Abstract
A 45-year-old man presented with follicular exanthema in his lower limbs, alternating bowel habits and significant weight loss. His medical history included seronegative arthritis, bipolar disease and an inconclusive diagnostic laparoscopy. Diagnostic work up revealed microcytic anaemia and multivitamin deficiency. Skin biopsy of the exanthema suggested scurvy. Owing to these signs of malabsorption, upper endoscopy with duodenal biopsies was performed, exhibiting villous atrophy and extensive periodic acid-Schiff-positive material in the lamina propria, therefore diagnosing Whipple's disease (WD). After starting treatment with ceftriaxone and co-trimoxazole, an impressive recovery was noted, as the wide spectrum of malabsorption signs quickly disappeared. After a year of antibiotics, articular and cutaneous manifestations improved, allowing the patient to stop taking corticosteroids and antidepressants. This truly unusual presentation reflects the multisystemic nature of WD, often leading to misdiagnosis of other entities. Scurvy is a rare finding in developed countries, but its presence should raise suspicion for small bowel disease.
Background
Almost a century has passed between the first description of Whipple's disease (WD) in 1907 and the identification of its causative agent, Tropheryma whipplei, in the 1990s. A plausible explanation for this observation is the elusive nature of this pathogen, which is extremely hard to culture, and its slow growth resembling Mycobacterium avium-intracellulare.1 Despite being commonly found in soil and sewage, with a presumed oral–faecal route of transmission, it is a rare cause of disease. This led to the conclusion that inherent immunological defects must play a decisive role in susceptibility to this disease.2
Likewise, from a clinical point of view, WD is a hard to diagnose condition, especially when gastrointestinal symptoms are absent, and it often has a protracted course and pleomorphic presentation. The first manifestations of this disease may precede the typical clinical picture of diarrhoea, abdominal pain and malabsorption by several years.
It is not unusual for a patient with WD to have a history of other apparently unrelated illnesses that in fact were all manifestations of this multifaceted entity. This seems to be the case of our patient, as he was previously diagnosed with bipolar disease and seronegative arthritis. Severe malabsorption is one of the hallmarks of WD. However, scurvy due to vitamin C deficiency, reflecting significant damage to small bowel mucosa, was described only twice in the medical literature.3 4
Case presentation
In July 2013, a 45-year-old man was admitted to our gastroenterology department due to alternating bowel movements, with a predominance of watery diarrhoea without blood or mucus discharge, significant weight loss (12 kg in about 2 weeks) and fatigue. The patient denied any vomiting, gastrointestinal bleeding or fever. The most relevant findings in his physical examination were pale skin, a distended abdomen, and perifollicular hyperkeratotic purpura and ecchymosis in legs and buttocks (figure 1). The exanthema preceded other manifestations for about 3 weeks. Neurological examination revealed no significant abnormalities.
Figure 1.
