Abstract
Brucella is among the most common zoonotic diseases affecting humans. Although musculoskeletal involvement is seen in a large proportion of patients, the disease is often diagnosed late or misdiagnosed due to its subtle nature and rarity, and lack of awareness among clinicians. In this report, a 12-year-old girl was diagnosed with acute septic arthritis of the hip based on clinico-radiological features, and managed with standard treatment, including arthrotomy. However, the child did not respond to the treatment. Based on the histopathology and local endemicity, Brucella was suspected, and confirmed after serological testing. The child subsequently responded to treatment and, at latest follow-up at 1 year, had a full painless range of motion, with no relapse.
Background
Human Brucellosis is among the most common zoonotic diseases and has an incidence of over 500 000 worldwide with a prevalence of more than 10/100 000 population in some endemic countries.1 Although the musculoskeletal system is frequently involved, Brucella can mimic various multisystem diseases, which frequently leads to misdiagnosis and delay in treatment, resulting in complications. Although, as reported in the literature, septic arthritis is most commonly caused by Staphylococcus aureus in all age groups, there are some cases where a strong clinical suspicion is needed based on the local epidemiology when patients do not respond to standard treatment, as seen in the present case.
Case presentation
A 12-year-old girl presented with sudden onset acute pain in the left hip for 10 days. The pain, associated with high grade fever with chills, was non-radiating, severe in intensity and aggravated by movements at the hip, and was only partially relieved with medication. The fever was remittent and was relieved with medication, only to reoccur. There was no history of any trauma, cough, anorexia or pain or swelling in any other joint of the body. The child's medical and family history were non-contributory. There was no significant past history.
On examination, the patient's vitals were stable. She was febrile and had pallor. There was no evidence of lymphadenopathy. During local examination, the child kept her left hip in a flexed position. There was tenderness at the anterior hip region and all movements, active and passive, were painfully restricted. There was fixed flexion deformity of 40°. The other hip joint, and spine and distal neurovascular status were within normal limits. Examination of the rest of the organ systems (abdomen, respiratory and nervous system) was normal.
Investigations
Laboratory examination revealed low haemoglobin (7.7 g/dL) and an increased leucocyte count of 14 400/mL. The differential count revealed 74% polymorphonuclear leucocytes, 14.7% lymphocytes, 8.4% monocytes, 0.8% basophils and 2.1% eosinophils. Erythrocyte sedimentation rate (33 mm Hg in the first hour) and highly sensitive C reactive protein (150.63 mg/L) were increased. The child underwent imaging studies including X-ray of the pelvis with both hips, X-rays of the chest and spine, Ultrasound of the hip region and MRI. The X-rays of the chest and spine were normal.
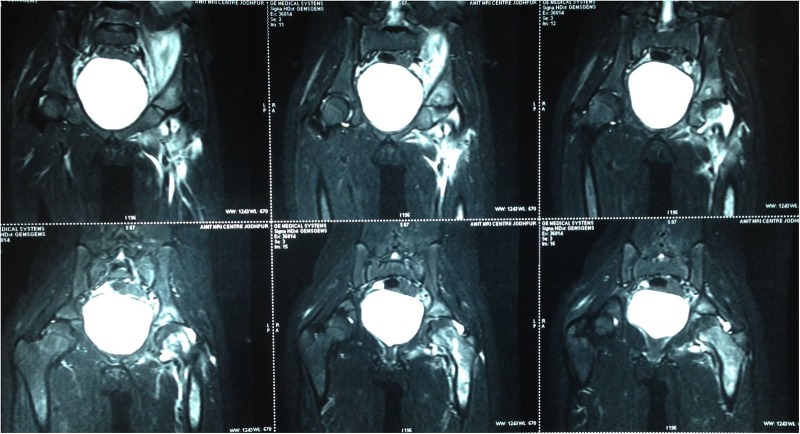
The X-ray of the pelvis with both hips showed decreased joint space with subchondral lesions on both sides of the left hip joint (figure 1). Ultrasonography of the left hip showed a heterogeneous hypoechoic collection in the iliopsoas muscle in the region of the iliac fossa, extending for a length of about 6.6 cm and measuring 2 cm in maximum thickness (figure 2). MRI showed an altered bone marrow signal intensity area in the left acetabulum, head, neck and shaft of femur, appearing hypointense on T1 and hyperintense on T2 weighted and fat suppressed inversion recovery images, and a collection in the left iliacus muscle extending from pelvis to upper thigh (figures 3 and 4). There was also associated mild left hip joint effusion.
