Abstract
Acute prosthetic valve thrombosis is a potentially serious complication with an incidence as high as 6% per patient-year for prostheses in the mitral position. Accurate diagnosis of the degree of obstruction and differentiation of pannus versus thrombus is critical in determination of the best mode of therapy. We discuss a case of a patient with multiple comorbidities who presented with mechanical mitral valve obstruction where both transthoracic and two-dimensional transesophageal echocardiography (TEE) were limited in making an accurate diagnosis regarding the mechanism of obstruction. Real-time 3D-TEE (RT-3DTEE) was critical in identifying a partial thrombus on the mechanical valve and guided the choice of thrombolysis as the most appropriate intervention, thus avoiding high-risk surgery in this patient with significant multiple comorbidities.
Background
Acute prosthetic valve thrombosis is a potentially grave complication with high mortality if left unrecognised and untreated.1 It is clinically important to differentiate prosthetic valve thrombosis from pannus formation. We present a case of an acute thrombus causing obstruction of a mechanical mitral valve. Although surgery is the standard intervention in these cases, our patient had multiple comorbidities that made her a high surgical risk. We believe that multimodality imaging (especially three-dimensional transesophageal echocardiography (RT-3DTEE)) helped us make an accurate diagnosis, enabling us to choose thrombolysis as a suitable intervention in this particular patient and mitigating the need for high-risk surgery.
Case presentation
A 55-year-old woman with progressive exertional dyspnoea, orthopnea and profound fatigue on minimal activity for 5 days, presented to our institution. Her medical history was notable for rheumatic fever with severe mitral regurgitation. She had undergone mitral valve repair 5 years earlier. Two years later, following the failure of the mitral repair, she had undergone a repeat sternotomy and mechanical mitral valve replacement (26 mm Carbomedics valve) due to worsening symptoms. She endorsed non-adherence to warfarin therapy. On her current presentation, she denied any recent infection or drug abuse. Examination on arrival revealed bi-basilar pulmonary crackles and bipedal oedema. Her cardiovascular examination was notable for a diastolic murmur at the apex with absence of audible prosthetic valve clicks.
Investigations
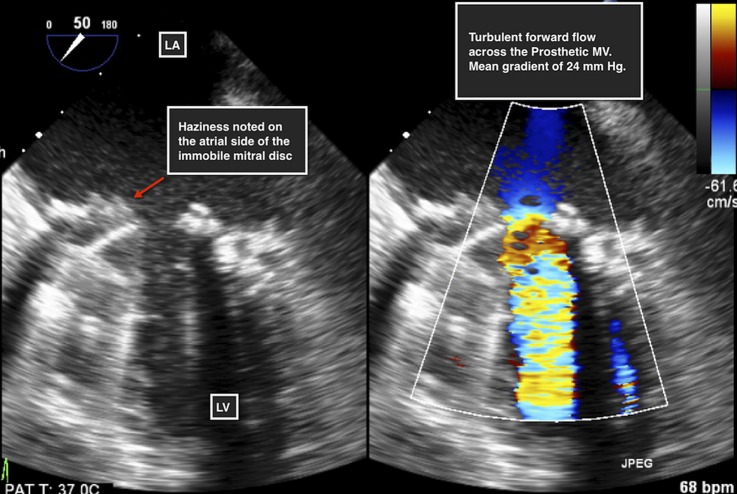
The results of routine biochemical tests were within normal limits with the exception of a subtherapeutic international normalised ratio (INR) of 1.2. A 12-lead ECG was performed, which did not reveal any acute ST or T wave changes. Chest X-ray revealed evidence of vascular congestion. A fluoroscopic view of the prosthesis indicated absent opening and closing of one mechanical disc (video 1). A transthoracic echocardiogram (TTE) revealed mitral valve obstruction with an increased mean trans-mitral gradient of 24 mm Hg. Two-dimensional (2D) TEE revealed an immobile disc with haziness noted on its atrial side (figure 1). Images obtained using RT-3DTEE demonstrated the presence of a mass, with texture and appearance suggestive of a partially obstructive thrombus attached to the atrial side of the mechanical valve restricting the antero-medial disc of the valve (figures 2, 3 and video 2).
Figure 1.
Two-dimensional transesophageal echocardiographic view of the prosthetic mitral valve (MV). Haziness noted over the atrial side of the prosthesis (left). Turbulent forward flow across the prosthetic mitral valve with measured mean gradient of 24 mm Hg (right).
Figure 2.
