Abstract
Stress urinary incontinence (SUI) and end-stage erectile dysfunction (ED) after radical prostatectomy (RP) can decrease a patient's quality of life (QoL). We describe a surgical technique involving scrotal incision for simultaneous dual implantation of an artificial urinary sphincter (AUS) and an inflatable penile prosthesis (IPP). Patients with moderate to severe SUI (>3 pads per day) and end-stage ED following RP were selected for dual implantation. An upper transverse scrotal incision was made, followed by bulbar urethra dissection and AUS cuff placement. Through the same incision, the corpora cavernosa was exposed, and an IPP positioned. Followed by extraperitoneal reservoirs placement and pumps introduced in the scrotum. Short-term, intra- and post-operative complications; continence status and erectile function; and patient satisfaction and QoL were recorded. A total of 32 patients underwent dual implantation. Early AUS-related complications were: AUS reservoir migration and urethral erosion. One case of distal corporal extrusion occurred. No prosthetic infection was reported. Over 96% of patients were socially the continent (≤1 pad per day) and > 95% had sufficient erections for intercourse. Limitations of the study were the small number of patients, the lack of the control group using a perineal approach for AUS placement and only a 12 months follow-up. IPP and AUS dual implantation using a single scrotal incision technique is a safe and effective option in patients with SUI and ED after RP. Further studies on larger numbers of patients are warranted.
Keywords: artificial urinary sphincter, erectile dysfunction, penile prosthesis, radical prostatectomy, single scrotal incision, urinary incontinence
INTRODUCTION
Prostate cancer is the second most common cause of cancer in men worldwide and is also the sixth leading cause of cancer death.1 There is an expected morbidity associated with radical prostatectomy (RP) for prostate cancer, including erectile dysfunction (ED) and urinary incontinence (UI).2,3 At 18 months or more following RP, a large patient series reports incidences of 8% UI and 60% ED.4
Nerve-sparing techniques and new effective oral medications for ED have reduced the incidence of end-stage ED, but there is still a subgroup of patients who do not respond to medical therapies. Inflatable penile prostheses (IPPs) were first described in 19735 and significant improvements in the mechanical properties, and surgical implantation techniques have taken place subsequently. These devices remain the standard of care for men with medically refractory ED.
Urinary incontinence is a serious postoperative adverse event with few medical options. When mild UI present 12 months after RP, male slings can be considered, but the gold standard in the past decade for severe UI remains artificial urinary sphincter (AUS) placement.6 In 2003, Wilson et al. described the placement of the AUS through a trans-scrotal incision,7 offering the possibility for concurrent IPP insertion via a single incision (dual implant). In 2010, further publication provided a technical enhancement that allowed proximal cuff placement, resulting in increased continence rates.8 There are a few published studies demonstrating the feasibility, safety and cost-effectiveness of simultaneous dual implantation and some have reported encouraging outcomes in terms of patient's satisfaction, quality of life (QoL) and functional results.9,10,11
We describe a surgical technique through a single trans-scrotal incision performed at two centers in Spain.
PATIENTS AND METHODS
Between September 2007 and October 2011, double implant surgery involving an AUS and an IPP was performed on 32 patients by two surgeons at two referral centers: Hospital Universitario Puerta de Hierro-Majadahonda and Hospital La Zarzuela. Patients had a surgical history of RP for organ-confined or locally advanced prostate cancer and had undetectable PSA levels during follow-up or stable PSA after salvage/adjuvant radiotherapy. The following patient inclusion criteria were applied: end-stage ED secondary to RP (failure of first-line [phosphodiesterase type 5 inhibitor] and second-line therapies [intracavernosal injection or MUSE], or nonacceptance of second-line therapies); moderate to severe UI secondary to RP (>3 pads per day or > 250 g day−1 on pad-test); free of prostate cancer biochemical recurrence (PSA < 0.2 ng ml−1); and primary implants (revisions and re-implantations were excluded). Patients with < 18 months follow-up following RP were excluded.
Due to the possibility of patients experiencing moderate UI post-RP and no clinically voiding symptoms having an urethrovesical anastomosis stricture,12 a detailed work-up was required prior to AUS placement. This includes: a 7–10 days voiding diary; 24-h pad test; flexible urethrocystoscopy; uroflowmetry (up to a full urodynamic study); and detailed physical examination. Patients identified as having an urethrovesical anastomosis stricture underwent internal urethrotomy under direct vision, followed by an outpatient dilatation program (twice every 2 weeks, 3 times every 3 weeks and 4 times every 4 weeks). Urethrovesical stricture was required to reach an acceptable diameter (14–16F) and be stable for at least 6 months before AUS implantation.