Figure 1.
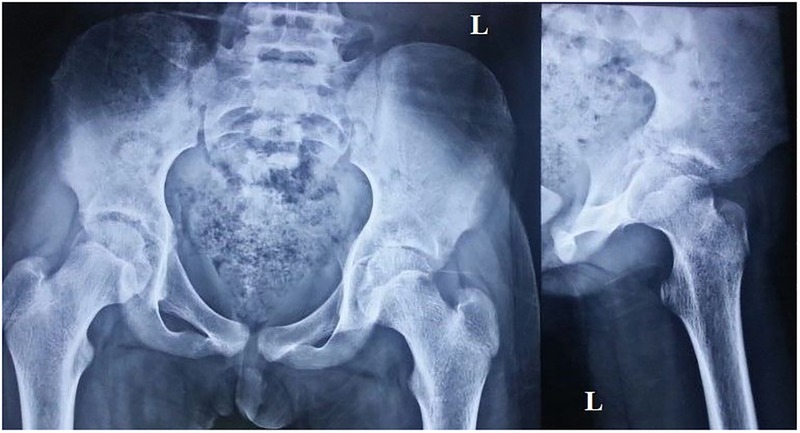
Radiograph of the pelvis with hips and lateral view of the left hip showing decreased joint space with subchondral lesion on either side of the left hip joint.
Figure 2.
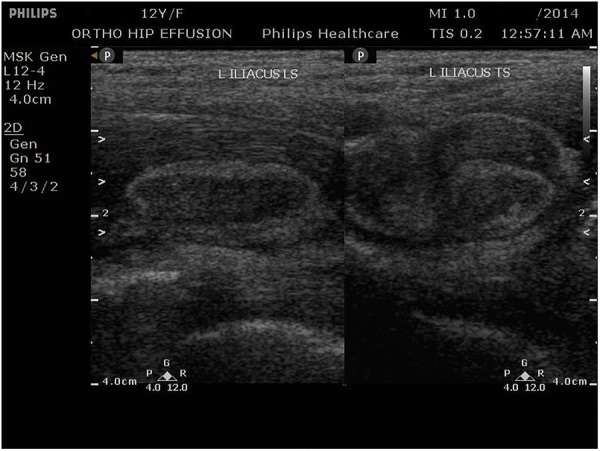
Ultrasonography of the left hip showing a heterogeneous hypoechoic collection in the iliopsoas muscle in the region of the iliac bone, measuring 2 cm in maximum thickness.
Figure 3.
MRI of the pelvis with both hips (coronal section) shows altered bone marrow signal intensity area in the left acetabulum, head, neck and shaft of the femur appearing hyperintense on T2-weighted images.
Figure 4.
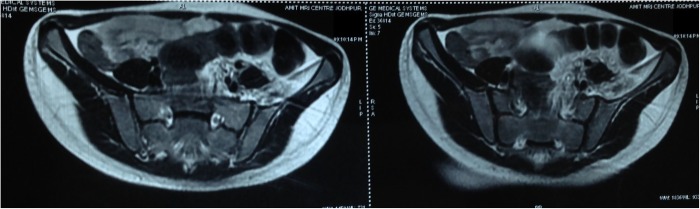
MRI of the pelvis with both hips (transverse section) showing collection in the left iliacus muscle.
Differential diagnosis
Acute pyogenic septic arthritis
Tubercular arthritis
Treatment
The child was put on empirical intravenous antibiotics (injection co-amoxiclav 1.2 g thrice daily and injection amikacin 250 mg, twice daily) and above knee skin traction. However, the clinical symptoms including low-grade fever persisted. Her hip movements, both active and passive, improved, but remained painful.
After 72 h of admission, the decision was taken to perform arthrotomy of the left hip after taking informed consent from the parents.
