Abstract
Background
About 8000 breast reconstructions after mastectomy are performed in Germany each year. It has become more difficult to advise patients because of the wide variety of heterologous and autologous techniques that are now available and because of changes in the recommendations about radiotherapy.
Methods
This article is based on a review of pertinent articles (2005–2014) that were retrieved by a selective search employing the search terms “mastectomy” and “breast reconstruction.”
Results
The goal of reconstruction is to achieve an oncologically safe and aestically satisfactory result for the patient over the long term. Heterologous, i.e., implant-based, breast reconstruction (IBR) and autologous breast reconstruction (ABR) are complementary techniques. Immediate reconstruction preserves the skin of the breast and its natural form and prevents the psychological trauma associated with mastectomy. If post-mastectomy radiotherapy (PMRT) is not indicated, implant-based reconstruction with or without a net/acellular dermal matrix (ADM) is a common option. Complications such as seroma formation, infection, and explantation are significantly more common when an ADM is used (15.3% vs. 5.4%). If PMRT is performed, then the complication rate of implant-based breast reconstruction is 1 to 48%; in particular, Baker grade III/IV capsular fibrosis occurs in 7 to 22% of patients, and the prosthesis must be explanted in 9 to 41%. Primary or, preferably, secondary autologous reconstruction is an alternative. The results of ABR are more stable over the long term, but the operation is markedly more complex. Autologous breast reconstruction after PMRT does not increase the risk of serious complications (20.5% vs. 17.9% without radiotherapy).
Conclusion
No randomized controlled trials have yet been conducted to compare the reconstructive techniques with each other. If radiotherapy will not be performed, immediate reconstruction with an implant is recommended. On the other hand, if post-mastectomy radiotherapy is indicated, then secondary autologous breast reconstruction is the procedure of choice. Future studies should address patients’ quality of life and the long-term aesthetic results after breast reconstruction.
Every year in Germany, mastectomy is required for 27% of the 75 000 women newly diagnosed with breast cancer. Approximately one-third of women choose ipsilateral breast reconstruction, and a further 1000 prophylactic mastectomy with reconstruction (n = 8000) (e1– e3). In the USA, where there are 230 000 new cases of breast cancer every year and a falling rate of breast-conserving surgeries, approximately 100 000 breast reconstructions are performed annually (1). These may be implant-based breast reconstruction (IBR), autologous breast reconstruction (ABR), or a combination of the two. Heated discussion regarding optimal surgical procedure and time of reconstruction is currently ongoing.
This review article provides a systematic overview of current options for breast reconstruction and their indications and contraindications.
Materials and methods
We performed a selective search of the literature using the search terms “mastectomy AND breast reconstruction” for the period between 1 January 2005 and 1 January 2015 in PubMed, the German S3 guideline Diagnosis and Treatment of Breast Cancer (Diagnostik und Therapie des Mammakarzinoms) (e4), the treatment recommendation of the AGO Breast Committee (Arbeitsgemeinschaft Gynäkologische Onkologie, Organgruppe Mamma) (e5), the guideline of American Society of Plastic Surgeons (1), the NCCN guideline (e6), and the Cochrane Library (e7). Statements were evaluated according to the Oxford Criteria (e8).
Background
The data on each type of reconstruction—particularly the increasingly common free flap plastic surgery—timing of reconstruction, and risk factors is limited by the absence of randomized trials and is mostly based on single-center, retrospective evaluations (e9). These show evidence of considerable bias, particularly regarding the use of meshes and implants, although this seems understandable given the individual nature of breast reconstruction (e10). Differences between health systems mean that there are differences in frequency and types of breast reconstruction, even within one country (2, 3, e11, e12).
Each and every patient must be given timely, detailed, comprehensive information on all breast reconstruction procedures (Table 1), expected outcomes, risks, and alternatives. According to the German Law on Patient Rights, this also includes the offer of a second opinion and information on surgical procedures that are not offered in the physician’s own hospital. If the tumor-to-breast volume ratio is unfavorable and chemotherapy is indicated, neoadjuvant chemotherapy must also be discussed, to improve the possibilities for breast-conserving surgery. The aim of reconstruction should be to achieve an oncologically safe and long-term aesthetically favorable outcome, with reasonable expenditure on surgery. The reconstructed breast should look natural and symmetrical and be soft and sensitive. Any surgeries necessary to achieve symmetry (reduction, lifting, etc.) must be discussed in advance. Reconstruction should never be the cause of any delay to the beginning of chemotherapy or radiotherapy, e.g. as a result of open wounds, within 8 to 12 weeks later (4, 5, e13, e14). Immediate reconstruction and the complications associated with it cause a mean treatment delay of three weeks (6), and a delay of more than 12 weeks for 20% of these patients (e15). Perioperative complications are reported in 9.5 to 19% of cases following ABR and in 4% following implant-based breast reconstruction (7, e16).
Table 1. Advantages and disadvantages of reconstruction techniques*.
| Technique | Advantages | Disadvantages |
|---|---|---|
| Implant-based reconstruction | ||
| Implant reconstruction |
|
|
| Implant± mesh/ADM ± autodermal graft |
|
(Except with autodermal graft)Meshes/ADM
|
| LADO ± implant |
|
|
| Tissue transfer from abdominal wall | ||
|
|
|
|
|
|
|
|
|
| Tissue transfer from other regions | ||
| Flaps from buttocks (SGAP, IGAP, FCI) |
|
|
| Gracilis flaps from thigh |
|
|
ADM, acellular dermal matrix; DIEP, deep inferior epigastric perforator; FCI, fasciocutaneous infragluteal flap; IGAP, inferior gluteal artery perforator; LADO, latissimus dorsi flap;
TRAM, transverse rectus abdominis muscle flap; NSM/SSM, nipple-sparing/skin-sparing mastectomy: SIEA, superficial inferior epigastric artery; SGOP, superior gluteal artery perforator*Modified according to (e5)
Autologous versus heterologous reconstruction
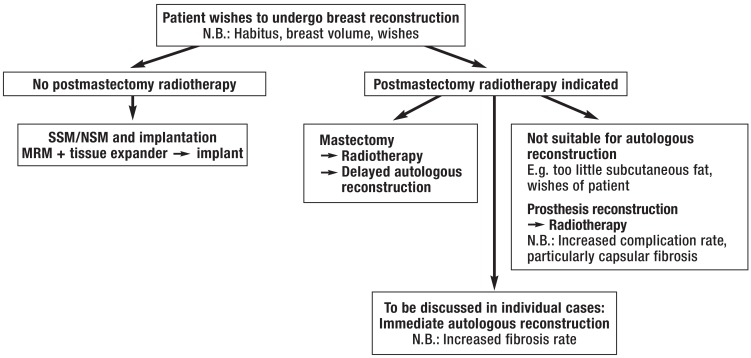
Immediate autologous and heterologous reconstruction are procedures that complement rather than oppose each other (Figure). The choice of procedure depends on habitus, the patient’s wishes and ideas, risk factors (smoking, diabetes mellitus, obesity, previous surgeries, radiation, history of thrombosis, cardiovascular disease, etc.), possible postmastectomy radiotherapy (PMRT), and the skills of the surgeon. In the USA, 83% of all immediate reconstructions involve implants. The percentage of implant-based immediate reconstructions there has increased from 20.8% to 37.8% in the last 10 years, while the rate of autologous breast reconstructions has remained roughly constant (8, e17). In England, the percentage of bilateral mastectomies has doubled in the last 10 years but remains substantially lower, at 4% (9). This increase is the result of the oncological safety of skin-/nipple-sparing mastectomy (SSM/NSM) and the improved quality of silicone implants (10, 11). On the other hand, women with unilateral cancer, and healthy women with an increased risk of breast cancer (the Angelina Jolie effect), increasingly wish to undergo prophylactic (contralateral) mastectomy; this has led to an increase in bilateral implant-based breast reconstructions, from 3% in 1998 to 18% in 2007 in the USA (12, 13). In a longitudinal study conducted in California and involving approximately 190 000 patients, there was an increase in the rate of bilateral mastectomies from 2% (1998) to 12.3% (2011). The increase in women aged under 40 years was particularly large, rising from 3.6% to 33% (14).
