Objectives:
To assess whether therapeutic vaccination with ALVAC-HIV ± Remune affects viral reservoir size in antiretroviral therapy–treated individuals.
Methods:
Participants in CTN 173, a multicentre, randomized, 3-arm, placebo-controlled, double-blind study, were vaccinated with ALVAC-HIV ± Remune (groups 1 and 2, respectively) or with placebos (group 3) over 20 weeks and assessed for changes in the size of their viral reservoirs from weeks 0 to 24.
Results:
Sixteen participants completed the viral reservoir substudy. The median sizes (interquartile range) of the viral reservoir at baseline (week 0) were 0.07 (0.03–0.37), 0.04 (0.02–0.33), and 0.13 (0.06–0.99) infectious units per million peripheral blood mononuclear cells for groups 1, 2, and 3, respectively; these baseline viral reservoir sizes were not significantly different (P = 0.37). By week 24, the median sizes of the viral reservoirs were 0.04 (0.01–2.16), 0.04 (0.01–0.34), and 0.12 (0.01–0.44) infectious units per million peripheral blood mononuclear cells for groups 1, 2, and 3 respectively; these week 24 viral reservoir sizes were not significantly different (P = 0.91). Furthermore, there were no statistically significant differences between baseline and week 24 reservoir sizes for any of the 3 groups (P = 0.88, P = 1.00, and P = 0.44, respectively).
Conclusions:
Despite evidence that ALVAC-HIV ± Remune was associated with a trend toward a delay in viral rebound and a smaller decrease in CD4 T-cell counts following antiretroviral therapy interruption, ALVAC-HIV ± Remune did not influence the size of the viral reservoir.
Key Words: ALVAC, Remune, viral reservoir, therapeutic vaccination
INTRODUCTION
The development of a therapeutic vaccine capable of inducing effective, sustained anti-HIV immunity in an infected individual would significantly improve HIV treatment by eliminating the need for antiretroviral therapy (ART),1–3 which is expensive, is associated with side effects, and must be taken for life.4,5 Ideally, such a therapeutic vaccine would induce immune responses that would control actively replicating virus and potentially reduce or eliminate latent but replication-competent virus2 that is sequestered in reservoirs.2,6,7 The persistence of viral reservoirs despite ART is considered a major barrier to HIV eradication.2,3,6–10
We previously reported the results of a multicentre, randomized, 3-arm, placebo-controlled, double-blind study (CTN 173) assessing the potential therapeutic benefits of immunizing effectively treated, chronically HIV-infected individuals with ALVAC-HIV ± Remune.11 ALVAC-HIV contains a modified, recombinant canarypox virus (vCP1452) that expresses multiple HIV proteins,12 whereas Remune consists of inactivated, gp120-depleted HIV.13 These vaccines induce HIV-specific CD8 and CD4 T-cell responses, respectively.
In that study, ALVAC-HIV and Remune were shown to be safe and immunogenic.11 Furthermore, the vaccines delayed the time until participants restarted therapy or met the predefined criteria to restart therapy. There was also a trend toward delayed viral load rebound, that is, time until the viral load exceeded 50 copies per milliliter, in vaccinated participants. In addition, participants who were vaccinated with ALVAC-HIV ± Remune had a smaller decrease in their CD4 T-cell counts from the time of ART interruption until the end of the study compared to participants who received placebos. These findings demonstrated that ALVAC-HIV ± Remune did, in fact, have biological activity, although any potential clinical benefits seem limited at best. ALVAC-HIV ± Remune had no effect on the following outcomes: (1) the mean viral load 12 weeks following ART interruption, which was almost identical to the mean viral load before ART was initiated; (2) the viral set point following ART interruption; (3) the proportion of participants who restarted ART; or (4) the change in the CD4 T-cell count, the CD4 T-cell percentage, or the CD4/CD8 ratio between week 0 and week 24.
In this report, we describe the viral reservoir substudy in which the effect of ALVAC-HIV ± Remune on the size of the viral reservoir between baseline (week 0) and the time following receipt of all vaccinations and before ART interruption (week 24) was investigated.
METHODS
Study Design
CTN 173 was a multicentre, randomized, 3-arm, double-blind, placebo-controlled trial that evaluated the efficacy of therapeutic vaccination with ALVAC-HIV ± Remune in HIV-infected participants receiving effective ART11,14; the study design has been previously described in detail.14 The study was registered with clinicaltrials.gov (NCT0021288). The participants involved in this substudy were from the McGill University Health Centre and the Centre de Recherche du Centre Hospitalier de l'Université de Montréal, both in Montréal, Quebec, Canada.