Surgical procedure
The child was placed in the supine position with a sandbag under the left pelvis. The Smith-Peterson anterior approach to the hip joint was used. An iliac crest incision was used to erase the iliacus subperiosteally from the iliac bone. The deeper and distal part of the iliacus revealed thick yellowish brown purulent material that appeared to be communicating with the hip joint. The hip joint capsule was exposed by making a plane between the sartorius and tensor fascia lata, and then erasing the straight and reflected head of the rectus femoris. The capsule was observed to be thickened. A T-shaped incision was made to expose the joint. The joint revealed granulation tissue and erosions of the femoral head and neck. Thorough lavage was performed and the wound was closed in layers over a suction drain. The pus was sent for Gram stain and acid fast bacilli (AFB) staining and culture while the granulation tissue was sent for histopathological and culture examinations.
Postoperatively, the child was continued on the same intravenous antibiotics and skin traction. The antibiotics were changed after 2 days when the culture and sensitivity report grew methicillin sensitive S. aureus sensitive to cefuroxime and amikacin. The child was afebrile for a period of 2 days after changing the antibiotics. However, she started experiencing low-grade fever again and the pain in the left hip persisted. The histopathology report showed non caseating granulomas that were Ziehl Neelsen (ZN) stain negative with secondary infection.
Histopathology interpretation
On gross examination, there were multiple reddish brown and reddish white soft tissue components altogether measuring 4.5×1.5×0.8 cms.
Microscopic examination showed extensive fibrinous neutrophilic exudate intermixed with lymphocytes, histiocytes including multinucleated histiocytes, macrophages and plasma cells. An occasional non-caseating well-defined granuloma was noted with giant cells (figures 5 and 6). Spicules of necrotic bone, haemorrhage and fibrocollagenous tissue were seen. ZN stain to demonstrate AFB was negative. No fungal profiles were noted.
Figure 5.
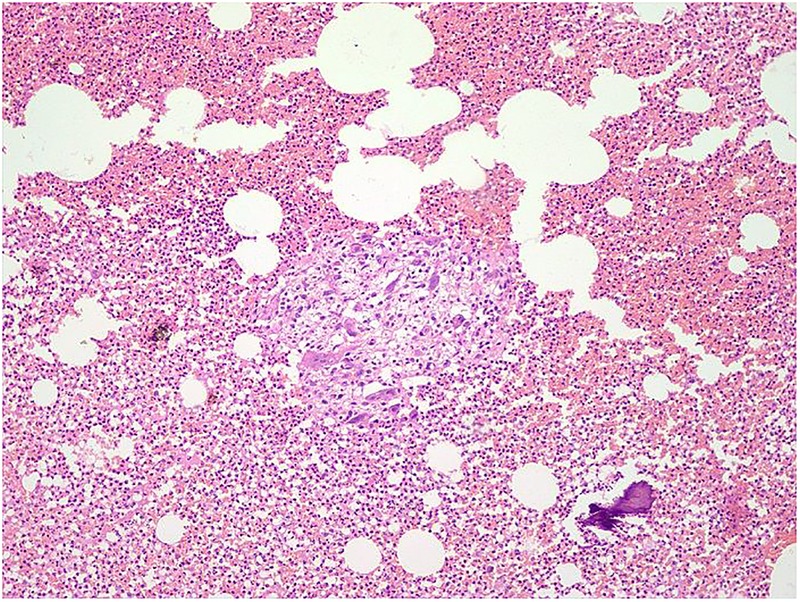
Epithelioid cell granuloma with spicule of necrotic bone and background dense neutrophilic inflammation, H&E stain, ×100.
Figure 6.
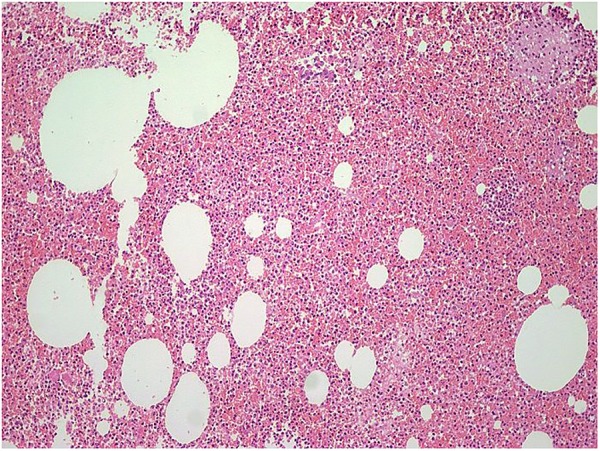
Dense neutrophilic inflammation, H&E stain ×100.
