCs in the peripheral and penile nerve system; (2) Wallerian degeneration and SC plastic change following peripheral and penile nerve injury; (3) how SCs promote peripheral and penile nerve regeneration by secreting neurotrophic factors; (4) and strategies targeting SCs to accelerate peripheral nerve regeneration. We searched PubMed for articles related to these topics in both animal models and human research and found numerous studies suggesting that SCs could be a novel target for treatment of nerve injury-induced ED.
Keywords: erectile dysfunction, growth factors, prostatectomy
INTRODUCTION
Although surgical techniques for radical prostatectomy for the treatment of prostate cancer have improved with the introduction of nerve-sparing techniques and robotic procedures, many patients still suffer from erectile dysfunction (ED) following surgery.1,2 During surgery trauma causes traction injury and local inflammatory changes in the neuronal microenvironment of the autonomic fibers innervating the penis resulting pathophysiological alterations in the end organ. The suggested pathophysiologic mechanisms responsible for radical prostatectomy (RP)-induced ED include: reduction in neuronal nitric oxide synthase (nNOS), a decrease in the number of cavernous endothelial and smooth muscle cells, increase in reactive oxygen species, and up-regulation of profibrotic factors and cavernous fibrosis as a result of cavernous hypoxia.3,4,5,6
Current research strategies to prevent ED following RP have focused on pharmacological interventions, such as PDE5 inhibitors, which prevent penile corporal fibrosis in an effort to preserve the hemodynamic mechanisms of penile erection. However, a number of patients with RP-induced ED do not respond well to such pharmacological intervention suggesting a need for novel therapies.7,8 Currently, there are no interventions targeted at cavernosal nerve regeneration because the mechanisms of regeneration including demyelination, axon degeneration, re-myelination, Schwann cell (SC) and neuron interaction, and the molecular signaling process are poorly understood.
For decades, SCs received relatively little scientific attention with poor understanding of their function despite the fact that they outnumber the neurons in the peripheral nerve system (PNS) and are involved in almost all neural functions. New technologies available to neurobiologists have now provided unexpected insight into SC function and have greatly expanded research. The aim of this review was to summarize contemporary evidence regarding: (1) the origin and development of SCs in the peripheral and penile nerve system; (2) Wallerian degeneration and SC plastic change following peripheral and penile nerve injury; (3) how SCs promote peripheral and penile nerve regeneration by secreting neurotrophic factors; (4) and strategies targeting SCs to accelerate peripheral nerve regeneration. We searched PubMed for articles related to these topics in both animal models and human research and found numerous studies suggesting that SCs could be a novel target for treatment of nerve injury-induced ED.
ORIGIN AND DEVELOPMENT OF SCS IN PERIPHERAL AND PENILE NERVE SYSTEM
Function of SCs
SCs in spinal nerves originate from the neural crest, although the origin of cells in spinal roots is more complex. The end point of SC development is the formation of myelinating or nonmyelinating cells which ensheath large and small diameter axons, respectively, throughout the PNS.9,10,11,12 SC formation is preceded by the generation of two other cell types: Schwann cell precursors (SCPs) and immature SCs. The postnatal fate of immature SCs is determined by which axons they randomly associate with myelination being selectively activated in those cells that happen to envelop single large diameter axons.13
SCs adopt one of the two distinct relationships with axons, either myelinating individual axons or ensheathing multiple, small axons in what are termed Remak bundles. In myelinated fibers, SCs form a compact myelin sheath around a large diameter axon, enabling rapid saltatory conduction. In Remak bundles, SCs segregate small diameter axons, such as nociceptive fibers, into separate pockets which conduct via cable propagation.
The development of SCs can be viewed as three main transitions: the transition from migrating neural crest stem cells to Schwann cell precursors (SCPs), from SCPs to immature SCs and the divergence of this population to form the two mature SC types. These events are strikingly dependent on survival factors, mitogens and differentiation signals from the axons with which SCPs and SCs continuously associate (Figure 1).10
Figure 1.
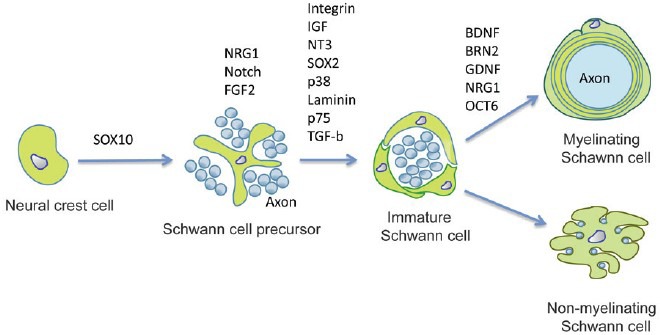
Lineage of SCs and controlling molecules.
Process of myelination and Remak bundle formation as well as associated signaling pathways
The reason why some axons are myelinated and others are not is unclear. New evidence indicates that various signaling pathways that inhibit myelin differentiation are active in immature SCs, and that these pathways are suppressed at the onset of myelination. For instance, the c-Jun-amino (N)-terminal kinase (JNK) pathway is active in SCs of mice from embryonic day 18 to birth and is required for NRG1 and TGFβ signaling.14 This pathway is inactivated in individual human cells as they start to myelinate by a mechanism that depends on the myelin-associated transcription factor KROX20. If this is prevented, and the JNK pathway remains active, myelination in neuron–SC co-cultures is blocked and myelin gene expression that would normally result from pro-myelin signals, such as KROX20 or the elevation of cyclic AMP is inhibited. Similarly, Notch signaling promotes proliferation of immature SCs but is suppressed as cells start to myelinate, and if this is prevented, myelination is blocked. An analogous pattern of action is seen in the transcription factors PAX3 and SOX2: these are expressed before myelination and are involved in proliferation. They are down-regulated in myelinating cells and exert a negative effect on myelin differentiation.15,16 In neuron-SC co-cultures, axon-derived ATP also delays myelination.17 These studies are starting to define the signals that determine the immature SC state. Myelination is activated by inhibition of these pathways together with activation of pro-myelin pathways, which involve the transcription factors KROX20, octamer-binding transcription factor 6 (OCT6), brain 2 class III POU-domain protein (BRN2), NGFI-A-binding proteins 1 and 2 (NAB1/2), phosphatidylinositol 3-kinase (PI3K) signaling and v-ski sarcoma viral oncogene homologue (SKI).18,19,20,21,22 The mechanism that initiates these switches remains unclear.
