Abstract
Heart failure (HF) is the leading cause of hospitalization among older adults and the prevalence is growing with the aging populations in the Western countries. Epidemiologic reports suggest that approximately 50% of patients who have signs or symptoms of HF have preserved left ventricular ejection fraction. This HF type predominantly affects women and the elderly with other co-morbidities, such as diabetes, hypertension, and overt volume status. Most of the current treatment strategies are based on morbidity benefits such as quality of life and reduction of clinical HF symptoms. Treatment of patients with HF with preserved ejection fraction displayed disappointing results from several large randomized controlled trials. The heterogeneity of HF with preserved ejection fraction, understood as complex syndrome, seems to be one of the primary reasons. Here, we present an overview of the current management strategies with available evidence and new therapeutic approach from drugs currently in clinical trials, which target diastolic dysfunction, chronotropic incompetence, and risk factor management. We provide an outline and interpretation of recent clinical trials that failed to improve outcome and survival in patients with HF with preserved ejection fraction.
Keywords: Diastolic dysfunction, Preserved ejection fraction, Co-morbidities, Clinical trials
Core tip: Heart failure (HF) has preserved left ventricular ejection fraction (HFpEF) accounts for approximately 50% of all patients diagnosed with HF, with similary poor outcomes. To date, only the prevention of HFpEF by treating the cardiovascular risk factors (coronary artery disease, atrial fibrillation, hypertension, diabetes, and obesity) has been shown to be efficient. This observation suggests that investigators in future trials should specify the indication of hospitalization for HF and may request to verify the details of patients’ admissions. We provide an outline and interpretation of recent clinical trials that failed to improve outcome and survival in patients with HF with preserved ejection fraction.
INTRODUCTION
Prevalence of heart failure (HF) has been rising in the recent past[1,2]. Epidemiologic reports suggest that approximately 50% of patients who have signs or symptoms of HF have preserved left ventricular ejection fraction (HFpEF)[3-5]. It has been observed that the morbidity and the mortality rates of HFpEF patients are significantly increased when compared to the reference population[3,6]. Moreover, it appears that the all-cause mortality of patients with HFpEF is comparable to patients with HF with reduced ejection fraction (HFrEF).
Patients with HFpEF are older, more likely women, and more often have hypertension[7,8]. Chronic hypertension is the most common cause in addition to age, with suggestion to 60% of patients suffering from HFpEF being hypertensive[7]. Diabetes and obesity also contribute independently to the development of diastolic and vascular dysfunction[9], both being important in the HFpEF pathophysiology. Most of the common treatment of HFpEF is based on morbidity benefits and reduction of clinical HF symptoms. Several co-morbidities are important drivers of the clinical outcome in the HFpEF population. Excluding patients with co-morbidities from clinical trials to enhance the specificity reduces clinical event rate and entails loss of statistical power to detect differences.
Current guidelines recommend the management of treating hypertension, heart rate reduction, volume status, and prevention of myocardial ischemia[10]. However, current intervention strategies available for HFrEF have not been supported by clinical trials for HFpEF[11,12].
Here, we present an overview of the current recommended therapeutic options with available evidence and new therapeutic approaches from drugs currently in clinical trials, which aim at impaired diastolic function, chronotropic incompetence, and risk factor management. We provide an outline and interpretation of previous clinical trials that failed to improve outcome and survival in the HFpEF population.
BETA-BLOCKERS
Study of effects of Nebivolol Intervention on outcomes and Rehospitalisation in Seniors with HF trial (SENIORS). The mechanism behind β-blockers’ therapeutic potential in enhancement diastolic function in patients with HFpEF is believed to be associated with negative chronotropic and inotropic properties in stabilizing heart rate and optimizing left ventricular (LV) relaxation[13].
The SENIORS trial (Study of effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with HF) enrolled 2128 patients aged greater than 75 years who had either an LVEF less than 35% or a hospitalization for HF in the previous year and randomly assigned them to placebo or nebivolol. In the SENIORS trial 752 patients displayed a preserved LVEF (mean 49.2%).
The SENIORS trial indicated that nebivolol significantly reduced the composite outcome of death and cardiovascular hospitalization. In detail, the SENIORS trial demonstrated a 15% reduction in the relative risk of the composite of all-cause mortality of cardiovascular admission in patients older than 70 years of age with history of congestive HF[14]. The investigators consumed two primary aims distinct from previous trials on β-blockers. First, was to demonstrate the safety and efficacy of nebivolol in elderly HF patients, a group that has been under-represented in previous clinical studies. Secondly, another goal, of this trial was to demonstrate nebivolol’s safety and efficiency across a broad range of LVEF, including the HFpEF population.
