Abstract
Tetrandrine (TET), a traditional Chinese medicine, exerts remarkable anticancer activity on various cancer cells. However, little is known about the effect of TET on human prostate cancer cells, and the mechanism of function of TET on prostate cancer has not yet been elucidated. To investigate the effects of TET on the suppression of proliferation, induction of apoptosis, and inhibition of migration and invasion in human prostate cancer cell lines, DU145 and PC-3. Inhibition of growth was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and clone formation assay, and flow cytometry analysis was performed to detect the induction of apoptosis. Activation of poly (ADP-ribose) polymerase, caspase-3, Akt, phospho-Akt, Bcl-2, and Bax was analyzed by Western blotting. Wound healing assay and transwell migration assay were used to evaluate the effect of TET on migration and invasion of cancer cells. TET inhibited the growth of DU145 and PC–3 cells in a dose- and time-dependent manner. Cell cloning was inhibited in the presence of TET in DU145 and PC-3 cells. TET suppressed the migration of DU145 and PC-3 cells. Transwell invasion assay showed that TET significantly weakened invasion capacity of DU145 and PC-3 cells. TET exhibited strong inhibitory effect on proliferation, migration, and invasion of prostate cancer cells. In addition, TET induced apoptosis in a dose-dependent manner by activating the caspase cascade and inhibiting phosphoinositide 3-kinase-Akt signal pathway. The accumulating evidence suggests that TET could be a potential therapeutic candidate against prostate cancer in a clinical setting.
Keywords: apoptosis, invasion, migration, proliferation, prostate cancer, tetrandrine
INTRODUCTION
Prostate cancer is one of the most common genitourinary malignancies in males. According to the epidemiology statistics of global cancer from World Health Organization (GLOBOCAN 2008), the morbidity from prostate cancer in 2008 was ranked 2nd, accounting for 14% of all cancer types in males.1 In the past few years, there is a significant upward trend in morbidity and mortality from prostate cancer in China. Its treatment has become a task of priority in modern medicine. At present, several methods are available to treat prostate cancer including endocrine therapy, surgery, radiation therapy, and chemotherapy. Among these methods, radical prostatectomy is still the most effective method for treating localized prostate cancer, but its clinical applications are restricted by tumor grading and staging. Therefore, there is a strong demand to develop new treatment regimens to suppress prostate cancer.
Tetrandrine (TET; International Union of Pure and Applied Chemistry name: 6,6’,7,12-tetramethoxy-2, 2’-dimethyl-1β-berbaman; molecular structural formula: C38H42N2O6; molecular weight: 622.74988 g mol−1), a bisbenzylisoquinoline alkaloid, is purified from the roots of Stephania tetrandra (or hang fang ji) (family: Menispermaceae). TET has been used as an effective constituent to treat patients with hypertension, arrhythmia, arthritis, inflammation, even silicosis in traditional Chinese medicine.2 There is accumulating evidence suggesting that TET presents anticancer effects against various cancers in vitro and to some extent, in vivo including leukemia,3 hepatocellular carcinoma,4,5 gastric cancer,6 colon cancer,7,8 lung cancer,9 glioma,10,11 nasopharyngeal carcinoma,12 bladder cancer,13 and renal cell carcinoma.14
However, little is known about the effect of TET on human prostate cancer cells. And the mechanism of function of TET on prostate cancer has not yet been elucidated. Hence, this study investigated the effect of TET on the suppression of proliferation, induction of apoptosis, and inhibition of migration and invasion in human prostate cancer cell lines, DU145 and PC-3.
MATERIALS AND METHODS
Cell culture
Human prostate cancer DU145 and PC-3 cell lines were from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco's Modified Eagle's medium/1640 supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA) and 1% penicillin-streptomycin, at 37°C, in humidified air containing 5% CO2.
Reagents
Tetrandrine (C38H42N2O6) and 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). TET was made into a fine suspension by dissolving the compound in 0.1 mol l−1 HCl at a concentration of 25 mg ml−1, which was diluted to desired concentrations in the medium immediately before each experiment. Antibodies against cleaved caspase-3, poly (ADP-ribose) polymerase (PARP), Akt, phospho-Akt, Bcl-2, Bax and peroxidase-conjugated secondary antibodies were from Cell Signaling Technology, Inc. (Beverly, MA, USA). Antibody against glyceraldehyde-3-phosphate dehydrogenase was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The enhanced chemiluminescence (ECL) detection system was obtained from Amersham Life Science, Inc. (Arlington Heights, IL, USA).