To determine the individual and additive effects of the 2 complementary vaccines on HIV-specific immunity, 52 participants were randomized into 3 treatment groups: group 1: ALVAC-HIV with Remune; group 2: ALVAC-HIV with Remune placebo; group 3: both placebos. Remune was administered at weeks 0, 12, and 20, whereas ALVAC-HIV was administered at weeks 8, 12, 16, and 20. At week 24, at which time 48 participants remained in the study, ART was interrupted and the participants were closely monitored for clinical, virologic, and immunologic outcomes until week 48.
A substudy, the results of which are presented in this report, was also performed to evaluate the effect of ALVAC-HIV ± Remune on the size of the viral reservoir that was assessed at baseline (week 0) and at week 24 after all vaccinations had been received.
Approval was obtained by the Research Ethics Boards of the 2 participating sites. This study was conducted in accordance with the ethical guidelines specified by Health Canada's Food and Drug Regulations and the Declaration of Helsinki. Written informed consent was obtained from each participant.
Vaccines
The ALVAC-HIV vaccine is a preparation of a modified recombinant canarypox virus (vCP1452) that expresses the HIV-1 Env and Gag proteins and a synthetic polypeptide that encompasses the known human cytotoxic T-cell (CTL) epitopes from the Nef and Pol proteins.12 ALVAC-HIV placebo was composed of Tris–HCl buffer, virus stabilizer, and freeze-drying media reconstituted in isotonic saline. Remune consists of chemically and physically inactivated, gp120-depleted HIV-1 that is processed and highly purified before formulation with incomplete Freund adjuvant.13 Remune placebo was incomplete Freund adjuvant.
Participant Characteristics
Eligible participants met the following criteria: (1) HIV-infected adults (>18 years); (2) receiving at least 3 antiretroviral agents; (3) viral load <50 copies per milliliter for more than 2 years; (4) CD4 T-cell count >500 cells per microliter and CD4:CD8 ratio higher >0.5 at screening; (5) nadir CD4 T-cell count >250 cells per microliter; and (6) no evidence of hepatitis B or hepatitis C coinfection.
Participants who reached week 24 and maintained a viral load of <50 copies per milliliter interrupted ART. The following protocol-defined criteria were used to decide if ART should be restarted: (1) viral load higher than 30,000 copies per milliliter on 2 occasions at least 4 weeks apart and not decreasing; OR (2) decrease in total CD4 T-cell counts >20% and CD4 T-cell percentage >5% points; OR (3) total CD4 T-cell counts <350 cells per microliter.
Laboratory Measures
CD4 T-Cell Counts and Viral Loads
CD4 T-cell counts and viral loads were measured at weeks −4, 0, 4, 8, 12, 16, 20, and 24 as previously described.11 Upon treatment interruption (week 24), CD4 T-cell counts continued to be performed every 4 weeks, whereas viral loads were measured twice weekly for 4 weeks (weeks 25–28 inclusive), weekly for 8 weeks (weeks 29–36 inclusive), then monthly, or until ART was restarted. Viral load was measured using the Chiron bDNA version 3.0 assay (Chiron Diagnostics, Emerville, CA).
Viral Outgrowth Assay to Quantify the Size of the Viral Reservoir
The size of the viral reservoir was assessed by quantifying replication-competent virus using a viral outgrowth assay.15 A 5-fold change in infectious units per million (IUPM) values from week 0 to week 24 was defined as being a biologically meaningful change in the size of the viral reservoir based on our previous observations that an individual's IUPM values may vary daily from 0 to 5-fold (unpublished data C. Tremblay [2008]). When a value below the limit of detection [<0.01 IUPM peripheral blood mononuclear cells (PBMCs)] was obtained, an imputed value of 0.01 was used to perform statistical analyses. Briefly, PBMCs were resuspended at a concentration of 1 × 106 cells per milliliter in RPMI 1640 medium (Sigma, St. Louis, MO) supplemented with 10% heat-inactivated fetal calf serum (Sigma), penicillin (50 U/mL) (Gibco, Burlington, ON), streptomycin (50 mg/mL) (Invitrogen, Burlington, Canada), l-glutamine (2 mM) (Gibco), HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer (10 mM) (Gibco), and recombinant human interleukin 2 (100 U/mL) (Hoffmann-La Roche, Nutley, NJ). Six 5-fold PBMC dilutions ranging from 25 × 106 to 8 × 103 PBMCs were made. The 25 × 106 and 5 × 106 PBMC dilutions were cultured in duplicate in T75 and T25 flasks, respectively. The other dilutions were cultured in quadruplicate in 24-well plates. A CD3/CD8-bispecific monoclonal antibody that selectively expands CD4 T-cells while depleting CD8 T-cells by redirected cell-mediated cytotoxicity16,17 was added to the PBMCs at a final concentration of 1 mg/mL. One million PBMC feeder cells obtained from a healthy donor were added to the 24-well plate cultures; feeder cells were not required for the 25 × 106 and 5 × 106 PBMC cultures because these contained sufficient numbers of uninfected cells that could be subsequently infected. The cultures were incubated at 37°C, 5% CO2 for 21 days with twice-weekly medium exchanges; the supernatant collected on day 21 was used for the measurement of HIV-1 p24 antigen by enzyme-linked immunosorbent assay (Vironostika; BioMérieux, Marcy l'Etoile, France). The number of IUPM PBMC was calculated from the pattern of positive wells by the method of maximum likelihood.
