Abstract
In most settings, approved medications for the treatment of opioid-use disorder include methadone and buprenorphine/naloxone, and in some settings, naltrexone. We present a case in which methadone administration was associated with an in-hospital episode of Torsades de Pointes in a patient who was subsequently maintained on sustained release oral morphine (SROM) for treatment of his opioid-use disorder. This transition was made in the context of long-term compliance to methadone maintenance, and with a previous adverse reaction to buprenorphine/naloxone precluding its use. The change to SROM, supported by emerging evidence, resulted in a reduction in the patient's measured QTc interval, prevention of further arrhythmias and continued abstinence from illicit opioid-use. In this context, we believe careful consideration should be given to the use of SROM.
Background
Opioid-use disorder is associated with significant morbidity and mortality.1 2 Currently, in most settings, approved pharmacotherapy includes methadone and buprenorphine/naloxone, both opioid agonists, and, in some settings, naltrexone, an opioid antagonist.3 Methadone, in particular, has been shown to reduce mortality4 5 and to reduce many of the health and social complications associated with opioid addiction through comparatively high treatment retention, and improved reduction in the use of illicit opioids.6 7 When prescribed according to guidelines, methadone has a relatively safe side effect profile.8 There can, however, be rare serious side effects. For example, cardiac QTc prolongation, though uncommonly dangerous, can occasionally be fatal in some patients who develop a subsequent arrhythmia, notably Torsades de Pointes (TdP).9 10 For patients with severe opioid-use disorder who suffer from this complication, options for treatment may be limited.
Alternatives to methadone for the treatment of opioid-use disorder have their own challenges. Though highly effective, buprenorphine/naloxone, a partial opioid agonist, has been associated with less treatment retention when compared to methadone.11–13 For patients maintained on methadone, conversion to buprenorphine/naloxone may involve challenging induction strategies,14 with little evidence to serve as guidance and the potential to cause adverse effects including opioid withdrawal symptoms.14–16 Similarly, oral naltrexone has only been found to be effective when adherence is forced.3
Thus, treating individuals who have a prolonged QTc and opioid-use disorder and who are seeking treatment can be particularly challenging. The previous literature has supported the use of an implantable cardiac defibrillator (ICD) while continuing methadone,17 18 though avoidance of an arrhythmia would be preferable. As such, emerging evidence suggests the use of sustained release oral morphine (SROM) as an alternative opioid agonist treatment in this context.19 20 We present a case in which methadone administration was associated with an in-hospital episode of TdP in a patient who was subsequently maintained on SROM for treatment of his opioid-use disorder.
Case presentation
A 53-year-old man was admitted to St Paul's Hospital in Vancouver, Canada, with a cardiac arrest initially thought secondary to cocaine-induced ventricular fibrillation. His past medical history was significant for hepatitis B and C co-infection, benzodiazepine-use disorder and cocaine-use disorder with a recent hospital stay (3 weeks prior to this admission) for management of a cocaine-induced myocardial infarction and concurrent leg cellulitis. At that time, his QTc was measured at 448 ms, and an echocardiogram revealed evidence of structural heart disease with left ventricular hypertrophy and an ejection fraction of 40%. He had never been formally diagnosed with long QT syndrome, however, there was an ECG from 2012 showing a QTc interval of 478. Furthermore, the patient had a history of opioid addiction dating back to childhood and had been maintained on methadone therapy at a stable dose of 220 mg daily for years by self-report. He reported over 20 years of abstinence from heroin and denied missing any recent methadone doses, including on the morning of admission. This was corroborated by his community pharmacy where he received daily witnessed ingestion. His other medications included quetiapine, rabeprazole and aspirin. He was living alone in an apartment in Vancouver's Downtown Eastside.
On the day of admission, the patient had a witnessed collapse outside his residence in Vancouver. Within 3 min, cardiopulmonary resuscitation was initiated by a bystander and was continued for a total of 10 min until emergency health service providers arrived. The patient's initial cardiac rhythm strip prior to hospitalisation revealed ventricular fibrillation (figure 1) with conversion to a normal sinus rhythm after the administration of a 200 J defibrillation. He was sedated, intubated and subsequently transferred to St Paul's Hospital.
Figure 1.
