Graphical abstract
Abbreviations: BSA, bovine serum albumin; BAG1, bradyzoite antigen 1; DA, dopamine; DDC, DOPA decarboxylase and AADC; EDTA, ethylenediaminetetraacetic acid; FACS, fluorescence-activated cell sorting; FBS, fetal bovine serum; FRET, fluorescence resonance energy transfer; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; HPLC, high-performance liquid chromatography; l-DOPA, l-3,4-dihydroxyphenylalanine; PBS, phosphate-buffered saline; PV, parasitophorous vacuole; T. gondii, Toxoplasma gondii; TH, tyrosine hydroxylase; VMAT1, vesicular monoamine transporter 1
Key words: apicomplexa, neurotransmitter, tyrosine hydroxylase, DOPA decarboxylase, manipulation
Highlights
-
•
Toxoplasma infection of neurosecretory cells increases the production of dopamine.
-
•
Infection did not change host TH or DDC.
-
•
Host DDC was observed in the parasitic vacuole in infected brain cells.
-
•
Import of host DDC may facilitate DA synthesis in the parasitic vacuole.
-
•
This would prevent toxicity due to cytosolic unpackaged dopamine.
Abstract
Infection by the neurotropic agent Toxoplasma gondii alters rodent behavior and can result in neuropsychiatric symptoms in humans. Little is understood regarding the effects of infection on host neural processes but alterations to dopaminergic neurotransmission are implicated. We have previously reported elevated levels of dopamine (DA) in infected dopaminergic cells however the involvement of the host enzymes and fate of the produced DA were not defined. In order to clarify the effects of infection on host DA biosynthetic enzymes and DA packaging we examined enzyme levels and activity and DA accumulation and release in T. gondii-infected neurosecretory cells. Although the levels of the host tyrosine hydroxylase (TH) and DOPA decarboxylase and AADC (DDC) did not change significantly in infected cultures, DDC was found within the parasitophorous vacuole (PV), the vacuolar compartment where the parasites reside, as well as in the host cytosol in infected dopaminergic cells. Strikingly, DDC was found within the intracellular parasite cysts in infected brain tissue. This finding could provide some explanation for observations of DA within tissue cysts in infected brain as a parasite-encoded enzyme with TH activity was also localized within tissue cysts. In contrast, cellular DA packaging appeared unchanged in single-cell microamperometry experiments and only a fraction of the increased DA was accessible to high potassium-induced release. This study provides some understanding of how this parasite produces elevated DA within dopaminergic cells without the toxic ramifications of free cytosolic DA. The mechanism for synthesis and packaging of DA by T. gondii-infected dopaminergic cells may have important implications for the effects of chronic T. gondii infection on humans and animals.
Introduction
Toxoplasma gondii (T. gondii) is a common parasite that has been found to infect all warm-blooded animals tested, including humans (Hill et al., 2005; Darde et al., 2007). During infection, the parasite forms tissue cysts predominantly in the brain and muscles generating a chronic infection (Hutchinson, 1966). Chronic infection with the encysted form of T. gondii has been associated with behavioral changes in rodents, with infected rodents showing higher activity levels and decreases in predator vigilance (Webster et al., 1994; Webster, 1994a,b; Flegr et al., 1996, 2000, 2002; Berdoy et al., 2000; Yereli et al., 2006; Vyas et al., 2007; Kocazeybek et al., 2009). These behavioral traits could facilitate parasite transmission from the rodent to its definitive feline host (Hutchinson, 1966) as rodents lose their strong innate aversion to cat odor upon infection and exhibit, to some degree, an attraction to cat urine-treated areas (Berdoy et al., 2000; Vyas et al., 2007).
It has been postulated that alterations to dopaminergic signaling mediate the behavioral changes seen in rodents following T. gondii infection, as high concentrations of dopamine (DA) were found to be localized in tissue cysts within infected mouse brains (Prandovszky et al., 2011). Further support is gained from the administration of DA receptor antagonists, such as DA D2 receptor antagonists haloperidol and GBR 12909, which block establishment of the behavioral changes in rats, furthermore haloperidol also has some antiparasitic activity (Jones-Brando et al., 2003; Skallova et al., 2006; Webster et al., 2006). Further supporting fact of behavior changes involvement in the facilitation of parasite transmission the activation of sexual arousal pathways in infected male rats when exposed to cat urine has been observed (House et al., 2011); interestingly the three regions identified with increased activity (medial amygdala, ventromedial hypothalamus, and basolateral amygdala) are DA responsive. In addition, T. gondii infection has been shown to increase the amount of DA in the striatum, another region of dense DA neurotransmission, of mice with a 38% greater level in infected mice relative to uninfected mice (Xiao et al., 2014).
The molecular mechanisms by which T. gondii infection could induce alterations to dopaminergic signaling are unclear, but based on our previous findings, infection of dopaminergic cells in vitro increased their DA content by threefold (Prandovszky et al., 2011), suggesting altered DA synthesis. DA synthesis involves two enzymatic steps from l-tyrosine to DA with coincident packaging. Firstly, l-3,4-dihydroxyphenylalanine (l-DOPA) is synthesized from l-tyrosine by tyrosine hydroxylase (TH) which is subsequently metabolized to DA by DOPA decarboxylase and AADC (DDC; also named aromatic-l-amino-acid decarboxylase). As DA is synthesized, it is rapidly imported into vesicles via vesicular monoamine transporters (Cartier et al., 2010) to prevent cellular damage via free-radical generation (Hald and Lotharius, 2005; Chuenkova and Pereiraperrin, 2006). T. gondii contains two genes that encode enzymes with TH activity (Gaskell et al., 2009) but has not been reported to possess a version of DDC enzyme and no sequences homologous to this enzyme are detectable in the T. gondii genome based on bioinformatic searches in Toxodb (version 24) (www.toxodb.org), NCBI (www.ncbi.nlm.nih.gov), and using a sensitive algorithm based on enzyme profiles for parasitic protozoa (http://www.bioinformatics.leeds.ac.uk/metatiger/). This raises several questions on the mechanism by which T. gondii is invoking its changes on dopaminergic cells. This study addresses the contribution of host proteins in DA biosynthesis and packaging and how the process is orchestrated for containment of DA to avoid cellular damage. We hypothesized that infection alters levels and properties of the host DA biosynthetic machinery. To assess the stimulatory activity of infection on amount of DA, changes in the amounts of the enzymes at the RNA and protein level were measured, enzyme activity, phosphorylation, and location were analyzed, and vesicular DA accumulation and release were quantitated.
Experimental procedures
Materials
All chemicals were of analytical grade or higher and from Fisher Scientific (Loughborough, UK) unless otherwise stated.
Cell and parasite culture
Rat pheochromocytoma (PC12 cells) cells from the European Collection of Cell Cultures (Salisbury, UK) were maintained as previously described (Prandovszky et al., 2011). For all experiments PC12 cells were plated in poly-d-lysine-coated flasks and plates. The T. gondii strain Prugniaud was used for all experiments except the fluorescence-activated cell sorting (FACS)/amperometry experiment that used Prugniaud KU80- kindly provided by Louis Weiss (Fox et al., 2011). Parasites were maintained in human foreskin fibroblasts as previously described (Gaskell et al., 2009).
Bradyzoite induction and PC12 infection
Bradyzoite induction was as previously described (Prandovszky et al., 2011). Briefly, liberated tachyzoites were incubated at 37 °C in RPMI supplemented with 1% fetal bovine serum (FBS) at pH 8 for 16–18 h in ambient CO2, then diluted with DMEM, isolated by centrifugation, and suspended in RPMI (pH 7.4) containing horse serum, FBS and penicillin/streptomycin. PC12 cells were plated at a density of 2.5 × 105 cells/well in 6-well plates (unless otherwise stated), the “induced tachyzoites” were added to PC12 cell cultures, and cultures were changed 3 days after infection with assays performed on the fifth day. Bradyzoite differentiation was monitored by detection of Bradyzoite antigen 1 (BAG1) expression as described below.
Measurement of DA levels
DA was measured by high-performance liquid chromatography (HPLC) with electrochemical detection using the method described by Prandovszky et al. (2011) with the cells detached from the wells by trypsin treatment, pelleted and an aliquot taken for measurement of cell number using the CyQuant kit (Invitrogen Gaithersburg, MD, USA) as per the manufacturer’s instructions (Prandovszky et al., 2011). All samples were run with three technical replicates and standards. Technical replicates were reproducible within 5% of each other and were within the linear range of detection.