Calculations and Statistical Analysis
GraphPad Prism versions 5.04 and 6 software (GraphPad Software, La Jolla, CA) were used for statistical analyses. Changes in the size of the viral reservoir from baseline (week 0) to week 24 for an experimental group were assessed using the Wilcoxon signed rank test.
To determine if the median values of various demographic and biological characteristics varied significantly among the 3 experimental groups, the Kruskall–Wallis test was performed; Dunn multiple comparison test was performed posttest to determine if the medians of any pairs of groups differed.
Correlations between the size of the viral reservoir at either week 0 or week 24 and the time to viral rebound, the time to meet at least one criterion to restart ART, the viral load at the time of viral rebound, the viral load 12 weeks following ART interruption, the CD4 T-cell count at baseline (week 0), the CD4 T-cell count at week 24, the nadir CD4 T-cell count, or the new viral set point following ART interruption were assessed using Spearman correlation analysis.
RESULTS
Participants
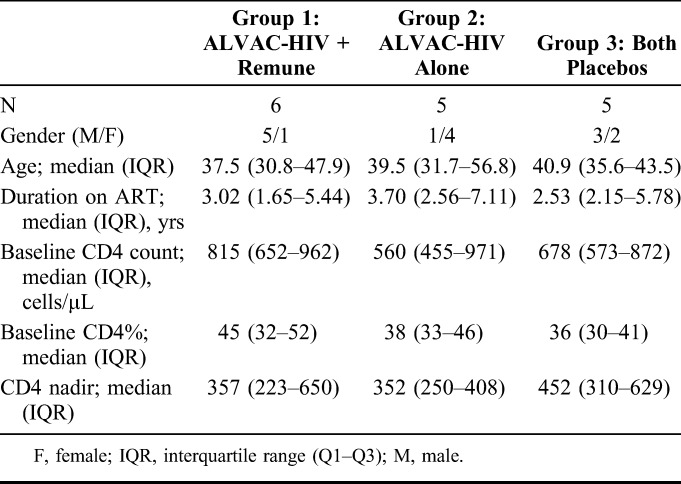
CTN 173 was conducted between May 2004 and May 2006 during which time 17 individuals participated in a viral reservoir substudy, 16 of whom completed the substudy. These 16 participants were matched with respect to age, CD4 nadir, CD4 count at baseline, CD4% at baseline, and years on ART with the other participants in the parent study. However, only 20% of the subgroup participants were male compared to 92% of the remaining participants in the parent study. Of the 16 participants, 6 were randomized to group 1 (ALVAC-HIV + Remune), 5 to group 2 (ALVAC-HIV alone), and 5 to group 3 (both placebos). With the exception of gender, all baseline characteristics (age, CD4 T-cell counts, and duration on ART) were balanced between the arms (Table 1).
TABLE 1.
Participant Demographics
Viral Reservoir Size
The effects of vaccination with ALVAC-HIV ± Remune on the size of the viral reservoir were assessed by a viral outgrowth assay using activated CD4 T-cells expanded from PBMCs obtained from each participant at baseline (week 0) and at week 24 following receipt of all vaccinations and before ART interruption. A meaningful change in the size of the viral reservoir for an individual was defined as a ≥5-fold difference in the number of IUPM PBMC between the 2 time-points. Virus was recovered from 14 participants (group 1: n = 5; group 2: n = 4; group 3: n = 5) at baseline and from 10 participants (group 1: n = 4; group 3: n = 3; group 3: n = 3) at week 24 (Table 2).
TABLE 2.