Figure.
Procedure for breast reconstruction SSM, skin-sparing mastectomy; NSM, nipple-sparing mastectomy; MRM, modified radical hysterectomy
Immediate autologous breast reconstruction is performed in 14% of cases in the USA. Most are performed in university facilities. Pedicle flaps, mainly from the abdomen, are the standard procedure, accounting for 68% of cases, while only around 28% of plastic surgeons who were questioned performed free flap plastic surgery. The most common free flap surgery is DIEP (deep inferior epigastric flap) (e17). The percentage of autologous breast reconstructions in the USA depends essentially on the local density of plastic surgeons, insurance status, financial resources, and planned radiotherapy (13, 15, e18).
The cost of free flap surgery with microsurgical anastomosis is 2.5 times higher than for implant-based reconstruction and rises further if there are complications (16). The aesthetic outcome of autologous breast reconstruction is more stable in the long term than that of implant-based breast reconstruction (17). In the long term, autologous breast reconstruction can even become more cost-effective than implant-based reconstruction (e19). On the other hand, following autologous breast reconstruction second operations are required in 100% of cases, third operations in 53%, and fourth operations in 12% (nipple reconstruction, delayed complications, surgery to achieve symmetry) (7).
Quality of life following breast reconstruction
Patient satisfaction with outcome one year after implant-based reconstruction was comparable to that of autologous (transverse rectus abdominis muscle flap, TRAM) reconstruction (satisfaction figure: 46.1% of all included patients), while after two years (satisfaction figure: 38% of patients) the figure was substantially higher (odds ratio 2.8; p <0.01) for autologous breast reconstruction (e20). A recent survey, using the BREAST-Q questionnaire, of 7619 women after a median of 6.7 years found the highest satisfaction rates in women who had undergone reconstruction using autologous tissue from the abdomen (free/pedicle TRAM, DIEP). This figure was even higher than for patients who had undergone breast-conserving surgery (18, e21, e22).
Patients’ quality of life and satisfaction with aesthetic outcome following reconstruction involving implants was significantly better when the following criteria were met:
The patient was involved in the decision to perform reconstruction (n= 325; 66.7% versus 38.9%, p = 0.020) (e23, e24).
Bilateral rather than unilateral implant-based reconstruction was performed (n = 294; 64.4% versus 54.9%; p <0.001) (e25).
Silicone implants were used rather than saline implants (n = 142; BREAST-Q score 63.8 versus 56.9; p = 0.0083) (e21, e26, e27).
The nipple–areola complex was successfully conserved (n = 108; 97% versus 86%; p <0.025) (10, e28).
No radiotherapy was administered following implant-based breast reconstruction (n = 633; 64% versus 58.3%; p <0.01) (19, e29).
Immediate, delayed-immediate, or delayed reconstruction
Immediate reconstruction has a number of advantages over delayed reconstruction (Table 2) (e30). Surveys of US plastic surgeons revealed that approximately 80% of breast reconstructions were immediate reconstructions (13, e17). When postmastectomy radiotherapy was indicated, delayed reconstruction was recommended in 81% of cases. Whether reconstruction can and should be autologous or heterologous following postmastectomy radiotherapy depends on the individual circumstances (skin reaction to radiation, subcutaneous fat, arm function, etc.) and the wishes of the patient.
Table 2. Time of reconstruction—advantages and disadvantages*.
| Oxford | AGO | ||
|---|---|---|---|
| LOE | GR | GR | |
Immediate reconstruction
|
3b | B | ++ |
Delayed breast reconstruction
|
3b | B | ++ |
| Delayed-immediate BR | 3b | B | +/− |
|
Oxford levels of evidence (LOEs) (e9) 1a: Systematic review (SR) (with homogeneity) of randomized controlled trials (RCTs) 1b: Individual RCTs (with narrow confidence interval [CI]) 1c: All or none 2a: Systematic review (with homogeneity) of cohort studies 2b: Individual cohort studies (including low-quality RCTs; e.g. <80% follow-up) 2c: outcomes research; ecological studies 3a: systematic review (with homogeneity) of case–control studies 3b: individual case–control studies 4: case series (and poor-quality cohort and case–control studies) 5: expert opinion Oxford grades of recommendation (GR) A: consistent level 1 studies B: consistent level 2 or 3 studies or extrapolation from level 1 studies C: consistent level 4 studies or extrapolation from level 2 or 3 studies D: level of evidence 5 or unclear or inconsistent data at any level Grades of recommendation of ago breast Committee Therapy/intervention is: ++ Of great benefit to patient, should be performed + Of limited benefit to patient, may be performed +/- Of no confirmed benefit to patient but may be performed in individual cases - Seems to be disadvantageous to patient, should not be performed - -Clearly disadvantageous to patient, should always be avoided | |||
RCT, randomized controlled trial; AGO, Working Group for Gynecological Oncology; BR, breast reconstruction; CHT, chemotherapy; GR, grade of recommendation; LOE, Oxford level of evidence; NSM, nipple-sparing mastectomy; RT, radiotherapy; SSM, skin-sparing mastectomy
*Modified according to (e5)
For delayed-immediate breast reconstruction, a placeholder prosthesis is inserted into the breast when skin-sparing mastectomy is performed. If no radiotherapy is indicated on the basis of final histology, the placeholder prosthesis is replaced with the final implant. If postmastectomy radiotherapy is indicated, the implant is replaced after radiotherapy. The complication rate was high in both groups: 32% (postmastectomy radiotherapy before implant-based breast reconstruction) and 44% (implant-based breast reconstruction before postmastectomy radiotherapy; p= 0.176), while satisfaction with aesthetic outcome was only 50% and 62% respectively (p = 0.238) (e31). In our opinion, delayed-immediate breast reconstruction is now acceptable only in exceptional cases.