Certain cell adhesion molecules, such as L1 and polysialylated NCAM (neural cell adhesion molecule), are known to be expressed on unmyelinated axons and to be down-regulated during axonal myelination.23,24 Other candidates for sensing axonally presented molecules on SCs include the integrins and neurofascin. Integrins appear to be most important for survival mechanisms in SCs involved in the radial sorting of unmyelinated axons.25 Laminins, the ligands for some integrins, also have an important role in the defasciculation of axons.26 Dystroglycan, the another laminin receptor on SCs, is not vital to the early stages of myelination, and seems to be more important for the organization of the nodal microvilli.27 The neurofascin gene and its products do play an important part in later stages of myelination, however, they do not seem to be necessary for initial ensheathment.
The role of neurotrophins in the earliest stages of axon engagement has attracted renewed interest. The prototypical neurotrophin is nerve growth factor (NGF), and studies on the ability of NGF to promote neuronal survival have a long history.28,29 It has recently been shown that NGF might also have a role in regulating myelination.30 NGF stimulates myelination by SCs but inhibits oligodendrocyte-mediated myelination. Antibody-blocking experiments showed that NGF exerts its effect through binding to tyrosine kinase TrkA receptors, which was later confirmed by the use of reagents that independently activate these receptors. Interestingly, the effects of NGF on the cell biology of myelin-forming SCs seem to be indirect that is, the signals that affect myelination arise from axons in response to the binding of NGF to axonal TrkA receptors.
The fact that NGF seems to act through axonal but not SCTrkA receptors begs the question as to the nature of the axonal signals that are produced in response to the binding of NGF and that influence the cell biology of SCs. Ligand binding to Trk receptors causes autophosphorylation, which is followed by binding of various adaptor proteins, including phospholipase C-γ1 (PLCγ1), Src homology 2 domain-containing transforming protein (SHC), phosphatidyl-inositol 3–kinase (PI3K) and extracellular signal-regulated kinase 1 (ERK1). Each of these has been implicated in signaling pathways that converge on the nucleus. It is, therefore, likely that receptor activation influences the transcription of neuronal genes that can modulate the ability of SCs to myelinate. Possible candidates for the neuronal effecter molecules that influence SCs to myelinate include secreted molecules such as neuregulins (NRG) and the small molecules known to act at glial purinergic receptors.31 NRG1 is a member of the epidermal growth factor (EGF) superfamily composed of a large number of alternatively spliced transmembrane isoforms. All NRG1 isoforms, including type III, are cleaved just extracellular to the plasma membrane, by metalloproteinase.32 In contrast, the type III isoform has a second, N-terminal hydrophobic sequence and is therefore retained on the axon membrane after cleavage, functioning as a juxtacrine signal.33 Type III NRG1 is the key axonal signal that activates the PI 3-kinase and MAPK pathways in SCs.33 Recent data suggests that type III NRG1 is an instructive signal: threshold levels are required to trigger SC myelination; above threshold, the amount of compact myelin formed is graded to the levels of type III NRG1 (Figure 2).
Figure 2.
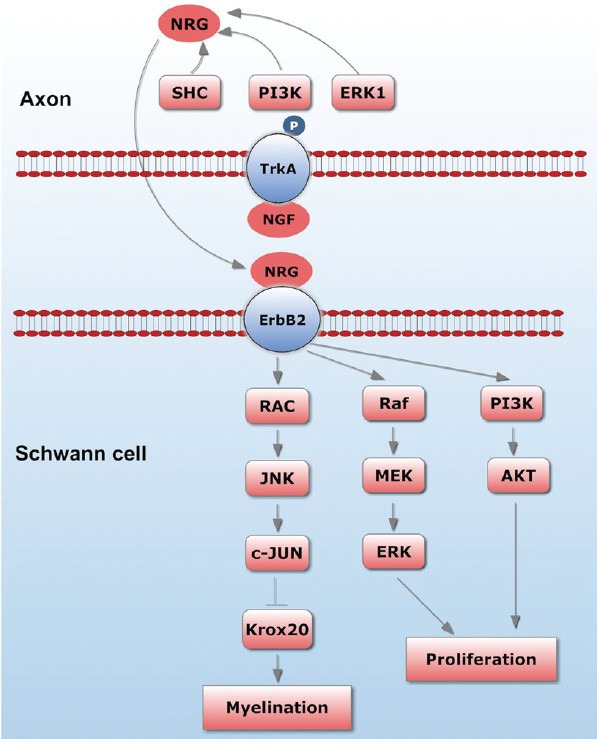
Axon-SC interaction and some associated molecules.
SC–axon interactions in peripheral nerves
One of the most striking examples of reciprocal, cell-cell interactions in cell biology is exemplified by the interactions between axons and SCs in the vertebrate peripheral nervous system. These interactions are essential for the formation and function of peripheral nerve fibers. During development, axons promote the generation of SCs via trophic, mitogenic and differentiative effects on precursor cells. SCs, in turn, regulate the integrity and functional organization of axons.12,34 The critical, mutual dependency of axons and SCs persists into adulthood and contributes to the vulnerability of myelinated fibers to a variety of clinical disorders as pathology of one partner invariably affects the function of the other.35 A major goal remains to elucidate the reciprocal signals that drive the formation of and maintain the integrity of mature myelinated axons. Elucidation of these interactions, and the intracellular signaling pathways they regulate may lead to new, rational therapies for the treatment of inherited and acquired neuropathies.