Conversely, in the SENIORS trial there was no difference in the primary outcome when patients were stratified according to preserved or reduced LVEF using a cut-off of > 35% to define preserved EF[14]. Subsequent analyses suggested no strong interaction between the therapeutic benefit of nebivolol and LVEF above or below 35%, but this does not entirely allay concerns that there might be no benefit in those with an LVEF greater than 45%.
Besides, patients with atrial fibrillation, a common co-morbidity of both HFrEF and HFpEF, do not appear to benefit whether or not LVEF is reduced[15]. In addition, it has to be mentioned that more than half of the patients, included in the SENIORS trial, had LVEF values ranging between 35%-50% and therefore would not be considered to have HFpEF.
However, in a separate analysis of patients with an LVEF cut-off greater than 40%, there was no statistical interaction, suggesting that nebivolol was of comparable benefit in reduced LVEF and preserved LVEF patients. The definition of HFpEF used a low cut-off LVEF of greater than 35% therefore making it difficult to extrapolate these findings to most patients with HFpEF who have a higher LVEF.
Furthermore, the SENIORS echocardiography substudy randomized 112 patients in 29 european centres, of whom 104 were evaluable for the study; 43 with LVEF ≤ 35% and 61 with an LVEF > 35%[16]. LV end-systolic volume (ESV), LVEF, mitral valve E/A ratio, and E-wave deceleration time were assessed at baseline and after 12 mo.
In the group with LVEF ≤ 35%, nebivolol reduced ESV and improved EF; no changes were observed in the E/A ratio or E-wave deceleration time. In LVEF > 35% group, no significant changes in either systolic or diastolic parameters were observed. This absence of detectable differences with standard echocardiography in patients with predominant diastolic dysfunction questions the mechanism of benefit on morbidity and/or mortality in this HF population. In the separate analysis of patients with an EF cut-off greater than 40%, there was no noted statistical interaction, suggesting that nebivolol was of comparable benefit in reduced EF and preserved EF patients.
Swedish HF registry
Lund et al[17] from the Karolinska Institute, Stockholm in Sweden, conducted a study to examine whether β-blocker therapy is associated with reduced mortality in patients with HFpEF.
The investigators used data from the Swedish HF Registry, which includes 67 hospitals with inpatient and outpatient units and 95 outpatient primary care clinics in Sweden. The analysis included 41976 patients, 19083 patients with HFpEF[17]. Of these, 8244 were matched 2:1 based on age and β-blocker use, yielding 5496 treated and 2748 untreated patients with HFpEF. Another analysis involved 22893 patients with HFrEF, of whom 6081 were matched, yielding 4054 treated with β-blockers and 2027 untreated patients.
In patients with HFpEF, use of β-blocker therapy was associated with lower all-cause mortality but not with lower combined all-cause mortality or HF hospitalization. In detail, in the matched HFrEF cohort, β-blockers were associated with reduced mortality (HR = 0.89; 95%CI: 0.82-0.97; P = 0.005) and also with reduced combined mortality or HF hospitalization (HR = 0.89; 95%CI: 0.84-0.95; P = 0.001).
This study provides a rationale for performing large-scale randomized trials with this inexpensive category of drugs.
However, because myocardial ischemia can drive the development of HFpEF, its presence should be detected and treated with anti-ischemic therapies, which still include β-blockers. Patients with evidence of myocardial ischemia could also be considered for revascularization with percutaneous coronary intervention or coronary artery bypass surgery.
However, current guidelines do not recommend the use of β-blockers solely for HFpEF, unless it is used to optimize treatment of comorbidity, such as controlling ventricular rate in atrial fibrillation or tachyarrhythmia, or hypertension.
Since cardiac output is the product of heart rate and stroke volume, patients with HFpEF are often dependent on augmentation of heart rate in order to increase cardiac output.
Negative chronotropic medications are recommended in HFpEF to increase the diastolic filling period, but slowing the heart rate in the absent of tachycardia tends to only prolong diastasis, where transmitral flow is minimal or absence[18]. More importantly, recent studies have repeatedly shown that chronotropic incompetence is highly prevalent and associated with exercise disability in HFpEF[19-21]. Indeed, in the setting of reduced systolic and diastolic reserve, chronotropic reserve may represent the only mechanism to augment cardiac output during exercise, although there is concern that inadequate ability to enhance relaxation with tachycardia may limit stroke volume responses. β-blockers, especially at high doses may aggravate rather than alleviate exercise intolerance.