Risk factors for postsurgical complications following autologous or heterologous breast reconstruction
Postmastectomy radiotherapy
Recommendations for or against postmastectomy radiotherapy as adjuvant therapy are still based on lymph node status, the significance of which must now be viewed critically (20, e32, e33).
Postmastectomy radiotherapy after or before implant-based radiotherapy increases the risk of complications (wound infections, explantation, skin necrosis, seroma formation, capsular contracture) significantly. For example, significantly more complications—in 41 to 48% of cases—were reported in patients who had undergone reconstruction involving implants and postoperative radiation than in patients who had not undergone radiotherapy (4 to 23% of cases) (10, 21– 23). In the long term, after postmastectomy radiotherapy there are Baker grade III/IV capsular contractures in 7 to 22% of cases and explantations in 9 to 41%; these occurred in 0.5 to 2% and 8 to 20% respectively of cases in which no radiotherapy was administered (24– 27).
Following prior irradiation of the chest wall too (e.g. following mastectomy, breast-conserving therapy, lymphoma), the risk of infection or capsular contracture following reconstruction using a tissue expander or implant is two to three times higher (e34– e36). In a multicenter Swedish cohort study with a median follow-up time of 43 months, after prosthesis reconstruction without postmastectomy radiotherapy (n = 386), with prior radiotherapy (n = 64), or with postoperative postmastectomy radiotherapy (n = 304) there were explantation rates of 6%, 25%, and 15% respectively (p <0.001) and subsequent surgeries in 44%, 66%, and 59% of cases (28). If implant-based breast reconstruction is indicated despite postmastectomy radiotherapy, radiotherapy should be performed before reconstruction.
In patients who have undergone prior radiotherapy, the implant loss rate of 30 to 42% can be significantly reduced using a combination of implant and autologous tissue (LADO flap: 15%, pedicle TRAM: 10%, free TRAM: 5%) (e37).
The long-term risk of complications is also significantly (four times) higher after irradiation of a tissue expander prosthesis than without irradiation (25, e38). Irradiation of tissue expander prostheses (n = 50) versus silicone implants (n = 109) led to significantly increased complication rates (40% versus 6.4%), mainly capsular fibrosis (e39). However, it should be noted that tissue expander prostheses were usually used following complete mastectomy and permanent implantation following skin-sparing mastectomy.
There is no guidance covering when a tissue expander prosthesis should be replaced with a permanent implant (delayed-immediate breast reconstruction) (e40). However, filling of the tissue expander during chemotherapy and replacement with a final implant before the beginning of radiotherapy seems to be associated with substantially fewer complications and better aesthetic outcome than replacement following radiotherapy (22, 23, 26, 28, 29).
When postmastectomy radiotherapy is indicated, autologous breast reconstruction should be performed after radiotherapy wherever possible (Table 3) (10). In a study by Berry et al. (21), there was no significant difference in severe complications following autologous reconstruction without or with radiotherapy: the rates were 17.9% and 20.5% respectively. However, the total number of complications was significantly higher with radiotherapy (31.5%) than without it (19.7%). In a meta-analysis of 13 nonrandomized trials, the rate of fibrosis following autologous breast reconstruction and radiotherapy was 36%, significantly higher than the comparator group’s rate of 2.7%; the authors therefore recommend autologous breast reconstruction after postmastectomy radiotherapy (22). On the other hand, in a series of 363 patients who underwent free flap reconstruction and subsequent radiotherapy there was no significant disadvantage in comparison to patients who had not undergone radiotherapy (7).
Table 3. Breast reconstruction following mastectomy, with and without PMRT*.
| Oxford | AGO | ||
|---|---|---|---|
| LOE | GR | GR | |
IBR
|
2a 2b 2a 3a 2a |
B B B B B |
++ +/– +/– + +/– |
Breast reconstruction with ABR
|
2a 2a 3b 3a 3a 3a 4 4 |
B B C B B C C C |
+/– + + +/– + +/– +/– +/– |
ABR, autologous breast reconstruction; AGO, Working Group for Gynecological Oncology; BCT, breast-conserving therapy; DIEP, deep inferior epigastric perforator; GR, grade of recommendation; IBR, implant-based breast reconstruction; IGAP, inferior gluteal artery perforator; LADO, latissimus dorsi flap; LOE, Oxford level of evidence; PMRT, postmastectomy radiotherapy; SIEA, superficial inferior epigastric artery; SGAP, superior gluteal artery perforator; TRAM, transverse rectus abdominis muscle flap; TGM, transverse myocutaneous gracilis*Modified according to (e5)
Whether postmastectomy radiotherapy should be followed by heterologous, autologous, or combined reconstruction depends on local skin status and the wishes of the patient. However, the grade of recommendation for autologous breast reconstruction is higher than that for prosthetic reconstruction (Table 3). Breast reconstruction should be performed no earlier than six months after the end of radiotherapy (e41).
Autologous reconstruction is preferable following postmastectomy radiotherapy and explantation as a result of capsular contracture.
Individual risk factors
Current and long-term nicotine abuse is associated with a deterioration in microcirculation and therefore a threefold to fivefold increase in the frequency of wound healing problems (e42, e43). A meta-analysis of 14 585 implant-based reconstructions following skin-sparing mastectomy showed a significant increase in the risk of early prosthesis loss for the following risk factors: age over 55 years (odds ratio: 1.66; p = 0.013), obesity (odds ratio: 2.14 to 3.17; p <0.014), current smoking (odds ratio: 2.95; p <0.001), and bilateral reconstruction (odds ratio: 1.67; p = 0.007) (30).
For implant-based reconstructions (n = 9305), in addition to the factors mentioned above, prolonged surgery time (odds ratio: 2.2; p = 0.002) and local wound infections (odds ratio: 4.0; p = 0.002) were found to increase the risk of implant loss (31). Regardless of the type of reconstruction, more surgical complications were reported when large breasts (mass over 600 g or cup size above C) were reconstructed (large breasts: 18%, medium-sized: 7%, small: 3%; p = 0.0003) (e44). Other risk factors for complications in all types of breast reconstruction were cardiovascular disease, intraoperative blood transfusion, American Society of Anaesthesiologists (ASA) score above 3, and major weight loss before surgery (e45, e46). In a series of 2138 autologous reconstructions, mainly DIEP flaps, the percentage of complete flap loss was 2.1%, independent of age, body mass index, smoking, prior postmastectomy radiotherapy, or chemotherapy (32). The rate of partial flap necrosis and wound healing problems, particularly in the donator area, was significantly increased when these risk factors were present (e47).