WALLERIAN DEGENERATION AND SC PLASTICITY FOLLOWING PERIPHERAL AND PENILE NERVE INJURY
The penile nerve system – myelinated and Remak fibers
Before the studies done by Walsh and Donker36 on fetal specimens, the cause of ED after radical prostatectomies was not well understood. By tracing the autonomic innervation of the corpora cavernosa, Lepor et al.37 showed that ED can occur secondary to injury to the cavernosal nerves. Classically, it was thought that these nerves branched from the pelvic plexus and ran as a plexus of small nerves within a prominent neurovascular bundle on the posterolateral border of the prostate, before piercing the urogenital diaphragm and descending along the lateral aspect of the urethra. They course outside the prostatic capsule and are intimately associated with capsular vessels of the prostate.37,38,39 These initial findings have since been supported by additional anatomic studies, which have further characterized the anatomy of the neurovascular bundle of the prostate. Detailed histological studies have revealed the cross-sectional profile of the neurovascular supply of the prostate and have shown that it runs through leaves of the lateral pelvic fascia. New advancements in surgery, including the use of laparoscopic/robotic modalities and magnifying visual devices in open surgery, have enabled very precise nerve dissection. Nerve grafting and interposition to realign the neurovascular bundle after neurovascular bundle resections are now offered to patients to maintain their potency.40,41 However, despite all these advancements in nerve preservation or restoration, potency rates have remained unsatisfactory.41,42 Therefore, the classical knowledge of the neurovascular bundle of the prostate and the cavernosal nerves was challenged and revisited. It has been suggested that the neurovascular bundle of the prostate may not cover all of the cavernosal nerves, and these unidentified nerves may be severed inadvertently during surgery.43,44,45
Allaf et al. studied the effects of erythropoietin on cavernous nerve regeneration after injury. Their study showed that daily recombinant human erythropoietin (rhEPO) treatment resulted in a greater recovery of axons per Remak bundle compared to that in saline-treated animals. In saline-treated animals, there were many empty SCs and some re-innervated SCs. In rhEPO treated animals, the regeneration rate was significantly higher for many SCs having regenerated axons. Such profiles were evident in all rhEPO treated animals, suggesting that rhEPO causes neuronal regeneration.46 FK506 (an immunosuppressant drug) has been linked to enhanced nerve regeneration after nerve injury and neurodegenerative diseases by binding to FK506-binding proteins (FKBPs), a select group of immunophilins. Lagoda et al. found that immunoreactivity for all the FKBPs and nNOS was localized to the periprostatic ganglia although FKBP12 was the only FKBP localized to nerve bundles in this location. In penile tissue, immunoreactivity for all five FKBPs and nNOS was localized to nerves, although immunoreactivity for FKBP38 was minimal.47 Sezen et al. subjected rats to a partial nerve-crush injury and then administered FK506 (1 mg kg−1 i.p.) at the same time as the crush and on successive days. They found that pronounced preservation of erectile function was noted in groups treated with FK506 and that FK506 exerts neuroprotection by increasing axonal survival rate.48
Wallerian degeneration postperipheral and penile nerve injury
Wallerian degeneration is a process that results when a nerve fiber is cut or crushed, in which the part of the axon separated from the neuron's cell body degenerates distal to the injury. This is also known as anterograde or orthograde degeneration. A related process known as “Wallerian-like degeneration” occurs in many neurodegenerative diseases, especially those where axonal transport is impaired.49 Primary culture studies suggest that failure to deliver sufficient quantities of the essential axonal protein NMNAT2 is a key initiating event.50 Wallerian degeneration occurs after axonal injury in both the peripheral nervous system and central nervous system. It occurs in the axon stump distal to a site of injury and usually begins within 24–36 h of a lesion. Prior to degeneration, distal axon stumps tend to remain electrically excitable. After injury, the axonal skeleton disintegrates, and the axonal membrane breaks apart. The axonal degeneration is followed by degradation of the myelin sheath and infiltration by macrophages. The macrophages, accompanied by SCs, serve to clear the debris from the degeneration.51 Our previous study showed that after cavernous nerve crush, distal nerve showed overall distortion of normal nerve anatomy, axonal swelling and axonal vacuolization which are microanatomical signs of Wallerian degeneration.52
SCs de-differentiation and plasticity
When exposed to harmful situations such as nerve injury, SCs stop expressing myelin genes and begin to actively degrade and remove their myelin sheath.53 Unidentified signals from damaged axons or physicochemical insults to the myelin sheath induce these SC responses.53 The demyelinating action of SCs following nerve damage is accompanied by cellular de-differentiation, which generally refers to a process by which fully differentiated cells revert to an immature phenotype.53,54,55 In de-differentiated states, SCs express proteins such as p75 neurotrophin receptor (p75), glial fibrillary acidic protein, and neuronal cell adhesion molecule, which are found in immature SCs but not in adult myelinating SCs.53,56 In addition, de-differentiated SCs proliferate in response to nerve injury.57 Although these are features of immature SCs, de-differentiated SCs exhibit several unique features that are not observed in immature SCs. For example, de-differentiated SCs express macrophage-2 antigens58 and produce enormous amounts of lysosomes, which correlate with the increased phagocytic activity of SCs.59 De-differentiated SCs also produce a large number of cytokines that regulate chemotaxis and infiltration of blood monocytes into lesion sites.60 In addition, these injury-induced SC responses include the induction of several growth factors for axonal regeneration and neuronal survival.61,62 Therefore, the overall responses of SCs following nerve injury represent a sophisticated SC plasticity that cannot simply be described as de-differentiation.