Present differential diagnosis
Brucellosis
Non-tuberculous mycobacteria (NTM)
Sarcoidosis
Considering that a large percentage of patients with Brucellosis present with musculoskeletal complications including septic arthritis, we had Brucella serology performed initially.2–5 The serology for Brucella melitensis was positive with a titre of 1:320 and IgM antibody level of 12.84 U/mL. In addition, the child was anaemic, which is also a common accompaniment of Brucella affection of the musculoskeletal system. The blood culture for NTM was negative and chest X-ray was normal.
Diagnosis
Acute septic arthritis of left hip with left iliac abscess with osteomyelitis of proximal left femur, caused by B. melitensis.
Outcome and follow-up
The child was started on intravenous gentamycin for 7 days and oral doxycycline and rifampicin, which were continued for 8 weeks. The child responded to the treatment and, at the latest follow-up at 1 year, was asymptomatic and had painless full range of motion of the involved hip.
Discussion
Brucella is among the most common zoonotic diseases, and presents with skeletal complications in 11–85% cases, in various studies.2–5 The most common presentation is with fever, arthralgia and monoarthritis.6 The peripheral joints, including hip and knee, are more commonly involved in children than is the axial skeleton.7–9 In the present case, the child presented with fever, arthralgia and peripheral monoarthritis of the hip. On imaging studies, there were features of acute osteomyelitis with septic arthritis of the hip. Contrary to the standard observation of increased medial joint space on radiography in acute septic arthritis of the hip, this patient had decreased joint space, probably an observational variation due to the flexion deformity of the hip. MRI was performed to determine the extent of involvement of the pathological process, as the duration of symptoms in the patient was almost 2 weeks, the sonography report predominantly showed an iliac abscess and the child did not respond to empirical antibiotic therapy. The diagnosis was suspected when the child did not respond to the emperical antibiotic therapy, the symptoms persisted even after arthrotomy and the histopathology showed granulomas without caseous necrosis. The diagnosis was, however, confirmed with serology showing positive titres for B. melitensis. It is to be noted that, although multiple different surgical treatment protocols have been mentioned in the literature for septic arthritis, such as daily ultrasound-guided aspirations, arthroscopic irrigation and drainage,10 we chose to perform an open arthrotomy and drainage, as the infection was not only limited to the hip but also involved the iliac region as well as the proximal third of the femur.
The differential diagnosis considered initially was acute pyogenic septic arthritis and tubercular arthritis. The short history, signs and symptoms, and imaging studies, were consistent with acute pyogenic septic arthritis. However, the child not responding to the definitive antibiotic therapy even after performing arthrotomy, and non-caseating granulomas on histopathology, were against this diagnosis. Tubercular arthritis was kept as a differential diagnosis as the disease is endemic in this country and the presentation can be acute in cases of associated secondary infection. However, ZN staining and culture of pus and granulation tissue were negative and the histopathology report was also not consistent with this diagnosis. Based on the histopathology interpretation, the differential diagnoses considered were Brucella, NTM and sarcoidosis. Although non-caseating granulomas can be seen in all the above diseases, a literature review showed that osteoarticular complications were most commonly associated with Brucella.2–5 NTM infection involving the musculoskeletal system is uncommon and usually acquired by direct inoculation of pathogen as a consequence of surgery, penetrating trauma or injections. The clinical course of the disease is typically protracted with average time from the onset of symptoms to diagnosis being 10 months.11 Clinically, patients present with signs and symptoms similar to tubercular arthritis, with pain, swelling, stiffness and constitutional symptoms. However, the definitive diagnosis requires a positive culture, which was negative in our case. Similarly, involvement of the musculoskeletal system in sarcoidosis is very rare, accounting for 3–13% cases, and most commonly involves the small bones of the hands and feet.12 When symptomatic, patients usually present with pain, palpable mass, chronic myopathy or acute myositis. In the majority of patients, chest involvement is also seen.13 In the present case, the history was not consistent with the diagnosis of sarcoidosis, and the chest X-ray was normal. ACE levels were not measured as investigations had already revealed positivity for Brucella serology.