Patient Outcome Measures
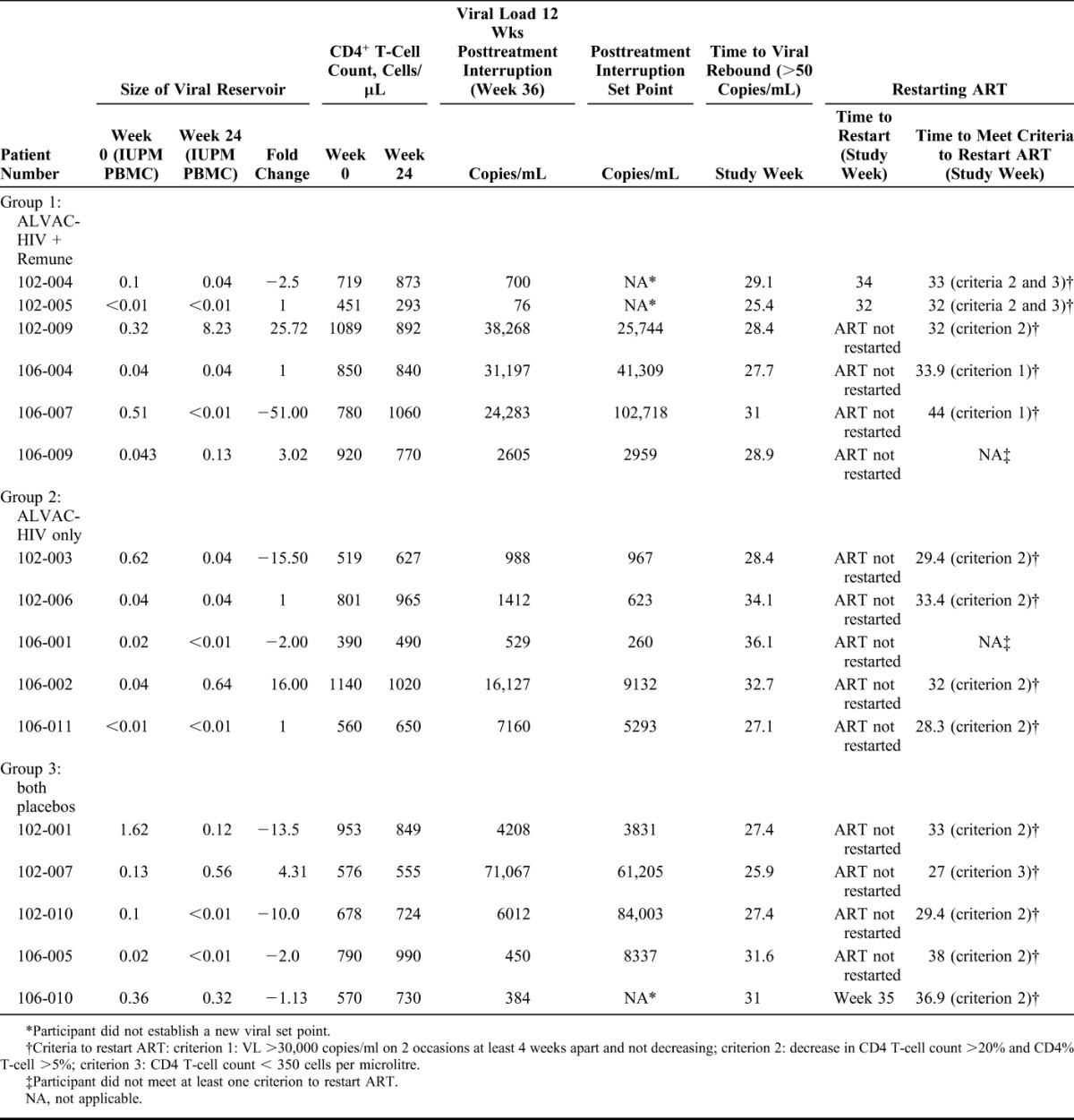
The median size of the viral reservoir of the 16 participants at baseline was 0.07 (0.03–0.35) IUPM PBMC. The median sizes of the viral reservoir at baseline were 0.07 (0.03–0.37), 0.04 (0.02–0.33), and 0.13 (0.06–0.99) IUPM PBMC for groups 1, 2, and 3, respectively, with no significant difference between these values (P = 0.37). By week 24 of the study, the median sizes of the viral reservoirs were 0.04 (0.01–2.16), 0.04 (0.01–0.34), and 0.12 (0.01–0.44) IUPM PBMC for groups 1, 2, and 3, respectively, with no significant differences between these values (P = 0.91).
A comparison within each group of the median IUPM values at baseline and week 24 revealed that there were no statistically significant differences between these 2 time-points (Table 2): group 1 (ALVAC-HIV + Remune): P = 0.88; group 2 (ALVAC-HIV alone): P = 1.0; and group 3 (both placebos): P = 0.44, suggesting that vaccination with ALVAC-HIV ± Remune did not affect the size of the viral reservoir.