However, slowing elevated heart rate can prolong LV filling time in abnormally stiff LV and also prolong coronary perfusion. As a result, we recommend the careful use of β-blockade to optimize chronotropic incompetence (induced by atrial fibrillation or tachyarrhythmia) by stabilizing heart rate and optimizing LV relaxation with regard to heart rate profile under basal and exercise conditions in patients with HFpEF. Moreover, additional benefical effects of β-blockers have to be reconsidered. In detail, nebivolol itself would possible confer additional effects due to the NO enhancing action of the drug. This action of nebivolol is exerted via a signaling pathway starting from the activation of β3-adrenergic receptors and leading to overexpression of inducible NO synthase. Cardiac NO production by nebivolol could participate in the cardiovascular effects of nebivolol treatment in patients affected by hypertension and HF.
Adequate prospective trial data regarding the effects of β-blockers in HFpEF are not currently available. In this regard it is interesting to know that Pieske et al (Charité - Berlin, Germany) are planning an additional large multicenter trial with about 2300 participants with preserved LVEF in order to investigate the effects of β-blockers treatment starting in 2015.
ANGIOTENSIN-CONVERTING ENZYME INHIBITORS AND ANGIOTENSIN RECEPTOR BLOCKERS
Perindopril in elderly people with chronic HF trial
The theoretical benefits of Angiotensin-converting enzyme inhibitors (ACEi) in HFpEF rest on pathophysiological basis that angiotensin II contributes to myocardial hypertrophy and adverse cardiac fibrosis. To date, only one substantial trial of ACEi has been conducted in the HFpEF population, the perindopril in elderly people with chronic HF (PEP-CHF). The PEP-CHF Trial included 850 patients, older than 70 years of age with HFpEF (LVEF > 45%) with echocardiographic evidence of diastolic dysfunction[22]. The primary endpoint of the trial was a composite of all-cause mortality or unplanned HF related hospitalization. A significant reduction in HF hospitalization rate was observed in posthoc analysis of the results at 1 year, when cross over rates to open label ACEi were used. However, early beneficial effects of perindopril treatment were lost by the end of the trial.
A major limitation of the trial was the high rate of discontinuation at 18 mo (62%), the majority of whom went on open-label ACEi (about 90%). In addition, the event rate in the trial was lower than expected, further reducing the power of the trial. Perindopril appeared favorable at 1-year follow-up when the large majority of patients were on study drug, although these data should not be considered definitive given the post-hoc nature of the analysis. Although the PEP-CHF trial also does not provide conclusive evidence that perindopril is of benefit in this population, the observed favourable trends on hospitalization and days in hospital for HF (early seen beneficial effects), combined with improvements in symptoms and functional capacity provide arguments for its use.
Effects of candesartan in patients with chronic HF and preserved left-ventricular ejection fraction: The CHARM-Preserved trial
In the effects of candesartan in patients with chronic HF and preserved left-ventricular ejection fraction: The CHARM-Preserved trial (CHARM-Preserved) trial, 3023 (mean age 67 years, 40% women) patients were randomly assigned to the angiotensin receptor blocker (ARB) candesartan or placebo and followed 37 mo[23]. Adequate patients were aged greater than 18 years, suffering from HF for more than 4 wk, were in NYHA class II-IV, had a history of hospital admission and had a greater LVEF than 40%. The primary outcome (cardiovascular death or HF admission) was neutral (P = 0.051), but only slightly short of the primary outcome. A possible explanation of this finding could be the rates of study-drug discontinuation due to adverse events or laboratory abnormalities, which were significantly higher in the candesartan group (17.8% vs 13.5%, P = 0.001). In detail, candesartan was discontinued in more patients due to hyperkalemia, worsening creatinine levels or hypotension. In an echocardiography substudy of CHARM-Preserved, only 44% had moderate or severe diastolic dysfunction, which conferred a 3-fold increased risk but it is not clear whether these patients obtained a greater benefit from candesartan. Overall, CHARM-Preserved results were related with reduced hospitalization with candesartan[23]. However, the LVEF cut-off value of 40% and a non-defined diastolic function identified the study population as not a true HFpEF population.
Irbesartan in patients with HF and preserved ejection fraction trial
The Irbesartan in patients with HF and preserved ejection fraction trial (I-Preserve), the largest trial in the HFpEF population so far, randomly assigned 4128 patients (mean age 72 years, 60% female) to irbesartan or placebo[24]. The observation period was about 49.5 mo (mean). All included patients were aged greater than 60 years, had symptoms of HF and had a greater LVEF than 40%. The primary outcome (death from any cause or hospitalization for cardiovascular cause) occurred 36% of patients in the irbesartan group and 37% in the placebo treated group[24]. There were no significant differences in the primary endpoints between the two groups. This trial also found no treatment benefit in any group and no significant difference in secondary endpoints such as CV death, HF death, exercise testing, NT-proBNP levels, and quality of life (Table 1).