Devices
The IPP used were a three-piece inflatable prosthesis, which was comprised of two intracorporeal cylinders, a fluid reservoir and a pump; the antibiotic-coated version was used in all patients. The AUS implanted were formed of an occlusive cuff, a fluid reservoir and a pump.
Surgical technique
Patients were placed in the supine position for surgery preparation. Shaving followed by an intensive scrub with chlorhexidine or povidone-iodine for 10 min was conducted in the OR. The bladder was completely drained using a 14F Foley catheter, which was then left in place. The patient was readjusted to the lithotomy position with legs gently abducted at a low level. This position permitted sufficient scrotal retraction to allow good access to the bulbar urethra. Antibiotic prophylaxis was given comprising amoxicilin-clavulanic acid 1 g and gentamicin 240 mg intravenous or in cases of allergy to betalactams, levofloxacin 500 mg.
A Scott retractor was placed, and an upper transverse scrotal incision made.13 The first stage of the procedure involved placement of the AUS. The tunica albuginea was dissected and the corpora cavernosa exposed. Deep exposure of the proximal corpora was needed for Deaver retraction, which exposed the bulbar urethra. Approaching the bulbar urethra through the scrotal incision allowed better mobilization and the bulbocavernosus muscle was then dissected distally under direct vision (Figure 1).12 A cuff sizer was used to measure the urethral circumference (Figure 2). Inexperienced surgeons might place cuffs over the bulbocavernosus muscle in order to protect the urethra. However, this was not recommended as long-term leakage due to muscle atrophy has been described.9 Once cuff size was determined, air was evacuated from the cuff before final placement.
Figure 1.
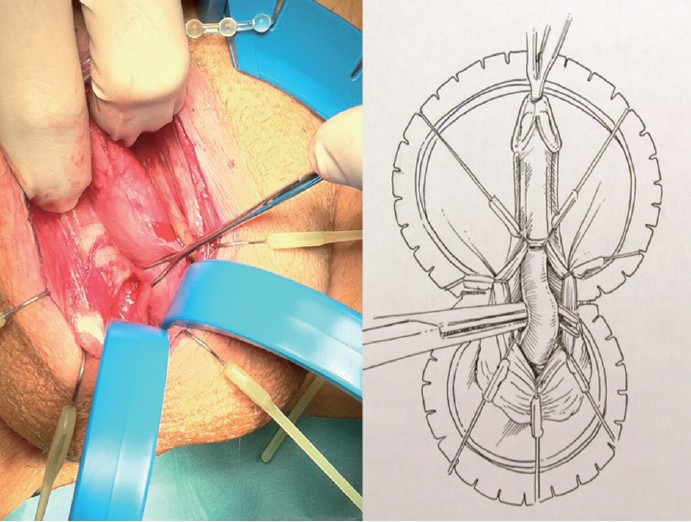
Urethral dissection. Corpus spongiosum is bluntly dissected from Buck's fascia.
Figure 2.
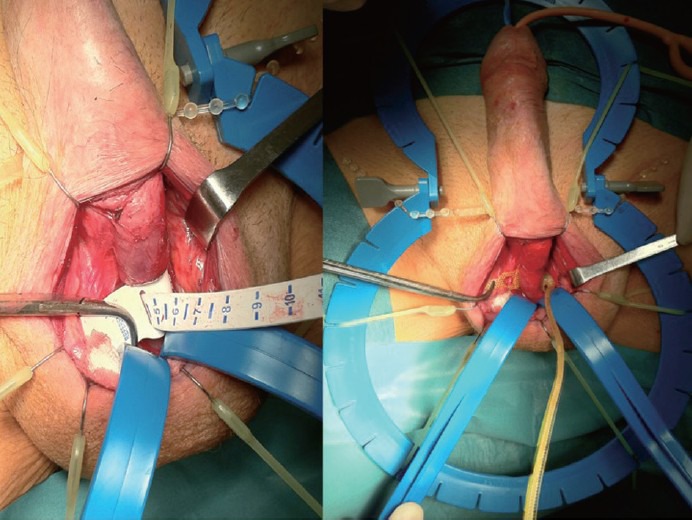
After measuring sphincter size, cuff placed where corpora decussate.