RNA extraction, RT-PCR and qPCR
Infected PC12 cells were detached by trypsin treatment and rinsed in phosphate-buffered saline (PBS). Cell pellets were then resuspended in Tri-reagent and total RNA extracted as per the manufacturer’s instructions (SigmaAldrich, St. Louis, MO, USA). RNA was quantified by Nanodrop (ThermoScientific, Waltham, MA, USA) and 500 ng of RNA was subjected to DNase treatment (Invitrogen, Gaithersburg, MD, USA) as per the manufacturer’s instructions. First strand cDNA was synthesized using random primers (Promega, Southampton, UK) and Superscript II (Invitrogen) as described by the manufacturer. For qualitative PCR 100-ng cDNA was amplified using GoTaq (Promega) as per the manufacturer’s instructions. For quantitative PCR amplification of 100-ng cDNA was undertaken using the Bio-Rad CFX96 (Hemel Hempstead, UK) and SYBR® Green PCR Master Mix (Applied Biosystems, Paisley, UK), as described by the manufacturers. Primer pairs were as follows: BAG1 Sense 5′ ATTCTTCTCAGGGCGGTGCT 3′, Anti-sense 5′ CCTTCTTTGTTTCATCGTCGTCC 3′; Rat GAPDH Sense 5′ GTGGACCTCATGGCCTACAT 3′, Anti-sense 5′ TGTGAGGGAGATGCTCAGTG 3′; Rat DDC Sense 5′ CGGAGAAGAGGGAAGGAGATGGT 3′, Anti-sense 5′ GCCGTGGGGAAGTAAGCGAAG 3′; Rat TH Sense 5′ CCCAAAGTCTCCATCCCCTTC 3′, Anti-sense 5′ GGTTGAGAAGCAGTGTTGGGA 3′. Fragment identities were confirmed by sequencing. Primer efficiencies for TH were 101% and DDC 97%. Fold change was measured as ΔΔCQ with raw values in the range of 20–30 cycles. All measurements were three independent experiments with three technical repeats per experiment and technical repeats within 10% of each other and GAPDH run in parallel.
Protein extraction and Western blotting
PC12 cells were plated in 6-well plates and infected as described above. Cells were detached by trypsinization and centrifuged at 2000 rpm for 5 min, followed by rinsing with PBS. Cell pellets were then resuspended in and sonicated as in Prandovszky et al. (2011). Protein concentration was assessed using Bradford reagent (SigmaAldrich) as per the manufacturer’s instructions. Western blotting was employed with 5–10 μg of protein extracts from cells sonicated in radioimmunoprecipitation assay buffer (Caymen Chemicals, Ann Arbor, MI, USA) and protease inhibitors (cOmplete Mini EDTA-free cocktail; Roche Life Sciences, Burgess Hill, UK) separated by SDS–PAGE, transferred to nitrocellulose membrane, blocked (with 5% milk or BSA) and probed overnight with primary antibody in 1% blocking solution. Protein transfer and amounts were verified on blots with Ponceau S staining. All experiments were performed with at least three independent experiments. The enzymes in this study are low abundance proteins that are in the dynamic linear range with experimental design for quantitation as described (Taylor and Posch, 2014).
The following day membranes were washed in PBS-Tween 20, incubated with appropriate secondary antibody, washed and visualized with SuperSignal West Pico Chemiluminescent Substrate (Pierce, Loughborough, UK). Primary antibodies were goat anti-rat TH (1:1000; SigmaAldrich), rabbit anti-DDC (1:5000; Abcam, Cambridge, UK), rabbit anti-TH phosphor ser19 (Millipore, Watford, UK), rabbit anti-TH phosphor ser40 (Millipore) and mouse anti-β-actin (1:25,000; SigmaAldrich). Secondary antibodies were donkey anti-goat HRP conjugate (1:2500; Abcam), goat anti-rabbit HRP conjugate (1:5000; SigmaAldrich) and goat anti-mouse HRP conjugate (1:10,000; Abcam). Control experiments demonstrated that these primary antibodies did not detect any proteins in purified parasite extracts.
TH activity
PC12 cells were plated in T25 flasks at a density of 1 × 106 cells/flask and infected with different ratios of induced tachyzoites of the Prugniaud strain. The enzymatic activity of TH was assessed using a previously described method (Naoi and Nagatsu, 1988) as follows: 1 × 106 PC12 cells were detached by scrapping, centrifuged at 800 rpm for 10 min, rinsed in PBS before being resuspended in 100 μl 10 mM potassium phosphate buffer (pH 7.4), sonicated and protein content measured by Bradford assay. Cell extracts (25 μg) were incubated at 37 °C for 5 min in 200 mM sodium acetate buffer (pH 6) containing 10 μg/ml catalase (SigmaAldrich) and 200 μM carbidopa (SigmaAldrich). Enzyme substrate and co-factor were then added, 1 μl of 20 mM l-tyrosine and 20 μl of 10 mM (6R)-5-methyl-5,6,7,8-tetrahydro-l-biopterin (Schicks Laboratories, Jona, Switzerland) in 1 M β-mercaptoethanol (SigmaAldrich) and the 100-μl reaction mixture incubated at 37 °C. The reaction was terminated at different time points by the addition of 100 μl 0.1 M perchloric acid (PCA). l-DOPA production was determined by HPLC as described for DA above, but with a flow rate of 0.8 ml/min.
Amperometry
PC12 cells were plated in T75 flasks at a density of 5 × 106 cells/flask and infected with 5 × 106 induced tachyzoites of the Prugniaud KU80 strain (Fox et al., 2011) as this strain contains GFP under the control of a bradyzoite-specific promoter. Dexamethasone (SigmaAldrich) was added to a final concentration of 1 μM. On the fourth day of infection cells were detached and resuspended in 1 ml PBS containing 25 mM HEPES pH 7.4, 2.5 mM EDTA and 1% FBS. GFP-positive cells were then isolated by FACS on a BDFACSAria IIu flow cytometer (Beckton Dickinson, Oxford, UK) controlled with BDFACSDiva 6 software. GFP was determined by fluorescence signal generated from a 488-nm laser excitation and signal collection through a bandpass emission filter 525/10 nm. GFP-positive cells were identified by sequential gating of cells (based on forward and side scatter profiles to exclude debris) and GFP sort gates were established using PC12 cells infected with non-GFP parasites based on a dot plot demonstrating GFP signal against side scatter. Typically GFP-positive cells were between 1% and 2% of the total cell population, and were subsequently enriched to >90%.
Control and GFP-positive cells were then plated onto poly-d-lysine-coated coverslips at a density of approximately 1 × 104 cells/cm2 and allowed to adhere overnight. Fragments of coverslip were then transferred to a recording chamber (volume ca 80 μl) and perfused with a solution of composition (135 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 2.5 mM CaCl2, 5 mM HEPES and 10 mM glucose 10 (pH 7.4). Carbon fiber microelectrodes (proCFE, Axon Instruments; diameter 5 μm) were positioned adjacent to individual PC12 cells and polarized to +800 mV to allow oxidation of released catecholamine. Currents were filtered at 1 kHz and digitized at 2 kHz before storage on computer. All data acquisition was performed using an Axopatch 200A amplifier, Digidata 1200 interface and Fetchex software from the pCLAMP 6.0.3 suite (Axon Instruments, Sunnyvale, CA, USA).
For each experiment, current was recorded for a control period (10–30 s), then the cell was mechanically stimulated with the electrode (Wightman et al., 1991), and the resultant quantal secretory events recorded (Wightman et al., 1991; Chow and Von Ruden, 1995). Spikes were analyzed for amplitude and also integrated to obtain charge, Q as previously described (Finnegan et al., 1996). All spike analysis was performed using Minian 16 software (Jaejin Software, Columbia, NY, USA). This allowed visual inspection of each event so that artifacts could be rejected from analysis. The sample numbers (n) are within the range typically studied in previous work (Taylor and Peers, 1999a,b; Taylor et al., 2000).
