Abstract
Objective
Mortality among adults of all ages diagnosed with impaired glucose regulation (IGR) in Tayside, Scotland, UK, was evaluated using routinely collected healthcare data sets.
Research design and methods
Using record-linked data in 2003–2008, all instances of blood glucose testing in the population defined 2 cohorts of patients aged 18+years: those with IGR (whether impaired fasting glucose or impaired glucose tolerance (IGT)) according to the WHO criteria, and those who were normoglycemic. They were followed in survival analyses for mortality or cardiovascular mortality (censoring deaths that occurred within a 30-day period of testing), to derive HRs (with 95% CI) for IGR status using Cox regression, adjusted for age, sex, and an area measure of deprivation.
Results
There were 2 372 712 tests for 214 094 patients, with 196 799 patients in the non-IGR cohort and 50 080 in the IGR cohort. During follow-up, 19 147 (9.7%) and 8397 (16.8%) patients died in 2 cohorts, respectively, with mortality rates of 33.2/1000 patient-years and 70.7/1000 patient-years. In multivariable analyses, the overall adjusted risk of mortality for IGR was 1.16 (95% CI 1.13 to 1.20). However, it was 2.59 (95% CI 2.17 to 3.10) for people aged <45 years, decreasing to 0.94 (95% CI 0.85 to 1.00) in those aged 85+years. The HRs for cardiovascular mortality were lower overall, but they followed the same pattern, with statistically significant increased risks for patients aged <64 years only. The mortality risk was highest among patients with IGT.
Conclusions
IGR is associated with an increased mortality risk which declines with age. It is therefore important to prioritize young people with IGR for prevention; but less important to be aggressive about risk factor modification in older people.
Keywords: Impaired Fasting Glucose, Impaired Glucose Tolerance, Epidemiology, Mortality
Key messages.
Impaired glucose regulation (IGR; impaired fasting glucose or impaired glucose tolerance) is associated with an increased risk of mortality and cardiovascular mortality.
This increased risk is higher among patients with impaired glucose tolerance.
The increased risk decreases as age increases; no increased risk of mortality associated with IGR is evident among patients over the age of 85 years.
It is important to prioritize young people with IGR for prevention; but less important to be aggressive about risk factor modification in older people with IGR, thereby limiting the risk of adverse side effects from inappropriate medications.
Diabetes mellitus increases the risk of all-cause and cardiovascular mortality.1 Impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), collectively known as impaired glucose regulation (IGR) or prediabetes, are diagnosed in patients whose blood glucose levels are above the normal range, but not high enough to be diagnostic of diabetes.2 IFG and IGT can occur separately or in combination, and are associated with an increased risk of type 2 diabetes.3 There is increasing evidence that these intermediate states of hyperglycemia are also associated with increased all-cause and cardiovascular mortality. Risks for adverse outcomes such as mortality or cardiovascular mortality are likely to be continuous across the glucose range, and diagnoses of IFG or IGT represent arbitrary cut-off points, depending also on whether fasting or 2 h plasma glucose tests have been carried out.3 Despite this, wide variation exists in estimates of the magnitude of these increased risks between different studies.
For example, the Whitehall study showed that IGT doubled the risk of mortality from coronary heart disease among men aged 40–64 years.4 Among older people (50–75 years) in the Hoorn study, all-cause and cardiovascular mortality increased as glycemic measures (2 h postload glucose and glycated hemoglobin (HbA1c)) increased.5 Recently, in the Australian Diabetes, Obesity and Lifestyle Study, the risk of mortality was increased by 1.6-fold and 1.5-fold for 610 adults over 25 years with IFG and 1298 with IGT, respectively, over mean follow-up of 5.2 years, when they were compared with normoglycemic adults,6 but the Cardiovascular Health Study found no independent increased risk of mortality associated with prediabetes among 4602 older people (>65 years) in the USA,7 neither did a German study of 1466 adults aged 55–74 years.8 In contrast, a US study using the National Health and Nutrition Examination Survey over an 18-year period showed that mortality was higher in patients with prediabetes aged 35–74 years, although glycemic status in this study was measured by HbA1c.9
It is likely that differences between studies are due to underlying differences in the study populations and also differences in measurement of IGR. While the definitions of IGT and IFG may be arbitrary, they do have meaning for clinicians and patients. In this 6-year study, we therefore evaluated the risk of mortality among all adults diagnosed with IGR in Tayside, Scotland. By including adults of all ages, we were able to examine differences by age in particular detail.