There are four Brucella species known to cause disease in humans; of these, B. melitensis is the most prevalent and virulent, and causes the most severe and acute cases of brucellosis.14 15 Childhood brucellosis accounts for 10–30% of all cases and most commonly involves children older than 5 years of age.16–20 The main source of infection is consumption of raw milk and milk products, and, to a lesser extent, contact with infected animals or their waste products. Clinically, the most common presentation is with fever, arthralgia, sweating and peripheral monoarthritis involving the hip or knee. The involvement of the axial skeleton and sacroiliac joint is rare in children as compared to in adults.17 21–24 The diagnosis of Brucella can be performed with certainty by isolating the species from blood, bone marrow and other tissue fluids. However, the rate of isolation in culture remains very low. So, the standard agglutination test remains the best diagnostic modality with titres >1:160 suggestive of acute infection. The treatment of Brucella requires a regimen consisting of a combination of agents that can penetrate the cells; prolonged treatment is suggested so as to achieve eradication of the disease. Treatment with a single agent or combination of agents given for less than 4 weeks is associated with a high risk of relapse.25–28 In children older than 8 years, the regimen involves doxycycline with either rifampicin, streptomycin or gentamycin.
Learning points.
Brucella is an unusual cause of acute septic arthritis of the hip in adolescents and should be considered in the differential diagnosis, particularly in endemic regions.
The present case report highlights the importance of sending tissue for culture as well as for histopathology in all cases of septic arthritis.
Treatment for Brucella should include more than one drug and be given for more than 4 weeks to prevent relapse.
Acknowledgments
The authors thank Devendra Singh Rathore and Deepak Maley Kumar, Senior Residents, Department of Orthopaedics, for assisting in the surgical procedure and collecting the data. The authors would also like to thank Pushpinder Khera and Neeraj, Department of Radiology, for imaging studies and interpretation.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Pappas G, Papadimitriou P, Akritidis N et al. The new global map of human brucellosis. Lancet Infect Dis 2006;6:91–9. 10.1016/S1473-3099(06)70382-6 [DOI] [PubMed] [Google Scholar]
- 2.Al-Eissa YA, Kambal AM, Alrabeeah AA et al. Osteoarticular brucellosis in children. Ann Rheum Dis 1990;49:896–900. 10.1136/ard.49.11.896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gür A, Geyik MF, Dikici B et al. Complications of brucellosis in different age groups: a study of 283 cases in southeastern Anatolia of Turkey. Yonsei Med J 2003;44:33–44. 10.3349/ymj.2003.44.1.33 [DOI] [PubMed] [Google Scholar]
- 4.Geyik MF, Gür A, Nas K et al. Musculoskeletal involvement of brucellosis in different age groups: a study of 195 cases. Swiss Med Wkly 2002;132:98–105. [DOI] [PubMed] [Google Scholar]
- 5.Rajapakse CN. Bacterial infections: osteoarticular brucellosis. Baillieres Clin Rheumatol 1995;9:161–77. 10.1016/S0950-3579(05)80153-0 [DOI] [PubMed] [Google Scholar]
- 6.Roushan MR, Amiri MJ. Update on childhood brucellosis. Recent Pat Antiinfect Drug Discov 2013;8:42–6. 10.2174/1574891X11308010008 [DOI] [PubMed] [Google Scholar]
- 7.Gotuzzo E, Seas C, Guerra JG et al. Brucellar arthritis: a study of 39 Peruvian families. Ann Rheum Dis 1987;46:506–9. 10.1136/ard.46.7.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gómez-Reino FJ, Mateo I, Fuertes A et al. Brucellar arthritis in children and its successful treatment with trimethoprimsulphamethoxazole (co-trimoxazole). Ann Rheum Dis 1986;45:256–8. 10.1136/ard.45.3.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bocanegra TS, Gotuzzo E, Castañeda O et al. Rheumatic manifestations of brucellosis. Ann Rheum Dis 1986;45:526 10.1136/ard.45.6.526-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutz E, Spoerri M. Septic arthritis of the paediatric hip—a review of current diagnostic approaches and therapeutic concepts. Acta Orthop Belg 2013;79:123–34. [PubMed] [Google Scholar]