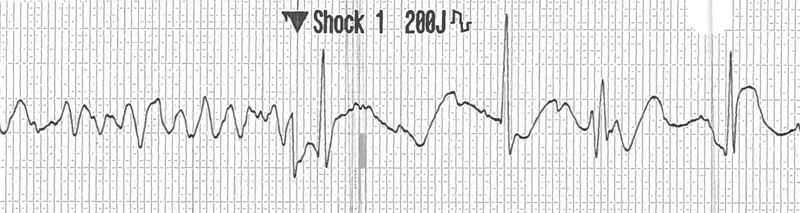
Prehospital rhythm strip recorded by emergency health service providers demonstrating ventricular fibrillation with restoration of a narrow complex rhythm after delivery of a 200 J defibrillation.
On arrival in the emergency department, the patient's ECG revealed sinus bradycardia with a rate of 45 bpm and his QTc was elevated at 589 ms shortly after presentation. He was transferred to the intensive care unit. All of his regular home medications, including quetiapine and rabeprazole, were discontinued and he received 12.5 mg total of intravenous haloperidol in the ensuing 24 h.
A few hours following admission, the patient was extubated, given an improvement in his level of consciousness, and remained on a cardiac monitor. The following morning, a repeat ECG once again showed sinus bradycardia with a heart rate of 48 bpm. His QTc was then measured at 650 ms (figure 2). Given his need for opioid replacement therapy, the in-hospital Addiction Medicine team was consulted. Discussion with the patient about the use of buprenorphine/naloxone for the treatment of his opioid dependence in the context of his prolonged QTc was unfortunately met with staunch resistance, as the patient had previously tried this and experienced uncomfortable withdrawal symptoms. In an effort to prevent a non-medically sanctioned discharge and manage impending opioid withdrawal symptoms, the Addiction Medicine team prescribed his methadone but in a split quarterly dose to allow for monitoring of symptoms and further QTc prolongation. An initial dose of 55 mg was given at 10:00 the morning following admission. Haloperidol 2.5 mg intravenously was subsequently given 30 min later for ongoing agitation. At 13:00 the patient developed a polymorphic wide complex tachycardia consistent with TdP (figure 3). This persisted for a total of 8 min during which he received 1 electrical defibrillation, 2 min of chest compressions (during a brief episode of pulselessness) and 5 g of intravenous magnesium sulfate. Following this, his cardiac rhythm reverted to sinus bradycardia. His electrolytes from earlier that morning revealed a potassium level of 4.3 mmol/L, magnesium of 0.75 mmol/L, phosphorus of 1.02 mmol/L and calcium of 2.20 mmol/L. Cardiology was consulted and diagnosed the patient with an acquired long QT syndrome. They initiated treatment with a β-blocker (esmolol) and all ongoing QTc prolonging medications, including methadone and haloperidol, were withheld. In an effort to prevent opioid withdrawal, the Addiction Medicine team prescribed regular oral morphine at an initial dose of 50 mg every 3 h, this was later increased to 100 mg every 4 h.
Figure 2.
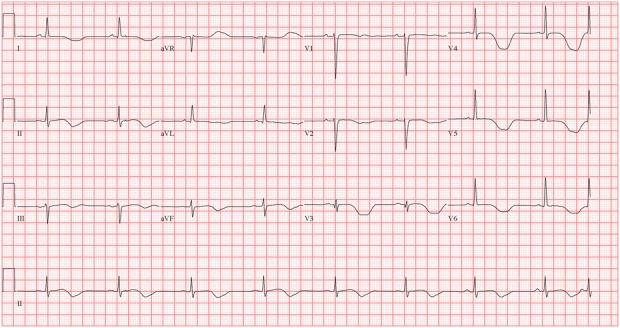
ECG taken 2 h prior to the development of a Torsades de Pointes arrest with a measured QTc of 650 ms.
Figure 3.
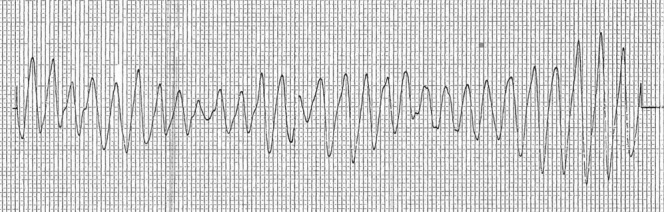
Waveform from the cardiac monitor during an episode of a Torsades de Pointes rhythm, exhibiting classic sinusoidal polymorphic morphology.