Research design and methods
This study was carried out using the data sources of the Health Informatics Centre (HIC), University of Dundee, for the population of Tayside, in Scotland, UK. HIC has developed the record linkage of multiple, routinely collected health data sets to carry out anonymized diabetes research in the population of Tayside. Electronic linkage is facilitated by the widespread use of a 10-digit Community Health Identifier (CHI) that is assigned universally to all patients in Scotland when they register with a general practitioner. Registration of these patients is held on a central index.10
The specific data sets used for this study included a biochemistry data set with records of all blood glucose testing results for the Tayside population, a comprehensive diabetes clinical information system (SCI-DC), records of death certification from the General Register Office for Scotland with a coded diagnosis for underlying cause of death, and demographic data held centrally on the CHI index. This includes a quintile of deprivation, known as Scottish Index of Multiple Deprivation (SIMD), which is calculated using 38 area indicators of access to services, education, employment, crime, housing, health, and income.11 The demographic breakdown of Tayside is broadly similar to that of the rest of Scotland, with low proportions of residents from ethnic minority groups.12
The 6-year study period was from January 2003 to December 2008. All instances of blood glucose testing in the population of Tayside during this time were identified, after excluding non-valid records (eg, damaged samples). Tests were classified as indicating a diagnosis of IGT or IFG if the patient had undergone two blood glucose tests on the same date (one coded in the database as a fasting test with the second test assumed to be carried out after an oral glucose tolerance test) that met the WHO (6) criteria for IGT and IFG (IGT: first test <7.0 mmol/L and second test 7.8–11.0 mmol/L; IFG: first test 6.1–6.9 mmol/L and second test <7.8 mmol/L). However, the majority of patients were found to have only taken a fasting test. The WHO criteria allow a single fasting test of 6.0–6.9 mmol/L to diagnose IFG (although IGT and type 2 diabetes cannot be ruled out).
From these testing results, two cohorts of patients were identified. They had to be at least 18 years at the time of testing. Patients whose first test during the study period was diagnostic of diabetes were not included in the study.
Non-IGR cohort: This cohort included all patients for whom there was a record of blood glucose testing during the 6-year study period that was not diagnostic of IGR. Their index date was the date of the first such test during the study period. If they subsequently developed IGR they moved into the IGR cohort.
IGR cohort: This cohort included all patients for whom there was a record of blood glucose testing during the 6-year study period that was diagnostic of IGR. Their index date was the date of the first such test during the study period.
Patients in the two cohorts were followed to the study end date (31 December 2008). If they left Tayside before the study end date, they were censored, as were patients in the non-IGR cohort who developed IGR (censored at date of first test diagnostic of IGR). Similarly patients who were diagnosed with type 2 diabetes were censored at the time of their diabetes diagnosis. Otherwise, the primary outcome variable was mortality that occurred at least 30 days after the index date. Thus, if the patient died within 30 days of the index date, they were censored at this date.
Using Cox regression to assess the risk of the primary outcome variable (>30 day mortality), hazard ratios (with 95% CIs) were determined in univariable analyses for non-IGR versus IGR cohort, age at testing, sex, and SIMD deprivation category. Those covariates that were statistically significant (p<0.05) in the univariable analyses were entered into a multivariable model. The analysis was then repeated for cardiovascular mortality only. To be defined as cardiovascular mortality, the underlying cause of death on the death certificate had to be ICD-10 coded as a cardiovascular diagnosis. The adjusted hazard ratios were then determined for these outcome variables in subgroups stratified by age.
The results for the fasting test were then extracted for patients in the IGR cohort who were categorized into four groups. The first group were those who were known to have IGT. The others were either diagnosed with IFG or undefined IGR (as previously described13). Their fasting test results were categorized as <6.4, 6.4 to <6.7, and 6.7+mmol/L. Hazard ratios for >30-day mortality and cardiovascular mortality were determined, adjusted for other covariates.
The Tayside Committee for Medical Research Ethics has granted general approval for studies using routinely collected, anonymized health data.