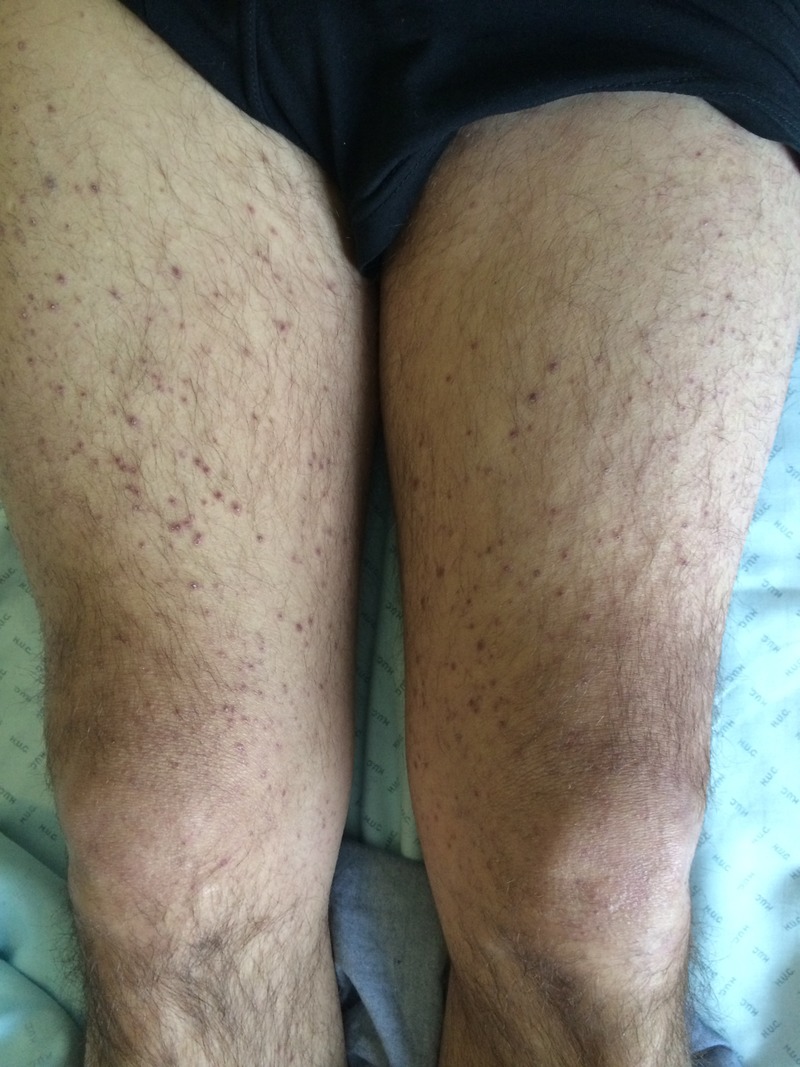
Perifollicular haemorrhage and corkscrew hair on our patient's legs were suggestive of scurvy.
About 3 years earlier, the patient had been diagnosed with seronegative arthritis. For this condition he was prescribed hydroxychloroquine, celecoxib and a low dose of prednisone. At this time, a CT scan was conducted, revealing abdominal adenopathies, which were biopsied during exploratory laparoscopy. The biopsy was not conclusive and the adenopathies were interpreted as a consequence of recent acute illness.
Two weeks prior to admission, the patient's long-standing articular problems also got worse—symmetric polyarthralgia affecting wrists, knees and ankles. An increase in prednisone dosage was suggested by his rheumatologist.
In 2008, following months of frequent mood changes, from serious depressive periods to markedly increased energy and inappropriate elation and euphoria, the patient was diagnosed with bipolar disease. When he was admitted to our department, he was medicated with olanzapine, lamotrigine and bupropion, and considered stable from a psychiatric point of view.
Diagnostic work up started with standard blood tests, which revealed microcytic anaemia (haemoglobin 10.4 g/dL; mean corpuscular volume 79 fL), hypoalbuminaemia (3 g/dL) and elevated inflammation markers (C reactive protein: 5.3 mg/dL—cut-off <0.5 mg/dL; erythrocyte sedimentation rate: 22 mm/h). Subsequent tests showed important folic acid, iron and vitamin D deficiencies. Thyroid hormones were normal and anti-transglutaminase antibody was negative. Stool culture and parasitological examination were also negative. Imaging included CT enterography to exclude inflammatory bowel disease with small bowel involvement. This examination did not show any thickening of bowel wall or any stenotic segments or fistulas, but revealed diffuse distension of the small bowel, multiple enlarged lymph nodes and a diffuse thickening of mesenteric fat (mesenteric panniculitis—figure 2). A few weeks before being admitted, the patient had undergone a colonoscopy, which had revealed an acute self-limited colitis. This could explain part of the clinical picture, but certainly not the significant signs of malabsorption.
Figure 2.