Cell viability assay
Cell viability was assessed using the MTT assay. DU145 and PC-3 cells were incubated with or without TET for various durations, and incubated with 0.5 mg ml−1 MTT at 37°C for 4 h. After incubation, cells were lysed with dimethyl sulfoxide. The absorbance was determined using a 96-well microplate reader at a wavelength of 490 nm (Bio-Rad, Hercules, CA, USA). The experiments were performed in triplicate.
Flow cytometry analysis
DU145 and PC-3 cells were exposed to different doses of TET (HCl, 2.5, 5.0, and 10.0 μmol l−1) for 48 h. Cells were stained with fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide (PI) using the Annexin V-FITC Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA), according to the manufacturer's protocol. Apoptotic cells were then analyzed by flow cytometry (BD FACScan Flow Cytometer; BD Biosciences, San Jose, CA, USA). The representative data presented in this study were reproduced in three independent experiments.
Clone formation assay
Prostate cancer cell lines (DU145 and PC-3) were seeded onto six-well plates (1000 per well). When cells were adherent, diverse doses of TET or solvent control containing diluted HCl were added to each well. When the cell density in solvent control reached > 50 per cluster, cells were washed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde, and stained with 0.1% crystal violet (Beyotime, Shanghai, China). After washing with PBS again, cloning of the cells was visible with the naked eye, and cells were counted from five randomly selected fields with microscopy at × 100 magnification. The experiments were performed in triplicates.
Western blotting
After treatment under each experimental condition, total cell lysates were denatured with lysis buffer (10 mmol l−1 Tris-HCl [pH 7.4], 150 mmol l−1 NaCl, 0.1% sodium dodecyl sulfate [SDS], 1 mmol l−1 ethylenediaminetetraacetic acid, 1 mmol l−1 ethylene glycol tetraacetic acid, 0.3 mmol l−1 phenylmethylsulfonyl fluoride, 0.2 mmol l−1 sodium orthovanadate, 1% NP-40, 10 mg ml−1 leupeptin, and 10 mg ml−1 aprotinin). Clarified protein lysates (30–60 μg, whole-cell lysates, mitochondrial and cytosolic fractions) were electrophoretically resolved on denaturing SDS-polyacrylamide gel (12%), transferred onto nitrocellulose membranes, and probed with primary antibodies against desired molecules overnight at 4°C followed by peroxidase-conjugated secondary antibody for 1 h at room temperature (25°C). Finally, proteins were visualized by ECL detection and exposure to X-ray film.
Wound healing assay
Prostate cancer DU145 or PC-3 cells were seeded onto six-well plates. When the cell density reached above 90%, scratch wounds were made by scraping the cell layer in each culture plate using the tip of a 200-μl pipette. Later the cells were washed with PBS. The wounded cultures were later incubated in a serum-free medium with various concentrations of TET at different times, and then five fields (×100) were randomly chosen from each scratch wound and visualized by microscopy to evaluate the ability of cell migration. The experiments were performed in triplicates.
Transwell invasion assay
Transwell membranes (polycarbonic membrane, diameter 6.5 mm, pore size 8 μm) were coated with matrigel in a total of 50 μl each transwell (matrigel: serum-free medium 1:5). After 5 h, cells (DU145: 6 × 104 or PC-3: 12 × 104 in 200 μl serum-free medium, respectively) with TET were seeded onto the upper chamber, and 800 μl medium with 10% fetal calf serum was added to the lower chamber. After certain duration of incubation, cells adhering to the upper surface of the membrane were removed with a cotton swab. The migrated cells, which adhered to the lower surface, were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet (Beyotime, Shanghai, China). The number of cells attached to the lower surface was counted from five randomly chosen visual fields with microscopy at × 100 magnification. Data were obtained from three independent experiments.
Statistical analysis
All statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Statistical differences among the control and various treatment groups were compared using one-way analysis of variance, followed by Dunnett's t-test for multiple comparisons. Student's t-test (two-sided) was used for comparisons involving only two groups. P < 0.05 was considered statistically significant.