Results
There were 2 372 712 tests in Tayside during the study period for a total of 214 094 patients. The majority (around 90%) were single fasting tests. The final non-IGR cohort comprised 196 799 patients of at least 18 years of age, of whom 32 785 had a subsequent test diagnostic of IGR, at which point they moved into the IGR cohort. The IGR cohort comprised 50 080 patients. The age and sex breakdown of the non-IGR and IGR cohorts are presented in table 1. There was a higher proportion of males in the IGR cohort, and higher proportions of patients from less affluent areas. The mean age of patients was 63 years (median 65 years), compared with 54 years in the non-IGR cohort (median 55 years). In total, 2381 patients in the IGR cohort developed type 2 diabetes within the study period.
Table 1.
Sociodemographic characteristics of non-IGR and IGR cohort
| Non-IGR cohort | IGR cohort | |
|---|---|---|
| Sex | ||
| Male | 82 417 (41.9%) | 23 858 (47.6%) |
| Female | 114 382 (58.1%) | 26 222 (52.4%) |
| Age category (years) | ||
| 18–34 | 38 620 (19.6%) | 3791 (7.6%) |
| 35–44 | 28 454 (14.5%) | 4206 (8.4%) |
| 45–54 | 32 599 (16.6%) | 6667 (13.3%) |
| 55–64 | 35 498 (18.1%) | 9849 (19.7%) |
| 65–74 | 31 381 (15.9%) | 10 966 (21.9%) |
| 75–84 | 22 503 (11.4%) | 10 266 (20.5%) |
| 85+ | 7742 (3.9%) | 4335 (8.7%) |
| SIMD category | ||
| 1 (least affluent) | 35 967 (18.8%) | 9302 (18.9%) |
| 2 | 38 082 (19.9%) | 10 006 (20.4%) |
| 3 | 39 632 (20.7%) | 10 069 (20.5%) |
| 4 | 39 950 (20.8%) | 10 027 (20.4%) |
| 5 (most affluent) | 38 192 (19.9%) | 9761 (19.9%) |
IGR, impaired glucose regulation; SIMD, Scottish Index of Multiple Deprivation.
During follow-up, 19 147 (9.7%) patients died in the non-IGR cohort; 8397 (16.8%) patients died in the IGR cohort. Mean (median) follow-up time among all patients was 1028 (1024) days: 1069 (1069) days for the non-IGR cohort and 866 (806) days for the IGR cohort. In the non-IGR cohort the mortality rate was 33.2 deaths per 1000 patient years of follow-up. In the IGR cohort, it was 70.7 deaths per 1000 patient years. A relatively high proportion of deaths (15%) occurred within 30 days of the index date, probably representing patients who had their blood glucose levels tested on presentation with a condition.
In Cox regression analyses (table 2), being male, increasing age, and living in less affluent areas were all strongly associated with increasing risks of mortality (when patients who died within 30 days of the index date were censored). Patients in the IGR cohort were nearly twice as likely to die compared with patients in the non-IGR cohort. In multivariable analyses that then adjusted for confounding by age, sex, and SIMD, the risk of IGR was still elevated, although lower. In analyses for cardiovascular mortality only, increasing age, and being male and less affluent were all still strongly associated with risk of mortality, but there was no increased risk associated with being in the IGR cohort.
Table 2.