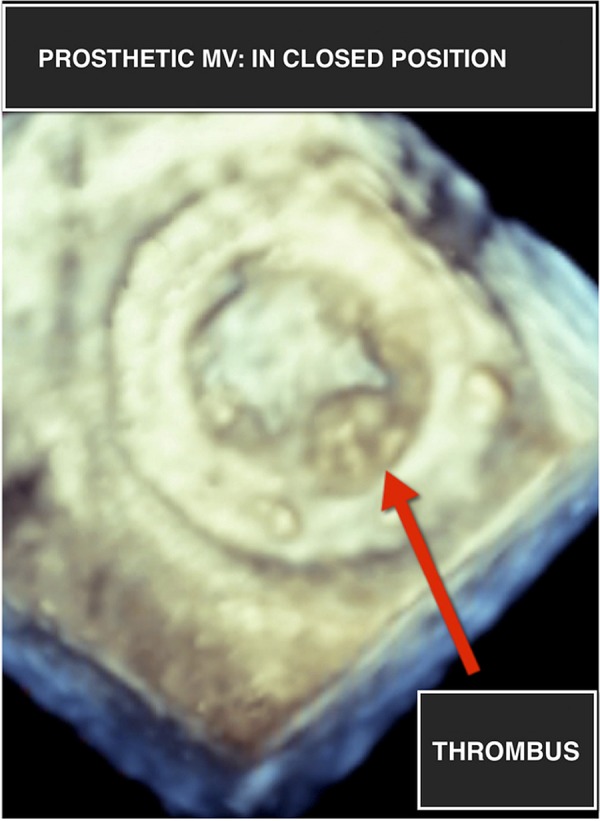
Real-time three-dimensional transesophageal echocardiogram of prosthetic mitral valve (MV) in closed position showing a mass suggestive of a partially obstructive thrombus attached to the atrial side of the mechanical valve, restricting the anteromedial disc of the valve.
Figure 3.
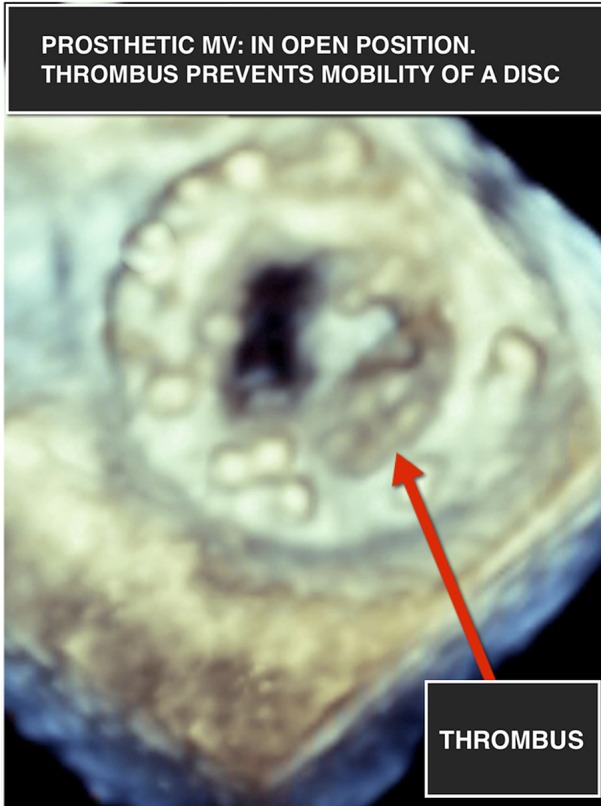
Real-time three-dimensional transesophageal echocardiogram of prosthetic mitral valve (MV) in open position showing a mass suggestive of a partially obstructive thrombus attached to the atrial side of the mechanical valve, restricting the anteromedial disc of the valve.
Video 1.
Cine-fluoroscopic view of the prosthesis (pre-thrombolysis) indicated absent opening and closing of one mechanical disc.
Video 2.
Real-time three-dimensional transesophageal echocardiography of the prosthetic mitral valve (view from the atrial side) showing restricted movement of the anteromedial disc due to a partially obstructing thrombus.
Differential diagnosis
The differential diagnosis included pannus formation, vegetation from prosthetic valve endocarditis and thrombus formation. Pannus is an insidious bioreaction to the valve prosthesis involving fibrous connective tissue ingrowth from the sewing ring. This process typically evolves over many years after valve implantation. Our patient did not have a high clinical likelihood for prosthetic valve endocarditis. In this patient, with a relatively short duration of symptoms and an INR of 1.2 (which is subtherapeutic for a mechanical mitral valve), thrombus formation was most likely.