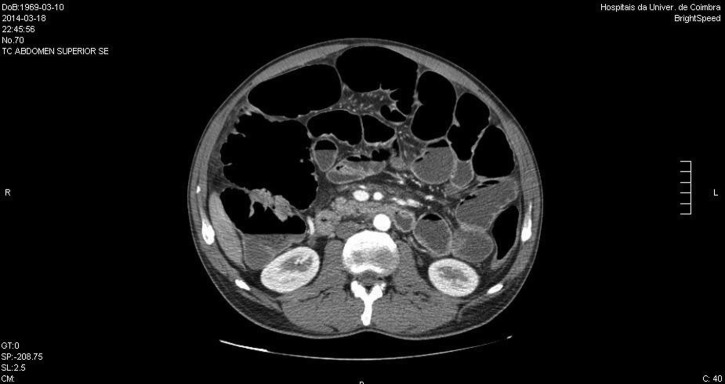
Diffuse distension of the small bowel, multiple enlarged lymph nodes and a diffuse thickening of mesenteric fat—mesenteric panniculitis—were evident in the CT enterography.
Our patient was examined in the dermatology department and a skin biopsy was performed, revealing erythrocyte extravasation, perifollicular inflammatory infiltrate, and torsion of vascular epithelium and of the hair shaft (figure 3). These findings pointed to vitamin C deficiency. Plasma levels confirmed vitamin C deficiency (1 µmol/L; normal range 11–114µmol/L).
Figure 3.
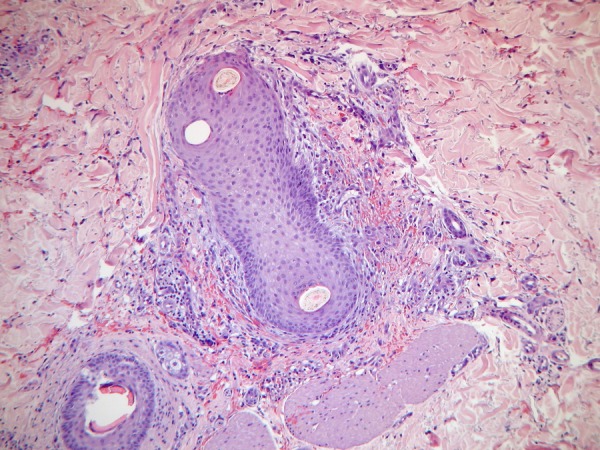
Skin biopsies revealed erythrocyte extravasation, perifollicular infiltrate and torsion of the hair shaft.
Facing such severe signs of vitamin deficiency, an upper digestive endoscopy was performed. In the second portion of the duodenum, oedematous and slightly atrophic duodenal villi and whitish dots were evident, suggesting WD (figure 4). Duodenal biopsies revealed significant villous atrophy, with a great number of histiocytes with ‘foamy’ cytoplasm and intracytoplasmic granules, with positive periodic acid-Schiff colouration (PAS+), therefore diagnosing WD.
Figure 4.
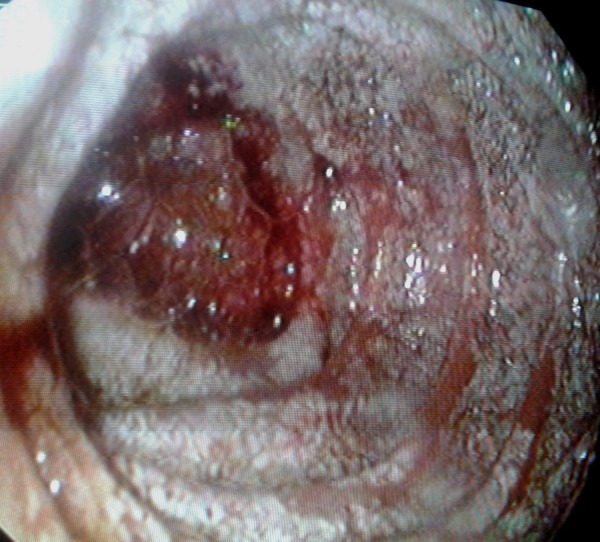
Upper endoscopy revealed friability and oedematous duodenal villi in the second portion of the duodenum.