Table 1.
Clinical trials in heart failure with preserved ejection fraction
| Acronym (yr) | Drug | Number of patients | Age (mean) | Percentage female (mean, %) | LVEF (mean, %) | Primary outcome | Follow up period |
| Swedish heart failure registry[17] | Beta-Blocker | 8244 | 78 | 45 | 40-49; > 50 | ACM, HFH | 24 mo |
| TOPCAT[33] | Aldactone | 3445 | 68.6 | 52 | 60.1 | CVD-HFH: NS | 27 mo |
| PARAMOUNT[51] | LCZ696 | 292 | 70.6 | 56 | 57.7 | Reductions in NT-proBNP levels | 36 wk |
| RELAX[43] | Sildenafil | 216 | 69 | 48 | 60 | EC-CS: NS | 24 wk |
| ALDO-DHF[27] | Spironolactone | 422 | 67 | 52 | 67 | Reduced E/É | 12 mo |
| I-Preserve[24] | Irbesartan | 4128 | 72 | 60 | 59.5 | D-CVH: NS | 49.5 mo |
| PEP-CHF[22] | Perindopril | 850 | 75 | 55.5 | 65 | D-HFH: NS | 26.2 mo |
| DIG[26] | Digoxin | 6800 | 63.8 | 22.7 | 28.6 | ACM: NS; improvements in DFWHF, HFWHF | 37 mo |
| SENIORS[14] | Nebivolol | 2128 | 76.1 | 38.4 | 36 | Improvements CVD, HFH | 21 mo |
| CHARM-Preserved[23] | Candesartan | 3023 | 67.1 | 40 | 54 | CVD-HFH: NS | 36.6 mo |
ALDO-DHF: Aldosterone Receptor Blockade in Diastolic Heart failure; CHARM-Preserved: Effects of candesartan in patients with chronic HF and preserved left-ventricular ejection fraction trial; DIG: The Effect of Digoxin on Mortality and Morbidity in Patients with HF trial; I-Preserve: The irbesartan in HF with preserved systolic function trial; PARADIGM: Angiotensin–Neprilysin Inhibition vs Enalapril in HF trial; PARAMOUNT: The angiotensin receptor neprilysin inhibitor LCZ696 in HF with preserved ejection fraction: a phase 2 double-blind randomised controlled trial; PEP-CHF: The perindopril in elderly people with chronic HF trial; RELAX: Phosphodiesterase-5 Inhibition in Diastolic HF: The RELAX Trial Rationale and Design; SENIORS: Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with HF trial; TOPCAT: Spironolactone for HF with Preserved Ejection Fraction trial; ACM: All-cause mortality; CS: Clinical status; CVA: Cardiovascular admission; CVD: Cardiovascular death; CVH: Cardiovascular hospilization; DFWHF: Death from worsening HF; EC: Exercise capacity; FHCVE: First hospitalization for a cardiovascular event; HF: Heart failure; HFAR: Hospitalization for any reason; HFH: Heart failure hospitalization; HFWHF: Hospitalization for worsening HF; NS: Not significant.
However, it is essential to mention that in this study a high percentage of patients were already receiving ACEi and spironolactone. The investigators speculated that the treatment of a large proportion of patients with multiple inhibitors of the RAS might have left reduced opportunity for further benefit from the addition of an angiotensin-receptor blocker. Furthermore, it seems to be possible that HFpEF does not appear to involve neurohormonal activation as a critical pathophysiologic mechanism in the same way that HFrEF does.
The rationale for using ACEi and ARBs in patients with HFpEF is blocking the neurohumoral signaling leading to HF progression and poor clinical outcomes. First, the CHARM-Preserved trial showed a significant reduction in hospitalization rate caused by HF, but failed to display a significant reduction in cardiovascular mortality. Moreover, in an echocardiography substudy of CHARM preserved, only 44% had moderate or severe diastolic dysfunction. Second, the I-Preserved trial failed to show a reduction in risk of the composite outcome, cardiovascular hospitalization and all-cause mortality. However, the not insignificant co-medication in this trial could be one reason for the neutral endpoints. Third, the PEP-CHF trial also failed to demonstrate a reduction in composite all-cause mortality and hospitalization caused by HF.