SCs plasticity is regulated by complex molecular mechanisms. A growing body of recent researches has shown that mitogen-activated protein kinase family proteins (MAP kinases) are important regulators for SC plastic changes. Interestingly, extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK) and p38 MAP kinase are all activated in SCs following nerve injury and play a specific or overlapping role in SC de-differentiation.21,22,23,24,25
In human, the glial growth factor NRG1, which is expressed at the axonal surface, is critical for SC precursor generation and myelination during peripheral nerve development.1,2,49 NRG may be upstream of MAP kinase activation in SC plasticity. NRG1-ErbB2 signaling regulates SC plasticity via c-jun expression which is mediated by JNK and p38 kinase pathways. C-jun increases the expression of glial cell line-derived neurotrophic factor (GDNF) and LIF but suppresses the crucial transcription factor for myelination, Kro × 20, thereby inducing SC de-differentiation. The Raf-ERK pathway is involved in the expression of cell cycle proteins and chemotactic factors such as Ccl2.
SC PROMOTE PERIPHERAL AND PENILE NERVE REGENERATION BY SECRETING NEUROTROPHIC FACTORS
Aliperti et al. examined the effect of pioglitazone on erectile function in a rat model of postprostatectomy erectile dysfunction. His study indicates that pioglitazone improves erectile function in rats undergoing bilateral nerve crush via a nitric oxide–mediated pathway including nNOS.63 Thus, nerve regeneration is very important to the function recovery in target organ.
Growth factors represent one potential group of environmental cues that could influence SC differentiation. GDNF is a growth factor present in the environment during nerve regeneration, and it has been shown to affect the behavior of SCs.64 In SC-neuron co-cultures, the presence of GDNF enhances the myelination of axons.65,66 GDNF exerts its effects on SCs by binding to the glycosylphosphatidyl-inositol-anchored GFRα-1.67 The GFRα-1 receptor does not have an intracellular domain and thus needs to couple with other proteins to induce downstream signaling. However, Ret is not expressed in SCs, therefore, GFRα-1 couples with neural cell adhesion molecule (NCAM), which can act as a co-receptor to facilitate GDNF-mediated signaling in SCs.66,68 GDNF stimulation of GFRα-1/NCAM triggers phosphorylation of Fyn, a Src family tyrosine kinase. Previous studies have shown that exogenous GDNF affects SC proliferation and differentiation through signaling pathways downstream of Fyn.66,68 Therefore, GDNF may be a cue that promotes re-differentiation of SCs in vivo to myelinate axons.
Neurotrophic factors, in particular, brain-derived neurotrophic factor (BDNF), play an essential role in promoting axonal regeneration and re-myelination when SCs were transplanted into nerve injury lesions.69,70 It has been widely reported that BDNF can support the survival of sensory neurons, retinal ganglion cells, and basal forebrain cholinergic neurons as well as regulate synaptic activity of developing neuromuscular synapses.71,72,73,74,75 In addition, recent work has shown that BDNF is a crucial factor for SCs polarization and initiation of myelination.70 All these findings indicate that regulation of BDNF expression and secretion is important for SCs to facilitate axonal regeneration and re-myelination. However, it has been recognized that the ability of SCs cultured in vitro to synthesize BDNF is at a relatively low level, which significantly limits the application of SCs in the repair of nerve injuries in both CNS and PNS. Therefore, up-regulation of BDNF production in SCs might lead to new avenues for the application of SCs in traumatic injuries and demyelinating lesions in the nervous system.
The ability of BDNF to prevent degeneration of neurons located in the major pelvic ganglion (MPG), which contain nNOS, along with its ability to enhance the recovery of erectile function in rat models of bilateral cavernous nerve injury, has been demonstrated using BDNF gene therapy with adeno-associated virus-mediated production of neurotrophic factors.76
In 2003, Lin et al.77 first observed the function of VEGF and BDNF in enhancing the outgrowth of nerve fibers on cultured MPG in vitro in serum-free medium supplemented with VEGF (50 ng ml−1), BDNF, and neurotrophins 3 and 4 (each at a concentration of 20 ng ml−1). After 2 days of culture, the specimens were stained for NOS, tyrosine hydroxylase, and acetylcholinesterase. Lin et al. found that BDNF and VEGF were both capable of inducing fibers that express NOS, tyrosine hydroxylase, and acetylcholinesterase. Many study groups have made remarkable contributions to other clinical applications. The retrograde transport of BDNF from axons to the neuronal cell body provided a theoretical basis for many of these studies.78 One group tried a new approach in which bilateral cavernous nerves were crushed with a hemostat for 2 min and then injected with BDNF (600 ng per rat) and VEGF (4000 ng per rat) to investigate their function in nerve regeneration and recovery of erectile function.79 They later suggested that the optimal dose of both factors for promoting MPG neurite growth was 25–50 ng ml−1 in the in vitro system.80 Neurite growth from aged rats was not as robust as growth from tissue from younger rats, which indicates that future clinical trials might reveal important differences in the responsiveness of aged human tissue to these types of treatments.80
STRATEGIES TARGETED AT SCS TO ACCELERATE PERIPHERAL NERVE REGENERATION
Delivery of neurotrophic factors with transplanted SCs
Use of localized cellular delivery approach that is, transplantation of genetically modified SCs that produce the desired types (and amounts) of NTFs, will likely represent an effective therapy to treat peripheral nerve defects. SCs can be modified genetically, both ex vivo and in vivo by viral and nonviral vectors carrying coding sequences of neuronal specific growth factors to achieve better effects. In a clinical context, adult facial nerve lesions were treated with adenovirus encoding for GDNF, BDNF, or transforming growth factor β2 (TGFβ2); the treatment significantly prevented the degeneration of facial motor neurons, improved choline acetyltransferase immunoreactivity, and prevented the induction of nitric oxide synthase activity in the affected neurons.81 A similar clinical success was achieved with adenoviral GDNF gene transfer into a crushed laryngeal nerve.82 After 2 and 4 weeks, the treatment had induced growth of axons with relatively large diameters, and improved re-myelination, motor nerve conduction velocity, and recovery of vocal folds movement. May and colleague showed that SC-mediated delivery of GDNF enhances regeneration of penile nerves and subsequently restores erectile function after cavernous nerve injury. Their studies indicate that SC-based therapy might be a viable approach for the treatment of ED after cavernous nerve injury. In terms of clinical application, this enhanced nerve repair might be critical for timely reinnervation of the corpus cavernosum as a prerequisite for functional recovery in men.