On the other hand, there are also studies that have found no increase in the rate of complications with increased age, body mass index, acellular dermal matrix (ADM) use, or smoking (21, e48).
Systemic diseases that affect microcirculation (diabetes mellitus, collagenosis, hypertension) can increase the risk of complications following breast reconstruction in line with their duration, severity, and treatment. For example, a significantly increased rate of surgical complications (odds ratio: 1.58) has been reported for diabetic patients following autologous breast reconstruction, although the complication rate following implant-based breast reconstruction was the same (33).
Antibiotic prophylaxis, drainage
Meta-analyses have shown no advantages for the administration or duration of antibiotic prophylaxis or drainage in terms of wound infections or outcome (34, 35). Nevertheless, surveys of 4669 plastic surgeons in North America found that 81% of respondents used drainage in the reconstructed breast, and 93% did not remove the drain until after a fluid quantity of less than 30 mL per 24 hours had been achieved (35, 36). For autologous breast reconstruction, drain insertion in the donator area and in the reconstructed breast is recommended (e49). Perioperative administration of antibiotics (97% cefazolin) was reported by 98% of respondents; the duration of administration ranged from a single dose administered over administration for 24 to 48 hours to administration up to drainage removal. Following autologous breast reconstruction, longer antibiotic administration was no more effective than administration for 24 hours (19.5% versus 15.5%; p = 0.47) (e50). Following ADM use, antibiotic prophylaxis lasting at least 48 hours significantly reduces complications (e51). Patients can shower on the first day after breast reconstruction.
Systemic oncological therapy
Neoadjuvant chemotherapy had no detectable effect on the rate of complications provided surgery was performed two to four weeks after the patient’s last chemotherapy session and thus white blood cell count nadir (e52). Breast-conserving surgeries and mastectomies four weeks after neoadjuvant administration of bevacizumab showed no significant increase in postoperative complications (37, e53), while after primary breast reconstruction there were significantly more complications, particularly protracted wound healing problems (38, e54).
When microsurgical reconstruction is performed, the increased risk of thrombosis caused by tamoxifen is important. Thus in 670 patients undergoing free flap surgery (205 receiving tamoxifen up to surgery, 465 until no later than four weeks before surgery) those who were still receiving tamoxifen had significantly more complications (odds ratio: 1.7, p = 0.015). Flap complications (p = 0.002) up to and including total flap loss (p= 0.041) were particularly common (e55).
Acellular matrices, synthetic (Vicryl) meshes, dermal fat flaps
Publications on the use of meshes suffer from considerable bias, as these patients are usually negatively selected (e56).
Textile meshes are used preferentially in immediate reconstruction in order to fix the pectoral muscle and the implant in position for subpectoral implant insertion. Acellular matrices are used if the patient wishes to have reconstruction with an implant and the subcutaneous fat layer is very thin, the patient has undergone or is scheduled to undergo radiotherapy, or in surgery for complications. These patients must be informed of the options for autologous reconstruction. Because authorization by the Food and Drug Administration (FDA) for meshes is pending, in the USA acellular matrices are used almost without exception (11, e57). Rates of complications (seroma, infection, implant removal) following reconstruction involving implants were significantly higher with acellular matrices (15.3%) than without them (5.4%) (39). Following irradiation of the chest wall, in smokers, in overweight patients, and for very large prostheses (more than 600 mL) complications were more common after the use of acellular matrices (46.2% versus 22.7%) (40, e58– e61). The use of sterile, ready-to-use acellular matrices can significantly reduce the risk of complications.
As an alternative to meshes and acellular matrices, patients with breast ptosis and/or who wish to undergo breast reduction can be offered a deepithelized lower breast skin graft (autodermal graft), which covers the implant together with the pectoral muscle.
Outlook
In recent years there have been significant advances in implant-based and autologous breast reconstructions. Randomized trials on breast reconstruction will be all but impossible, for ethical reasons and due to their individual nature. Future studies should provide long-term findings on individual procedures, particularly the use of meshes, and patient satisfaction. Costs, complications, and subsequent surgeries, as well as care structures, should also be analyzed.
key messages.
The aim is an oncologically safe and long-term aesthetically favorable outcome with reasonable expenditure on surgery.
Patients must be comprehensively informed of all procedures and their advantages and disadvantages. Patient wishes, habitus, and risk factors must be taken into account when selecting a surgical procedure.
Autologous and heterologous reconstructions complement each other in immediate breast reconstruction.
If no postmastectomy radiotherapy is planned, immediate skin-sparing/nipple-sparing reconstruction involving implants is preferable.
If postmastectomy radiotherapy is planned, patients must be informed in detail of the advantages and disadvantages of immediate versus delayed and heterologous versus autologous reconstruction.
Acknowledgments
Translated from the original German by Caroline Shimakawa-Devitt, M.A.
Footnotes
Conflict of interest statement
Prof. Faridi has received consultancy fees and reimbursement of conference fees from pfm medical ag, Cologne and DIZGg GmbH, Berlin.
Prof. Gerber has received consultancy and lecture fees from AstraZeneca, Novartis, Roche, TEVA, JanssenCilag, Celgene, and Pfizer.
Prof. Marx and Prof. Untch declare that no conflict of interest exists.