Of the 16 participants in this substudy, a meaningful decline in IUPM values (defined as ≥5-fold) from baseline to week 24 was observed in 4 participants, an increase was observed in 2 participants, and no change was observed in 10 participants (Table 2 and Fig. 1).
FIGURE 1.
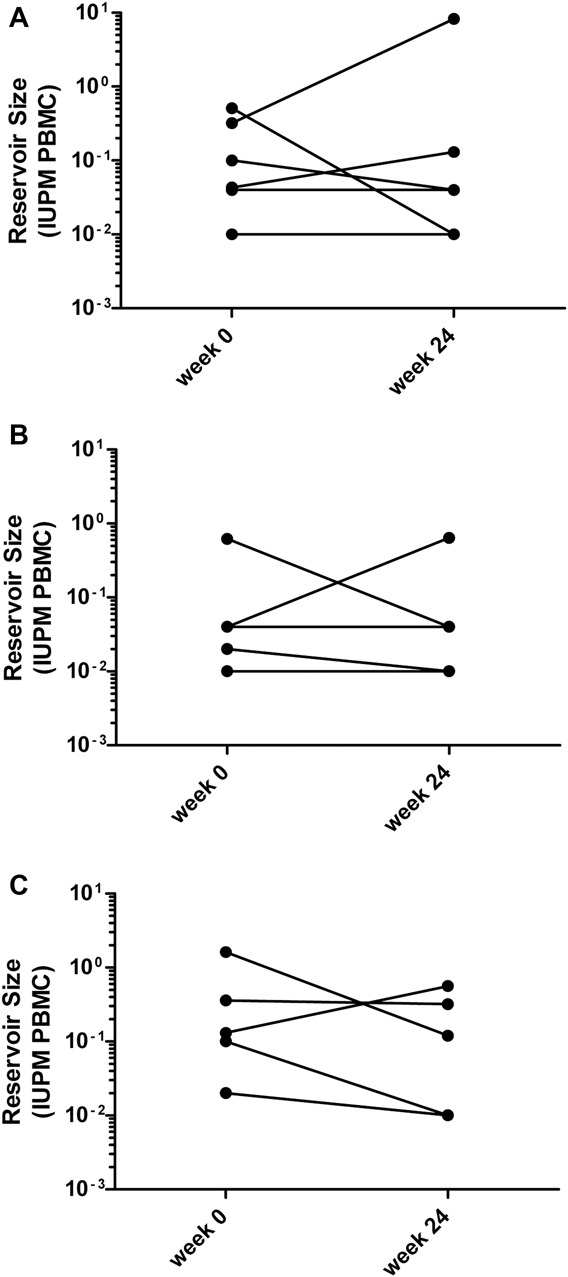
ALVAC-HIV ± Remune does not affect the size of the viral reservoir from week 0 to week 24. Changes in the size of the viral reservoir from week 0 to week 24 were assessed by quantifying replication-competent virus using an ultrasensitive viral outgrowth assay. The number of IUPM PBMC was calculated from the pattern of positive wells by the method of maximum likelihood: (A) group 1: ALVAC-HIV + Remune; (B) group 2: ALVAC-HIV alone; (C) group 3: both placebos.
Of the 4 participants who experienced a decrease in the size of their viral reservoir from baseline to week 24, 1 received ALVAC-HIV + Remune (1/6 = 17% of participants in group 1), 1 received ALVAC-HIV alone (1/5 = 20% of participants in group 2), and 2 received both placebos (2/5 = 40% of participants in group 3) (Table 2 and Fig. 1). Of the 2 participants who experienced an increase in the size of their viral reservoirs from week 0 to week 24, one received ALVAC-HIV + Remune (1/6 = 17% of participants in group 1) and the other received ALVAC-HIV alone (1/5 = 20% of participants in group 2) (Table 2 and Fig. 1).
These observations further indicate that ALVAC-HIV ± Remune had no effect on the size of the viral reservoir. In contrast, the recipients of both placebos had the highest number of participants experiencing a decrease in the size of their viral reservoir (40%) and the fewest number of participants experiencing an increase in the size of their viral reservoir (0%), although there were no statistically significant differences between groups with respect to the number of participants with decreases or increases in the size of their viral reservoir (P = 0.66 and P = 0.61, respectively). Furthermore, there were no statistically significant differences with respect to the change in the size of the viral reservoir from week 0 to week 24 for any of the groups.