Increase BDNF release from SC by electrical stimulation
Beier and colleague showed that electrical stimulation can activate SCs to secrete BDNF, which requires the involvement of calcium influx through T-type voltage-gated calcium channel and calcium mobilization from internal calcium stores. In addition, activation of calcium-calmodulin dependent protein kinase IV (CaMK IV), MAPK, and CREB was also involved in the electro stimulation- induced BDNF release. These findings indicate that electrical stimulation can improve the neurotrophic ability in SCs and raise the possibility of developing electrically stimulated SCs as a source of cell therapy for nerve injury in both peripheral and central nervous systems.83
Low energy shockwave treatment and SC
Rompe et al. evaluated the safety of shock wave treatment for sciatic nerve lesions in rabbits. They applied low-energetic shockwave (0.08 mJ mm−2) treatment to normal rabbit sciatic nerve. There were no cases of nerve disruption or neuropraxia suggesting that shock wave is safe and does not threaten peripheral nerve integrity in an animal model.84
Hausner and colleagues85 made an 8 mm long homotopic nerve autograft into the right sciatic nerve, fixed with epineurial sutures in Sprague-Dawley rats. The group 1 animals received extracorporeal shock wave therapy (ESWT) (300 impulses, 3 Hz) immediately after nerve grafting whereas the group 2 (control) animals received only nerve autografts. At 6 to 8 weeks of survival, the ESWT group of animals exhibited a significantly improved functional recovery relative to the controls. Electrophysiological observations at 3 weeks after surgery revealed marked values of nerve action potential amplitude and compound nerve action potential in the ESWT group, whereas there were no detectable nerve action potential amplitudes in the control group. This finding was accompanied by significantly greater numbers of myelinated nerve fibers in the middle of the graft and in the distal stump of ESWT animals relative to the controls 3 weeks after surgery. Three weeks after surgery the nerve grafts of control animals contained great numbers of phagocytes and unmyelinated nerve fibers while the ESWT nerve grafts were filled with well-myelinated regenerating axons. These results suggest that ESWT induces an improved rate of axonal regeneration, this phenomenon probably involving faster Wallerian degeneration, the improved removal of degenerated axons and a greater capacity of the injured axons to regenerate.85 Mense and Hoheisel showed similar results in a rodent model of nerve compression with accelerated recovery of muscle sensitivity and functionality and regeneration of injured nerve fibers after treatment with focused shock waves.86 Marata and colleagues investigated the dorsal root ganglion neurons of rats following shockwave exposure to the footpad to elucidate its effect on the peripheral nervous system. They used activating transcription factor 3 (ATF3) and growth-associated phosphoprotein (GAP-43) as markers for nerve injury and axonal regeneration, respectively. The average number of neurons immunoreactive for ATF3 increased significantly in the treated rats at all experimental time points, with 78.3% of those neurons also exhibiting the immunoreactivity for GAP-43. It is to be expected that ESWT will become more widely used in the treatment of injuries and pathological conditions affecting peripheral nerves.
PERSPECTIVES
Investigations into SC regulation of nerve regeneration are providing new insights into pro-regeneration pathways whereas those focused on injury models are elucidating novel de-differentiative and regeneration pathways. Together, these studies hold the promise of identifying novel targets for the therapy of cavernous nerve injury.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Walsh PC, Mostwin JL. Radical prostatectomy and cystoprostatectomy with preservation of potency. Results using a new nerve-sparing technique. Br J Urol. 1984;56:694–7. doi: 10.1111/j.1464-410x.1984.tb06149.x. [DOI] [PubMed] [Google Scholar]
- 2.Noldus J, Michl U, Graefen M, Haese A, Hammerer P, et al. Patient-reported sexual function after nerve-sparing radical retropubic prostatectomy. Eur Urol. 2002;42:118–24. doi: 10.1016/s0302-2838(02)00219-1. [DOI] [PubMed] [Google Scholar]
- 3.Lagoda G, Jin L, Lehrfeld TJ, Liu T, Burnett AL. FK506 and sildenafil promote erectile function recovery after cavernous nerve injury through antioxidative mechanisms. J Sex Med. 2007;4(4 Pt 1):908–16. doi: 10.1111/j.1743-6109.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 4.Yin GN, Kim WJ, Jin HR, Kwon MH, Song KM, et al. Nerve injury-induced protein 1 (Ninjurin-1) is a novel therapeutic target for cavernous nerve injury-induced erectile dysfunction in mice. J Sex Med. 2013;10:1488–501. doi: 10.1111/jsm.12129. [DOI] [PubMed] [Google Scholar]
- 5.User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;169:1175–9. doi: 10.1097/01.ju.0000048974.47461.50. [DOI] [PubMed] [Google Scholar]
- 6.Leungwattanakij S, Bivalacqua TJ, Usta MF, Yang DY, Hyun JS, et al. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernosum. J Androl. 2003;24:239–45. doi: 10.1002/j.1939-4640.2003.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Jabaloyas JM, Gil-Salom M, Villamón-Fort R, Pastor-Hernández F, Martínez-García R, et al. Prognostic factors for response to sildenafil in patients with erectile dysfunction. Eur Urol. 2001;40:641–6. doi: 10.1159/000049850. [DOI] [PubMed] [Google Scholar]