Immunocytochemistry
In vitro
PC12 cells were plated and infected with Prugniaud as described. On the fifth day on infection cells were fixed in 4% paraformaldehyde for 15 min, rinsed with PBS and incubated with AlexaFluor 633-conjugated wheat germ agglutinin (5 μg/mL in PBS; Invitrogen) for 20 min. Cells were permeabilized and non-specific sites blocked with 1% BSA, 10% normal goat serum (Vector Labs, Burlingame, CA, USA) and 0.3 M glycine in PBS-Tween 0.1% for 1 h. This was followed by overnight incubation at 4 °C with rabbit anti-vesicular monoamine transporter 1 (VMAT1) antibody (1:200; Millipore) Cells were then rinsed with PBS, incubated for an hour with an AlexaFluor 555-conjugated anti-rabbit secondary (1: 2500 in PBS; Invitrogen), rinsed with PBS and incubated with Hoechst 33342 (5 μg/mL) for 20 min. Following final rinses with PBS coverslips were mounted with Fluromount G (Southern Biotech, Birmingham, UK) and imaged by confocal microscopy (LSM 510, Carl Zeiss, Hertfordshire, UK). For fluorescence resonance energy transfer (FRET) analysis, cells were stained with rabbit anti-T. gondii TH (Prandovszky et al., 2011) with overnight incubation at 4 °C followed by anti-rabbit conjugated with biotin as secondary antibody (1:500, Vector Labs) and streptavidin conjugated with FITC (Vector Labs) at 1:100 dilution as described by the manufacturer. Sections were then stained with sheep anti-DDC (LifeSpan Biosciences, Inc., Seattle, USA) primary antibody at 1:200 dilution for several hours at 4 °C followed by anti-sheep IgG polyclonal antibody conjugated with rhodamine (GTX27108, GeneTex, Inc., Irvine) at a dilution of 1:100. FRET analysis was performed with the ImageJ PixFRET plug-in. Resulting FRET images were pseudocolored with the Fire look up table.
In vivo
Immunofluorescence of DDC was performed on female Swiss Webster mouse brain sections. Mice were infected with T. gondii VEG strain oocysts 6–8 weeks prior to processing (kind gift of J.P. Dubey, United States Department of Agriculture). Brains (n = 2) were collected, formalin-fixed and paraffin-embedded using standard protocols and following approved guidelines. Sections for immune fluorescence staining were processed as previously described (Prandovszky et al., 2011) except rabbit anti-mouse DDC (1:500, Abcam) was used as the primary antibody with FITC-conjugated streptavidin (Vector Labs). Immunohistochemical assays were performed as previously described (Prandovszky et al., 2011) with primary antibody rabbit anti mouse DDC (1:500; Abcam) staining overnight at 4 °C.
Statistical analysis
Data were analyzed in GraphPad Prism (Version 5.04 for Windows, GraphPad Software, San Diego, CA, USA). The data were initially tested for Gaussian distribution. Statistical significance was determined using an ANOVA or student t-test for parametric data and Kruskal–Wallis with Dunn’s post-hoc test for non-parametric data. A p value of <0.05 was considered significant. All values expressed are mean ± SEM.
Results
Impact of infection on DA levels and DA synthesis in dopaminergic cells
Initially, experiments were performed to reproduce the increase in DA synthesis and release during infection of the dopaminergic PC12 cell line (Westerink and Ewing, 2008) observed in a prior study (Prandovszky et al., 2011). The cells were infected with different numbers of parasites (expressed as parasite:PC12 cell ratio) and DA synthesis was measured by HPLC-ED. In all experiments liberated tachyzoites were stressed exogenously (see Experimental procedures) prior to infection and assayed at day five of infection as previously. Bradyzoite induction in these cultures, monitored by RT-PCR, was confirmed (data not shown). Spontaneous release of DA was not influenced by infection (Kruskal–Wallis test, n = 5, p = 0.19). T. gondii infection increased total DA amounts in PC12 cells 2–3-fold of the uninfected controls with the amount increasing proportionally to the number of parasites in the infection (ie. increasing parasite:PC12 cell ratio) (Fig. 1A). Levels of both high potassium-released and total DA were statistically increased with infection (Kruskal–Wallis test, n = 6, p = 0.017 and p = 0.0041, respectively). Biological repeats varied in amplitude of increase due to differing properties with different batches and passages of PC12 cells (Green et al., 2001). These increases in DA levels in infected cells here with several biological repeats concur with our previous observations (Prandovszky et al., 2011). Next we compared the amount of DA released by induction with a high potassium buffer to total amount of DA per cell with infected and uninfected cells to extend our prior study (Prandovszky et al., 2011). The infected cells released up to twice the amount of DA compared to the uninfected controls with a significant correlation between infective dose and amount of DA released over several biological repeats (n = 5–6, Spearman correlation p < 0.05; Fig. 1B). Infection did not trigger spontaneous DA release shown by the lack of significant difference in the amount of DA in the wash buffer compared to uninfected cells as in earlier work (Prandovszky et al., 2011). Hence DA upregulation and release are reproducibly increased with infection of PC12 cells with a lower increase in percent released compared to total DA synthesis.
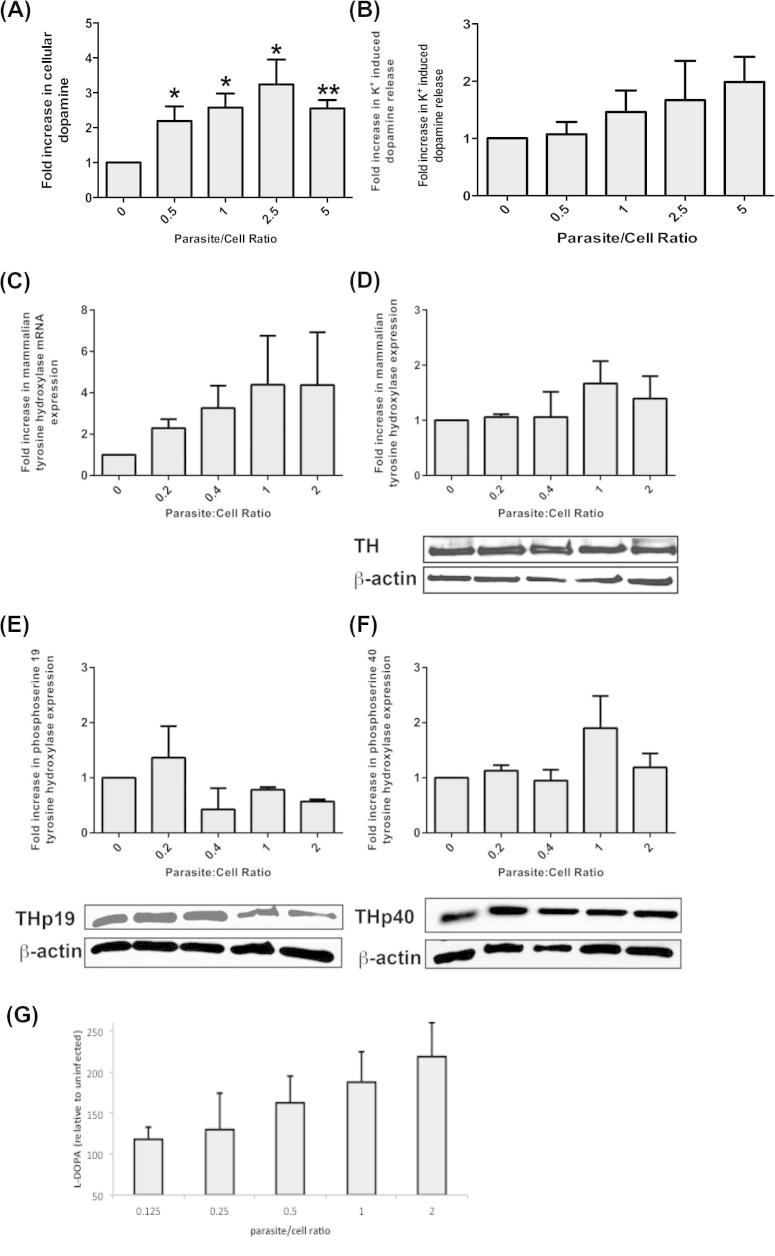
Fig. 1.