Correlations Between the Size of the Viral Reservoir and Various Participant Characteristics and Outcome Measures
There were no correlations between the size of the viral reservoir at baseline and the time to viral rebound (rs = −0.06; P = 0.80), the time to meet at least one criterion to restart ART (rs = 0.10; P = 0.74), the viral load at the time of viral rebound (rs = −0.38; P = 0.15), the viral load 12 weeks following ART interruption (rs = 0.26; P = 0.32), the CD4 T-cell count at baseline (rs = 0.23; P = 0.39), the CD4 T-cell count at week 24 (rs = 0.20; P = 0.45), the nadir CD4 T-cell count (rs = 0.04; P = 0.88), or the new viral set point following ART interruption (rs = 0.11; P = 0.75). The viral set point was defined as the geometric mean of 3 consecutive weekly viral load values in which the slope between the first 2 and last 2 values was between −0.2 and 0.2 log10 copies per milliliter per week.18
Furthermore, there were no correlations between the size of the viral reservoir at week 24 and the time to viral rebound (rs = 0.02; P = 0.93), the time to meet at least one criterion to restart ART (rs = −0.15; P = 0.50), the viral load at the time of viral rebound (rs = 0.23; P = 0.39), the viral load 12 weeks following ART interruption (rs = 0.39; P = 0.14), the CD4 T-cell count at week 24 (rs = 0.21; P = 0.43), the nadir CD4 T-cell count (rs = 0.34; P = 0.19), or the new viral set point following ART interruption (rs = −0.13; P = 0.63) (data not shown). However, a correlation was observed between the size of the viral reservoir at week 24 and the CD4 T-cell count at baseline (rs = 0.57; P = 0.02).
DISCUSSION
The viral reservoir represents a major barrier to HIV eradication.2,3,6–10,18,19 Resting CD4 T-cells constitute the primary viral reservoir,20–23 particularly central and transitional memory CD4 T cells24 which are the most studied and best characterized HIV cellular reservoirs.8,10
The reservoir is established during primary HIV infection,25 and its stability is thought to reflect the homeostatic proliferative capacity and longevity of memory T cells.7 Although early initiation of ART before the reservoir is fully established, appears to reduce its size26–28; once established, these reservoirs are not eradicated by ART.20,21 As a result, when ART is interrupted, viral rebound occurs within days to weeks because of reseeding from the viral reservoirs.29
CTN 173 was a multicentre, randomized, 3-arm, placebo-controlled, double-blind study of a therapeutic vaccine in participants on suppressive ART. This study included a substudy that examined the effects of vaccination with ALVAC-HIV ± Remune on the size of the viral reservoir. Overall, the vaccine regimen had no demonstrable impact on the size of the viral reservoir as measured by viral outgrowth assay.
Although polymerase chain reaction (PCR)-based quantification of cell-associated HIV-1 DNA is the most common technique for measuring the size of the viral reservoir7 because of the relative simplicity of this method,6,7,9,10 the viral outgrowth assay is currently considered the “gold standard” for measuring cells harboring latent virus able to replicate.7,10,30 The use of this assay in this viral reservoir substudy is, therefore, one of the strengths of this substudy. Important advantages of this infectivity-based assay over PCR-based assays are its sensitivity7,9 and its ability to distinguish between replication-competent HIV DNA versus defective, replication incompetent HIV DNA.6,7,9 Because PCR-based assays cannot distinguish between replication competent and defective, replication incompetent HIV DNA, PCR-based assays can overestimate the size of the viral reservoir, possibly by more than 2 logs.7,9,10 In contrast, the viral outgrowth assay, which measures the minimum frequency of latently infected cells, underestimates the size of the viral reservoir.6,9 It was recently shown by Ho et al31 that the extent of this underestimate, which is because of the presence of noninduced, but replication-competent, provirus, is at least 60-fold. Given that no single biomarker or assay has yet been identified that accurately quantifies the size of the replication-competent HIV reservoir,7,9,30–32 the use of a single assay to measure the size of the viral reservoir is a limitation of this substudy.
The failure of ALVAC-HIV ± Remune to affect the size of the viral reservoir in our substudy is consistent with the results obtained by Casazza et al33 in a randomized, placebo-controlled trial, in which effectively treated participants were vaccinated with an HIV DNA vaccine encoding multiple HIV antigen, followed by boosting with a replication-deficient adenovirus type 5 vaccine expressing multiple HIV antigen.
In a therapeutic vaccine trial by Persaud et al,34 although a modest, transient decrease in the size of the viral reservoir was observed, by the end of the study, the reservoir size was no different than that observed at baseline. In this trial, effectively treated participants were vaccinated with a modified vaccinia Ankara vector-based vaccine, followed by boosting with the same vaccine and with a Fowlpox vector–based HIV vaccine.