- 11.Piersimoni C, Scarparo C. Extrapulmonary infections associated with nontuberculous mycobacteria inimmunocompetent persons. Emerg Infect Dis 2009;15:1351–8. 10.3201/eid1509.081259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcox A, Bharadwaj P, Sharma OP. Bone sarcoidosis. Curr Opin Rheumatol 2000;12:321–30. 10.1097/00002281-200007000-00016 [DOI] [PubMed] [Google Scholar]
- 13.Zisman DA, Biermann JS, Martinez FJ et al. Sarcoidosis presenting as a tumorlike muscular lesion. Case report and review of the literature. Medicine (Baltimore) 1999;78:112–22. 10.1097/00005792-199903000-00002 [DOI] [PubMed] [Google Scholar]
- 14.Pappas G, Akritidis N, Bosilkovski M et al. Brucellosis. N Engl J Med 2005;352:2325–36. 10.1056/NEJMra050570 [DOI] [PubMed] [Google Scholar]
- 15.Ariza J, Bosilkovski M, Cascio A et al. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med 2007;4:e317 10.1371/journal.pmed.0040317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendricks MK, Perez EM, Burger PJ et al. Brucelosis in childhood in the Western Cape. S Afr Med J 1995;85:176–8. [PubMed] [Google Scholar]
- 17.Tanir G, Tufekci SB, Tuygun N. presentation, complications, and treatment outcome of brucellosis in Turkish children. Pediatr Int 2009;51:114–19. 10.1111/j.1442-200X.2008.02661.x [DOI] [PubMed] [Google Scholar]
- 18.Shaalan MA, Memish ZA, Mahmoud SA et al. Brucellosis in children: clinical observations in 115 cases. Int J lnfec Dis 2002;6:182–6. 10.1016/S1201-9712(02)90108-6 [DOI] [PubMed] [Google Scholar]
- 19.Sharda DC, Lubani M. A study of brucellosis in childhood. Clin Pediatr 1986;25:492–5. 10.1177/000992288602501002 [DOI] [PubMed] [Google Scholar]
- 20.Caksen H, Arslan S, Onor AF et al. Childhood brucellosis is still a severe problem in the eastern region of Turkey. Trop Doct 2002;32:91–2. [DOI] [PubMed] [Google Scholar]
- 21.Buzgan T, Karahocagil MK, Irmak H et al. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Intern J Infect Dis 2010;14:e469–78. 10.1016/j.ijid.2009.06.031 [DOI] [PubMed] [Google Scholar]
- 22.Roushan MR, Ahmadi SA, Gangi SM et al. Childhood brucellosis in Babol, Iran. Trop Doct 2005;35:229–3. 10.1258/004947505774938693 [DOI] [PubMed] [Google Scholar]
- 23.Roushan MR, Mohraz M, Janmohammadi N et al. Efficacy of cotrimoxazole and rifampin for 6 or 8weeks of therapy in childhood brucellosis. Pediatr Infect Dis J 2006;25:544–5. 10.1097/01.inf.0000219403.91975.ce [DOI] [PubMed] [Google Scholar]
- 24.Ulug M, Yaman Y, Yapici F et al. Clinical and laboratory features, complications and treatment outcome of brucellosis in childhood and review of the literature. Turk J Pediatr 2011;53:413–24. [PubMed] [Google Scholar]
- 25.Gottesman G, Vanunu D, Maayan MC et al. Childhood brucellosis in Israel. Pediatr Infect Dis J 1996;15:610–15. 10.1097/00006454-199607000-00010 [DOI] [PubMed] [Google Scholar]
- 26.Abramson O, Abu-Rashid M, Gorodischer R et al. Failure of short antimicrobial treatments for human brucellosis. Antimicrob Agents Chemother 1997;41:1621–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubani MM, Dudin KI, Sharda DC et al. A multicenter therapeutic study of 1100 children with brucellosis. Pediatr Infect Dis J 1989;8:75–8. 10.1097/00006454-198901001-00025 [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Tamayo T, Colmenero JD, Martinez-Cortes F et al. Failure of short term anti microbial therapy in childhood brucellosis. Pediatr Infect Dis J 1997;16:323–4. 10.1097/00006454-199703000-00012 [DOI] [PubMed] [Google Scholar]