Effect of infection on dopamine production and tyrosine hydroxylase in dopaminergic cells. Levels of total dopamine (A) and potassium-induced dopamine release (B) from cells infected with increasing numbers of T. gondii parasites were assessed by HPLC-ED (n = 6 independent experiments). A one-way ANOVA was performed (total DA, F(4,25) = 3.78 p = 0.015; released DA, F(4,25) = 0.988 p = 0.43). Statistically significant differences are denoted by *for p < 0.02 and **for p < 0.0001 compared to uninfected cells (Student t-test). Tyrosine hydroxylase (TH) amounts in PC12 cells infected with increasing numbers of induced tachyzoites monitored by qPCR (C) and (n = 3 independent experiments, p > 0.5, ANOVA F(4,10) = 0.845) Western analysis (D) (n = 3 independent experiments, p > 0.5) with a representative blot are shown. Rat actin served as a control. Western blot analysis of phosphorylation at rat TH ser19 and ser40 (n = 3 independent experiments) that contribute to TH activation shown on a representative blots (E, F). l-DOPA synthetic activity was measured in extracts derived from PC12 cells infected with increasing doses. The graph shows the mean log of the increased l-DOPA measured by HPLC-ED with the SEM (n = 5 independent experiments, p value = 0.03, Student t-test) (G).
As TH is the key regulatory enzyme for DA synthesis, we tested whether infection altered the level of TH activity in PC12 cells as an increase in activity could explain the increased amount of DA synthesized with infection. The TH activity in extracts from cells infected with different numbers of parasites, as measured by synthesis of l-DOPA synthesis in cell extracts, was determined. l-DOPA amounts were significantly increased with infection (ANOVA p < 0.005; Fig. 1G) with the changes with infective dose reflecting that observed with DA levels (Fig. 1A, B).
We hypothesized that an increase in the amount of PC12 TH could explain the increased TH activity. Hence, the effect of infection on the host cell TH was evaluated. PC12 cells infected with increasing doses of induced T. gondii were allowed to develop for 5 days, as before, and host TH expression was assessed by RT-qPCR and western analysis. A trend toward increased TH is apparent but this was not statistically significant (Kruskal–Wallis test, n = 3–5, p > 0.1) (Fig. 1C, D).
TH activity is highly regulated by post-translational modifications with rat TH regulated by phosphorylation at residues serine 19 and serine 40 (Sura et al., 2004; Wang et al., 2011). T. gondii infection may alter phosphorylation of the rat TH. Therefore, we examined the effect of infection on phosphorylation of ser19 and ser40 using antibodies specific for these phosphorylated residues. There were no observable differences (Kruskal–Wallis test, n = 3, p > 0.4) in amounts of enzyme with phosphorylation at either of these two residues (Fig. 1E, F).
Intriguingly, infection increased DA amounts and global l-DOPA synthetic activity but significant changes in rat TH amount or modulation were not observed. A plausible explanation is significant contribution from the parasite-encoded aromatic amino acid hydroxylases (TgTH) previously described that are expressed at day five of infection and have TH activity (Gaskell et al., 2009; Prandovszky et al., 2011).
Expression and localization of DDC in T. gondii-infected dopaminergic cells
Although TH is the rate-limiting enzyme in DA synthesis, DDC, DDC, is necessary to complete DA synthesis by converting l-DOPA to DA. The effect of infection on levels of DDC was assessed in infected PC12 cells. DDC expression appears to be slightly increasing with infection but this difference is not statistically significant (Kruskal–Wallis test, n = 3, p > 0.3) at the mRNA and protein levels (Fig. 2A, B). Hence, T. gondii changes in expression of host cell TH or DDC could not be detected in these dopaminergic cells upon infection. Indeed, the only change in DA biosynthetic enzymes observed with infection is the expression of a parasite TH. Prior research showed that this enzyme is restricted to the parasitophorous vacuole (PV) in vitro and parasite cysts in the brain (Prandovszky et al., 2011).
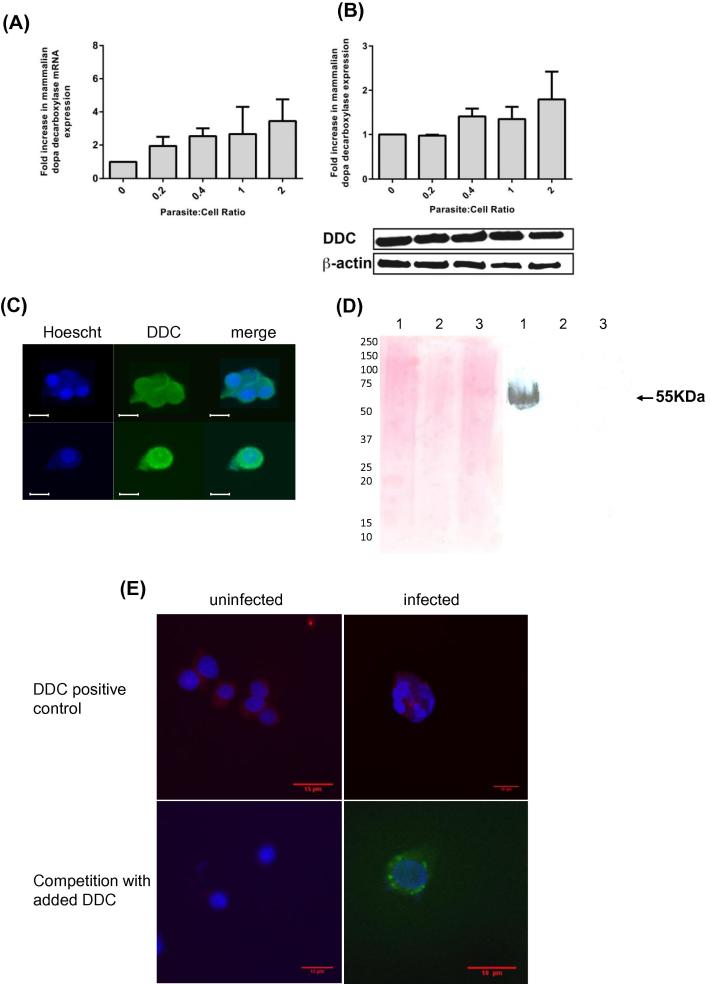
Fig. 2.
Effect of infection on dopa decarboxylase in dopaminergic cells. DOPA decarboxylase (DDC) levels in PC12 cells infected with increasing numbers of induced tachyzoites monitored by qPCR (A) and Western analysis (B) (n = 3 independent experiments) as in Fig. 1. (C) T. gondii-infected (top) and -uninfected (lower) PC12 cells were double-stained with Hoechst (blue) and DDC antibody with FITC secondary antibody (green). The panels (from left to right) show Hoechst, DDC and both channels. Hoechst stains nuclei of PC12 cells (large blue circles) and parasites (multiple small blue dots). DDC is excluded from the PC12 nuclei (lower panels) but stains similarly throughout cytosol including where the parasites reside. (D) Western blot analysis (right panel) showing lack of cross-reactivity of DDC antibody with T. gondii proteins (purified T. gondii Prugniard extract in lanes 2 and 3) and uninfected PC12 cell extract (lane 1) as a positive control. Ponceau S staining of the blot is on the left. (E) Competition experiment with purified DDC added with DDC antibody to infected and uninfected cells. Indirect DDC antibody staining (red) and Hoechst in positive controls (top) and in competition with added DDC (bottom) with anti-BAG1 antibody staining (green) to visualize the parasites are shown with merge of all three channels. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The requirement for DDC to complete DA synthesis led us to probe the location of rat DDC in infected PC12 cells as this enzyme is reported to be cytosolic and associated with cytosolic vesicles (Cartier et al., 2010). Staining with anti-DDC staining was found both in the host cytosol and parasitic compartment (Fig. 2C), i.e., the PV where T. gondii resides and replicates following infection. This was surprising as host proteins are primarily excluded from the PV. The DDC antibody used did not cross-react with any parasite proteins in Western analysis and did not stain parasite-infected human fibroblasts (Fig. 2D) demonstrating that this antibody does not recognize any parasite proteins. The specificity of the DDC antibody was further confirmed by competition assays. Primary DDC antibody staining of tissue sections was performed in the presence of exogenous recombinant DDC followed by secondary staining with fluorescein-labeled antibody. The added recombinant DDC eliminated staining as visualized by loss of fluorescence (Fig. 2E).