Unadjusted and adjusted HRs (with 95% CI) for deaths after 30 days for all covariates: all-cause and cardiovascular mortality
| Total (n) | Number of deaths* (%) | Univariate | Multivariate | |
|---|---|---|---|---|
| All-cause mortality | ||||
| Sex | ||||
| Male | 106 275 | 13 033 (12.3) | 1.20 (1.17 to 1.24) | 1.38 (1.35 to 1.42) |
| Female | 140 609 | 14 511 (10.3) | 1.00 | 1.00 |
| Age category (years) | ||||
| 18–34 | 42 411 | 287 (0.7) | 1.00 | 1.087† (1.086 to 1.088) |
| 35–44 | 32 660 | 536 (1.6) | 2.43 (2.07 to 2.85) | |
| 45–54 | 39 266 | 1226 (3.1) | 4.72 (4.10 to 5.48) | |
| 55–64 | 45 347 | 2960 (6.5) | 9.68 (8.45 to 11.08) | |
| 65–74 | 42 349 | 6059 (14.3) | 21.54 (18.87 to 24.60) | |
| 75–84 | 32 769 | 9999 (30.5) | 51.30 (44.98 to 58.51) | |
| 85+ | 12 077 | 6476 (53.6) | 115.39 (101.06 to 131.74) | |
| SIMD category | ||||
| 1 (least affluent) | 45 269 | 5782 (12.8) | 1.23 (1.18 to 1.29) | 1.70 (1.63 to 1.77) |
| 2 | 48 088 | 5481 (11.4) | 1.10 (1.06 to 1.15) | 1.32 (1.26 to 1.37) |
| 3 | 49 701 | 5357 (10.8) | 1.06 (1.01 to 1.10) | 1.18 (1.13 to 1.23) |
| 4 | 49 977 | 5704 (11.4) | 1.13 (1.09 to 1.18) | 1.13 (1.09 to 1.18) |
| 5 (most affluent) | 47 953 | 4843 (10.1) | 1.00 | 1.00 |
| IGR status | ||||
| No | 196 799 | 19 147 (9.7) | 1.00 | 1.00 |
| Yes | 50 080 | 8397 (16.8) | 1.93 (1.87 to 1.98) | 1.16 (1.13 to 1.20) |
| Cardiovascular mortality | ||||
| Sex | ||||
| Male | 106 275 | 6573 (6.2 | 1.19 (1.14 to 1.25) | 1.50 (1.43 to 1.67) |
| Female | 140 609 | 7451 (5.3) | 1.00 | 1.00 |
| Age category (years) | ||||
| 18–34 | 42 411 | 28 (0.1) | 1.00 | 1.116† (1.113 to 1.119) |
| 35–44 | 32 660 | 137 (0.4) | 10.85 (4.31 to 27.34) | |
| 45–54 | 39 266 | 403 (1.0) | 31.32 (12.85 to 76.29) | |
| 55–64 | 45 347 | 1190 (2.6) | 86.79 (35.97 to 209.40) | |
| 65–74 | 42 349 | 3024 (7.1) | 239.69 (99.61 to 576.75) | |
| 75–84 | 32 769 | 5784 (17.7) | 745.27 (310.0 to 1791.7) | |
| 85+ | 12 077 | 3458 (28.6) | 1910.1 (794.2 to 4593.4) | |
| SIMD category | ||||
| 1 (least affluent) | 45 269 | 2463 (5.4) | 0.94 (0.89 to 1.10) | 1.54 (1.42 to 1.67) |
| 2 | 48 088 | 2686 (5.6) | 0.99 (0.94 to 1.05) | 1.28 (1.19 to 1.39) |
| 3 | 49 701 | 2985 (6.0) | 1.06 (1.01 to 1.12) | 1.29 (1.20 to 1.40) |
| 4 | 49 977 | 3006 (6.0) | 1.09 (1.03 to 1.15) | 1.14 (1.06 to 1.23) |
| 5 (most affluent) | 47 953 | 2681 (5.6) | 1.00 | 1.00 |
| IGR status | ||||
| No | 196 799 | 9897 (5.0) | 1.00 | 1.00 |
| Yes | 50 080 | 4127 (8.2) | 1.82 (1.72 to 1.92) | 1.01 (0.96 to 1.07) |
*Total includes all deaths (including those prior to 30 days).
†Age entered as continuous variable.
IGR, impaired glucose regulation; SIMD, Scottish Index of Multiple Deprivation.
A formal test of interaction indicated that there was interaction between age and IGR status (p<0.01); therefore, the results were stratified by age (table 3). The increased mortality risks associated with IGR were strongest in younger age groups. Patients aged <45 years had over twice the risk of mortality if they were in the IGR cohort. This risk decreased with increasing age and IGR conferred no excess mortality to patients aged 85 years or over. Although there was no increased risk for cardiovascular mortality overall that was associated with IGR, there was an increased risk evident for younger patients (aged<65 years).
Table 3.