Unfortunately, 4 days after the patient's admission to hospital, he discharged himself against medical advice. The risks of cocaine use as it pertains to long QT syndrome and ischaemia were discussed with the patient before he left hospital. Fortunately, he returned for continued work up and treatment 2 days later. His work up and management are as below.
Investigations
Cardiac CT angiogram demonstrated mild non-obstructive disease of the left anterior descending artery.
Gadolinium contrast-enhanced cardiomyopathy protocol MRI showed left ventricular systolic dysfunction with an ejection fraction of 46% but no myocardial scarring.
Treatment
After excluding other possible causes for a polymorphic ventricular tachycardia (ischaemia) with the unremarkable angiogram and MRI, and given the normal electrolytes on the day of the cardiac arrest, cardiology carried forward the diagnosis of acquired long-QT syndrome secondary to methadone treatment. Given the patient's myocardial infarction 3 weeks earlier and mild coronary artery disease seen on the cardiac CT angiogram, ramipril, bisoprolol and simvastatin were initiated, and the patient underwent insertion of a dual-chamber ICD 10 days following his initial cardiac arrest.
Prior to his final discharge, the patient remained clinically stable and denied the development of any opioid withdrawal symptoms while being prescribed oral morphine at 100 mg every 4 h. Given this, and the need to avoid the use of QTc prolonging medications, his community methadone prescription was replaced with 12 h SROM. 24 h SROM was briefly trialled but did not maintain the patient for a full day. As such, he was prescribed a total of 700 mg of the SROM morphine sulfate (M-Eslon) daily, with twice daily dosing of 350 mg, with the morning dose requiring daily witnessed ingestion at a community pharmacy given the high daily dosage and potential for drug diversion.
Outcome and follow-up
The patient was discharged from hospital 12 days after his initial admission and followed up with his family physician in the community. A repeat ECG 12 days following his TdP arrest revealed a QTc of 520 ms. Two months after hospitalisation, the patient remained asymptomatic and interrogation of his ICD revealed no documented arrhythmias. Furthermore, he maintains that treatment with SROM for his heroin addiction has been successful in managing drug cravings and preventing a relapse to the use of illicit opioids. His treating community physician has corroborated this. Further therapy to address his chronic hepatitides is an ongoing consideration for this patient.
Discussion
We have described a case of an individual being switched from methadone to SROM as an alternative treatment for opioid-use disorder due to a life threatening adverse event. Methadone, a μ-opioid receptor agonist, is known to act on the delayed rectifier repolarising potassium current IKr in ventricular myocytes and delays ventricular repolarisation. This manifests as a dose-dependent prolongation of the QTc interval demonstrated on an ECG tracing.21 22 Methadone is a racemic mixture consisting of an R and an S enantiomer, and it is thought that the S enantiomer has greater action on the potassium channel IKr and so greater influence on prolonging the QTc interval.23 QTc prolongation can predispose to the ventricular arrhythmia TdP with subsequent sudden cardiac death. This association is often taken into consideration by clinicians when formulating a patient's medical treatment plan, and frequently results in physicians opting for medications that have less of an impact on the QTc interval.10 24 Beyond methadone, some factors associated with an increased risk of TdP include concomitant hypokalaemia, liver disease, the use of known QTc prolonging medications and certain ECG features, including bradycardia and T wave changes.10 Our patient had normal electrolytes and no change in the status of his liver disease, however, he did use QT-prolonging medications in addition to methadone, including quetiapine for sleep, before his hospitalisation and haloperidol in hospital for severe agitation. Interestingly, the ECG taken just before his cardiac arrest (figure 2) does exhibit bradycardia and T-wave changes. More specific to this case, it is known that cocaine use increases the QTc interval,25 and there is evidence that hepatitis C infection can also have the same effect.26 A QTc interval above 500 ms is the accepted cut-off for increasing the risk for the development of TdP,27 28 and existing evidence suggests that treatment with methadone does significantly increase the proportion of patients found above this cut-off.9