RESULTS
Tetrandrine inhibits the growth of prostate cancer DU145 and PC-3 cells
First, the growth inhibitory effects of TET on prostate cancer DU145 and PC-3 cells by the MTT assay and clone formation assay were investigated. As shown in Figure 1, TET inhibited the growth of DU145 and PC-3 cells in a dose- and time-dependent manner. Since TET treatment at 10, 15, 20, and 30 μmol l−1 showed strong inhibitory effects on the proliferation of prostate cancer DU145 and PC-3 cells, TET at 2.5, 5.0, and 10.0 μmol l−1 was chosen as the representative doses for the in vitro treatment in the subsequent studies. Meanwhile, cell cloning was inhibited in the presence of TET in DU145 and PC-3 cells (Figure 2).
Figure 1.
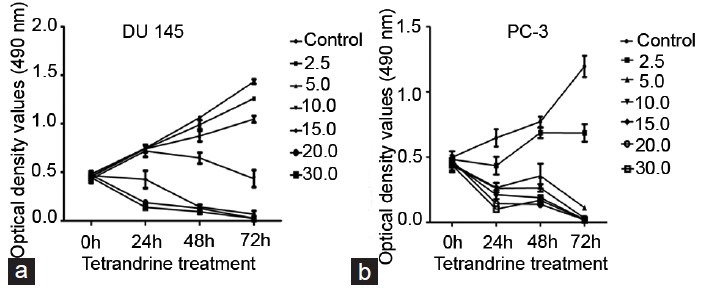
The inhibitory effect of tetrandrine (TET) on proliferation of (a) DU145 and (b) PC-3 cells. DU145 and PC-3 cells were treated with indicated concentrations of TET for three periods (24 h, 48 h and 72 h). The optical density values (490 nm) and viability of cells were measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay using HCl as a blank. Each assay was performed in triplicate. The data represent mean ± s.d.; s.d.: standard deviation.
Figure 2.
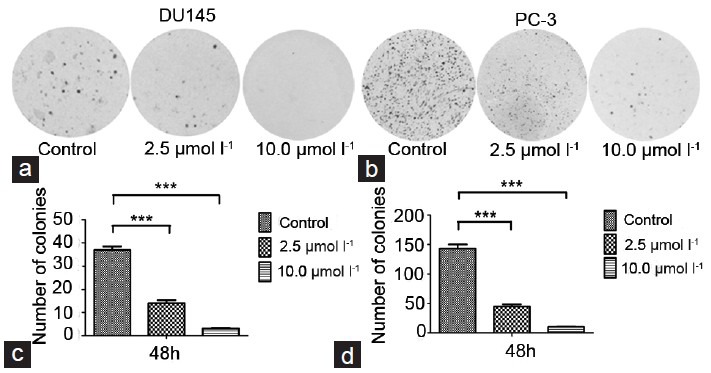
Cloning formation assay. DU145 cells were treated with indicated doses of tetrandrine for 48 h (a and c). PC-3 cells were treated for 48 h (b and d). Later the cells were subjected to 0.1% crystal violet as described in the materials and methods.
Tetrandrine induces apoptosis in prostate cancer DU145 and PC-3 cells
Since a significant inhibitory effect of TET on prostate cancer DU145 and PC-3 cells was observed, whether TET could induce apoptosis in prostate cancer cells by annexin V and PI double staining was investigated. The effect of TET on the apoptosis of PC-3 cells, as detected by flow cytometry, is shown in Figure 3. The TET treatments at 2.5, 5, and 10 μmol l−1 for 48 h resulted in 12.01%, 16.56%, and 69.24% of apoptotic cells, respectively, and the baseline apoptosis of the solvent control cells was 5.08% (P < 0.05). Similar effects were observed in DU145 cells (data not shown). These results indicated that TET could induce apoptosis in human prostate cancer DU145 and PC-3 cells in a dose-dependent manner. Moreover, as shown in (Figure 4), TET could activate the cleavage of caspase-3 (17 kDa and 19 kDa) and PARP (89 kDa) in PC-3 cells in a dose-dependent manner, which indicated that TET triggered caspase cascade. Similar effects were observed in DU145 cells (Figure 4).
Figure 3.