Treatment
Owing to the patient's comorbidities, two prior sternotomies and high Society of Thoracic Surgeons (SCS) score of 17%, our multidisciplinary team recommended she undergo thrombolysis with systemic tissue plasminogen activator (tPa). She received 10 mg intravenous bolus alteplase, followed by 90 mg infused over one and half hours and subsequently intravenous heparin infusion.
Outcome and follow-up
Post-thrombolysis, the patient's symptoms resolved and a follow-up TTE revealed reduction in mitral valve gradient to 6 mm Hg. The full excursion of the disc was confirmed on fluoroscopy, suggesting resolution of obstruction (video 3).
Video 3.
Fluoroscopic video of the opening and closing of the prosthetic mitral valve, post-thrombolysis. The full excursion of the valve is appreciated here.
The patient was discharged home in stable condition. During the 4-week outpatient follow-up, she reported resolution of all her symptoms. An outpatient TTE was performed 4 weeks after the thrombolysis, confirming full excursion of the discs and a normal mitral valve gradient.
Discussion
The overall incidence of prosthetic valve thrombosis is 0.1–6% per patient-year for left-sided heart valves, most notably within the first postoperative year.1 The mechanism of valve obstruction can be explained by the hypercoagulable state postoperatively along with contact of the bloodstream with non-endothelialised thrombogenic surfaces on suture sites and prosthesis material. The major associations for prosthetic valve obstruction are mitral location of the prosthesis and inadequate anticoagulation therapy. However, pannus formation is an insidious bioreaction that involves fibrous connective tissue ingrowth from the sewing ring, typically evolving over many years after valve implantation, and is unaffected by routine anticoagulation.2
Accurate diagnosis of prosthetic valve obstruction is crucial as its management will differ depending on the size, location, degree of obstruction and whether the obstruction is due to pannus or thrombus formation. Cine-fluoroscopy, transthoracic and TEE represent the major diagnostic modalities. Cine-fluoroscopy provides excellent visualisation of mechanical prosthetic heart valve leaflet motion and allows for correct evaluation of the opening and closing angles; however, it is limited in identifying non-obstructive lesions or differentiation of pannus from thrombus2 and thus additional imaging modalities are often necessary. TTE allows for direct prosthesis visualisation and measurement of pertinent haemodynamic parameters, most notably, the transvalvular gradient, abnormal transprosthetic flow and central regurgitation.3 Limitations include poor quality of acoustic window and artefacts associated with the highly reflective surface of the prosthesis. In the case of a non-obstructive prosthetic valve thrombus, TTE may often be normal.3 2D-TEE will provide a greater degree of detail when it comes to leaflet morphology and visualising the obstruction to make a definite diagnosis in most cases. It is, however, limited in differentiation of pannus formation versus thrombus, as well as obstruction location on the ventricular side of the prosthetic valve. Lastly, 2D-TEE is limited in delineating small thrombi from sutures or strands, which are fibrinous mobile filaments often considered to have no embolic risk.4 RT3D-TEE is increasingly used because of its superior imaging quality. It provides a unique and accurate visualisation of both native and prosthetic heart valves, and is a promising diagnostic tool for exact localisation of the obstruction.5 Three-dimensional views of both bioprosthetic and mechanical heart valve components, including leaflets, rings and struts, are displayed with great detail. The predictors for obstructive thrombus (compared with pannus) are leaflet restriction, softer echo density, larger mass and rapid development of gradient across the valve.6 Although infective endocarditis is in the differential diagnosis for any mass associated with prosthetic valves, there should be a high index of clinical suspicion. RT3D-TEE is well suited to detect any peri-valvular leaks or abscess formation, which can occur in prosthetic valve endocarditis especially in visualisation of the mitral valve.7–9 Cardiac CT angiography (CTA) with gating can be used to visualise the thrombus due to its excellent temporal resolution to detect obstruction during valve motion. However, the need for retrospective gating to image the valve motion during the various cardiac phases results in increased radiation exposure, which is a major limitation. In addition, CTA can result in attenuation artefacts from the metallic valves, limiting the characterisation of thrombus.