References
- 1.Alderman A, Gutowski K, Ahuja A, Gray D. ASPS clinical practice guideline summary on breast reconstruction with expanders and implants. Plast Reconstr Surg. 2014;134:648e–655e. doi: 10.1097/PRS.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 2.Covelli AM, Baxter NN, Fitch MI, Wright FC. Increasing mastectomy rates-the effect of environmental factors on the choice for mastectomy: a comparative analysis between Canada and the United States. Ann Surg Oncol. 2014;21:3173–3184. doi: 10.1245/s10434-014-3955-4. [DOI] [PubMed] [Google Scholar]
- 3.Albornoz CR, Cordeiro PG, Hishon L, et al. A nationwide analysis of the relationship between hospital volume and outcome for autologous breast reconstruction. Plast Reconstr Surg. 2013;132:192e–200e. doi: 10.1097/PRS.0b013e31829586c1. [DOI] [PubMed] [Google Scholar]
- 4.Knauerhase H, Strietzel M, Gerber B, Reimer T, Fietkau R. Tumor location, interval between surgery and radiotherapy, and boost technique influence local control after breast-conserving surgery and radiation: retrospective analysis of monoinstitutional long-term results. Int J Radiat Oncol Biol Phys. 2008;72:1048–1055. doi: 10.1016/j.ijrobp.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Lohrisch C, Paltiel C, Gelmon K, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2006;24:4888–4894. doi: 10.1200/JCO.2005.01.6089. [DOI] [PubMed] [Google Scholar]
- 6.Vandergrift JL, Niland JC, Theriault RL, et al. Time to adjuvant chemotherapy for breast cancer in National Comprehensive Cancer Network institutions. J Natl Cancer Inst. 2013;105:104–112. doi: 10.1093/jnci/djs506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang EI, Liu TS, Festekjian JH, Da Lio AL, Crisera CA. Effects of radiation therapy for breast cancer based on type of free flap reconstruction. Plast Reconstr Surg. 2013;131:1e–8e. doi: 10.1097/PRS.0b013e3182729d33. [DOI] [PubMed] [Google Scholar]
- 8.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in US. Breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131:15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 9.Neuburger J, Macneill F, Jeevan R, van der Meulen JH, Cromwell DA. Trends in the use of bilateral mastectomy in England from 2002 to 2011: retrospective analysis of hospital episode statistics. BMJ open. 2013 doi: 10.1136/bmjopen-2013-003179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber B, Krause A, Dieterich M, Kundt G, Reimer T. The oncological safety of skin-sparing mastectomy with conservation of the nipple-areola complex and immediate reconstruction: an extended follow-up study. Ann Surg. 2009;249:461–468. doi: 10.1097/SLA.0b013e31819a044f. [DOI] [PubMed] [Google Scholar]
- 11.Dieterich M, Faridi A. Biological matrices and synthetic meshes used in implant-based breast reconstruction - a review of products available in Germany. Geburtshilfe Frauenheilkd. 2013;73:1100–1106. doi: 10.1055/s-0033-1350930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cemal Y, Albornoz CR, Disa JJ, et al. A paradigm shift in US. breast reconstruction: Part 2. The influence of changing mastectomy patterns on reconstructive rate and method. Plast Reconstr Surg. 2013;131:320e–326e. doi: 10.1097/PRS.0b013e31827cf576. [DOI] [PubMed] [Google Scholar]
- 13.Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol. 2014;32:919–926. doi: 10.1200/JCO.2013.52.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurian AW, Lichtensztajn DY, Keegan TH, Nelson DO, Clarke CA, Gomez SL. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998-2011. JAMA. 2014;312:902–914. doi: 10.1001/jama.2014.10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurian AW, Mitani A, Desai M, et al. Breast cancer treatment across health care systems: linking electronic medical records and state registry data to enable outcomes research. Cancer. 2014;120:103–111. doi: 10.1002/cncr.28395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albornoz CR, Cordeiro PG, Mehrara BJ, et al. Economic implications of recent trends in US . immediate autologous breast reconstruction. Plast Reconstr Surg. 2014;133:463–470. doi: 10.1097/PRS.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 17.Yueh JH, Slavin SA, Adesiyun T, et al. Patient satisfaction in postmastectomy breast reconstruction: a comparative evaluation of DIEP, TRAM, latissimus flap, and implant techniques. Plast Reconstr Surg. 2010;125:1585–1595. doi: 10.1097/PRS.0b013e3181cb6351. [DOI] [PubMed] [Google Scholar]
- 18.Scott AM, Mehrara BJ, Pusic AL, Matros E, McCarthy CM, Disa JJ. Patient-reported satisfaction and health related-quality of life in patients converting from prosthetic to autologous breast reconstruction. Plast Reconstr Surg. 2014;134 [Google Scholar]
- 19.Albornoz CR, Matros E, McCarthy CM, et al. Implant breast reconstruction and radiation: a multicenter analysis of long-term health-related quality of life and satisfaction. Ann Surg Oncol. 2014;21:2159–2164. doi: 10.1245/s10434-014-3483-2. [DOI] [PubMed] [Google Scholar]
- 20.Reimer T, Hartmann S, Stachs A, Gerber B. Local treatment of the axilla in early breast cancer: concepts from the national surgical adjuvant breast and bowel project B-04 to the planned intergroup sentinel mamma trial. Breast Care (Basel) 2014;9:87–95. doi: 10.1159/000360411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry T, Brooks S, Sydow N, et al. Complication rates of radiation on tissue expander and autologous tissue breast reconstruction. Ann Surg Oncol. 2010;17(Suppl 3):202–210. doi: 10.1245/s10434-010-1261-3. [DOI] [PubMed] [Google Scholar]
- 22.Berbers J, van Baardwijk A, Houben R, et al. Reconstruction: before or after postmastectomy radiotherapy?’ A systematic review of the literature. Eur J Cancer. 2014;50:2752–2762. doi: 10.1016/j.ejca.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Momoh AO, Ahmed R, Kelley BP, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol. 2014;21:118–124. doi: 10.1245/s10434-013-3284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ASPS (American Society of Plastic Surgeons) Evidence-based clinicalpractice guideline: Breast reconstruction with expanders and implants. www.plasticsurgery.org/reconstructive-procedures/breast-reconstruction.html; 2013. (last accessed on 1 July 2015)