Also because of the neutral results of these three main outcome trials the current guidelines do not recommend the use of ACEi and ARBs for HFpEF. Nevertheless, when hypertension and other co-morbidities like LV hypertrophy and atherosclerotic vascular disease are involved ACEi and ARBs are first-line therapy and should also be given to patients with HFpEF. A possible mechanism for potential benefit of ACEi and ARBs could be afterload reduction and reduced and reduced wall tension, leading to improved diastolic function.
MINERALOCORTICOID RECEPTOR ANTAGONISTS
Randomized controlled aldosterone receptor blockade in diastolic HF trial
Series of RCTs[25,26] have shown that treatment with mineralocorticoid-receptor antagonists (MRAs) improved some properties of cardiac performance in patients suffering from HFpEF. The randomized controlled aldosterone receptor blockade in diastolic HF (ALDO-HF) trial displayed an improvement in ejection fraction, E/É relation, LV mass and LV end-diastolic volume[27]. However, these findings were not related with an enhancement in exercise capacity.
In the ALDO-HF trial, treatment with MRAs decreases renal function. Therefore, MRAs cannot be recommended based on the mentioned results. Physicians treating patients with MRAs should carefully monitor renal function and potassium levels. Whether the improved left ventricular function observed in the ALDO-HF trial is of clinical significance requires further investigation in larger HFpEF populations.
Treatment of preserved cardiac function HF with an aldosterone antagonist trial
The rationale to use MRAs for HFpEF therapy has been initially generated in experimental studies. These studies suggested that a blockade of the aldosterone-induced signaling may lead to anti-hypertrophic and anti-fibrotic effects[28]. Moreover, clinical trials EPHESUS and EMPHASIS-HF demonstrated significant reductions in risk of death from cardiovascular causes or first hospitalization for HF in patients after myocardial infarction and mild HF symptoms. However, in these trials solely patients with reduced LVEF were included.
MRAs such as spironolactone are highly effective in patients with HF accompanied with reduced LVEF[29-32].
In the treatment of preserved cardiac function HF with an aldosterone antagonist (TOPCAT) trial, patients with at least one symptom of HF were included if those patients had an ejection fraction greater than or equal to 45%[33].
Moreover, increased natriuretic peptide levels in the foregoing 60 d or a hospital admission in the previous year (with management of HF a major component of the care provided) were required, and these eligibility criteria were used for stratification of patients at randomization of this study[33]. Three thousand four hundred and forty-five patients undertook randomization in 6 different countries (United States, Argentina, Brazil, Canada, Russia and Georgia) to spironolactone or placebo.
Regarding a mean follow-up of 3.3 years (mean), the incidence rate of the primary composite outcome of death from cardiovascular causes, cardiac arrest, or hospitalization for HF was 5.9 events per 100 person-years in the spironolactone group and 6.6 events per 100 person-years in the placebo group.
Overall, the TOPCAT trial showed neutral results. There was a significant reduction in the secondary outcome of hospitalization for HF with spironolactone treatment.
Patients randomized to treatment with spironolactone had a fewer admission rate for HF, but an increased risk for renal dysfunction and hyperkalemia[34].
The majority of patients from Russia and Georgia were included in the hospitalization stratum (therefore no increased NT-proBNP was present) and thus were at lower cardiovascular risk, whereas patients from the United States were further balanced between the two mentioned strata. However, a post hoc analysis showed, that spironolactone treatment seemed to benefit patients in the United States but not those patients in Russia or Georgia. In detail, a total of 3445 subjects were recruited over a period of 4 years from 270 clinical centers in the United States (1151), Russia (1066), Georgia (612), Canada (326), Brazil (167) and Argentina (123), and were randomized on 1:1 basis to either spironolactone (target dose of 30 mg daily) or placebo. Patients with uncontrolled hypertension, those with infiltrative or hypertrophic cardiomyopathy and patients with elevated baseline serum potassium levels (> 5.0 mmol/L) were excluded. The overall event rate was low, with 3-year mortality being 10.2%. This is in sharp contrast with the previously reported annual mortality rates of 22%-29% in large community-based studies[35]. This concern is further intensified by a primary event rate (in the placebo group) of 8.4% in Russia and the Republic of Georgia: A rate which not only is unheard of in HF studies, but also one that is remarkably less than that observed in the “American” arm of the same study (31.8%).
It is remarkable that geographic differences in outcome have been a significant relevance in previous trials involving patients with HF. Possible factors in such geographic variation include differences in the clinical characteristics of the patient population, standards of care and methodological knowledge of clinical trials[34].