- 8.Park NC, Kim TN, Park HJ. Treatment strategy for non-responders to PDE5 inhibitors. World J Mens Health. 2013;31:31–5. doi: 10.5534/wjmh.2013.31.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grim M, Halata Z, Franz T. Schwann cells are not required for guidance of motor nerves in the hindlimb in Splotch mutant mouse embryos. Anat Embryol (Berl) 1992;186:311–8. doi: 10.1007/BF00185979. [DOI] [PubMed] [Google Scholar]
- 10.Jessen KR, Mirsky R. Schwann cells and their precursors emerge as major regulators of nerve development. Trends Neurosci. 1999;22:402–10. doi: 10.1016/s0166-2236(98)01391-5. [DOI] [PubMed] [Google Scholar]
- 11.Corfas G, Velardez MO, Ko CP, Ratner N, Peles E. Mechanisms and roles of axon-Schwann cell interactions. J Neurosci. 2004;24:9250–60. doi: 10.1523/JNEUROSCI.3649-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–90. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Sanchez JA, Lopez de Armentia M, Lujan R, Kessaris N, Richardson WD, et al. Sustained axon-glial signaling induces Schwann cell hyperproliferation, Remak bundle myelination, and tumorigenesis. J Neurosci. 2009;29:11304–15. doi: 10.1523/JNEUROSCI.1753-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parkinson DB, Bhaskaran A, Droggiti A, Dickinson S, D’Antonio M, et al. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol. 2004;164:385–94. doi: 10.1083/jcb.200307132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kioussi C, Gross MK, Gruss P. Pa×3: a paired domain gene as a regulator in PNS myelination. Neuron. 1995;15:553–62. doi: 10.1016/0896-6273(95)90144-2. [DOI] [PubMed] [Google Scholar]
- 16.Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, et al. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies So×2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci U S A. 2005;102:2596–601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fields RD, Stevens B. ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci. 2000;23:625–33. doi: 10.1016/s0166-2236(00)01674-x. [DOI] [PubMed] [Google Scholar]
- 18.Maurel P, Salzer JL. Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J Neurosci. 2000;20:4635–45. doi: 10.1523/JNEUROSCI.20-12-04635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wegner M. Transcriptional control in myelinating glia: the basic recipe. Glia. 2000;29:118–23. [PubMed] [Google Scholar]
- 20.Wegner M. Transcriptional control in myelinating glia: flavors and spices. Glia. 2000;31:1–14. doi: 10.1002/(sici)1098-1136(200007)31:1<1::aid-glia10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Jaegle M, Ghazvini M, Mandemakers W, Piirsoo M, Driegen S, et al. The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev. 2003;17:1380–91. doi: 10.1101/gad.258203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atanasoski S, Notterpek L, Lee HY, Castagner F, Young P, et al. The protooncogene Ski controls Schwann cell proliferation and myelination. Neuron. 2004;43:499–511. doi: 10.1016/j.neuron.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Charles P, Reynolds R, Seilhean D, Rougon G, Aigrot MS, et al. Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? Brain. 2002;125(Pt 9):1972–9. doi: 10.1093/brain/awf216. [DOI] [PubMed] [Google Scholar]
- 24.Haney CA, Sahenk Z, Li C, Lemmon VP, Roder J, et al. Heterophilic binding of L1 on unmyelinated sensory axons mediates Schwann cell adhesion and is required for axonal survival. J Cell Biol. 1999;146:1173–84. doi: 10.1083/jcb.146.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feltri ML, Graus Porta D, Previtali SC, Nodari A, Migliavacca B, et al. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156:199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang D, Bierman J, Tarumi YS, Zhong YP, Rangwala R, et al. Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J Cell Biol. 2005;168:655–66. doi: 10.1083/jcb.200411158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito F, Moore SA, Barresi R, Henry MD, Messing A, et al. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–58. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 28.Cohen S, Levi-Montalcini R. Purification and properties of a nerve growth-promoting factor isolated from mouse sarcoma 180. Cancer Res. 1957;17:15–20. [PubMed] [Google Scholar]
- 29.Levi-Montalcini R, Cohen S. Effects of the extract of the mouse submaxillary salivary glands on the sympathetic system of mammals. Ann N Y Acad Sci. 1960;85:324–41. doi: 10.1111/j.1749-6632.1960.tb49963.x. [DOI] [PubMed] [Google Scholar]
- 30.Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, et al. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004;43:183–91. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–68. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 33.Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–94. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–82. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 35.Scherer SS, Wrabetz L. Molecular mechanisms of inherited demyelinating neuropathies. Glia. 2008;56:1578–89. doi: 10.1002/glia.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–7. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 37.Lepor H, Gregerman M, Crosby R, Mostofi FK, Walsh PC. Precise localization of the autonomic nerves from the pelvic plexus to the corpora cavernosa: a detailed anatomical study of the adult male pelvis. J Urol. 1985;133:207–12. doi: 10.1016/s0022-5347(17)48885-9. [DOI] [PubMed] [Google Scholar]
- 38.Lue TF, Zeineh SJ, Schmidt RA, Tanagho EA. Neuroanatomy of penile erection: its relevance to iatrogenic impotence. J Urol. 1984;131:273–80. doi: 10.1016/s0022-5347(17)50344-4. [DOI] [PubMed] [Google Scholar]