Despite knowledge that methadone can prolong the QTc interval, the value of QTc screening for patients receiving the medication has been the subject of ongoing controversy. A review of the existing literature and guidelines in 2009 resulted in a US recommendation for baseline and routine QTc monitoring using ECG in an effort to identify patients on methadone therapy at high risk for the development of a cardiac arrhythmia.29 Conversely, a subsequent Cochrane Review published in 2013 suggests no evidence exists for this practice.30 More recent studies have demonstrated the difficulty in obtaining accurate and reliable QT measurements (due to fluctuating intrinsic factors and poor accuracy of automated QT measurements),31 and the inability of a prolonged QTc, to independently predict with accuracy the subsequent development of a ventricular arrythmia.32 Furthermore, given the potential QTc screening may have in delaying or hindering the initiation of methadone therapy, a treatment proven to reduce morbidity and mortality for opioid-use disorder, the procedure is not without risk.33
The use of buprenorphine/suboxone as an alternative to methadone to reduce the risk of cardiac arrhythmia from long QT syndrome has been supported by case reports, which show improvement in the QT interval following transition to buprenorphine.11 34 Limited alternatives exist for the treatment of opioid-use disorder when contraindications to methadone exist and patients are unwilling to switch to buprenorphine/naloxone. Furthermore, adverse side effects, patient intolerance and/or sociopolitical barriers impeding one's access to potential substitutes (as can be the case for the use of morphine or diacetylmorphine in certain settings) can further restrict the successful use of these alternatives.35 There are case reports and case series supporting the use of ICD insertion for secondary prevention of TdP in patients on methadone,17 18 however, avoidance of an arrhythmia is preferable. In this case, oral morphine was prescribed to prevent opioid withdrawal following the development of a serious adverse event while on methadone. Of note, the dose of SROM prescribed is considered to be in the regular range, with the mean dose of SROM in a large controlled clinical trial being 791 mg daily.19 20 Given its success, objectively and subjectively, the patient was subsequently transitioned to SROM for long-term maintenance therapy. Although a Cochrane Review published in 2013 concluded that there was insufficient evidence to address the effectiveness of SROM for the treatment of opioid-use disorder,36 a large controlled clinical trial comparing methadone to SROM in this context demonstrated numerous favourable outcomes.19 20 More specifically, the use of SROM was associated with a shorter mean QTc interval, higher treatment satisfaction, fewer cravings for heroin, reduced dysthymic symptoms and less heroin use when compared with methadone.19 20
This case highlights the challenges associated with the management of opioid-use disorder in patients with a substantially prolonged QTc. While pharmacological therapy is the gold standard of treatment,37 with methadone being the most extensively researched and supported,6 12 certain patients with medical circumstances precluding its use (or that of buprenorphine/naloxone) should be considered for an effective alternative. In this context, we believe careful consideration should be given to the use of SROM and support further research being carried out to determine its overall effectiveness in this regard.
Patient's perspective.
“My life changed, a 100% turnaround. I don't use anything anymore and I've gained 27 pounds since the summer (8 months). I feel like a million dollars, I feel so much better. I don't even notice I'm taking it (the sustained release oral morphine) and I don't have the side effects I had before.”
Learning points.
Methadone can contribute to an increased QTc interval and increase the proportion of patients with a QTc interval over 500 ms, which is established as the cut-off for an increased risk of Torsades de Pointes.21 22 27 28
Transitioning patients from methadone to sustained release oral morphine (SROM) results in a decrease in their QTc interval.19
SROM is a safe and effective alternative to methadone, and its use should be considered in some settings for opioid-use disorder.
Acknowledgments
The study was supported by the US National Institutes of Health (R25DA037756). This research was undertaken, in part, thanks to funding for a Tier 1 Canada Research Chair in Inner City Medicine, which supports Evan Wood, who was also involved to some degree in the editing of this project.