To conclude, TOPCAT was a neutral study. Spironolactone failed to reduce the primary outcome compared to placebo in patients with HFpEF. However, it did reduce the rate of HF hospitalizations. A signal of benefit was also seen in patients with elevated natriuretic peptides and in a geographical subset of patients. Based upon these findings, a mixed response from the medical community is expected: Some clinicians will not prescribe spironolactone for HFpEF patients, while others will continue using it especially in patients with elevated natriuretic peptides and/or in those with objective evidence of diastolic dysfunction. Finally, we prescribe spironolactone for HFpEF patients during carefully monitoring of renal function and serum potassium levels given the overall positive data from the Americas in TOPCAT.
DIGITALIS THERAPY
Digitalis investigation group ancillary trial
It has been shown that treatment with digoxin has beneficial effects on hospitalization in patients with HFrEF. Treatment with digoxin reduced the total number of hospitalizations. In the digitalis investigation group ancillary trial 988 patients suffering from chronic HF and ejection fraction greater than 45% were randomized to treatment with digoxin or placebo[36].
After 37 mo (mean follow-up), patients treated with digoxin or placebo had similar rates of the primary composite of hospitalization of HF or cardiovascular death[36]. However, an early benefit in patients with digoxin treatment was lost by the end of follow-up of the trial.
In ambulatory patients with chronic mild to moderate diastolic HF and normal sinus rhythm receiving angiotensin-converting enzyme inhibitor and diuretics, digoxin had no effect on natural history end points such as mortality and all-cause or cardiovascular hospitalizations[36].
To conclude, there is fragile evidence of digoxin in patients with HFpEF. Similar to β-blockers, guidelines do not recommend the use of digoxin solely for HFpEF, unless for treatment of co-morbidities, such as atrial fibrillation or tachyarrhythmia. However, common use of digoxin in the elderly HFpEF population with increased renal dysfunction seems not to be advisable.
INHIBITION OF THE LATE CURRENT OF THE CARDIAC ACTION POTENTIAL (LATE INA)
RAnoLazIne for the treatment of diastolic HF in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study
In a small, randomized (phase II) trial 18 patients were included who received ranolazine infusion followed by 2 wk of oral application[37]. It was shown by the investigators that left ventricular end-diastolic pressure and pulmonary capillary wedge pressure were reduced in patient with ranolazine treatment whereas in patients with placebo treatment there were no significant effects seen (clinicaltrails.gov NCT01163734). However, at the end of the trial no significant differences were observed by echocardiography and exercise capacity. In addition, a planned multi-center trial has been abandoned due to low recruitment. Finally, results of two ongoing studies are earliest expected in 2016.
PHOSPHODIESTERASE-5 INHIBITION
Sildenafil, a phosphodiesterase-5 (PDE-5) inhibitor is currently approved for treatment of pulmonary arterial hypertension (PAH)[38-40]. A small clinical trial observed improvements in pulmonary pressure, right ventricular (RV) function and LV relaxation after treatment with sildenafil in patients suffering from HFpEF. In a phase III ongoing trial the effect of sildenafil on patients suffering from HFpEF and PAH will be studied[41]. Moreover, sildenafil treatment led to an enhancement of systolic and diastolic LV function in a one-year randomized double-blind study placebo controlled study in patients suffering from stable HF and reduced ejection fraction[42].
PhosphdiesteRasE-5 inhibition to improve clinical status and exercise capacity in diastolic HF trial
Controversial findings have been oberserved from the PhosphdiesteRasE-5 inhibition to improve clinical status and EXercise Capacity in Diastolic Heart Failure (RELAX) trial[43] with HFpEF patients. Here, no significant improvement in diastolic function, exercise capacity and quality of life was observed.
In addition, in a multi-center study 216 patients with HFpEF and increased pulmonary artery pressures did not affect exercise capacity or clinical constitution over a time period of 24 wk[44]. Furthermore, longterm analyses of NT-proBNP and endothelin-1 displayed no significant changes between sildenafil and placebo treated groups. However, in a one-year single center trial of sildenafil in patients with HFpEF described significant improvements in with sildenafil treated patients when compared to placebo treated patients[41].
The lack of benefit of sildenafil treatment could be because the inclusion and exclusion criteria. In this trial, the included patients did not have pulmonary hypertension and suggested by highly increased NT-proBNP levels had advanced HF; this could explain the less-responding to sildenafil-treatment.
Furthermore, in a small clinical trial including 44 patients suffering from HFpEF (LVEF > 50%) and PAH inhibition by PDE-5 displayed a significant improvement in diastolic dysfunction, pulmonary pressures and right ventricular performance over an oberservation period of 12 mo[41]. Given the results, PDE-5 inhibition for HFpEF without proven increased PAP should not be used.
DEVICE THERAPY
No substantial clinical trials of implantable cardiac defibrillators or cardiac resynchronization therapy exist in the HFpEF population.