- 39.Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998;160(6 Pt 2):2418–24. doi: 10.1097/00005392-199812020-00010. [DOI] [PubMed] [Google Scholar]
- 40.Kim ED, Scardino PT, Hampel O, Mills NL, Wheeler TM, et al. Interposition of sural nerve restores function of cavernous nerves resected during radical prostatectomy. J Urol. 1999;161:188–92. [PubMed] [Google Scholar]
- 41.Kim ED, Nath R, Kadmon D, Lipshultz LI, Miles BJ, et al. Bilateral nerve graft during radical retropubic prostatectomy: 1-year followup. J Urol. 2001;165(6 Pt 1):1950–6. doi: 10.1097/00005392-200106000-00024. [DOI] [PubMed] [Google Scholar]
- 42.Anastasiadis AG, Benson MC, Rosenwasser MP, Salomon L, El-Rashidy H, et al. Cavernous nerve graft reconstruction during radical prostatectomy or radical cystectomy: safe and technically feasible. Prostate Cancer Prostatic Dis. 2003;6:56–60. doi: 10.1038/sj.pcan.4500613. [DOI] [PubMed] [Google Scholar]
- 43.Takenaka A, Murakami G, Soga H, Han SH, Arai Y, et al. Anatomical analysis of the neurovascular bundle supplying penile cavernous tissue to ensure a reliable nerve graft after radical prostatectomy. J Urol. 2004;172:1032–5. doi: 10.1097/01.ju.0000135648.33110.df. [DOI] [PubMed] [Google Scholar]
- 44.Costello AJ, Brooks M, Cole OJ. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int. 2004;94:1071–6. doi: 10.1111/j.1464-410X.2004.05106.x. [DOI] [PubMed] [Google Scholar]
- 45.Takenaka A, Murakami G, Matsubara A, Han SH, Fujisawa M. Variation in course of cavernous nerve with special reference to details of topographic relationships near prostatic apex: histologic study using male cadavers. Urology. 2005;65:136–42. doi: 10.1016/j.urology.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 46.Allaf ME, Hoke A, Burnett AL. Erythropoietin promotes the recovery of erectile function following cavernous nerve injury. J Urol. 2005;174:2060–4. doi: 10.1097/01.ju.0000176808.94610.dd. [DOI] [PubMed] [Google Scholar]
- 47.Lagoda G, Sezen SF, Liu T, Höke A, Burnett AL. FK506-binding protein localizations in human penile innervation. BJU Int. 2008;101:604–9. doi: 10.1111/j.1464-410X.2007.07290.x. [DOI] [PubMed] [Google Scholar]
- 48.Sezen SF, Hoke A, Burnett AL, Snyder SH. Immunophilin ligand FK506 is neuroprotective for penile innervation. Nat Med. 2001;7:1073–4. doi: 10.1038/nm1001-1073. [DOI] [PubMed] [Google Scholar]
- 49.Coleman MP, Freeman MR. Wallerian degeneration, wld (s), and nmnat. Annu Rev Neurosci. 2010;33:245–67. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8:e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coleman MP, Conforti L, Buckmaster EA, Tarlton A, Ewing RM, et al. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proc Natl Acad Sci U S A. 1998;95:9985–90. doi: 10.1073/pnas.95.17.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albersen M, Fandel TM, Zhang H, Banie L, Lin G, et al. Pentoxifylline promotes recovery of erectile function in a rat model of postprostatectomy erectile dysfunction. Eur Urol. 2011;59:286–96. doi: 10.1016/j.eururo.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–65. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- 54.Mirsky R, Woodhoo A, Parkinson DB, Arthur-Farraj P, Bhaskaran A, et al. Novel signals controlling embryonic Schwann cell development, myelination and dedifferentiation. J Peripher Nerv Syst. 2008;13:122–35. doi: 10.1111/j.1529-8027.2008.00168.x. [DOI] [PubMed] [Google Scholar]
- 55.Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- 56.Lee HK, Shin YK, Jung J, Seo SY, Baek SY, et al. Proteasome inhibition suppresses Schwann cell dedifferentiation in vitro and in vivo. Glia. 2009;57:1825–34. doi: 10.1002/glia.20894. [DOI] [PubMed] [Google Scholar]
- 57.Yang DP, Zhang DP, Mak KS, Bonder DE, Pomeroy SL, et al. Schwann cell proliferation during Wallerian degeneration is not necessary for regeneration and remyelination of the peripheral nerves: axon-dependent removal of newly generated Schwann cells by apoptosis. Mol Cell Neurosci. 2008;38:80–8. doi: 10.1016/j.mcn.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reichert F, Saada A, Rotshenker S. Peripheral nerve injury induces Schwann cells to express two macrophage phenotypes: phagocytosis and the galactose-specific lectin MAC-2. J Neurosci. 1994;14(5 Pt 2):3231–45. doi: 10.1523/JNEUROSCI.14-05-03231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung J, Cai W, Jang SY, Shin YK, Suh DJ, et al. Transient lysosomal activation is essential for p75 nerve growth factor receptor expression in myelinated Schwann cells during Wallerian degeneration. Anat Cell Biol. 2011;44:41–9. doi: 10.5115/acb.2011.44.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jang SY, Shin YK, Lee HY, Park JY, Suh DJ, et al. Local production of serum amyloid a is implicated in the induction of macrophage chemoattractants in Schwann cells during Wallerian degeneration of peripheral nerves. Glia. 2012;60:1619–28. doi: 10.1002/glia.22382. [DOI] [PubMed] [Google Scholar]