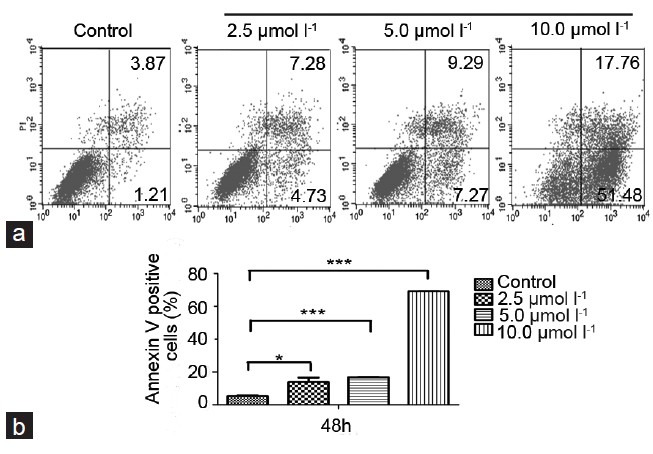
(a) Flow cytometry analysis to detect the apoptotic effect of tetrandrine (TET) on PC-3 cells. After treatment with TET for 48 h, cells were harvested and stained with annexin-V/propidium iodide (PI) followed by FACS analysis as described in the Materials and methods. (b) PC-3 stained with annexin V/PI after treatment with various concentrations of TET for 48 h. Data represent mean ± s.d.; s.d.: standard deviation.
Figure 4.
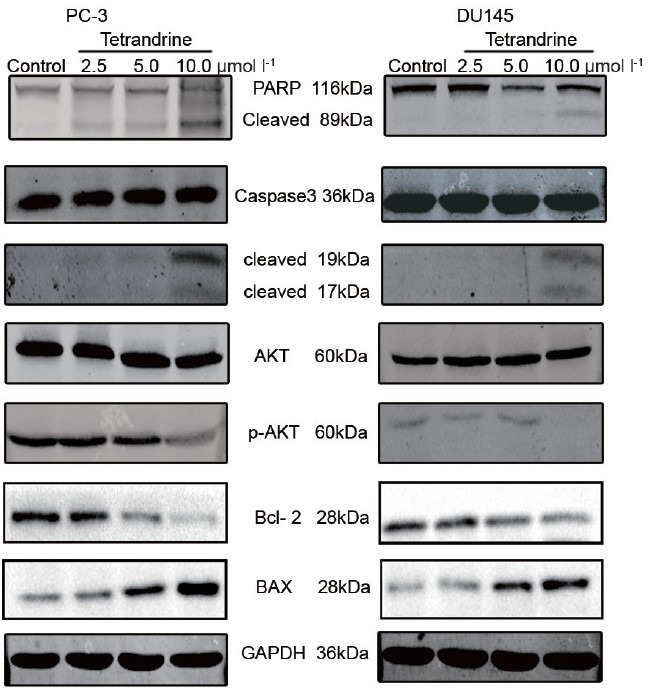
Western blotting was used to detect the caspase cascade and Akt pathway-related protein with tetrandrine (TET) treatment in PC-3 and DU145 cells. Activation of caspase-3 and poly (ADP-ribose) polymerase, downregulation of phospho-Akt and Bcl-2, and upregulation of Bax were observed with 48 h treatment of TET. Glyceraldehyde-3-phosphate dehydrogenase was used as a loading control.
The phosphoinositide 3-kinase (PI3K)-Akt signal pathway is a critical pathway that regulates various biological processes including apoptosis, cell cycle, proliferation, etc., Western blotting was used to detect the expression of Akt-related protein, including Akt, phospho-Akt, Bcl-2, and Bax. The results showed that there is a significant concentration-dependent decrease of phosphorylated Akt and Bcl-2; Bax showed the opposite effect (Figure 4).
Tetrandrine inhibits migration and invasion in prostate cancer DU145 and PC-3 cells
To evaluate the impact of TET on cell migration, the wound healing assay and transwell migration assay were performed. It was found that TET suppressed the migration of DU145 and PC-3 cells (Figure 5). Consistent with this finding, transwell invasion assay showed that TET significantly weakened invasion capacity of DU145 cells (Figure 6). These results suggested that TET played an important role in inhibiting migration and invasion potential of human prostate cancer cells.
Figure 5.