- 25.Ho AL, Bovill ES, Macadam SA, Tyldesley S, Giang J, Lennox PA. Postmastectomy radiation therapy after immediate two-stage tissue expander/implant breast reconstruction: a University of British Columbia perspective. Plast Reconstr Surg. 2014;134:1e–10e. doi: 10.1097/PRS.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 26.Cordeiro PG, Albornoz CR, McCormick B, Hu Q, van Zee K. The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: An analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plast Reconstr Surg. 2014;134:588–595. doi: 10.1097/PRS.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 27.Lam TC, Hsieh F, Boyages J. The effects of postmastectomy adjuvant radiotherapy on immediate two-stage prosthetic breast reconstruction: a systematic review. Plast Reconstr Surg. 2013;132:511–518. doi: 10.1097/PRS.0b013e31829acc41. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson M, Anveden L, Celebioglu F, et al. Radiotherapy in implant-based immediate breast reconstruction: risk factors, surgical outcomes, and patient-reported outcome measures in a large Swedish multicenter cohort. Breast Cancer Res Treat. 2013;142:591–601. doi: 10.1007/s10549-013-2770-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhong T, McCarthy CM, Price AN, Pusic AL. Evidence-based medicine: breast reconstruction. Plast Reconstr Surg. 2013;132:1658–1669. doi: 10.1097/PRS.0b013e3182a80836. [DOI] [PubMed] [Google Scholar]
- 30.Fischer JP, Wes AM, Tuggle CT, 3rd, Serletti JM, Wu LC. Risk analysis of early implant loss after immediate breast reconstruction: a review of 14,585 patients. J Am Coll Surg. 2013;217:983–990. doi: 10.1016/j.jamcollsurg.2013.07.389. [DOI] [PubMed] [Google Scholar]
- 31.Fischer JP, Nelson JA, Serletti JM, Wu LC. Peri-operative risk factors associated with early tissue expander (TE) loss following immediate breast reconstruction (IBR): a review of 9305 patients from the 2005-2010 ACS-NSQIP datasets. J Plast Reconstr Aesthet Surg. 2013;66:1504–1512. doi: 10.1016/j.bjps.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 32.Chang EI, Chang EI, Soto-Miranda MA, et al. Comprehensive evaluation of risk factors and management of impending flap loss in 2138 breast free flaps. Ann Plast Surg. 2014 doi: 10.1097/SAP.0000000000000263. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Qin C, Vaca E, Lovecchio F, Ver Halen JP, Hansen NM, Kim JY. Differential impact of non-insulin-dependent diabetes mellitus and insulin-dependent diabetes mellitus on breast reconstruction outcomes. Breast Cancer Res Treat. 2014;146:429–438. doi: 10.1007/s10549-014-3024-5. [DOI] [PubMed] [Google Scholar]
- 34.Stojkovic CA, Smeulders MJ, van der Horst CM, Khan SM. Wound drainage after plastic and reconstructive surgery of the breast. Cochrane Database Syst Rev. 2013;3 doi: 10.1002/14651858.CD007258.pub2. Cd007258. [DOI] [PubMed] [Google Scholar]
- 35.Phillips BT, Bishawi M, Dagum AB, Khan SU, Bui DT. A systematic review of antibiotic use and infection in breast reconstruction: what is the evidence? Plast Reconstr Surg. 2013;131:1–13. doi: 10.1097/PRS.0b013e3182729c39. [DOI] [PubMed] [Google Scholar]
- 36.Brahmbhatt RD, Huebner M, Scow JS, et al. National practice patterns in preoperative and postoperative antibiotic prophylaxis in breast procedures requiring drains: survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2012;19:3205–3211. doi: 10.1245/s10434-012-2477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerber B, von Minckwitz G, Eidtmann H, et al. Surgical outcome after neoadjuvant chemotherapy and bevacizumab: results from the GeparQuinto study (GBG 44) Ann Surg Oncol. 2014;21:2517–2524. doi: 10.1245/s10434-014-3606-9. [DOI] [PubMed] [Google Scholar]
- 38.Kansal KJ, Dominici LS, Tolaney SM, et al. Neoadjuvant bevacizumab: surgical complications of mastectomy with and without reconstruction. Breast Cancer Res Treat. 2013;141:255–259. doi: 10.1007/s10549-013-2682-z. [DOI] [PubMed] [Google Scholar]
- 39.Weichman KE, Wilson SC, Weinstein AL, et al. The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:1049–1058. doi: 10.1097/PRS.0b013e31824a2acb. [DOI] [PubMed] [Google Scholar]
- 40.Valdatta L, Cattaneo AG. Acellular dermal matrices and radiotherapy in breast reconstruction: a systematic review and meta-analysis of the literature. Plast Surg Int. 2014 doi: 10.1155/2014/472604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Robert-Koch-Institut. Evaluation of cancer incidence in Germany (2007-2010) www.rki.de/DE/Home/homepage_node.html. (last accessed on 2 January 2015)
- e2.Westdeutsches Brust-Centrum. www.doc-holding.de/2014. last accessed on 2 January 2015.
- e3.Deutsche Krebshilfe Brustkrebs. www.krebshilfe.de/wir-informieren/fuer-aerzte/betriebsaerzte/materialien/gesundheitsfoerderung-brustkrebs.html?L=0. 2015. last accessed on 2 January 2015.
- e4.Deutsche Gesellschaft für Senologie. 2012. Interdisziplinäre S3-Leitlinie für die Diagnostik, Therapie und Nachsorge des Mammakarzinoms. [Google Scholar]
- e5.AGO. Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer. http://www.ago-online.de/en/guidelines-mamma/march-2015/ (last accessed on 2 January 2015)
- e6.NNCC. Practice guidelines in oncology: breast cancer. ww.nccn.org/professionals/physician_gls/f_guidelines.asp#site. (last accessed on 2 January 2015)
- e7.Cochrane Libary. http://onlinelibrary.wiley.com/cochranelibrary/search. (last accessed on 2 January 2015)
- e8.Oxford Center for Evidence based Medicine. Levels of evidence and grades of recommendation. www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ 2014. (last accessed on 2 January 2015)
- e9.Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124:345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- e10.eD’Souza N, Darmanin G, Fedorowicz Z. Immediate versus delayed reconstruction following surgery for breast cancer. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD008674.pub2. Cd008674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Habermann EB, Thomsen KM, Hieken TJ, Boughey JC. Impact of availability of immediate breast reconstruction on bilateral mastectomy rates for breast cancer across the United States: data from the nationwide inpatient sample. Ann Surg Oncol. 2014;21:3290–3296. doi: 10.1245/s10434-014-3924-y. [DOI] [PubMed] [Google Scholar]
- e12.Hershman DL, Richards CA, Kalinsky K, et al. Influence of health insurance, hospital factors and physician volume on receipt of immediate post-mastectomy reconstruction in women with invasive and non-invasive breast cancer. Breast Cancer Res Treat. 2012;136:535–545. doi: 10.1007/s10549-012-2273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e13.Tsoutsou PG, Koukourakis MI, Azria D, Belkacemi Y. Optimal timing for adjuvant radiation therapy in breast cancer: a comprehensive review and perspectives. Crit Rev Oncol Hematol. 2009;71:102–116. doi: 10.1016/j.critrevonc.2008.09.002. [DOI] [PubMed] [Google Scholar]