Adjusted HRs (with 95% CI) for deaths after 30 days for all covariates, stratified by age group: all-cause and cardiovascular mortality
| <45 years | 45–64 years | 65–84 years | 85+years | |
|---|---|---|---|---|
| All-cause mortality | ||||
| Total deaths >30 days | 667 | 3388 | 13 436 | 5778 |
| Sex | ||||
| Male | 2.00 (1.71 to 2.34) | 1.41 (1.31 to 1.50) | 1.42 (1.37 to 1.47) | 1.26 (1.18 to 1.33) |
| Female | 1.00 | 1.00 | 1.00 | 1.00 |
| Age | 1.06 (1.05 to 1.07) | 1.073 (1.068 to 1.080) | 1.099 (1.096 to 1.103) | 1.08 (1.07 to 1.09) |
| SIMD category | ||||
| 1 (least affluent) | 2.79 (2.12 to 3.68) | 2.34 (2.11 to 2.60) | 1.65 (1.56 to 1.74) | 1.26 (1.15 to 1.38) |
| 2 | 2.06 (1.56 to 2.78) | 1.63 (1.46 to 1.81) | 1.29 (1.23 to 1.37) | 1.16 (1.07 to 1.27) |
| 3 | 1.09 (0.79 to 1.50) | 1.14 (1.01 to 1.28) | 1.19 (1.12 to 1.25) | 1.18 (1.08 to 1.28) |
| 4 | 1.16 (0.84 to 1.61) | 1.07 (0.95 to 1.21) | 1.17 (1.11 to 1.24) | 1.04 (0.90 to 1.13) |
| 5 (most affluent) | 1.00 | 1.00 | 1.00 | 1.00 |
| IGR status | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 2.59 (2.17 to 3.10) | 1.69 (1.56 to 1.82) | 1.12 (1.08 to 1.17) | 0.94 (0.89 to 1.00) |
| Cardiovascular mortality | ||||
| Total deaths >30 days | 49 | 604 | 3862 | 2076 |
| Sex | ||||
| Male | 1.43 (0.81 to 2.52) | 2.28 (1.93 to 2.69) | 1.53 (1.44 to 1.63) | 1.19 (1.08 to 1.32) |
| Female | 1.00 | 1.00 | 1.00 | 1.00 |
| Age | 1.21 (1.13 to 1.29) | 1.11 (1.09 to 1.12) | 1.13 (1.12 to 1.114) | 1.08 (1.07 to 1.09) |
| SIMD category | ||||
| 1 (least affluent) | 4.63 (1.60 to 13.41) | 3.28 (2.52 to 4.27) | 1.50 (1.36 to 1.66) | 1.09 (0.93 to 1.66) |
| 2 | 2.77 (0.90 to 8.50) | 2.19 (1.66 to 2.89) | 1.25 (1.13 to 1.37) | 1.11 (0.95 to 1.29) |
| 3 | 0.83 (0.21 to 3.31) | 1.58 (1.19 to 2.11) | 1.26 (1.14 to 1.40) | 1.30 (1.14 to 1.49) |
| 4 | 0.91 (0.23 to 3.63) | 1.25 (0.92 to 1.70) | 1.15 (1.04 to 1.27) | 1.10 (0.97 to 1.26) |
| 5 (most affluent) | 1.00 | 1.00 | 1.00 | 1.00 |
| IGR status | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 2.20 (1.12 to 4.33) | 1.22 (1.01 to 1.48) | 1.03 (0.96 to 1.10) | 0.92 (0.83 to 1.01) |
IGR, impaired glucose regulation; SIMD, Scottish Index of Multiple Deprivation.
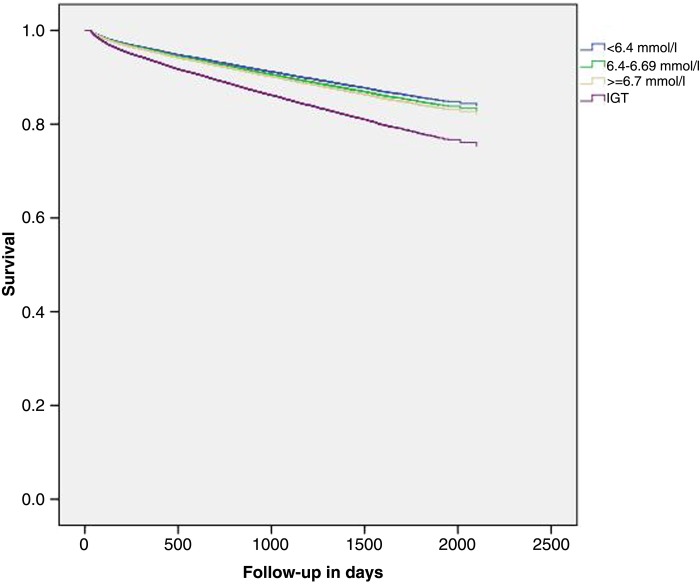
Among 50 080 patients with IGR, glucose values were not available for 2244. Among the rest, there were 1891 patients in the IGR cohort who were diagnosed as having IGT. Compared with patients with a fasting blood glucose of <6.4 mmol/L (n=20 249), the adjusted hazard ratio for >30-day mortality among patients with IGT was 1.61 (95% CI 1.45 to 1.79). For cardiovascular mortality, it was 1.49 (95% CI 1.20 to 1.85). The risks for IFG are presented in table 4, and increased slightly with increasing blood glucose levels. A Kaplan-Meier survival plot for overall mortality among these patients is shown in figure 1, clearly showing the increased mortality conferred by IGT.