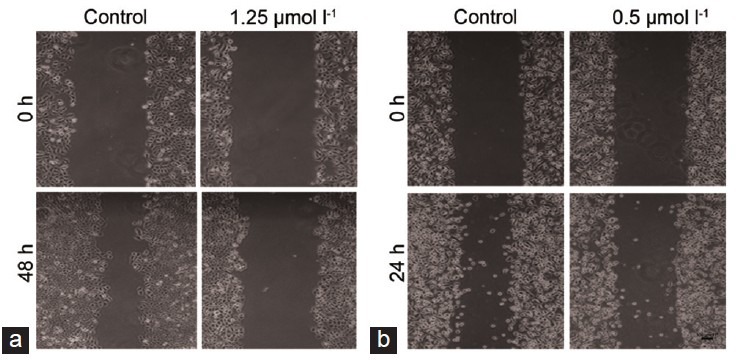
Tetrandrine inhibits cell migration in (a) DU145 and (b) PC-3 cells. The cell viability was measured by wound healing assay. Five random views were chosen along the scratch wound in each well at × 100.
Figure 6.
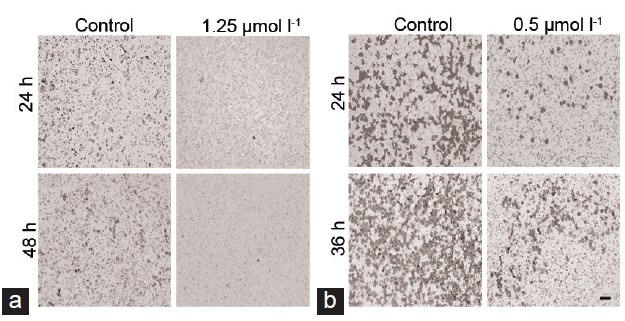
Transwell invasion assay. (a) DU145 and (b) PC-3 cells which penetrated the membrane were fixed and stained after 48 h as described in the materials and methods. Scale bar = 100 μm.
DISCUSSION
Tetrandrine has a long history of use in traditional Chinese medicine.15 There is accumulating evidence that TET partly exhibited antitumor activity against various cancer cells in vitro and vivo. However, whether TET could function on human prostate cancer cells remains under investigation. In this study, the effect of TET on human prostate cancer cells was demonstrated. It was observed that treatment with TET could trigger the inhibition of growth, induction of apoptosis, and inhibition of migration and invasion in DU145 and PC-3 cell lines; in addition, induction of apoptosis may be at least partially associated with the activation of caspase. To date, this is the first report about the effects of TET on human prostate cancer cells.
Tetrandrine elicited a concentration- and time-dependent inhibition of proliferation in DU145 and PC-3 cells. The drug concentration causing 50% inhibition of the desired activity (IC50) value decreased from 18.87 to 5.84 μmol l−1 for DU145 cells at different incubation times (PC-3 cells: from 6.95 to 1.42 μmol l−1). The low value of IC50 suggested that TET may play an important role as a growth inhibitor in prostate cancer. The availability of TET in vivo remains unknown. Hence, there is an urgent need to establish an animal model to confirm the inhibition of growth of TET on prostate cancer.
In the present study, TET showed a remarkable effect in inducing apoptosis in prostate cancer cells. Compared with DU145, PC-3 cells are more sensitive. As known, apoptosis consists of two diverse pathways: the extrinsic and the intrinsic pathways.16 Both pathways triggered caspase cascade, converged on caspase-3, and ultimately led to the morphologic characteristics of apoptosis such as DNA fragmentation, chromatin condensation, cell shrinkage, and membrane blebbing.17 This is the first study that reported that TET could induce activation of caspase-3 and cleavage of PARP on prostate cancer. Akt, as critical kinase, is implicated in cancer cell apoptosis by regulating downstream signal transduction cascade. The downregulation of phospho-Akt and Bcl-2 and the upregulation of Bax suggested that the apoptosis induced by TET on prostate cancer may be at least in part mediated by Akt pathway, although further investigation is required.
Additionally, wound healing assay and transwell assay were used to evaluate the function of TET as a migration or invasion inhibitor. Significant inhibition was observed with the treatment of TET. Thus, molecular mechanisms of TET in regulating the migration and invasion of cancer cells should be focused further.
The present study has several limitations. On the one hand, the lack of animal models is a shortcoming, and the function of TET on animal models should be considered in future studies. On the other hand, the function of TET on prostate cancer cells was alone observed. Whether TET could function on normal cells, or it has cancer specificity has not yet been elucidated. Hence, it is necessary to resolve these questions in further studies.
In summary, this study provided the first evidence that TET inhibited proliferation, migration, and invasion, and presented apoptotic capacity in human prostate cancer DU145 and PC-3 cells. The apoptosis induced by TET might be associated with PI3K-Akt pathway and activation of caspases. These findings suggest that TET may be used as a potential anticancer candidate against prostate cancer in clinical setting in the future.