- e14.Yu KD, Huang S, Zhang JX, Liu GY, Shao ZM. Association between delayed initiation of adjuvant CMF or anthracycline-based chemotherapy and survival in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2013;13 doi: 10.1186/1471-2407-13-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e15.Barry PN, Riley EC, Pan J, et al. Delay of adjuvant chemotherapy after elective mastectomy and immediate reconstruction in breast-conservation candidates: a matched-pair analysis. Am J Clin Oncol. 2014;37:575–579. doi: 10.1097/COC.0b013e318280d79f. [DOI] [PubMed] [Google Scholar]
- e16.Jagsi R, Jiang J, Momoh AO, et al. Complications after mastectomy and immediate breast reconstruction for breast cancer: A claims-based analysis. Ann Surg. 2015 doi: 10.1097/SLA.0000000000001177. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e17.Gurunluoglu R, Gurunluoglu A, Williams SA, Tebockhorst S. Current trends in breast reconstruction: survey of American Society of Plastic Surgeons 2010. Ann Plast Surg. 2013;70:103–110. doi: 10.1097/SAP.0b013e31822ed5ce. [DOI] [PubMed] [Google Scholar]
- e18.Sando IC, Chung KC, Kidwell KM, Kozlow JH, Malay S, Momoh AO. Comprehensive breast reconstruction in an academic surgical practice: an evaluation of the financial impact. Plast Reconstr Surg. 2014;134:1131–1139. doi: 10.1097/PRS.0000000000000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e19.Fischer JP, Wes AM, Nelson JA, et al. Propensity-matched, longitudinal outcomes analysis of complications and cost: comparing abdominal free flaps and implant-based breast reconstruction. J Am Coll Surg. 2014;219:303–312. doi: 10.1016/j.jamcollsurg.2014.02.028. [DOI] [PubMed] [Google Scholar]
- e20.Alderman AK, Kuhn LE, Lowery JC, Wilkins EG. Does patient satisfaction with breast reconstruction change over time? Two-year results of the Michigan Breast Reconstruction Outcomes Study. J Am Coll Surg. 2007;204:7–12. doi: 10.1016/j.jamcollsurg.2006.09.022. [DOI] [PubMed] [Google Scholar]
- e21.McCarthy CM, Mehrara BJ, Long T, et al. Chest and upper body morbidity following immediate postmastectomy breast reconstruction. Ann Surg Oncol. 2014;21:107–112. doi: 10.1245/s10434-013-3231-z. [DOI] [PubMed] [Google Scholar]
- e22.Atisha DM, Rushing CN, Samsa GP, et al. A national snapshot of satisfaction with breast cancer procedures. Ann Surg Oncol. 2015;22:361–369. doi: 10.1245/s10434-014-4246-9. [DOI] [PubMed] [Google Scholar]
- e23.Ho AL, Klassen AF, Cano S, Scott AM, Pusic AL. Optimizing patient-centered care in breast reconstruction: the importance of preoperative information and patient-physician communication. Plast Reconstr Surg. 2013;132:212e–220e. doi: 10.1097/PRS.0b013e31829586fa. [DOI] [PubMed] [Google Scholar]
- e24.Ashraf AA, Colakoglu S, Nguyen JT, et al. Patient involvement in the decision-making process improves satisfaction and quality of life in postmastectomy breast reconstruction. J Surg Res. 2013;184:665–670. doi: 10.1016/j.jss.2013.04.057. [DOI] [PubMed] [Google Scholar]
- e25.Koslow S, Pharmer LA, Scott AM, et al. Long-term patient-reported satisfaction after contralateral prophylactic mastectomy and implant reconstruction. Ann Surg Oncol. 2013;20:3422–3429. doi: 10.1245/s10434-013-3026-2. [DOI] [PubMed] [Google Scholar]
- e26.Macadam SA, Ho AL, Cook EF, Jr., Lennox PA, Pusic AL. Patient satisfaction and health-related quality of life following breast reconstruction: patient-reported outcomes among saline and silicone implant recipients. Plast Reconstr Surg. 2010;125:761–771. doi: 10.1097/PRS.0b013e3181cb5cf8. [DOI] [PubMed] [Google Scholar]
- e27.Macadam SA, Ho AL, Lennox PA, Pusic AL. Patient-reported satisfaction and health-related quality of life following breast reconstruction: a comparison of shaped cohesive gel and round cohesive gel implant recipients. Plast Reconstr Surg. 2013;131:431–441. doi: 10.1097/PRS.0b013e31827c6d55. [DOI] [PubMed] [Google Scholar]
- e28.Chattopadhyay D, Gupta S, Jash PK, Murmu MB, Gupta S. Skin sparing mastectomy with preservation of nipple areola complex and immediate breast reconstruction in patients with breast cancer: a single centre prospective study. Plastic Surgery International. 2014;2014 doi: 10.1155/2014/589068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e29.Albornoz CR, Matros E, McCarthy CM, et al. Implant breast reconstruction and radiation: a multicenter analysis of long-term health-related quality of life and satisfaction. Ann Surg Oncol. 2014;21:2159–2164. doi: 10.1245/s10434-014-3483-2. [DOI] [PubMed] [Google Scholar]
- e30.Susarla SM, Ganske I, Helliwell L, Morris D, Eriksson E, Chun YS. Comparison of clinical outcomes and patient satisfaction in immediate single-stage versus two-stage implant-based breast reconstruction. Plast Reconstr Surg. 2015;135:1e–8e. doi: 10.1097/PRS.0000000000000803. [DOI] [PubMed] [Google Scholar]
- e31.Adesiyun TA, Lee BT, Yueh JH, et al. Impact of sequencing of postmastectomy radiotherapy and breast reconstruction on timing and rate of complications and patient satisfaction. Int J Radiat Oncol Biol Phys. 2011;80:392–397. doi: 10.1016/j.ijrobp.2010.02.039. [DOI] [PubMed] [Google Scholar]
- e32.McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e33.Selz J, Le Scodan R, Menard J, Hennequin C, Quero L. [Indication of radiotherapy after neoadjuvant chemotherapy in breast cancer] Cancer Radiother. 2014;18:229 LP>234. doi: 10.1016/j.canrad.2013.12.009. [DOI] [PubMed] [Google Scholar]
- e34.Francis SH, Ruberg RL, Stevenson KB, et al. Independent risk factors for infection in tissue expander breast reconstruction. Plast Reconstr Surg. 2009;124:1790–1796. doi: 10.1097/PRS.0b013e3181bf80aa. [DOI] [PubMed] [Google Scholar]
- e35.Colwell AS, Damjanovic B, Zahedi B, Medford-Davis L, Hertl C, Austen WG., Jr. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg. 2011;128:1170–1178. doi: 10.1097/PRS.0b013e318230c2f6. [DOI] [PubMed] [Google Scholar]
- e36.Cordeiro PG, McCarthy CM. A single surgeon’s 12-year experience with tissue expander/implant breast reconstruction: part II. An analysis of long-term complications, aesthetic outcomes, and patient satisfaction. Plast Reconstr Surg. 2006;118:832–839. doi: 10.1097/01.prs.0000232397.14818.0e. [DOI] [PubMed] [Google Scholar]