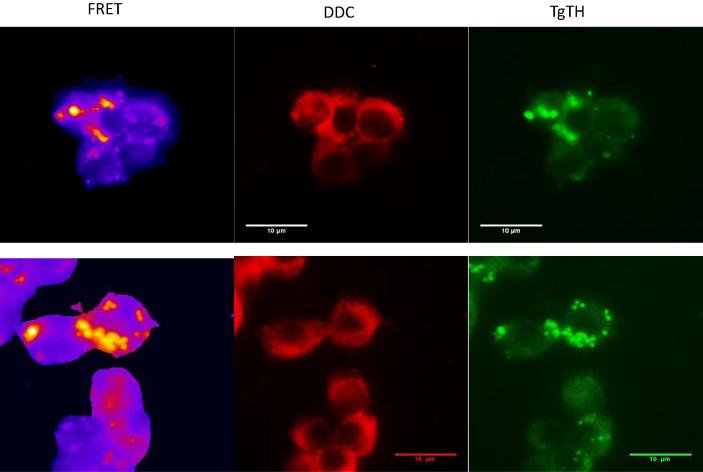
The location of rat DDC and TgTH was explored to examine whether there is potential for an interaction between the two enzymes. Double-staining, two color immunofluorescence found DDC staining throughout the cell cytoplasm and the PV as above (Fig. 2C), while TgTH staining was restricted to the PV. The specific antibody for TgTH (raised against a peptide from the amino-terminal domain without homology to any protein in the predicted rat proteome) was described in our earlier work (Prandovszky et al., 2011). FRET experiments were undertaken using fluorophore-labeled antibodies and analysis of the images to compare locations of the two enzymes. Infected cells were indirectly labeled with the TgTH antibody tagged with FITC and the DDC antibody tagged with rhodamine. FRET emission from the FITC donor activating the rhodamine acceptor was observed (Fig. 3) with co-localization quantitated (Welch’s test p < 0.0001). This indicates the two enzymes are located near each other, either both within the parasite compartment or DDC adjacent to the PV membrane, Other approaches are necessary to evaluate whether there are protein–protein interactions. Thirdly, immunoelectron microscopy was performed with antibodies against DDC and TgTH with secondary gold-conjugated antibodies finding gold particles in similar proximity (data not shown). Hence both enzymes are co-positioned in or adjoining the PV where the parasites reside.
Fig. 3.
FRET analysis of host cell DDC and a parasite-encoded tyrosine hydroxylase localization. Two representative fields of infected cells stained with DDC antibody with rhodamine-conjugated secondary antibody and TgTH antibody with FITC-conjugated secondary antibody are shown with pseudocolor of the FRET image (left), rhodamine excitation/emission (center), and fluorescein excitation/emission (right). Statistical analysis of FRET images was performed comparing to sections stained without a primary antibody (Mann Whitney test, p value <0.005).
Location of DDC in T. gondii-infected brain tissue
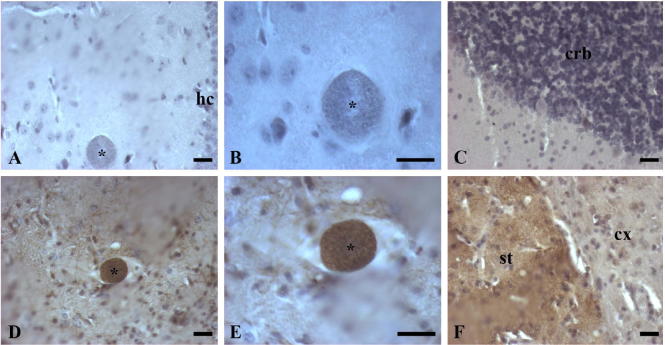
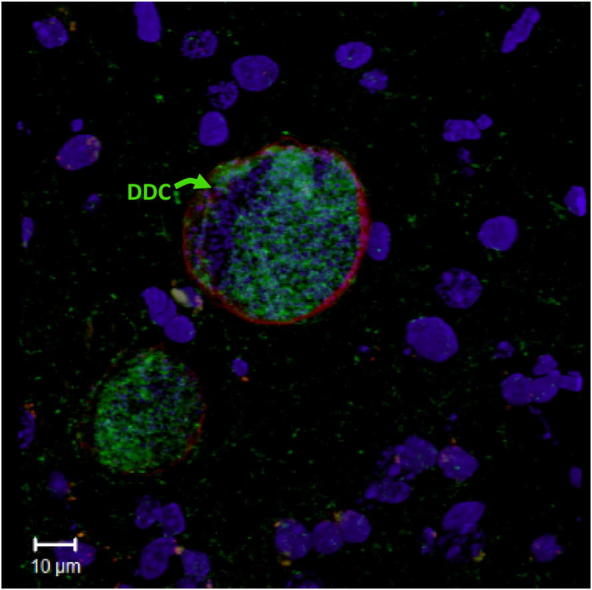
As DDC is associated with the PV in the dopaminergic cell line in vitro, we examined the location of DDC in infected brain tissue. DDC was localized in the brains of female Swiss Webster mouse (n = 2) chronically infected with T. gondii VEG strain oocysts 6–8 weeks prior to processing. The VEG chronic infection model was chosen because of the higher proposed cyst number in the brain. Using indirect labeling with horseradish peroxidase we demonstrated the presence of DDC in various brain regions. As seen in these images (Fig. 4), cysts stained brown indicating DDC within the cysts by immunohistochemical staining. To confirm immunohistochemical staining, immunofluorescence staining of DDC with nuclear (DAPI) and tissue cyst (lectin) counterstaining was also performed on consecutive sections. 3D confocal images (Fig. 5F–I) gave us insight into the inner environment of a bisected cyst. DDC staining was found inside the tissue cysts in different monoaminergic brain regions (Fig. 5). DDC was found in scattered monoaminergic neurons (Fig. 5C), as expected, as in normal mice. DDC is within the lectin-stained cyst reiterating the findings of the in vitro experiments.
Fig. 4.
Immunohistochemical localization of DDC in brain sections of chronically infected mice with horseradish peroxidase labeling. Brown staining indicates the presence of DDC. Hematoxylin was used to counterstain the sections and highlight cell nuclei blue. T. gondii tissue cysts (labeled with an asterisk) containing multiple individual parasites are large and circular. (A, B) show negative AADC staining (no primary antibody, secondary only was applied) in the hippocampus (hc) at 20× and 40× magnification, respectively. (D, E) show positive DDC staining in the hippocampus at 20× and 40× magnification, respectively. (C) shows negative DDC tissue staining in the cerebellum (crb), while (F) displays a positive tissue control for DDC staining at the border of the striatum (st) and cortex (cx). Regions of hippocampus (hc), cerebellum (crb), striatum (st), and cortex (cx) are labeled. Scale bars = 50 μm.
Fig. 5.
Immunofluorescence of DDC in the brains of chronically infected mice. 3D confocal projections of cysts (labeled with asterisks in the left panel) in the brain tissue of T. gondii-infected mice triple stained with DAPI (blue), DDC antibody (green), and lectin (red). The four panels (from left to right) show DAPI, DDC, and lectin staining separately, while the fourth panel shows a merged image of all three channels. DAPI identifies nuclei of cells and individual parasites, while lectin stains the T. gondii tissue cyst wall. In A–E the section is from the cortex and in F–I the section is from the hippocampus. The inset C shows the surrounding cortical area under higher magnification with arrows pointing to DDC positive neurons. Images J–M display control staining without primary DDC antibody. Scale bars = 10 μm.