Table 4.
Adjusted HRs (with 95% CI) for deaths after 30 days associated with fasting glucose levels/IGT
| IGR category | Total | Number of >30 deaths | Adjusted HRs (with 95% CI) |
|---|---|---|---|
| All cause mortality (mmol/L) | |||
| <6.4 | 20 249 | 2581 (12.7%) | 1.00 |
| 6.4–6.69 | 14 380 | 2040 (14.2%) | 1.07 (1.01 to 1.14) |
| ≥6.7 | 11 316 | 1717 (15.2%) | 1.12 (1.06 to 1.19) |
| IGT | 1891 | 397 (21.0%) | 1.61 (1.45 to 1.79) |
| Cardiovascular mortality (mmol/L) | |||
| <6.4 | 20 249 | 666 (3.3%) | 1.00 |
| 6.4–6.69 | 14 380 | 584 (4.1%) | 1.17 (1.05 to 1.31) |
| ≥6.7 | 11 316 | 470 (4.2%) | 1.16 (1.03 to 1.31) |
| IGT | 1891 | 95 (5.0%) | 1.49 (1.20 to 1.85) |
IGR, impaired glucose regulation; IGT, impaired glucose tolerance.
Figure 1.
Kaplan-Meier survival plot showing risk of mortality stratified by fasting glucose levels/impaired glucose tolerance (IGT).
Conclusions
This study in Tayside, Scotland, UK, shows that a diagnosis of IGR or prediabetes in a patient is associated with an increased risk of mortality, when compared with individuals who have had a blood glucose test but who are found to be normoglycemic. This excess risk decreases as age increases, and is absent among people aged over 85 years. This probably explains some of the conflicting evidence found on this association thus far. Several studies that have identified no increased risk have been restricted to older people.5 6 In this study, the increased risk evident for cardiovascular mortality is lower than for all-cause mortality, and no such increased risk is evident for people older than 65 years.
It is important to note that the comparator population of normoglycemic individuals in this study is not a healthy control group. They are people who have had a blood glucose test as part of their clinical care, for whatever reason (blood glucose tests are not carried out routinely in the UK), and are likely to be less healthy and more medicalized than the general population. For this reason, the risk ratios are probably all slightly underestimated. We have no information on possible lifestyle-related confounding factors, such as smoking or body mass index, that are associated with increased mortality and may be unequally distributed between the IGR and the non-IGR cohort. Although adjustment for SIMD may account for some of these differences (as adverse lifestyle factors are often patterned by SIMD), it is difficult to predict whether adjustment for specific factors would increase or decrease estimates of the HRs. Despite these limitations, we still see clear patterns emerging, particularly by age. Of relevance to clinicians and public health practitioners is that the effects on mortality of IGR status, and also deprivation, are more pronounced for younger patients (according with a previous study in Tayside that showed that a diagnosis of diabetes over the age of 65 years had a comparatively weak association with mortality14). The association between IGR and cardiovascular mortality appears to be less strong than that for overall mortality, and there is a less pronounced gradient for fasting plasma glucose levels.