- e37.Chang DW, Barnea Y, Robb GL. Effects of an autologous flap combined with an implant for breast reconstruction: an evaluation of 1000 consecutive reconstructions of previously irradiated breasts. Plast Reconstr Surg. 2008;122:356–362. doi: 10.1097/PRS.0b013e31817d6303. [DOI] [PubMed] [Google Scholar]
- e38.Drucker-Zertuche M, Bargallo-Rocha E, Zamora-Del RR. Radiotherapy and immediate expander/implant breast reconstruction: should reconstruction be delayed? Breast J. 2011;17:365–370. doi: 10.1111/j.1524-4741.2011.01090.x. [DOI] [PubMed] [Google Scholar]
- e39.Nava MB, Pennati AE, Lozza L, Spano A, Zambetti M, Catanuto G. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg. 2011;128:353–359. doi: 10.1097/PRS.0b013e31821e6c10. [DOI] [PubMed] [Google Scholar]
- e40.Peled AW, Foster RD, Esserman LJ, Park CC, Hwang ES, Fowble B. Increasing the time to expander-implant exchange after postmastectomy radiation therapy reduces expander-implant failure. Plast Reconstr Surg. 2012;130:503–509. doi: 10.1097/PRS.0b013e31825dbf15. [DOI] [PubMed] [Google Scholar]
- e41.Momoh AO, Colakoglu S, de Blacam C, Gautam S, Tobias AM, Lee BT. Delayed autologous breast reconstruction after postmastectomy radiation therapy: is there an optimal time? Ann Plast Surg. 2012;69:14–18. doi: 10.1097/SAP.0b013e31821ee4b6. [DOI] [PubMed] [Google Scholar]
- e42.Pluvy I, Panouilleres M, Garrido I, et al. Smoking and plastic surgery, part II. Clinical implications. A systematic review with meta-analysis. Ann Chir Plast Esthet. 2015;60:e15–e49. doi: 10.1016/j.anplas.2014.09.011. [DOI] [PubMed] [Google Scholar]
- e43.Hirsch EM, Seth AK, Kim JY, et al. Analysis of risk factors for complications in expander/implant breast reconstruction by stage of reconstruction. Plast Reconstr Surg. 2014;134:692e–699e. doi: 10.1097/PRS.0000000000000607. [DOI] [PubMed] [Google Scholar]
- e44.Duggal CS, Grudziak J, Metcalfe DB, Carlson GW, Losken A. The effects of breast size in unilateral postmastectomy breast reconstruction. Ann Plast Surg. 2013;70:506–512. doi: 10.1097/SAP.0b013e318263f1f8. [DOI] [PubMed] [Google Scholar]
- e45.Fischer JP, Nelson JA, Au A, Tuggle CT, 3rd, Serletti JM, Wu LC. Complications and morbidity following breast reconstruction—a review of 16,063 cases from the 2005-2010 NSQIP datasets. J Plast Surg Hand Surg. 2014;48:104–114. doi: 10.3109/2000656X.2013.819003. [DOI] [PubMed] [Google Scholar]
- e46.Hanwright PJ, Davila AA, Mioton LM, Fine NA, Bilimoria KY, Kim JY. A predictive model of risk and outcomes in tissue expander reconstruction: a multivariate analysis of 9786 patients. J Plast Surg Hand Surg. 2013;47:513–518. doi: 10.3109/2000656X.2013.789436. [DOI] [PubMed] [Google Scholar]
- e47.Zhong T, Novak CB, Bagher S, et al. Using propensity score analysis to compare major complications between DIEP and free muscle-sparing TRAM flap breast reconstructions. Plast Reconstr Surg. 2014;133:774–782. doi: 10.1097/PRS.0000000000000024. [DOI] [PubMed] [Google Scholar]
- e48.Reish RG, Damjanovic B, Austen WG, Jr., et al. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plast Reconstr Surg. 2013;131:1223–1230. doi: 10.1097/PRS.0b013e31828bd377. [DOI] [PubMed] [Google Scholar]
- e49.Turner EJ, Benson JR, Winters ZE. Techniques in the prevention and management of seromas after breast surgery. Future Oncol. 2014;10:1049–1063. doi: 10.2217/fon.13.257. [DOI] [PubMed] [Google Scholar]
- e50.Liu DZ, Dubbins JA, Louie O, Said HK, Neligan PC, Mathes DW. Duration of antibiotics after microsurgical breast reconstruction does not change surgical infection rate. Plast Reconstr Surg. 2012;129:362–367. doi: 10.1097/PRS.0b013e31823ae8ce. [DOI] [PubMed] [Google Scholar]
- e51.Avashia YJ, Mohan R, Berhane C, Oeltjen JC. Postoperative antibiotic prophylaxis for implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;131:453–461. doi: 10.1097/PRS.0b013e31827c6d90. [DOI] [PubMed] [Google Scholar]
- e52.Schaverien MV, Munnoch DA. Effect of neoadjuvant chemotherapy on outcomes of immediate free autologous breast reconstruction. Eur J Surg Oncol. 2013;39:430–436. doi: 10.1016/j.ejso.2013.02.015. [DOI] [PubMed] [Google Scholar]
- e53.Kansal KJ, Dominici LS, Tolaney SM, et al. Neoadjuvant bevacizumab: surgical complications of mastectomy with and without reconstruction. Breast Cancer Res Treat. 2013;141:255–259. doi: 10.1007/s10549-013-2682-z. [DOI] [PubMed] [Google Scholar]
- e54.Golshan M, Garber JE, Gelman R, et al. Does neoadjuvant bevacizumab increase surgical complications in breast surgery? Ann Surg Oncol. 2011;18:733–737. doi: 10.1245/s10434-010-1366-8. [DOI] [PubMed] [Google Scholar]
- e55.Kelley BP, Valero V, Yi M, Kronowitz SJ. Tamoxifen increases the risk of microvascular flap complications in patients undergoing microvascular breast reconstruction. Plast Reconstr Surg. 2012;129:305–314. doi: 10.1097/PRS.0b013e31823ae86c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e56.Hill JL, Wong L, Kemper P, Buseman J, Davenport DL, Vasconez HC. Infectious complications associated with the use of acellular dermal matrix in implant-based bilateral breast reconstruction. Ann Plast Surg. 2012;68:432–434. doi: 10.1097/SAP.0b013e31823b6ac6. [DOI] [PubMed] [Google Scholar]
- e57.Tessler O, Reish RG, Maman DY, Smith BL, Austen WG., Jr. Beyond biologics: absorbable mesh as a low-cost, low-complication sling for implant-based breast reconstruction. Plast Reconstr Surg. 2014;133:90e–99e. doi: 10.1097/01.prs.0000437253.55457.63. [DOI] [PubMed] [Google Scholar]
- e58.Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg. 2010;125:1606–1614. doi: 10.1097/PRS.0b013e3181d4fb2a. [DOI] [PubMed] [Google Scholar]
- e59.Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg. 2010;64:674–678. doi: 10.1097/SAP.0b013e3181dba892. [DOI] [PubMed] [Google Scholar]
- e60.Pannucci CJ, Antony AK, Wilkins EG. The impact of acellular dermal matrix on tissue expander/implant loss in breast reconstruction: an analysis of the tracking outcomes and operations in plastic surgery database. Plast Reconstr Surg. 2013;132:1–10. doi: 10.1097/PRS.0b013e318290f917. [DOI] [PubMed] [Google Scholar]
- e61.Clemens MW, Kronowitz SJ. Acellular dermal matrix in irradiated tissue expander/implant-based breast reconstruction: evidence-based review. Plast Reconstr Surg. 2012;130:27s–34s. doi: 10.1097/PRS.0b013e318265f690. [DOI] [PubMed] [Google Scholar]