Packaging of DA in infected cells
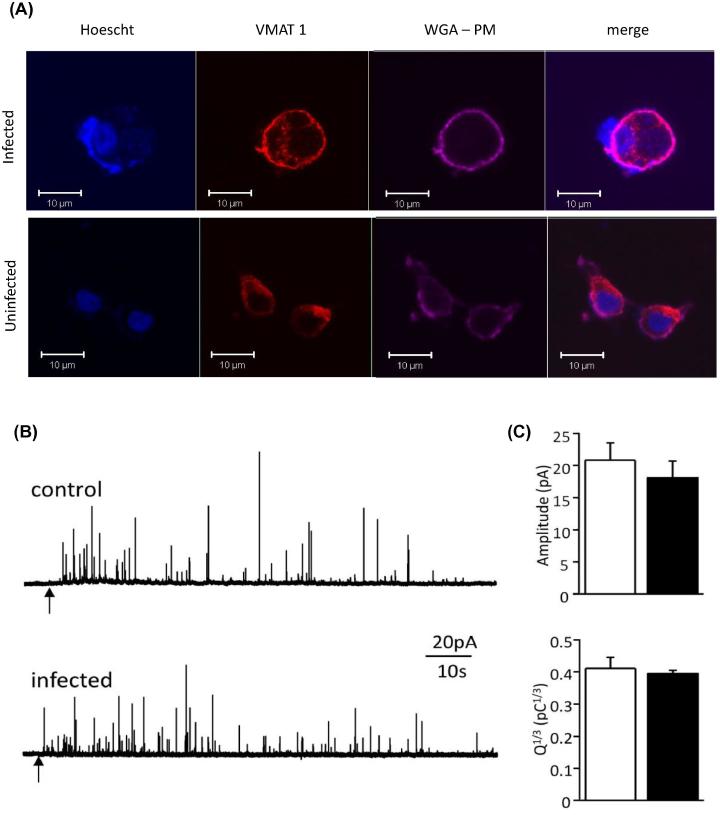
From the above data it is clear that the capacity exists for DA synthesis within the parasite compartment but the fate of any synthesized DA is unknown. As dopaminergic cells package DA into vesicles as it is synthesized, we examined the effect of infection on the DA packaging process. Firstly the distribution of monoamine vesicles within infected cells was examined using the vesicular monoamine transporter 1 (VMAT1) as a marker. In uninfected cells VMAT1 was observed with punctate staining throughout the cell cytoplasm and staining along the plasma membrane (Fig. 6A). The plasma membrane was visualized by staining with wheat germ agglutinin. In infected cells, VMAT1 staining shows a similar pattern of staining, located in the cytoplasm, along the plasma membrane and excluded from the vacuolar area containing the parasites.
Fig. 6.
Dopamine packaging in infected dopaminergic cells. (A) Immunofluorescent localization of vesicles in triple stained T. gondii-infected PC12 cells stained with VMAT1 (red) antibody, Hoechst (blue), and wheat germ agglutinin (WGA; violet). The panels (from left to right) show Hoechst, VMAT1, and WGA staining separately and all three channels. Hoechst staining identified PC12 and parasite nuclei and WGA stains the plasma membrane of PC12 cells. Note the parasitophorous vacuole containing a group of stained parasites (blue dots in infected cell) and exclusion of VMAT1 staining from this vacuole. (B) Amperometric recordings from PC12 cells infected with T. gondii ku80 Prugniaud bradyzoites. FACS selected bradyzoite infected cells were mechanically stimulated and secretion measured. Representative amperometric recordings from a control (upper trace) and infected (lower trace) PC12 cell. At the points indicated by the arrows, the cells were mechanically stimulated to initiate exocytosis, detected as spike-like events. (C) Bar graphs indicating mean (±SEM) spike amplitude and Q1/3 values determined in control cells (open bars, n = 7) and infected cells (solid bars, n = 8). Values were not statistically significantly different between the two groups. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Next we examined the effect of infection on vesicle parameters using single cell amperometry on infected PC12 cells. For these experiments, we compared uninfected PC12 cells with FACS-isolated cells containing bradyzoites to determine whether vesicle release parameters were altered. Mechanical agitation of cells led to the rapid appearance of spike-like events in both control cells and infected cells with each spike representing individual events of vesicle fusion with cell membrane and DA release (see Fig. 6B for example traces). Analysis of 998 spike events from seven control cells and 799 events from eight infected cells identified no significant difference in amplitude or Q1/3 values (i.e., the cube root of the integral of the spike charge as the current relative to time) between control and infected groups (Fig. 6C). Although increased DA is released from infected cultures (Fig. 1A), there is not an observable difference in DA packaging at the single-cell level.
Discussion
In this study we sought to clarify the impact of infection with the parasite T. gondii on the synthesis of the neurotransmitter DA, as increased levels of DA have been previously reported by our group and others in infected dopaminergic cells both in vitro and in vivo (Skallova et al., 2006; Webster et al., 2006; Prandovszky et al., 2011). Inherently, one might expect a decrease in DA synthesis with infection due to destruction of the host cell or stress on the cell rather than an increase. A recent study found that infection increased DA concentrations in vivo and altered DA receptor signaling (Xiao et al., 2014). Infection of dopaminergic cells with other pathogens that infect the brain, pseudorabies and Trypanosoma cruzi, found infection increased DA synthesis with observed increases in host cell TH levels and, therefore, we performed similar analyses (Schilter and Marchand, 1991; Chuenkova and Pereiraperrin, 2006). We found that the TH activity increased but, in contrast to the observations with pseudorabies and T. cruzi, expression levels of host TH were not significantly changed with T. gondii infection (Fig. 1). The additional TH activity could readily be due to a parasite-encoded aromatic amino acid hydroxylase with TH activity is expressed in the parasitic compartment (Gaskell et al., 2009). In this study we found that host DDC, the enzyme required for metabolizing l-DOPA to DA, is associated with the parasite’s compartment providing a mechanism for the parasite enzyme TgTH to contribute to DA synthesis.
Host DDC was found associated with the parasite’s compartment both in dopaminergic cells in vitro and in monoaminergic neurons in vivo while the amount of DDC expressed was not significantly changed. The presence of DDC within the PV was unexpected as most host proteins are excluded from the PV. There is some precedent for import of selected host components into the PV; the PV is permeable to nutrients and T. gondii has been observed to import endo-lysosomes into the PV (Coppens et al., 2006). Further evidence for import of proteins comes from a related apicomplexan parasite, Plasmodium falciparum, where the import of d-aminolevulinate dehydratase from the cytoplasm of erythrocytes into parasites has been reported (Bonday et al., 1997). The reason for the import of DDC into the PV, rather than export of l-DOPA is unclear. Perhaps DDC is imported to prevent l-DOPA degradation in the cytosol or to facilitate DA synthesis since in dopaminergic cells this process is closely co-ordinated in a spatial manner. Indeed TH and DDC have been found together on vesicles in PC12 cells (Cartier et al., 2010). This spatial co-ordination in DA synthesis is borne out in the FRET analysis used in this study showing both DDC and parasite-encoded TH in the parasite compartment. The FRET emission and immunoelectron microscopy data support the co-localization of the enzymes, but cannot distinguish whether a protein–protein interaction is occurring because of the size of secondary antibodies (Konig et al., 2006). However, regardless of whether the proteins are interacting, synthesis of DA, is unlikely to require the enzymes being less than hundreds of nanometers apart. Further work is required to determine whether TgTH and DDC are in a multi-protein complex.
The increased total cellular DA observed correlates with the presence of DA in parasite cysts and the co-localization of the synthesizing enzymes in the PV. However, only a portion of this extra DA is released upon induction with high potassium concentrations. Still it must be remembered that in vitro only a few to a dozen bradyzoites develop within the PV within a cell whereas tissue cysts contain hundreds of bradyzoites and hence will have an amplified effect on the cell. In our single cell studies, increased mechanically evoked vesicular release in infected cells was not observed but this may be due to the use of the Prugniaud ku80 strain in this study. A recent study using this strain was unable to demonstrate increase DA release in PC12 cells although their experimental conditions differed significantly from those used in this study (Wang et al., 2015). Wang et al. cultured PC12 cells in stressed conditions (pH 8.1, without CO2) for 48 h severely damaging the ability of PC12 cells to synthesize DA (Table 1 in Wang et al.) (Taylor et al., 1999). In light of these differences in Experimental procedures and parasite strain used, the role of the parasite-encoded aromatic amino acid hydroxylase in DA synthesis cannot be evaluated in their study. Future work should assess the effect of different parasite strains. Thus a considerable portion of the additional DA is not available for release; this may be due to sequestering in the PV or due to inefficient transport of DA to vesicles. It is important that future work addresses the localization of the unreleased DA as free cytosolic DA is reactive; readily undergoing auto-oxidation to form reactive quinones and semi-quinones that with enzymatic metabolism generate H2O2 (Hald and Lotharius, 2005). Indeed, this would be a reason for DA synthesis to occur outside the bradyzoite cell membrane but still encapsulated within the cyst.