Footnotes
Contributors: The first author wrote the manuscript and made revisions according to the other co-author's edits. He was involved in the interpretation of the clinical scenario. The other authors all were heavily involved in editing the manuscript of the case report. They helped with the interpretation of the case in the context of the existing literature and medical practices.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bargagli AM, Hickman M, Davoli M et al. Drug-related mortality and its impact on adult mortality in eight European countries. Eur J Public Health 2006;16:198–202. 10.1093/eurpub/cki168 [DOI] [PubMed] [Google Scholar]
- 2.Darke S, Hall W. Heroin overdose: research and evidence-based intervention. J Urban Health 2003;80:189–200. 10.1093/jurban/jtg022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minozzi S, Amato L, Vecchi S et al. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev 2011(4):Cd001333 10.1002/14651858.CD001333.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degenhardt L, Randall D, Hall W et al. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend 2009;105:9–15. 10.1016/j.drugalcdep.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 5.Brugal MT, Domingo-Salvany A, Puig R et al. Evaluating the impact of methadone maintenance programmes on mortality due to overdose and aids in a cohort of heroin users in Spain. Addiction 2005;100:981–9. 10.1111/j.1360-0443.2005.01089.x [DOI] [PubMed] [Google Scholar]
- 6.Mattick RP, Breen C, Kimber J et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2009;(3):Cd002209 10.1002/14651858.CD002209.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gowing L, Farrell MF, Bornemann R et al. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev 2011;(8):Cd004145 10.1002/14651858.CD004145.pub4 [DOI] [PubMed] [Google Scholar]
- 8.Dyer KR, White JM. Patterns of symptom complaints in methadone maintenance patients. Addiction 1997;92:1445–55. 10.1046/j.1360-0443.1997.921114456.x [DOI] [PubMed] [Google Scholar]
- 9.Ehret GB, Voide C, Gex-Fabry M et al. Drug-induced long QT syndrome in injection drug users receiving methadone: high frequency in hospitalized patients and risk factors. Arch Intern Med 2006;166:1280–7. 10.1001/archinte.166.12.1280 [DOI] [PubMed] [Google Scholar]
- 10.Krantz MJ, Lewkowiez L, Hays H et al. Torsade de pointes associated with very-high-dose methadone. Ann Intern Med 2002;137:501–4. 10.7326/0003-4819-137-6-200209170-00010 [DOI] [PubMed] [Google Scholar]
- 11.Krantz MJ, Garcia JA, Mehler PS. Effects of buprenorphine on cardiac repolarization in a patient with methadone-related torsade de pointes. Pharmacotherapy 2005;25:611–14. 10.1592/phco.25.4.611.61020 [DOI] [PubMed] [Google Scholar]
- 12.Mattick RP, Breen C, Kimber J et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014;(2):Cd002207 10.1002/14651858.CD002207.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrieri MP, Roux P, Cohen J et al. Self-reported side effects in buprenorphine and methadone patients receiving antiretroviral therapy: results from the MANIF 2000 cohort study. Addiction 2010;105:2160–8. 10.1111/j.1360-0443.2010.03108.x [DOI] [PubMed] [Google Scholar]
- 14.Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2004. [PubMed] [Google Scholar]
- 15.Lee JD, Vocci F, Fiellin DA. Unobserved “home” induction onto buprenorphine. J Addict Med 2014;8:299–308. 10.1097/ADM.0000000000000059 [DOI] [PubMed] [Google Scholar]
- 16.Fudala PJ, Bridge TP, Herbert S et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med 2003;349:949–58. 10.1056/NEJMoa022164 [DOI] [PubMed] [Google Scholar]
- 17.Pimentel L, Mayo D. Chronic methadone therapy complicated by torsades de pointes: a case report. J Emerg Med 2008;34:287–90. 10.1016/j.jemermed.2007.03.053 [DOI] [PubMed] [Google Scholar]
- 18.Patel AM, Singh JP, Ruskin JN. Role of implantable cardioverter-defibrillators in patients with methadone-induced long QT syndrome. Am J Cardiol 2008;101:209–11. 10.1016/j.amjcard.2007.07.068 [DOI] [PubMed] [Google Scholar]