A large trial of cardiac resynchronization therapy (CRT) in patients suffering from an LVEF between 36% to 50% has been stopped due to poor outcome[45].
CRT is currently limited to those patients with LVEF < 35%, sinus rhythm, QRS > 150 ms, and left bundle branch block (LBBB) pattern. A retrospective analysis of the predictors of response to CRT has shown that CRT may offer a valuable option for these patients[46,47]. However, this finding has to be proven in a prospective, randomized multicenter trial. To date, CRT should not be used as matter of routine in patients with HFpEF. Furthermore, a current small clinical trial used a cardiovascular simulation to provide insights into the potential effects of an inter-atrial shunt on rest and exercise hemodynamics in patients suffering from HFpEF[48]. The principal finding of this study is that the inter-atrial shunt lowers left atrial (LA) pressure and that this effect is particularly pronounced during the marked increase in LA pressure and increased left-to-right atrial pressure gradient during exercise in this patient population.
However, the marked reduction in LA pressure (and pulmonary capillary pressure) could allow patients to exercise longer, potentially resulting in higher heart rates and higher values of cardiac output.
There exist currently two different devices in clinical development to create a device to make a precisely sized interatrial septal defect that will maintain patency for this purpose. Whether the findings of this theoretical simulation provide insights into patient selection criteria and the expected magnitude of hemodynamic improvement has to be proven in further clinical trials.
Possible optimizations of clinical trials for HFpEF in the future
For future clinical trials in HFpEF better matching of treatments for the precise type of HFpEF seems to be necessary (Figure 1).
Figure 1.
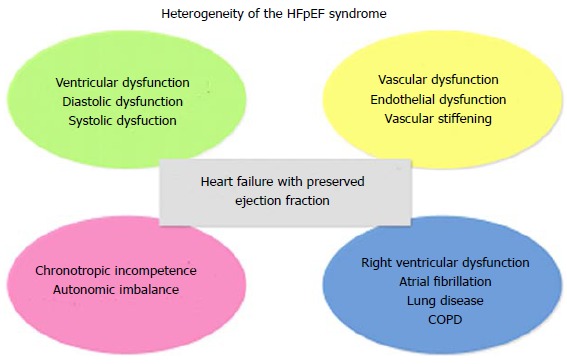
Overview of multiple effectors for the heterogeneity of the heart failure has preserved left ventricular ejection fraction syndrome. COPD: Chronic obstructive pulmonary disease; HFpEF: Heart failure has preserved left ventricular ejection fraction.
However, in retrospect it has been elucidated that the type of therapy tested in previous clinical trials may not be the correct match for the type of HF population included. This line of argument incorporates the ALDO-DHF trial, which included patients with early-stage HFpEF and not manifest volume overload.
Moreover, in the RELAX trial, which enrolled symptomatic HF patients with volume overload but not necessarily those with overt PAH and RV dysfunction. However, the inclusion and exclusion criteria should focus on patients with early HFpEF, in whom exercise intolerance is one of the main indicators and in whom there is objective evidence of exercise-induced increase in LV filling pressures. Excluding patients with co-morbidity to try to increase the specifity of HFpEF may purely make matters worse by excluding those patients at high risk. If co-morbidities drive the clinical course of the patient, then treatment directed only at cardiac function may be ineffective. In addition, diagnosis of HFpEF should not be based solely on clinical criteria and the absence of HFrEF (Figure 2).
Figure 2.
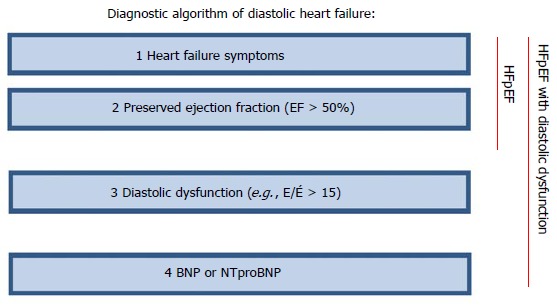
Diagnostic algorithm of diastolic heart failure. BNP: B-type natriuretic peptide; NT-proBNP: N-terminal of the B-type natriuretic peptide; E/É: Pulsed-wave Doppler E wave velocity divided by tissue Doppler E wave velocity; HFpEF: Heart failure has preserved left ventricular ejection fraction.
Natiuretic peptides provide considerable confidence for improved clinical trial design. HFpEF is a heterogeneous and a complex syndrome and only specific phenotypes may respond to a particular therapeutic intervention (Figure 1).