It has been shown that excess DA is a plausible mechanism to explain the changes in rodent behavior, (Webster et al., 2006) however, further studies are required to determine what other mediators of the behavior changes exist and how they may interact with the increased DA, for example, the involvement of the immune and/or endocrine response to infection (Dass and Vyas, 2014). During the immune response to T. gondii infection, tryptophan is degraded and degradation products accumulate that could modulate neurotransmitter levels (Gupta et al., 1994). Recent work finding that an attenuated strain of T. gondii, lacking a key protein that activates the host immune response, maintains the loss of aversion to feline odor fits well with a mechanism involving neurotransmitter changes (Ingram et al., 2013). There are several examples of long-lasting effects of exposure to increased DA; such as DA agonists (e.g. cocaine, methamphetamine) inducing long-lasting neurological and behavioral changes (Seiden et al., 1976; Jing et al., 2014). Even a transient DA increase can cause permanent changes in behavior (Calcagno et al., 2013). T. gondii seropositivity has been linked to several pathological states that include altered monoamines (Flegr et al., 1996; Cook et al., 2015), including Parkinson’s disease (Celik et al., 2010; Miman et al., 2010) and most robustly with schizophrenia as shown by a recent meta-analysis of 38 studies (Torrey et al., 2012).
Conclusion
T. gondii infection of dopaminergic cells alters the host dopaminergic machinery. The import of host DDC into the parasite’s compartment without altering the amount of host DA biosynthetic enzymes or the phosphorylation status of TH may be a simple yet elegant mechanism for specific regulation of DA synthesis in host cells without the detrimental effects of unpackaged cytosolic DA. The import of DDC into the PV may shed light on the mechanisms for specific behavior changes as previously proposed. Future studies are needed to further delineate and dissect the full complement of molecular mechanisms involved in the behavioral responses of rodents to T. gondii infection and how these may be relevant to the associations with human neurological disorders.
Acknowledgments
We gratefully acknowledge support for this research from the Dunhill Medical Trust (Grant SA11/0510) and the Stanley Medical Research Institute (Grant 08R-1938). I. Alsaady acknowledges support of the Saudi Arabian Cultural Bureau for PhD training.
References
- Berdoy M., Webster J.P., Macdonald D.W. Fatal attraction in rats infected with Toxoplasma gondii. Proc Biol Sci. 2000;267:1591–1594. doi: 10.1098/rspb.2000.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonday Z.Q., Taketani S., Gupta P.D., Padmanaban G. Heme biosynthesis by the malarial parasite. Import of delta-aminolevulinate dehydrase from the host red cell. J Biol Chem. 1997;272:21839–21846. doi: 10.1074/jbc.272.35.21839. [DOI] [PubMed] [Google Scholar]
- Calcagno B., Eyles D., van Alphen B., van Swinderen B. Transient activation of dopaminergic neurons during development modulates visual responsiveness, locomotion and brain activity in a dopamine ontogeny model of schizophrenia. Transl Psychiatry. 2013;3:e206. doi: 10.1038/tp.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier E.A., Parra L.A., Baust T.B., Quiroz M., Salazar G., Faundez V., Egana L., Torres G.E. A biochemical and functional protein complex involving dopamine synthesis and transport into synaptic vesicles. J Biol Chem. 2010;285:1957–1966. doi: 10.1074/jbc.M109.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik T., Kamisli O., Babur C., Cevik M.O., Oztuna D., Altinayar S. Is there a relationship between Toxoplasma gondii infection and idiopathic Parkinson’s disease? Scand J Infect Dis. 2010;42:604–608. doi: 10.3109/00365541003716500. [DOI] [PubMed] [Google Scholar]
- Chow R., Von Ruden L. Electrochemical detection of secretion from single cells. In: Sakmann B., Neher E., editors. Single channel recording. Plenum; London: 1995. pp. 247–275. [Google Scholar]
- Chuenkova M.V., Pereiraperrin M. Enhancement of tyrosine hydroxylase expression and activity by Trypanosoma cruzi parasite-derived neurotrophic factor. Brain Res. 2006;1099:167–175. doi: 10.1016/j.brainres.2006.04.128. [DOI] [PubMed] [Google Scholar]
- Cook T.B., Brenner L.A., Cloninger C.R., Langenberg P., Igbide A., Giegling I., Hartmann A.M., Konte B., Friedl M., Brundin L., Groer M.W., Can A., Rujescu D., Postolache T.T. “Latent” infection with Toxoplasma gondii: association with trait aggression and impulsivity in healthy adults. J Psychiatr Res. 2015;60:87–94. doi: 10.1016/j.jpsychires.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Coppens I., Dunn J.D., Romano J.D., Pypaert M., Zhang H., Boothroyd J.C., Joiner K.A. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell. 2006;125:261–274. doi: 10.1016/j.cell.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Darde M.L., Ajzenberg D., Smith J. Population structure and epidemiology of Toxoplasma gondii. In: Kim K., Weiss L.M., editors. Toxoplasma gondii the model Apicomplexan: perspectives and methods. Academic Press; London, UK: 2007. pp. 49–80. [Google Scholar]
- Dass S.A.H., Vyas A. Toxoplasma gondii infection reduces predator aversion in rats through epigenetic modulation in the host medial amygdala. Molec Ecol. 2014;23:6114–6122. doi: 10.1111/mec.12888. [DOI] [PubMed] [Google Scholar]
- Finnegan J.M., Pihel K., Cahill P.S., Huang L., Zerby S.E., Ewing A.G., Kennedy R.T., Wightman R.M. Vesicular quantal size measured by amperometry at chromaffin, mast, pheochromocytoma, and pancreatic beta-cells. J Neurochem. 1996;66:1914–1923. doi: 10.1046/j.1471-4159.1996.66051914.x. [DOI] [PubMed] [Google Scholar]
- Flegr J., Zitková S., Kodym P., Frynta D. Induction of changes in human behavior by the parasitic protozoan Toxoplasma gondii. Parasitology. 1996;113:49–54. doi: 10.1017/s0031182000066269. [DOI] [PubMed] [Google Scholar]
- Flegr J., Kodym P., Tolarová V. Correlation of duration of latent Toxoplasma gondii infection with personality changes in women. Biol Psychol. 2000;53:57–68. doi: 10.1016/s0301-0511(00)00034-x. [DOI] [PubMed] [Google Scholar]
- Flegr J., Havlicek J., Kodym P., Malý M., Smahel Z. Increased risk of traffic accidents in subjects with latent toxoplasmosis: a retrospective case-control study. BMC Infect Dis. 2002;2:11. doi: 10.1186/1471-2334-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B.A., Fella A., Rommereim L.M., Tomita T., Gigley J.P., Mercier C., Cesbron-Delauw M.F., Weiss L.M., Bzik D.J. Type II Toxoplasma gondii KU80 knockout strains enable functional analysis of genes required for cyst development and latent infection. Eukaryot Cell. 2011;10:1193–1206. doi: 10.1128/EC.00297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell E.A., Smith J.E., Pinney J.W., Westhead D.R., McConkey G.A. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS One. 2009;4:e4801. doi: 10.1371/journal.pone.0004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K.N., Taylor S.C., Smith I.F., Peers C. Differential coupling of voltage-gated Ca(2+) channels to catecholamine secretion from separate PC12 cell batches. Neurosci Lett. 2001;301:13–16. doi: 10.1016/s0304-3940(01)01594-4. [DOI] [PubMed] [Google Scholar]
- Gupta S.L., Carlin J.M., Pyati P., Dai W., Pfefferkorn E.R., Murphy M.J., Jr. Antiparasitic and antiproliferative effects of indoleamine 2,3-dioxygenase enzyme expression in human fibroblasts. Infect Immun. 1994;62:2277–2284. doi: 10.1128/iai.62.6.2277-2284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald A., Lotharius J. Oxidative stress and inflammation in Parkinson’s disease: is there a causal link? Exp Neurol. 2005;193:279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Hill D.E., Chirukandoth S., Dubey J.P. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev. 2005;6:41–61. doi: 10.1079/ahr2005100. [DOI] [PubMed] [Google Scholar]