AUTHOR CONTRIBUTIONS
WL and BK participated in the design of the trial, conducted the data acquisition, interpreted and statistically analyzed the data and drafted the manuscript. ZKM and XST designed the study and offered the study materials. CL, MY and JQC conducted the data acquisition, interpreted and statistically analyzed the data. LL and XYW conducted the data acquisition. DLH designed the study, interpreted the data and drafted the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare no competing interests.
ACKNOWLEDGEMENTS
This work was partially supported by the National Natural Science Foundation of China (81130041 to DLH, 81072107 to LL).
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Wang G, Lemos JR, Iadecola C. Herbal alkaloid tetrandrine: fron an ion channel blocker to inhibitor of tumor proliferation. Trends Pharmacol Sci. 2004;25:120–3. doi: 10.1016/j.tips.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Lai YL, Chen YJ, Wu TY, Wang SY, Chang KH, et al. Induction of apoptosis in human leukemic U937 cells by tetrandrine. Anticancer Drugs. 1998;9:77–81. doi: 10.1097/00001813-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Gong K, Mao X, Li W. Tetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinoma. Int J Cancer. 2011;129:1519–31. doi: 10.1002/ijc.25817. [DOI] [PubMed] [Google Scholar]
- 5.Yoo SM, Oh SH, Lee SJ, Lee BW, Ko WG, et al. Inhibition of proliferation and induction of apoptosis by tetrandrine in HepG2 cells. J Ethnopharmacol. 2002;81:225–9. doi: 10.1016/s0378-8741(02)00082-x. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Lu X, Xu H, Zhu Z, Yin H, et al. Paclitaxel/tetrandrine coloaded nanoparticles effectively promote the apoptosis of gastric cancer cells based on “oxidation therapy”. Mol Pharm. 2012;9:222–9. doi: 10.1021/mp2002736. [DOI] [PubMed] [Google Scholar]
- 7.He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, et al. Tetrandrine inhibits Wnt/ß-catenin signaling and suppresses tumor growth of human colorectal cancer. Mol Pharmacol. 2011;79:211–9. doi: 10.1124/mol.110.068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu JM, Chen Y, Chen JC, Lin TY, Tseng SH. Tetrandrine induces apoptosis and growth suppression of colon cancer cells in mice. Cancer Lett. 2010;287:187–95. doi: 10.1016/j.canlet.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Cho HS, Chang SH, Chung YS, Shin JY, Park SJ, et al. Synergistic effect of ERK inhibition on tetrandrine-induced apoptosis in A549 human lung carcinoma cells. J Vet Sci. 2009;10:23–8. doi: 10.4142/jvs.2009.10.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Tseng SH. The potential of tetrandrine against gliomas. Anticancer Agents Med Chem. 2010;10:534–42. doi: 10.2174/187152010793498609. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Chen JC, Tseng SH. Tetrandrine suppresses tumor growth and angiogenesis of gliomas in rats. Int J Cancer. 2009;124:2260–9. doi: 10.1002/ijc.24208. [DOI] [PubMed] [Google Scholar]
- 12.Wang TH, Wan JY, Gong X, Li HZ, Cheng Y. Tetrandrine enhances cytotoxicity of cisplatin in human drug-resistant esophageal squamous carcinoma cells by inhibition of multidrug resistance-associated protein 1. Oncol Rep. 2012;28:1681–6. doi: 10.3892/or.2012.1999. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Su B, Liu R, Wu D, He D. Tetrandrine induces apoptosis and triggers caspase cascade in human bladder cancer cells. J Surg Res. 2011;166:e45–51. doi: 10.1016/j.jss.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 14.Chen T, Ji B, Chen Y. Tetrandrine triggers apoptosis and cell cycle arrest in human renal cell carcinoma cells. J Nat Med. 2014;68:46–52. doi: 10.1007/s11418-013-0765-0. [DOI] [PubMed] [Google Scholar]
- 15.Parekh HS, Liu G, Wei MQ. A new dawn for the use of traditional Chinese medicine in cancer therapy. Mol Cancer. 2009;8:21. doi: 10.1186/1476-4598-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55:178–94. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 17.Kuranaga E. Caspase signaling in animal development. Dev Growth Differ. 2011;53:137–48. doi: 10.1111/j.1440-169X.2010.01237.x. [DOI] [PubMed] [Google Scholar]