In non-obstructive left-sided prosthetic valve thrombosis, treatment currently consists of a short course of intravenous heparin, minimum of 100 mg of daily aspirin and necessary adjustment of warfarin dose.10 Obstructed prosthetic valves require aggressive treatment, surgery or fibrinolysis, as anticoagulant treatment alone will usually be insufficient. Owing to the lack of randomised studies, recommendations are sparse. According to the American College of Cardiology/American Heart Association guidelines, surgery is the preferred treatment for left-sided prosthetic valve thrombosis. Fibrinolysis is considered when surgery is not possible due to advanced age, previous surgeries or in right-sided prosthetic heart valve in patients with New York Heart Association (NYHA) class III-IV symptoms and/or a large clot burden.11
As with our patient described above, RT3D-TEE provided an en face view of the mitral prosthesis that was otherwise not obtainable with routine 2D-TEE. In addition, this allowed visualisation of the mass from multiple angles, which provided a more comprehensive assessment of its exact location, size and morphology. Accurate diagnosis and confirmation of the partial thrombus, using RT3D-TEE, was crucial in determining the best management strategy for this high-risk patient, who had undergone two prior sternotomies. The decision to attempt thrombolysis allowed for avoidance of high-risk surgery.
Learning points.
Prosthetic valve obstruction due to thrombus is a rare and potentially devastating condition.
Proper identification of such obstruction is vital in preventing morbidity and mortality.
Multimodality imaging including transthoracic echocardiogram, transesophageal echocardiogram or CT angiography are required to properly identify the aetiology of the obstruction and its extent.
Treatment options include appropriate anticoagulation and ensuring full adherence to treatment.
Footnotes
Contributors: KJ and KL conceived the manuscript. AK and RJ wrote and finalised the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Deviri E, Sareli P, Wisenbaugh T et al. Obstruction of mechanical heart valve prostheses: clinical aspects and surgical management. J Am Coll Cardiol 1991;17:646–50. 10.1016/S0735-1097(10)80178-0 [DOI] [PubMed] [Google Scholar]
- 2.Bonou M, Lampropoulos K, Barbetseas J. Prosthetic heart valve obstruction: thrombolysis or surgical treatment? Eur Heart J Acute Cardiovasc Care 2012;1:122–7. 10.1177/2048872612451169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pibarot P, Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation 2009;119:1034–48. 10.1161/CIRCULATIONAHA.108.778886 [DOI] [PubMed] [Google Scholar]
- 4.Habib G, Cornen A, Mesana T et al. Diagnosis of prosthetic heart valve thrombosis. The respective values of transthoracic and transoesophageal Doppler echocardiography. Eur Heart J 1993;14:447–55. 10.1093/eurheartj/14.4.447 [DOI] [PubMed] [Google Scholar]
- 5.Roudaut R, Serri K, Lafitte S. Thrombosis of prosthetic heart valves: diagnosis and therapeutic considerations. Heart 2007;93:137–42. 10.1136/hrt.2005.071183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanis W, Habets J, van den Brink RB et al. Differentiation of thrombus from pannus as the cause of acquired mechanical prosthetic heart valve obstruction by non-invasive imaging: a review of the literature. Eur Heart J Cardiovasc Imaging 2014;15:119–29. 10.1093/ehjci/jet127 [DOI] [PubMed] [Google Scholar]
- 7.Ozkan M, Gunduz S, Yildiz M et al. Diagnosis of the prosthetic heart valve pannus formation with real-time three-dimensional transoesophageal echocardiography. Eur J Echocardiogr 2010;11:E17 10.1093/ejechocard/jep206 [DOI] [PubMed] [Google Scholar]
- 8.Butler TC, Sedgwick JF, Platts D et al. Obstructive mechanical valve thrombosis: utility of 3D trans-oesophageal echocardiography. Eur Heart J Cardiovasc Imaging 2015;16:230 10.1093/ehjci/jeu204 [DOI] [PubMed] [Google Scholar]
- 9.Ozkan M, Gursoy OM, Astarcioglu MA et al. Real-time three-dimensional transesophageal echocardiography in the assessment of mechanical prosthetic mitral valve ring thrombosis. Am J Cardiol 2013;112:977–83. 10.1016/j.amjcard.2013.05.032 [DOI] [PubMed] [Google Scholar]
- 10.Grunkemeier GL, Li HH, Naftel DC et al. Long-term performance of heart valve prostheses. Curr Probl Cardiol 2000;25:73–154. 10.1053/cd.2000.v25.a103682 [DOI] [PubMed] [Google Scholar]
- 11.Bonow RO, Carabello BA, Chatterjee K, et al. , American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006;48:e1–148. 10.1016/j.jacc.2006.05.021 [DOI] [PubMed] [Google Scholar]