- 61.Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–47. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fontana X, Hristova M, Da Costa C, Patodia S, Thei L, et al. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J Cell Biol. 2012;198:127–41. doi: 10.1083/jcb.201205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aliperti LA, Lasker GF, Hagan SS, Hellstrom JA, Gokce A, et al. Efficacy of pioglitazone on erectile function recovery in a rat model of cavernous nerve injury. Urology. 2014;84:1122–7. doi: 10.1016/j.urology.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 64.Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 65.Höke A, Ho T, Crawford TO, LeBel C, Hilt D, et al. Glial cell line-derived neurotrophic factor alters axon schwann cell units and promotes myelination in unmyelinated nerve fibers. J Neurosci. 2003;23:561–7. doi: 10.1523/JNEUROSCI.23-02-00561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iwase T, Jung CG, Bae H, Zhang M, Soliven B. Glial cell line-derived neurotrophic factor-induced signaling in Schwann cells. J Neurochem. 2005;94:1488–99. doi: 10.1111/j.1471-4159.2005.03290.x. [DOI] [PubMed] [Google Scholar]
- 67.Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci. 1997;9:1450–60. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 68.Paratcha G, Ledda F, Ibáñez CF. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113:867–79. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- 69.Bamber NI, Li H, Lu X, Oudega M, Aebischer P, et al. Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur J Neurosci. 2001;13:257–68. [PubMed] [Google Scholar]
- 70.Tep C, Kim ML, Opincariu LI, Limpert AS, Chan JR, et al. Brain-derived neurotrophic factor (BDNF) induces polarized signaling of small GTPase (Rac1) protein at the onset of Schwann cell myelination through partitioning-defective 3 (Par3) protein. J Biol Chem. 2012;287:1600–8. doi: 10.1074/jbc.M111.312736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwon YW, Gurney ME. Brain-derived neurotrophic factor transiently stabilizes silent synapses on developing neuromuscular junctions. J Neurobiol. 1996;29:503–16. doi: 10.1002/(SICI)1097-4695(199604)29:4<503::AID-NEU7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 72.Ward NL, Hagg T. BDNF is needed for postnatal maturation of basal forebrain and neostriatum cholinergic neurons in vivo. Exp Neurol. 2000;162:297–310. doi: 10.1006/exnr.1999.7346. [DOI] [PubMed] [Google Scholar]
- 73.Parrilla-Reverter G, Agudo M, Sobrado-Calvo P, Salinas-Navarro M, Villegas-Pérez MP, et al. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: a quantitative in vivo study. Exp Eye Res. 2009;89:32–41. doi: 10.1016/j.exer.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 74.Geremia NM, Pettersson LM, Hasmatali JC, Hryciw T, Danielsen N, et al. Endogenous BDNF regulates induction of intrinsic neuronal growth programs in injured sensory neurons. Exp Neurol. 2010;223:128–42. doi: 10.1016/j.expneurol.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 75.Wang T, Cong R, Yang H, Wu MM, Luo N, et al. Neutralization of BDNF attenuates the in vitro protective effects of olfactory ensheathing cell-conditioned medium on scratch-insulted retinal ganglion cells. Cell Mol Neurobiol. 2011;31:357–64. doi: 10.1007/s10571-010-9626-5. [DOI] [PubMed] [Google Scholar]
- 76.Bakircioglu ME, Lin CS, Fan P, Sievert KD, Kan YW, et al. The effect of adeno-associated virus mediated brain derived neurotrophic factor in an animal model of neurogenic impotence. J Urol. 2001;165(6 Pt 1):2103–9. doi: 10.1097/00005392-200106000-00078. [DOI] [PubMed] [Google Scholar]
- 77.Lin G, Chen KC, Hsieh PS, Yeh CH, Lue TF, et al. Neurotrophic effects of vascular endothelial growth factor and neurotrophins on cultured major pelvic ganglia. BJU Int. 2003;92:631–5. doi: 10.1046/j.1464-410x.2003.04439.x. [DOI] [PubMed] [Google Scholar]
- 78.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsieh PS, Bochinski DJ, Lin GT, Nunes L, Lin CS, et al. The effect of vascular endothelial growth factor and brain-derived neurotrophic factor on cavernosal nerve regeneration in a nerve-crush rat model. BJU Int. 2003;92:470–5. doi: 10.1046/j.1464-410x.2003.04373.x. [DOI] [PubMed] [Google Scholar]
- 80.Lin G, Shindel AW, Fandel TM, Bella AJ, Lin CS, et al. Neurotrophic effects of brain-derived neurotrophic factor and vascular endothelial growth factor in major pelvic ganglia of young and aged rats. BJU Int. 2010;105:114–20. doi: 10.1111/j.1464-410X.2009.08647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sakamoto T, Kawazoe Y, Shen JS, Takeda Y, Arakawa Y, et al. Adenoviral gene transfer of GDNF, BDNF and TGF beta 2, but not CNTF, cardiotrophin-1 or IGF1, protects injured adult motoneurons after facial nerve avulsion. J Neurosci Res. 2003;72:54–64. doi: 10.1002/jnr.10558. [DOI] [PubMed] [Google Scholar]
- 82.Araki K, Shiotani A, Watabe K, Saito K, Moro K, et al. Adenoviral GDNF gene transfer enhances neurofunctional recovery after recurrent laryngeal nerve injury. Gene Ther. 2006;13:296–303. doi: 10.1038/sj.gt.3302665. [DOI] [PubMed] [Google Scholar]
- 83.Luo B, Huang J, Lu L, Hu X, Luo Z, et al. Electrically induced brain-derived neurotrophic factor release from Schwann cells. J Neurosci Res. 2014;92:893–903. doi: 10.1002/jnr.23365. [DOI] [PubMed] [Google Scholar]
- 84.Rompe JD, Bohl J, Riehle HM, Schwitalle M, Krischek O. Evaluating the risk of sciatic nerve damage in the rabbit by administration of low and intermediate energy extracorporeal shock waves. Z Orthop Ihre Grenzgeb. 1998;136:407–11. doi: 10.1055/s-2008-1053676. [DOI] [PubMed] [Google Scholar]
- 85.Hausner T, Pajer K, Halat G, Hopf R, Schmidhammer R, et al. Improved rate of peripheral nerve regeneration induced by extracorporeal shock wave treatment in the rat. Exp Neurol. 2012;236:363–70. doi: 10.1016/j.expneurol.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 86.Mense S, Hoheisel U. Shock wave treatment improves nerve regeneration in the rat. Muscle Nerve. 2013;47:702–10. doi: 10.1002/mus.23631. [DOI] [PubMed] [Google Scholar]