The second stage of the procedure was implantation of the IPP. The tunica albuginea of both corpora cavernosa was exposed using the same “dartos window.” Two stitches with absorbable 3/0 suture material were placed side by side into both corpora followed by cold scalpel 2 cm vertical corporotomy (Figure 3). Corpora dilatation was avoided so as to reduce postoperative pain and preservation of residual erectile tissue.14 A Furlow meter was inserted sliding laterally through the corpora cavernosa at an angle of 45°, to avoid urethral injury. Once measured proximally and distally, using the Keith needles through the Furlow inserter to facilitate distal placement, intracorporal cylinders were passed. Corporotomies were then closed and tighten in order to avoid bleeding or hematoma.
Figure 3.
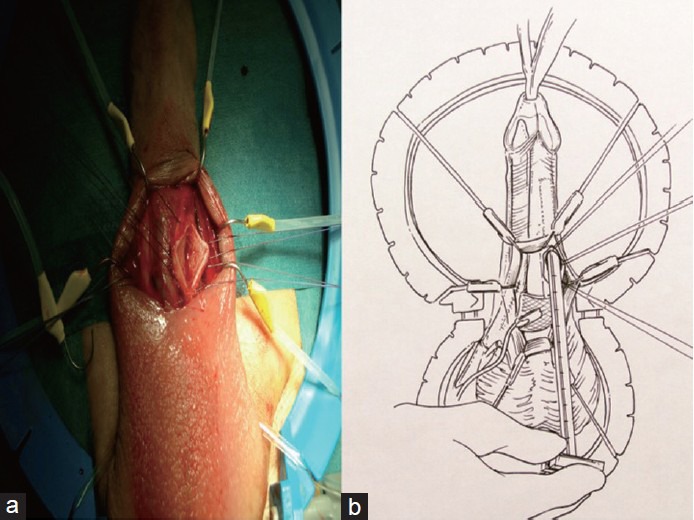
(a) Vertical corporotomy; (b) Corpora dilatation is avoided and Furlow meter is inserted sliding laterally at an angle of 45°.
The Scott retractor was removed, and reservoir placement undertaken. Digital dissection was made reaching the inguinal canal and piercing its posterior wall, the transversalis fascia. The fluid reservoirs were placed within the Retzius space (laterally to the bladder), placing the IPP reservoir on one side and the AUS reservoir on the other. The IPP pump was then placed in between both testicles with the button facing forwards while the AUS pump was placed under a sub-dartos muscle pouch laterally on the scrotum.
The components of both devices were connected, and reservoirs filled according to the manufacturer's recommendations (Figure 4). The correct functioning of the IPP was tested, evaluating penile curvature, and the AUS was deactivated.
Figure 4.
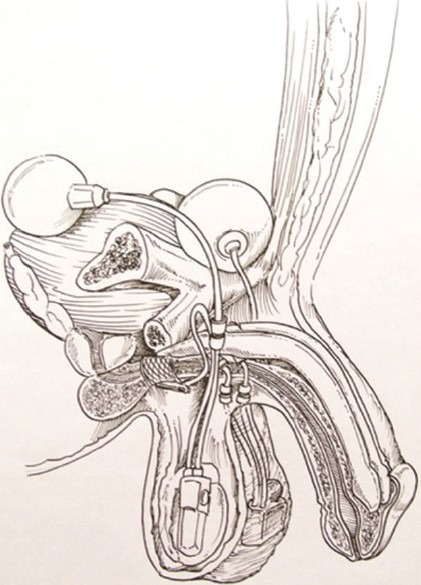
Positioning of all components of the artificial urinary sphincter and inflatable penile prosthese devices.
In order to avoid scrotal hematoma, drain and compression bandaging was placed for all patients for a 24 h period and observation then conducted. Broad-spectrum antibiotics were given postoperatively for 7 d. Patients were followed every week in the office during the first month in order to detect any early complications. Activation and device training was done sequentially at 4–6 weeks, starting first with the AUS and then the IPP some weeks later. Patients were required to use incontinence pads during the period leading up to AUS activation.