Outcome and follow-up
After reaching the correct diagnosis, immediate treatment with intravenous ceftriaxone was started (2 g four times a day). After about 2 weeks, significant clinical improvement was noted, with normalisation of bowel habits and improvement of the polyarthralgia. The patient was then discharged after initiating treatment with oral co-trimoxazole (960 mg two times a day) and supplementation with folic acid, ferrous sulfate and vitamin C. After 1 month of treatment, blood tests showed an impressive improvement of haemoglobin levels (from 10.4 to 16.9 g/dL). Folic acid and albumin levels returned to normal as well.
During follow-up, the attending rheumatologist attributed the patient's long-standing polyarthralgia to WD and prednisone was successfully interrupted. Moreover, despite not being certain if the patient's bipolar disease was in fact an early manifestation of WD, bupropion was successfully interrupted as well.
After about a year of treatment with co-trimoxazole, the patient completely recovered, with no gastrointestinal or articular problems. Despite the known tendency of WD to relapse, so far our patient has shown no signs of recrudescence.
Discussion
From a microbiologic standpoint, T. whipplei is a Gram-positive bacillus commonly found in soil and untreated water deposits. Unique characteristics include glutaraldehyde resistance, which may explain its abundance in the environment.5 This may be a concern as this agent has a probable faecal–oral route of transmission and this chemical agent is often used in disinfection. Resembling its slow-growing distant relative, M. avium-intracellulare, successful culture was hard to achieve, as it was described only in the 1990s. The most significant advance in documenting T. whipplei has been PCR.6
It is now known that T. whipplei is found in 1–11% of stool specimen of European adults, reaching even higher prevalence in Africa and in certain professional groups such as farmers and sewage workers (12–26%).7 These numbers are in sharp contrast to the very modest count of WD reports in the medical literature (around 1000, with most being European middle-aged men). An immediate assumption comes to mind—there must be a host immune deficiency predisposing certain individuals to the disease. A number of observations support this theory. Antigen presentation by the MHC class II apparatus is absent or diminished in WD, but normalises with treatment, suggesting immune downregulation by T. whipplei. Inherited characteristics also play a role, as HLA alleles DRB1*13 and DQB1*06 have shown to be risk factors for WD.8
Our case describes an extremely uncommon skin manifestation of WD due to vitamin C deficiency. In fact, scurvy has become a rarity in developed countries, and represents the terminal stage of prolonged and severe ascorbic acid depletion. Facing low serum levels, healthy intestinal mucosa can increase absorption through ascorbate-specific transporters (SVCT), and there is an increment of retention by the kidneys, prolonging vitamin C half-life up to 83 days after the onset of the first symptoms of scurvy.9 Therefore, clinical signs of such deficiency are an unusual finding even in the presence of a malabsorption syndrome, and imply a complete disruption of the normal absorption process.
It is well known that WD has frequent articular, psychiatric, central nervous system (CNS) and even ocular manifestations.10 Interestingly, our patient had a history of both articular and psychiatric disease. Joint involvement in WD usually consists of mild to moderate, migratory and symmetrical, intermittent, non-erosive polyarthritis affecting large joints such as knees or ankles. It usually responds very well to antibiotic treatment for WD. As was the case in our patient, median time from the first symptoms to diagnosis can reach 5 years or even more.11
Likewise, CNS and psychiatric involvement may be the first or even only manifestation of WD. The most common presentation is cognitive impairment, but oculomasticatory myorhythmia (continuous rhythmic movements of the eyes convergent with concurrent contractions of the masticatory muscles) and oculo-facial-skeletal myorhythmia are considered pathognomonic.12 It seems reasonable to admit that bipolar disease was a manifestation of WD in our patient. As he was stable from a psychiatric point of view, with no recent depressive or euphoric episodes, we decided not to perform lumbar puncture to confirm central involvement, considering risks involved and bearing in mind that rt-PCR assay of cerebrospinal fluid may be negative in patients with CNS-WD. According to proposed guidelines,13 a patient with possible CNS-WD should present with one of four systemic symptoms (diarrhoea, arthralgia, fever or adenopathies), and one of four neurological signs and/or symptoms, such as dementia with psychiatric symptoms. To the best of our knowledge, bipolar disease was only once described as an early manifestation of CNS-WD.14 The reversibility of the bipolar disease has to be evaluated after a longer time span.