- House P.K., Vyas A., Sapolsky R.M. Predator cat odors activate sexual arousal pathways in brains of Toxoplasma gondii infected rats. PLoS One. 2011;6:e23277. doi: 10.1371/journal.pone.0023277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson W.M. Recent observations on the biology of Toxoplasma gondii. Trans Ophthalmol Soc UK. 1966;86:185–189. [PubMed] [Google Scholar]
- Ingram W.M., Goodrich L.M., Robey E.A., Eisen M.B. Mice infected with low-virulence strains of Toxoplasma gondii lose their innate aversion to cat urine, even after extensive parasite clearance. PLoS One. 2013;8:e75246. doi: 10.1371/journal.pone.0075246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L., Zhang M., Li J.X., Huang P., Liu Q., Li Y.L., Liang H., Liang J.H. Comparison of single versus repeated methamphetamine injection induced behavioral sensitization in mice. Neurosci Lett. 2014;560:103–106. doi: 10.1016/j.neulet.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Brando L., Torrey E.F., Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62:237–244. doi: 10.1016/s0920-9964(02)00357-2. [DOI] [PubMed] [Google Scholar]
- Kocazeybek B., Oner Y.A., Turksoy R., Babur C., Cakan H., Sahip N., Unal A., Ozaslan A., Kilic S., Saribas S., Aslan M., Taylan A., Koc S., Dirican A., Uner H.B., Oz V., Ertekin C., Kucukbasmaci O., Torun M.M. Higher prevalence of toxoplasmosis in victims of traffic accidents suggest increased risk of traffic accident in Toxoplasma-infected inhabitants of Istanbul and its suburbs. Forensic Sci Int. 2009;187:103–108. doi: 10.1016/j.forsciint.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Konig P., Krasteva G., Tag C., Konig I.R., Arens C., Kummer W. FRET-CLSM and double-labeling indirect immunofluorescence to detect close association of proteins in tissue sections. Lab Invest. 2006;86:853–864. doi: 10.1038/labinvest.3700443. [DOI] [PubMed] [Google Scholar]
- Miman O., Kusbeci O.Y., Aktepe O.C., Cetinkaya Z. The probable relation between Toxoplasma gondii and Parkinson’s disease. Neurosci Lett. 2010;475:129–131. doi: 10.1016/j.neulet.2010.03.057. [DOI] [PubMed] [Google Scholar]
- Naoi M.T., Nagatsu T. Simple assay procedure for tyrosine hydroxylase activity by high-performance liquid chromatography employing coulometric detection with minimal sample preparation. J Chromatogr. 1988;427:229–238. doi: 10.1016/0378-4347(88)80125-7. [DOI] [PubMed] [Google Scholar]
- Prandovszky E., Gaskell E.A., Martin H., Dubey J.P., Webster J.P., McConkey G.A. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One. 2011;6:e232866. doi: 10.1371/journal.pone.0023866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilter B., Marchand C.M. Effects of pseudorabies virus on the neuronal properties of PC12 cells. J Neurochem. 1991;56:898–906. doi: 10.1111/j.1471-4159.1991.tb02006.x. [DOI] [PubMed] [Google Scholar]
- Seiden L.S., Fischman M.W., Schuster C.R. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend. 1976;1:215–219. doi: 10.1016/0376-8716(76)90030-2. [DOI] [PubMed] [Google Scholar]
- Skallova A., Kodym P., Frynta D., Flegr J. The role of dopamine in Toxoplasma-induced behavioural alterations in mice: an ethological and ethopharmacological study. Parasitology. 2006;133:525–535. doi: 10.1017/S0031182006000886. [DOI] [PubMed] [Google Scholar]
- Sura G.R., Daubner S.C., Fitzpatrick P.F. Effects of phosphorylation by protein kinase A on binding of catecholamines to the human tyrosine hydroxylase isoforms. J Neurochem. 2004;90:970–978. doi: 10.1111/j.1471-4159.2004.02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.C., Peers C. Store-operated Ca2+ influx and voltage-gated Ca2+ channels coupled to exocytosis in pheochromocytoma (PC12) cells. J Neurochem. 1999;73:874–880. doi: 10.1046/j.1471-4159.1999.0730874.x. [DOI] [PubMed] [Google Scholar]
- Taylor S.C., Peers C. Chronic hypoxia enhances the secretory response of rat phaeochromocytoma cells to acute hypoxia. J Physiol. 1999;514(Pt 2):483–491. doi: 10.1111/j.1469-7793.1999.483ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.C., Posch A. The design of a quantitative Western blot experiment. Biomed Res Int. 2014;2014 doi: 10.1155/2014/361590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.C., Roberts M.L., Peers C. Acid-evoked quantal catecholamine secretion from rat phaeochromocytoma cells and its interaction with hypoxia-evoked secretion. J Physiol. 1999;519(Pt 3):765–774. doi: 10.1111/j.1469-7793.1999.0765n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.C., Shaw S.M., Peers C. Mitochondrial inhibitors evoke catecholamine release from pheochromocytoma cells. Biochem Biophys Res Commun. 2000;273:17–21. doi: 10.1006/bbrc.2000.2894. [DOI] [PubMed] [Google Scholar]
- Torrey E.F., Bartko J.J., Yolken R.H. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38:642–647. doi: 10.1093/schbul/sbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A., Kim S.K., Giacomini N., Boothroyd J.C., Sapolsky R.M. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci U S A. 2007;104:6442–6447. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Lasagna M., Daubner S.C., Reinhart G.D., Fitzpatrick P.F. Fluorescence spectroscopy as a probe of the effect of phosphorylation at serine 40 of tyrosine hydroxylase on the conformation of its regulatory domain. Biochemistry (Mosc) 2011;50:2364–2370. doi: 10.1021/bi101844p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.T., Harmon S., O’Malley K.L., Sibley L.D. Reassessment of the role of aromatic amino acid hydroxylases and the effect of infection by Toxoplasma gondii on host dopamine. Infect Immun. 2015;83:1039–1047. doi: 10.1128/IAI.02465-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster J.P. The effect of Toxoplasma gondii and other parasites on activity levels in wild and hybrid Rattus norvegicus. Parasitology. 1994;109(Pt 5):583–589. doi: 10.1017/s0031182000076460. [DOI] [PubMed] [Google Scholar]
- Webster J.P. Prevalence and transmission of Toxoplasma gondii in wild brown rats, Rattus norvegicus. Parasitology. 1994;108(Pt 4):407–411. doi: 10.1017/s0031182000075958. [DOI] [PubMed] [Google Scholar]
- Webster J.P., Brunton C.F., MacDonald D.W. Effect of Toxoplasma gondii upon neophobic behaviour in wild brown rats, Rattus norvegicus. Parasitology. 1994;109(Pt 1):37–43. doi: 10.1017/s003118200007774x. [DOI] [PubMed] [Google Scholar]
- Webster J.P., Lamberton P.H., Donnelly C.A., Torrey E.F. Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite medication on Toxoplasma gondii’s ability to alter host behaviour. Proc Biol Sci. 2006;273:1023–1030. doi: 10.1098/rspb.2005.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink R.H., Ewing A.G. The PC12 cell as model for neurosecretion. Acta Physiol (Oxf) 2008;192:273–285. doi: 10.1111/j.1748-1716.2007.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R.M., Jankowski J.A., Kennedy R.T., Kawagoe K.T., Schroeder T.J., Leszczyszyn D.J., Near J.A., Diliberto E.J., Jr., Viveros O.H. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci U S A. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Li Y., Prandovszky E., Karuppagounder S.S., Talbot C.C., Jr., Dawson V.L., Dawson T.M., Yolken R.H. MicroRNA-132 dysregulation in Toxoplasma gondii infection has implications for dopamine signaling pathway. Neuroscience. 2014;268:128–138. doi: 10.1016/j.neuroscience.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yereli K., Balcioğlu I.C., Ozbilgin A. Is Toxoplasma gondii a potential risk for traffic accidents in Turkey? Forensic Sci Int. 2006;163:34–37. doi: 10.1016/j.forsciint.2005.11.002. [DOI] [PubMed] [Google Scholar]