This study suggests that patients with IGT are the highest risk group of patients with IGR, but that increasing fasting plasma glucose levels among patients with IFG are associated with increasing mortality. However, a limitation to this study is the possibility of misclassification of IGR diagnoses. Although it was assumed that two blood glucose results recorded on the same day represented a 2 h oral glucose tolerance test, this may not have been true for every patient. It is also possible that some IGT diagnoses were missed because the majority of patients only underwent one test, and those who had two may well have been sicker patients. The WHO criteria allow a single fasting test of 6.0–6.9 mmol/L to diagnose IFG (although IGT cannot then be ruled out). Misclassification of IGT in the IFG cohort would result in a dilution of differences between the two groups, yet we still see higher mortality in the IGT group. More detailed analysis of the associations between fasting and 2 h plasma glucose tests, whether they are independent of each other, and how they translate into excess mortality, is beyond the scope of this study, particularly given the frequency of single fasting tests only. This large record-linkage study has been carried out using well-validated data sets. The biochemistry database is a secure and complete record of all biochemical investigations for Tayside since 1993 and contains over 25 million data items. Identification of mortality relied on a national data set of death certification. We are therefore confident in the robustness of the data and study methods. The study highlights how important it is to prioritize young people with IGR for prevention, not only to reduce their risk of progression to type 2 diabetes13 but also to reduce their risk of mortality. Conversely, it is less important to be aggressive about risk factor modification in older people with IGR, thus limiting the risk of adverse side effects from inappropriate medications.
Acknowledgments
The authors acknowledge the Health Informatics Centre, University of Dundee, for provision of anonymized data for research.
Footnotes
Contributors: JMME, GPL and CEE designed the project. CEE acquired the data, identified patients for inclusion in the study and conducted preliminary analyses. JMME conducted the main analyses and wrote the paper. All three authors contributed to the discussion, reviewed/edited the manuscript and approved the final version. JMME is the guarantor.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–22. 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO/IDF Consultation Group. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. Geneva: World Health Organisation, 2006. [Google Scholar]
- 3.Unwin N, Shaw J, Zimmet P et al. . Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002:708–23. [DOI] [PubMed] [Google Scholar]
- 4.Fuller JH, Shipley MJ, Rose G et al. . Coronary heart disease risk and impaired glucose tolerance: the Whitehall Study. Lancet 1980;315:1373–6. 10.1016/S0140-6736(80)92651-3 [DOI] [PubMed] [Google Scholar]
- 5.De Vegt F, Dekker JM, Ruhe HG et al. . Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia 1999;42:926–31. 10.1007/s001250051249 [DOI] [PubMed] [Google Scholar]
- 6.Barr ELM, Zimmet PZ, Welborn TA et al. . Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance. The Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007;115:151–7. 10.1161/CIRCULATIONAHA.106.685628 [DOI] [PubMed] [Google Scholar]
- 7.Deedwania P, Patel K, Fonarow GC et al. . Prediabetes is not an independent risk factor for incident heart failure, other cardiovascular events or mortality in older adults: findings from a population-based cohort study. Int J Cardiol 2013;168:3616–22. 10.1016/j.ijcard.2013.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowall B, Rathmann W, Heier M et al. . Categories of glucose tolerance and continuous glycemic measures and mortality. Eur J Epidemiol 2011;26:637–45. 10.1007/s10654-011-9609-y [DOI] [PubMed] [Google Scholar]
- 9.Stokes A, Mehta NK. Mortality and excess risk in US adults with pre-diabetes and diabetes: a comparison of two nationally representative cohorts, 1988–2006. Popul Health Metr 2013;11:5 10.1186/1478-7954-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris AD, Boyle DI, MacAlpine R et al. . The diabetes audit and research in Tayside Scotland (DARTS) study: electronic record linkage to create a diabetes register. DARTS/MEMO Collaboration. BMJ 1997;315:524–8. 10.1136/bmj.315.7107.524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Using the Scottish Index of Multiple Deprivation 2006: Guidance leaflet. http://www.scotland.gov.uk/Publications/2006/10/13142841/1
- 12.NHS Tayside. Director of Public Health 2013–2014. Annual Report, 2014.
- 13.Eades C, Leese GP, Evans JMM. Incidence of impaired glucose regulation and progression to type 2 diabetes mellitus in the Tayside region of Scotland. Diabetes Res Clin Pract 2014;104:e16–19. 10.1016/j.diabres.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 14.Tan HH, Ritchie RR, James P et al. . Diagnosis of type 2 diabetes at an older age. Diabetes Care 2004;27:2797–9. 10.2337/diacare.27.12.2797 [DOI] [PubMed] [Google Scholar]