Patient outcome assessment
Under an Institutional Review Board approved protocol, prospective data were collected on patients at baseline and at 12 months follow-up. Urinary function was assessed using the International Consultation on Incontinence Questionnaire Urinary Incontinence Short Form (ICIQ-SF)15 and patient's QoL relating to UI by the incontinence QoL instrument (I-QoL).16 Social continence was defined as previously reported as the use of ≤ 1 pad per day.17 Sexual function was evaluated using the International Index of Erectile Function 5 (IIEF-5) questionnaire (sexual health inventory for men).18 Global satisfaction was assessed with a single question.
RESULTS
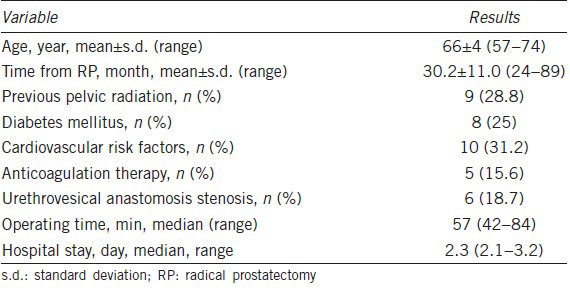
Baseline characteristics of the 32 patients are shown in Table 1. The minimum follow-up after RP was 2 years. Six patients (18.7%) were diagnosed preoperatively with urethrovesical stenosis and underwent the outpatient dilatation program. Of the 32 patients, 25 experienced failure of first- and second-line treatment of ED and seven rejected medical second-line treatment. Operating times and duration of hospital stay are described in Table 1.
Table 1.
Baseline characteristics and intra- and post-operative data on 32 patients treated with the double implantation technique
Safety
No intra-operative complications were identified. Postoperative drainage was used in 30 patients, and both drain and Foley catheter were removed on the first postoperative day. Median (range) pain score (Visual Analog Scale range 0–10) at 24 h postsurgery was 2.3 (1–3).
During the first-month follow-up, one patient experienced a urinary tract infection that was treated with oral antibiotics. No wound or device-related infections were recorded. Four device-related complications occurred: (1) AUS reservoir migration, (2) urethral erosion and (3) distal corporal extrusion. AUS reservoir migration occurred before AUS activation, and early replacement surgery was performed. The two urethral erosions occurred at 8 and 10 months and required the removal of the AUS. The distal corporal extrusion occurred at 48 h, resulting in the removal of the IPP device. The three patients undergoing device removal had a history of previous adjuvant/salvage radiotherapy. At median follow-up of 28 months (range 18–66), no further complications have been described.
Efficacy
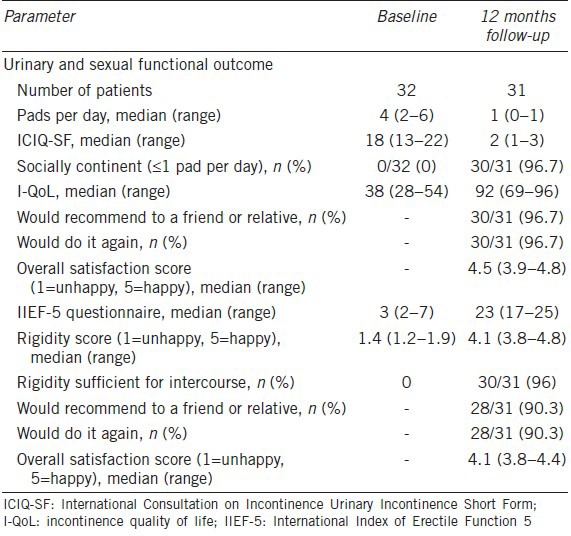
Urinary and sexual function at baseline and 12 months follow-up are shown in Table 2. Over 96% of patients were socially the continent (≤1 pad per day) and 96% had erections sufficient for intercourse. Patient satisfaction was high in regard to both urinary and sexual function.
Table 2.