- 19.Hammig R, Kohler W, Bonorden-Kleij K et al. Safety and tolerability of slow-release oral morphine versus methadone in the treatment of opioid dependence. J Subst Abuse Treat 2014;47:275–81. 10.1016/j.jsat.2014.05.012 [DOI] [PubMed] [Google Scholar]
- 20.Beck T, Haasen C, Verthein U et al. Maintenance treatment for opioid dependence with slow-release oral morphine: a randomized cross-over, non-inferiority study versus methadone. Addiction 2014;109:617–26. 10.1111/add.12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katchman AN, Koerner J, Tosaka T et al. Comparative evaluation of HERG currents and QT intervals following challenge with suspected torsadogenic and nontorsadogenic drugs. J Pharmacol Exp Ther 2006;316:1098–106. 10.1124/jpet.105.093393 [DOI] [PubMed] [Google Scholar]
- 22.Krantz MJ, Kutinsky IB, Robertson AD et al. Dose-related effects of methadone on QT prolongation in a series of patients with torsade de pointes. Pharmacotherapy 2003;23:802–5. 10.1592/phco.23.6.802.32186 [DOI] [PubMed] [Google Scholar]
- 23.Lin C, Somberg T, Molnar J et al. The effects of chiral isolates of methadone on the cardiac potassium channel IKr. Cardiology 2009;113:59–65. 10.1159/000167043 [DOI] [PubMed] [Google Scholar]
- 24.Chugh SS, Socoteanu C, Reinier K et al. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med 2008;121:66–71. 10.1016/j.amjmed.2007.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan IA. Long QT syndrome: diagnosis and management. Am Heart J 2002;143:7–14. 10.1067/mhj.2002.120295 [DOI] [PubMed] [Google Scholar]
- 26.Gholami N, Boesch L, Falcato L et al. QTc prolongation in methadone maintenance--the role of HCV infection. Swiss Med Wkly 2013;143:w13852. [DOI] [PubMed] [Google Scholar]
- 27.Priori SG, Schwartz PJ, Napolitano C et al. Risk stratification in the long-QT syndrome. N Engl J Med 2003;348:1866–74. 10.1056/NEJMoa022147 [DOI] [PubMed] [Google Scholar]
- 28.Makkar RR, Fromm BS, Steinman RT et al. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA 1993;270:2590–7. 10.1001/jama.1993.03510210076031 [DOI] [PubMed] [Google Scholar]
- 29.Krantz MJ, Martin J, Stimmel B et al. QTc interval screening in methadone treatment. Ann Intern Med 2009;150:387–95. 10.7326/0003-4819-150-6-200903170-00103 [DOI] [PubMed] [Google Scholar]
- 30.Pani PP, Trogu E, Maremmani I et al. QTc interval screening for cardiac risk in methadone treatment of opioid dependence. Cochrane Database Syst Rev 2013;(6):Cd008939 10.1002/14651858.CD008939.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Worster A, Varenbut M, Daiter J et al. Variability of QT interval measurements in opioid-dependent patients on methadone. Can J Addict 2014;5:10–16. [Google Scholar]
- 32.Shah RR. Drug-induced QT dispersion: does it predict the risk of torsade de pointes? J Electrocardiol 2005;38:10–18. 10.1016/j.jelectrocard.2004.09.001 [DOI] [PubMed] [Google Scholar]
- 33.Nolan S, Wood E. Methadone and QTc screening: Weighing the risks and benefits. Can J Addict 2014;5:17–19. [Google Scholar]
- 34.Esses JL, Rosman J, Do LT et al. Successful transition to buprenorphine in a patient with methadone-induced torsades de pointes. J Interv Card Electrophysiol 2008;23:117–19. 10.1007/s10840-008-9280-8 [DOI] [PubMed] [Google Scholar]
- 35.Oviedo-Joekes E, Brissette S, Marsh DC et al. Diacetylmorphine versus methadone for the treatment of opioid addiction. N Engl J Med 2009;361:777–86. 10.1056/NEJMoa0810635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferri M, Minozzi S, Bo A et al. Slow-release oral morphine as maintenance therapy for opioid dependence. Cochrane Database Syst Rev 2013;(6):Cd009879 10.1002/14651858.CD009879.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dole VP, Nyswander M. A medical treatment for diacetylmorphine (heroin) addiction. A clinical trial with methadone hydrochloride. JAMA 1965;193:646–50. 10.1001/jama.1965.03090080008002 [DOI] [PubMed] [Google Scholar]