In the study by Li et al,35 immunization of effectively treated participants with a replication-defective recombinant adenovirus type 5 HIV-1 gag vaccine had no significant effect on the size of the viral reservoir as determined in a randomized, placebo-controlled trial.
Finally, in an open-label, randomized trial by Herasimtschuk et al,36 immunization of effectively treated participants with GTU-MultiHIV DNA vaccine with or without subsequent administration of interleukin 2, granulocyte–macrophage colony-stimulating factor, and recombinant human growth hormone did not significantly change the size of the viral reservoir.
Although therapeutic vaccination alone might not be capable of reducing viral reservoir size, it might induce HIV-specific CTLs that could kill cells whose latent virus has been reactivated by pharmacological approaches,8,19 thus accelerating the decay of the viral reservoir that does occur during the early phase of ART initiation.19 Evidence for the involvement of stimulated, HIV-specific CTLs in eliminating CD4 T-cells whose latent HIV has been reactivated was provided recently in in vitro experiments with the HDAC inhibitor suberoylanilide hydroxamic acid.37 Although suberoylanilide hydroxamic acid treatment of CD4 T-cells obtained from ART-treated patients resulted in reactivation of latent virus, the CD4 T-cells did not die, despite the induction of HIV-induced cytopathic effects and the presence of autologous, HIV-specific CTLs in the cultures. However, the addition of gag-stimulated CTLs to the cell cultures did result in the death of infected CD4 T-cells.
We acknowledge that the small number of individuals who participated in this substudy is one of its limitations. Unfortunately, the complexity and demands of the substudy precluded the recruitment of large numbers of participants. However, the small number of participants did not likely prevent the observation of a vaccine effect because there was not even a trend toward decreased viral reservoir size in response to vaccination. Furthermore, these disappointing results are consistent with those of other trials of therapeutic vaccine candidates tested in effectively treated participants. As a next step, novel therapeutic vaccines containing immunogens capable of inducing functional CTL responses to HIV epitopes will need to be developed.19,38 When combined with pharmacological agents that reverse HIV latency, these next generation therapeutic vaccines could play a critical role in reducing the size of the viral reservoir.
ACKNOWLEDGMENTS
The authors would like to thank Dr Annie Chamberland and Mohamed Sylla for processing the blood samples and for performing the viral outgrowth assays, and Stéphanie Matte for enrolling participants.
The authors would also like to thank Dr Johnson Wong for the kind gift of CD3/CD8-bispecific monoclonal antibody.
Footnotes
Supported by the Canadian Institutes of Health Research (CIHR) Canadian HIV Trials Network (CTN 173). This study was also supported by operating grants to J.B.A. from the CIHR Grant (FRN 44179) and the Ontario HIV Treatment Network Grant (ROGA103). J.-P.R. and C.L.T. are supported by Fonds de recherché du Québec—Santé awards. J.-P.R. holds the Louis Lowenstein Chair in Hematology & Oncology, McGill University. C.L.T. holds the Pfizer/University of Montréal Chair on HIV Translational Research. Immune Response Corporation provided study materials, and Sanofi-Pasteur provided financial support and study materials.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Harari A, Rozot V, Cavassini M, et al. NYVAC immunization induces polyfunctional HIV-specific T-cell responses in chronically-infected, ART-treated HIV patients. Eur J Immunol. 2012;42:3038–3048. [DOI] [PubMed] [Google Scholar]
- 2.Towards an HIV Cure; Full Recommendations (1st ed). The International AIDS Society Scientific Working Group on HIV Cure. 2012. Available at: http://www.iasociety.org/Web/WebContent/File/HIV_Cure_Full_recommendations_July_2012.pdf. Accessed July 8, 2014. [Google Scholar]
- 3.Vanham G, Van GE. Can immunotherapy be useful as a “functional cure” for infection with human immunodeficiency virus-1? Retrovirology. 2012;9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Routy JP, Boulassel MR, Nicolette CA, et al. Assessing risk of a short-term antiretroviral therapy discontinuation as a read-out of viral control in immune-based therapy. J Med Virol. 2012;84:885–889. [DOI] [PubMed] [Google Scholar]
- 6.Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity. 2012;37:377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouzioux C, Richman D. How to best measure HIV reservoirs? Curr Opin HIV AIDS. 2013;8:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen TA, Tolstrup M, Winckelmann A, et al. Eliminating the latent HIV reservoir by reactivation strategies: advancing to clinical trials. Hum Vaccin Immunother. 2013;9:790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriksson S, Graf EH, Dahl V, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013;9:e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan L, Siliciano RF. From reactivation of latent HIV-1 to elimination of the latent reservoir: the presence of multiple barriers to viral eradication. Bioessays. 2013;35:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angel JB, Routy JP, Tremblay C, et al. A randomized controlled trial of HIV therapeutic vaccination using ALVAC with or without Remune. AIDS. 2011;25:731–739. [DOI] [PubMed] [Google Scholar]