Clinical manifestations of WD have been typically described in two (or three) stages—a long non-specific prodromal phase, often manifested by arthralgia or neuropsychiatric disease, and a later steady stage, which occurs about 5 years later and includes typical abdominal symptoms followed by systemic consequences of the disease, such as steatorrhoea, cachexia, lymphadenopathy and multisystemic dysfunction. Why this transition is so long and why it occurs are not yet fully understood. Owing to common joint involvement, almost 50% of WD cases are initially misdiagnosed with an inflammatory rheumatoid disease and treated with immunosuppressants (including tumour necrosis factor inhibitor), which are often responsible for rapid clinical progression.15 In our patient, an increase in corticosteroid dosage may have been a trigger for the transition to overt clinical manifestations of WD.
Another interesting aspect is that he was on hydroxychloroquine, a common anti-inflammatory drug used in rheumatism disorders. Hydroxychloroquine has also been described as an effective alternative in T. whipplei infection, especially in association with doxycycline.16 This antimalarial drug leads to alkalinisation of acid vesicles, which restores intracellular pH, inhibiting growth of intracellular agents and allowing antibiotic efficacy. This mechanism is the basis for the use of hydroxychloroquine in Q fever.
Infection by T. whipplei has been compared to Actinomyces genus or atypical Mycobacterium. This similarity is underlined by the need for a prolonged antibiotic regimen. This is probably explained by the immune reaction to T. whipplei. Resembling Th1/Th2 response, it is now thought that most infectious diseases exhibit both macrophage M1 and M2 response (M1—‘killing’, protective; M2—‘repair’, bacterial persistence). WD is an exception to this equilibrium, as it is one of the rare examples of almost exclusive M2 polarisation,17 which allows T. whipplei to pass under the immune system radar and accumulate at different sites, without exerting visible cytotoxic effects on host cells, at least in the first years of disease.
Learning points.
Whipple's disease (WD) is a rare entity caused by a ubiquitous organism that should be included in the differential diagnosis of any patient with chronic diarrhoea and evidence of malabsorption.
WD is a remarkable example of interaction between host and pathogen, as Tropheryma whipplei benefits from immunological deficiency and induces downregulation of immune response, undermining antigen presentation in intestinal epithelial cells.
It is known that WD is not exclusively gastrointestinally involved, but what our case exemplifies is that extra-intestinal manifestations often precede typical symptoms by several years and may even be confused with other entities, such as rheumatoid arthritis or bipolar disease. Immunosuppression prescribed for a supposed rheumatic disease is often the trigger for typical WD manifestations to take place.
Cutaneous involvement of WD is uncommon and scurvy is an extremely rare form of presentation. Currently, when facing signs of vitamin C deficiency such as scurvy in developed countries, we should consider investigating small bowel disease.
Treatment for WD is generally effective and often leads to spectacular improvement, as in our patient, but it requires a long course of antibiotic therapy and close follow-up monitoring due to the significant risk of relapse.