Baseline and postsurgery urinary and sexual functional outcomes
DISCUSSION
Inflatable penile prosthesis is the standard of care for patients whose erectile function does not respond to first- and second-line therapies for ED.19 These devices are associated with patient satisfaction rates as high as 70%–87%.20 Recovery of nerve function can occur following RP4 and for this reason the IPP was not offered to patients in the current study who were < 18 months post-RP. The EAU recommends different surgical approaches for men with stress UI (SUI) post-RP dependent on whether mild, moderate or severe SUI is present 12 months after RP.6 The fixed male sling is an accepted technique for mild SUI, and has been used in simultaneous implantations with the IPP.10 The AUS has been used for more than 30 years and is considered the standard treatment for moderate to severe SUI.6
Surgical treatment of sequelae following RP can be approached simultaneously or in a two-step procedure. Patients presenting with both end-stage ED and UI can benefit of a simultaneous approach. Different reports on published series reflect safety of the procedure and patient satisfaction.21,22,23 An asynchronous approach to dual implantation of the AUS and IPP was reported by Alechinsky et al.24 This retrospective analysis included five patients who were implanted with an IPP at a median time of 50 months following the implantation of an AUS. It was concluded that the two-step procedure was an efficacious option in patients with concomitant refractory UI and ED. Rolle et al. compared the simultaneous and the two-stage surgical implantation procedures, with data available for 15 and eight patients, respectively.9 Results in terms of QoL were similar if devices were placed simultaneously or in a two-stage procedure. There were no differences in patient satisfaction or functional outcome between the two groups. Segal et al. reported results from a retrospective study of penoescrotal simultaneous implantation versus single prosthesis implantation at a single institution (transperineal incisions for AUS cuff placement).22 With 55 double implants in their series, they concluded operative time significantly longer for the simultaneous procedure than for single implant procedure (P < 0.0001), although the rate of erosion, device infection or malfunction was not increased irrespective of combined or staged procedures (P > 0.05).
Over 96% of patients in the current study achieved social continence (≤1 pad per day) and median daily pad usage decreased from four to one pad. In a dual implant approach involving 95 men, Mancini et al. reported a socially continent rate of 72.7% and a reduction in daily pad usage from 6.1 to 1.3 pads at a mean postoperative time of 21.6 months.25 Urinary functional outcome as assessed with the ICIQ-SF in the current study improved as evidenced by a reduction in the median score from 18 to 2. Patients’ QoL improved with an increase in median I-QoL score from 38 to 92. Previous series describe safe and satisfactory functional results in the single penoescrotal access, but other publications concluded that perineal cuff placement results in better continence than the trans-scrotal approach.26 Henry et al.26 described single AUS implantation; self-reported dry rate was 28.0% and 56.7% for the penoscrotal and perineal approach, respectively (P = 0.03). This information is of importance; when completing urethral dissection through penoesrcotal access it is vital to place the AUS cuff as proximal as possible in the bulbar urethra in order to increase continence rates. Mancini et al.25 and our study report higher continence rates than Henry et al.26 through the trans-scrotal approach, 72.7%, 96% and 28%, respectively.
Sexual function was also ameliorated with an increase in median IIEF-5 Questionnaire score from 3 to 23. In total, 96% of patients achieving rigidity sufficient for intercourse, which is similar to the 97% rate reported in the Mancini dual implant study at 21.6 months follow-up.21
According to Mancini et al.25 patients treated with IPP alone reported less overall satisfaction than only AUS or double implant patients, and were less willing to recommend the surgery to a friend or relative. In the dual implant approach, 94% of patients would recommend an approach. In the current study, >90% of the population would recommend both AUS and IPP to a friend or relative or have the procedure performed again. Overall satisfaction scores for dual implant in the Mancini study were 4.4 and 4.2 for IPP and AUS function, respectively.25 This compares with 4.1 and 4.5 in the current study.
The complications associated with the synchronous implantation of an IPP and AUS using a trans-scrotal approach was reported by Kendirci et al.21 This multi-institutional, retrospective analysis included 22 patients implanted between 2000 and 2003; mean follow-up was 17 months. No mechanical failures were reported. Urethral erosion and reservoir migration occurred in two (9%) patients for each; the overall revision rate was 14%. Urethral atrophy is a long-term complication in AUS implantation, and the risk can be lessened with proximal bulbar placement.25 The scrotal incision approach used in the current study for the placement of the AUS provides rapid access to the proximal bulbar urethra. Improved exposure of the bulbar urethra in our surgical approach may avoid posterior urethral injury. Distal cuff placement can also result in a higher urethral erosion rate, which occurs in approximately 5% of patients in single AUS placement13,26,27 and 9% with dual implantation.21 In the current series, 2 (6.2%) patients experienced urethral erosion and in the future, the cuff will be implanted more proximally. Previous irradiation, surgery or trauma, which decreases the blood loss flow of these tissues, can also increase the risk of erosion.21 The other AUS-related complications reported was AUS reservoir migration, which was reported in 1 (3%) patient, which is < 9% previously reported in a dual implantation approach.21 The surgical approach presented here provides an adequate insertion of the AUS pump in the scrotum, reducing the risk of pump retraction into the upper groin.7,11 The only complication associated with the IPP in the present study was one case of distal corporal extrusion that required device removal.