- 12.Aventis Pasteur. ALVAC-HIV (vCP1452). 7th ed Lyon, France: 2003. [Google Scholar]
- 13.Immune Response Corporation. Remune (HIV Immunogen). 6th ed Carlsbad, CA: 2004. [Google Scholar]
- 14.Spaans JN, Routy JP, Tremblay C, et al. Optimizing the efficiency of therapeutic HIV vaccine trials: a case for CTN 173. Trials Vaccinol. 2012;1:21–26. [Google Scholar]
- 15.Tremblay CL, Giguel F, Merrill DP, et al. Marked differences in quantity of infectious human immunodeficiency virus type 1 detected in persons with controlled plasma viremia by a simple enhanced culture method. J Clin Microbiol. 2000;38:4246–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson CC, Wong JT, Girard DD, et al. Ex vivo expansion of CD4 lymphocytes from human immunodeficiency virus type 1-infected persons in the presence of combination antiretroviral agents. J Infect Dis. 1995;172:88–96. [DOI] [PubMed] [Google Scholar]
- 17.Routy JP, Tremblay CL, Angel JB, et al. Valproic acid in association with highly active antiretroviral therapy for reducing systemic HIV-1 reservoirs: results from a multicentre randomized clinical study. HIV Med. 2012;13:291–296. [DOI] [PubMed] [Google Scholar]
- 18.Kilby JM, Bucy RP, Mildvan D, et al. A randomized, partially blinded phase 2 trial of antiretroviral therapy, HIV-specific immunizations, and interleukin-2 cycles to promote efficient control of viral replication (ACTG A5024). J Infect Dis. 2006;194:1672–1676. [DOI] [PubMed] [Google Scholar]
- 19.Barouch DH, Deeks SG. Immunologic strategies for HIV-1 remission and eradication. Science. 2014;345:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. [DOI] [PubMed] [Google Scholar]
- 21.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. [DOI] [PubMed] [Google Scholar]
- 22.Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997;94:13193–13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun TW, Finzi D, Margolick J, et al. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–1290. [DOI] [PubMed] [Google Scholar]
- 24.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chun TW, Engel D, Berrey MM, et al. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95:8869–8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ananworanich J, Schuetz A, Vandergeeten C, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One. 2012;7:e33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hocqueloux L, Avettand-Fenoel V, Jacquot S, et al. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother. 2013;68:1169–1178. [DOI] [PubMed] [Google Scholar]
- 28.Chun TW, Justement JS, Moir S, et al. Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus. J Infect Dis. 2007;195:1762–1764. [DOI] [PubMed] [Google Scholar]
- 29.Joos B, Fischer M, Kuster H, et al. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc Natl Acad Sci U S A. 2008;105:16725–16730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams JP, Hurst J, Stohr W, et al. HIV-1 DNA predicts disease progression and post-treatment virological control. Elife. 2014;3:e03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho YC, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cockerham LR, Deeks SG. Biomarker reveals HIV's hidden reservoir. Elife. 2014;3:e04742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casazza JP, Bowman KA, Adzaku S, et al. Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire. J Infect Dis. 2013;207:1829–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Persaud D, Luzuriaga K, Ziemniak C, et al. Effect of therapeutic HIV recombinant poxvirus vaccines on the size of the resting CD4+ T-cell latent HIV reservoir. AIDS. 2011;25:2227–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li JZ, Heisey A, Ahmed H, et al. Relationship of HIV reservoir characteristics with immune status and viral rebound kinetics in an HIV therapeutic vaccine study. AIDS. 2014;28:2649–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herasimtschuk A, Downey J, Nelson M, et al. Therapeutic immunisation plus cytokine and hormone therapy improves CD4 T-cell counts, restores anti-HIV-1 responses and reduces immune activation in treated chronic HIV-1 infection. Vaccine. 2014;32:7005–7013. [DOI] [PubMed] [Google Scholar]
- 37.Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shapiro SZ. A proposal to use iterative, small clinical trials to optimize therapeutic HIV vaccine immunogens to launch therapeutic HIV vaccine development. AIDS Res Hum Retroviruses. 2014;31:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]