Footnotes
Contributors: DFB gathered the data and drafted the manuscript. MG made significant contributions to the content of the manuscript. SM was involved in critically reviewing the data. CS gave final approval of the version to be published.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ratnaike RN. Whipple's disease. Postgrad Med J 2000;76:760–6. 10.1136/pmj.76.902.760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marth T, Kleen N, Stallmach A et al. Dysregulated peripheral and mucosal Th1/Th2 response in Whipple's disease. Gastroenterology 2002; 123:1468 10.1053/gast.2002.36583 [DOI] [PubMed] [Google Scholar]
- 3.Juárez Y, España S, Fernández-Díaz M et al. Scurvy and acquired ichthyosis associated to Whipple's disease. Actas Dermosifiliogr 2006;97:587–90. 10.1016/S0001-7310(06)73472-X [DOI] [PubMed] [Google Scholar]
- 4.Hmamouchi I, Costes V, Combe B et al. Scurvy as the presenting illness of Whipple's disease exacerbated by treatment with etanercept in a patient with ankylosing spondylitis. J Rheumatology 2010;37:1077–8. 10.3899/jrheum.091301 [DOI] [PubMed] [Google Scholar]
- 5.La Scola B, Rolain JM, Maurin M et al. Can Whipple's disease be transmitted by gastroscopes? Infect Control Hosp Epidemiol 2003;24:191–4. 10.1086/502188 [DOI] [PubMed] [Google Scholar]
- 6.Fenollar F, Fournier PE, Raoult D et al. Quantitative detection of Tropheryma whipplei DNA by real-time PCR. J Clin Microbiol 2002;40:1119–20. 10.1128/JCM.40.3.1119-1120.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keita AK, Bassene H, Tall A et al. Tropheryma whipplei: a common bacterium in rural Senegal. PLoS Negl Trop Dis 2011;5:e1403 10.1371/journal.pntd.0001403 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Martinetti M, Biagi F, Badulli C et al. The HLA alleles DRB1*13 and DQB1*06 are associated to Whipple's disease. Gastroenterology 2009;136:2289 10.1053/j.gastro.2009.01.051 [DOI] [PubMed] [Google Scholar]
- 9.Grosso G, Bei R, Mistretta A et al. Effects of vitamin C on health: a review of evidence. Front Biosci (Landmark Ed) 2013;18:1017–29. [DOI] [PubMed] [Google Scholar]
- 10.Lo Monaco A, Govoni M, Zelante A et al. Whipple disease: unusual presentation of a protean and sometimes confusing disease. Semin Arthritis Rheum 2009;38:403–6. 10.1016/j.semarthrit.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 11.Durand DV, Lecomte C, Cathébras P et al. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore) 1997;76:170–84. [DOI] [PubMed] [Google Scholar]
- 12.Compain C, Sacre K, Puéchal X. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore) 2013;92:324–30. 10.1097/MD.0000000000000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis ED, Lynch T, Kaufmann P et al. Diagnostic guidelines in central nervous system Whipple's disease. Ann Neurol 1996;40:561–8. [DOI] [PubMed] [Google Scholar]
- 14.Verhagen W, Huygen PL, Dalman JE et al. Whipple's disease and the central nervous system. A case report and a review of the literature . Clin Neurol Neurosurg 1996;98:299–304. 10.1016/0303-8467(96)00035-2 [DOI] [PubMed] [Google Scholar]
- 15.Lagier JC, Lepidi H, Raoult D et al. Systemic Tropheryma whipplei: clinical presentation of 142 patients with infections diagnosed or confirmed in a reference center. Medicine (Baltimore) 2010;89:337–45. 10.1097/MD.0b013e3181f204a8 [DOI] [PubMed] [Google Scholar]
- 16.Boulos A, Rolain JM, Raoult D. Antibiotic susceptibility of Tropheryma whipplei in MRC5 cells. Antimicrob Agents Chemother 2004;48:747–52 10.1128/AAC.48.3.747-752.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mignane B, Daumas A, Textoris J. Phenotypic diversity and emerging new tools to study macrophage activation in bacterial infectious diseases. Front Immunol 2014;5:500 10.3389/fimmu.2014.00500 [DOI] [PMC free article] [PubMed] [Google Scholar]