Dual implantation has the benefit of a shorter operating time than the two implantations conducted in separate surgeries with the potential for lower infection rates.28 A review by Al-Shaiji of dual implant series reports infection rates of 0%–11%.23 No infections were reported in the current study to date. The reduction in operating time also provides the added benefit of cost savings, which were estimated to be of the order of $7000 compared with the individual procedures in a series reported in 2005.29
When considering a double implant, benefits of a single incision are reflected in the present study but lack of the control group on a perineal approach limit our results. We acknowledge certain limitations to the study including a relatively small number of patients, the lack of control group using a perineal approach for AUS placement and only 12 months follow-up.
CONCLUSION
Double implantation of the IPP and AUS through a single scrotal incision is a safe and effective option in patients with ED and SUI post-RP. Consideration should be given to previous treatment with radiotherapy as this can impact on AUS erosion rates. Further studies on larger numbers of patients are warranted.
AUTHOR CONTRIBUTIONS
JIMS participated in the design of study, carried out the surgeries and follow-up of patients, as well as the draft of the manuscript. ELE participated in surgeries and follow-up of patients, acquisition of data and drafting of the manuscript. IM and LPS carried out the surgeries and participated in the coordination of the study. JCR participated in the coordination and critical revision of the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
The authors thank Christine Mckillop, for editing the manuscript and editorial assistance and Javier González, MD, for original drawings.
REFERENCES
- 1.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Ficarra V, Novara G, Rosen RC, Artibani W, Carroll PR, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012;62:405–17. doi: 10.1016/j.eururo.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 3.Ficarra V, Novara G, Ahlering TE, Costello A, Eastham JA, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol. 2012;62:418–30. doi: 10.1016/j.eururo.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 4.Stanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the prostate cancer outcomes study. JAMA. 2000;283:354–60. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- 5.Scott FB, Bradley WE, Timm GW. Management of erectile impotence. Use of implantable inflatable prosthesis. Urology. 1973;2:80–2. doi: 10.1016/0090-4295(73)90224-0. [DOI] [PubMed] [Google Scholar]
- 6.Lucas MG, Bosch RJ, Burkhard FC, Cruz F, Madden TB, et al. EAU guidelines on surgical treatment of urinary incontinence. Eur Urol. 2012;62:1118–29. doi: 10.1016/j.eururo.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Wilson S, Delk J, 2nd, Henry GD, Siegel AL. New surgical technique for sphincter urinary control system using upper transverse scrotal incision. J Urol. 2003;169:261–4. doi: 10.1016/S0022-5347(05)64082-7. [DOI] [PubMed] [Google Scholar]
- 8.Wilson SK, Aliotta PJ, Salem EA, Mulcahy JJ. New enhancements of the scrotal one-incision technique for placement of artificial urinary sphincter allow proximal cuff placement. J Sex Med. 2010;7:3510–5. doi: 10.1111/j.1743-6109.2010.01933.x. [DOI] [PubMed] [Google Scholar]
- 9.Rolle L, Ceruti C, Sedigh O, Timpano M, Destefanis P, et al. Surgical implantation of artificial urinary device and penile prosthesis through trans-scrotal incision for postprostatectomy urinary incontinence and erectile dysfunction: synchronous or delayed procedure? Urology. 2012;80:1046–50. doi: 10.1016/j.urology.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Lee D, Romero C, Alba F, Westney OL, Wang R. Simultaneous penile prosthesis and male sling/artificial urinary sphincter. Asian J Androl. 2013;15:10–5. doi: 10.1038/aja.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morey AF, Cefalu CA, Hudak SJ. High submuscular placement of urologic prosthetic balloons and reservoirs via transscrotal approach. J Sex Med. 2013;10:603–10. doi: 10.1111/jsm.12000. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Salamanca JI, Moncada I, del Portillo L, Sola I, Martínez-Ballesteros C, et al. Simultaneous double prosthesis implant more artificial urinary sphincter via transscrotal: surgical technique. Arch Esp Urol. 2011;64:311–9. [PubMed] [Google Scholar]
- 13.Montague DK, Angermeir KW. Surgical approaches for penile prosthesis implantation: penoscrotal vs infrapubic. Int J Impot Res. 2003;15(Suppl 5):S134–5. doi: 10.1038/sj.ijir.3901089. [DOI] [PubMed] [Google Scholar]
- 14.Moncada I, Martínez-Salamanca JI, Jara J, Cabello R, Moralejo M, et al. Inflatable penile prosthesis implantation without corporeal dilation: a cavernous tissue sparing technique. J Urol. 2010;183:1123–6. doi: 10.1016/j.juro.2009.11.048. [DOI] [PubMed] [Google Scholar]
- 15.Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, et al. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23:322–30. doi: 10.1002/nau.20041. [DOI] [PubMed] [Google Scholar]
- 16.Patrick DL, Martin ML, Bushnell DM, Yalcin I, Wagner TH, et al. Quality of life of women with urinary incontinence: further development of the incontinence quality of life instrument (I-QOL) Urology. 1999;53:71–6. doi: 10.1016/s0090-4295(98)00454-3. [DOI] [PubMed] [Google Scholar]
- 17.Hussain M, Greenwell TJ, Venn SN, Mundy AR. The current role of the artificial urinary sphincter for the treatment of urinary incontinence. J Urol. 2005;174:418–24. doi: 10.1097/01.ju.0000165345.11199.98. [DOI] [PubMed] [Google Scholar]
- 18.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 19.Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010;57:804–14. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Montague DK, Angermeier KW. Penile prosthesis implantation. Urol Clin North Am. 2001;28:355–61, x. doi: 10.1016/s0094-0143(05)70144-0. [DOI] [PubMed] [Google Scholar]
- 21.Kendirci M, Gupta S, Shaw K, Morey A, Jones L, et al. Synchronous prosthetic implantation through a transscrotal incision: an outcome analysis. J Urol. 2006;175:2218–22. doi: 10.1016/S0022-5347(06)00345-4. [DOI] [PubMed] [Google Scholar]
- 22.Segal RL, Cabrini MR, Harris ED, Mostwin JL, Bivalacqua TJ, et al. Combined inflatable penile prosthesis-artificial urinary sphincter implantation: no increased risk of adverse events compared to single or staged device implantation. J Urol. 2013;190:2183–8. doi: 10.1016/j.juro.2013.06.084. [DOI] [PubMed] [Google Scholar]
- 23.Al-Shaiji TF. Dual implantation of artificial urinary sphincter and inflatable penile prostheses for concurrent male urinary incontinence and erectile dysfunction. Adv Urol. 2011;2011:178312. doi: 10.1155/2011/178312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alechinsky L, Phé V, Yates DR, Bourgade V, Parra J, et al. Asynchronous implantation of a penile prosthesis (AMS 700) in patients with an artificial urinary sphincter (AMS 800): what functional outcomes can we expect from the AMS 1500. ? Prog Urol. 2010;22:354–9. doi: 10.1016/j.purol.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Mancini JG, Kizer WS, Jones LA, Mora RV, Morey AF. Patient satisfaction after dual implantation of inflatable penile and artificial urinary sphincter prostheses. Urology. 2008;71:893–6. doi: 10.1016/j.urology.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Henry GD, Graham SM, Cleves MA, Simmons CJ, Flynn B. Perineal approach for artificial urinary sphincter implantation appears to control male stress incontinence better than the transscrotal approach. J Urol. 2008;179:1475–9. doi: 10.1016/j.juro.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 27.Montague DK, Angermeier KW. Postprostatectomy urinary incontinence: the case for artificial urinary sphincter implantation. Urology. 2000;55:2–4. doi: 10.1016/s0090-4295(99)00413-6. [DOI] [PubMed] [Google Scholar]
- 28.Kumar R, Nehra A. Dual implantation of penile and sphincter implants in the post-prostatectomy patient. Curr Urol Rep. 2007;8:477–81. doi: 10.1007/s11934-007-0052-2. [DOI] [PubMed] [Google Scholar]
- 29.Sellers CL, Morey AF, Jones LA. Cost and time benefits of dual implantation of inflatable penile and artificial urinary sphincter prosthetics by single incision. Urology. 2005;65:852–3. doi: 10.1016/j.urology.2004.11.017. [DOI] [PubMed] [Google Scholar]