Sufficient diagnosis and phenotyping seems to be essential. The disappointment in the last clinical trials that have proven so effective in treating HFrEF supports an urgent need for novel drug approaches to HFpEF (Figure 3). Underpowered clinical trials should be avoided and study designs need a focus on more consistent patient populations to control the impact cardiovascular co-morbidities. To conclude, ethnicity, cultural differences, co-medication, cut-off values and local clinical practice might influence results of clinical trials. Additional endpoints that include for example, quality of life evaluation and correct timing for effective therapeutic intervention must be kept in mind when planning expensive multicenter RCTs.
Figure 3.
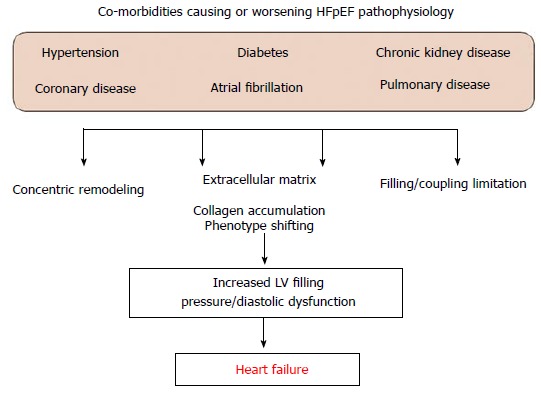
Scheme of co-morbidities causing or worsening heart failure has preserved left ventricular ejection fraction pathophysiology. LV: Left ventricular; HFpEF: Heart failure has preserved left ventricular ejection fraction.
PROMISING NEW THERAPY STRATEGIES
Soluble guanylate cyclase inhibitors
Solid evidence supports augmentation of (cGMP) signaling as a potential therapeutic strategy for HFpEF[49]. Direct soluble guanylate cyclase stimulators target reduced cGMP generation due to insufficient sGC stimulation and represent a promising method for cGMP enhancement.
In the SOCRATES-Preserved trial (soluble guanylate cyclase stimulator in HF patients with PRESERVED EF; clinicaltrial.org NCT01951638) stimulation of the soluble guanylate cyclase by the oral soluble guanylate cyclase stimulator BAY1021189 is currently being investigated over 12 wk in patients with worsening HFpEF.
Iƒ channel inhibition
The SHIFT trial demonstrated that significant heart rate reduction via ivabradine, inhibitor of the Iƒ channel of the sinoatrial node, led to a significant reduction in hospitalization caused by HF and cardiovascular mortality in the HFrEF population[50]. Interestingly, the effects of ivabradine in HFpEF have been studied in a small recent trial of 61 patients, randomized to placebo or ivabradine (5 mg twice a day). Treatment with ivabradine showed an enhancement in exercise capacity and an improvement in LV filling pressures. In addition, a larger multi-center study enrolling about 400 patients is going to evaluate the properties of ivabradine concerning diastolic function, NT-proBNP levels and exercice capacity (www.clinicaltrialsregister.eu-EUCTR2012-002742-20-DE).
Dual angiotensin receptor blocker-neutral endopeptidase inhibitors
Although studies conducted with ARBs or ACEi alone did not display enhancements in HFpEF patients, pathophysiological evidence support the rationale for targeting the renin angiotensin system (RAS) in this population of patients.
The Prospective comparison of ARNI with ARB on Management of HF with preserved ejectionN fraction (PARAMOUNT) study[51], a phase II trial conducted in 308 patients in 13 countries, compared the effects of LCZ696 and the ARB valsartan on the concentrations of natriuretic peptides. The natriuretic peptide investigated in this study, NT-proBNP, is a marker of cardiac wall stress, and levels are increased in patients with HF[51].
The agent LCZ696 in the PARAMOUNT study is the first compound to show both reductions in NT-proBNP and left atrial size (LA) in HFpEF patients, powerful predictors of outcome in HF. The favorable effects of LCZ696 seen in patients with HFpEF in the PARAMOUNT trial are encouraging, and further testing of this agent in this patient population is warranted.
LCZ696 acts by inhibiting both the angiotensin receptor and the enzyme responsible for the breakdown of the natriuretic peptides (neprilysin). LCZ696’s dual mechanism of action thus acts to restore the altered neurohormonal balance in HFpEF[52]. These dual effects may be important in the treatment of HFpEF. Moreover, the large outcome trial PARAGON-HF will test the efficacy and safety in HFpEF patients (clinicaltrials.gov NCT01920711).
CONCLUSION
HFpEF accounts for approximately 50% of all patients diagnosed with HF, with similary poor outcomes. To date, only the prevention of HFpEF by treating the cardiovascular risk factors (coronary artery disease, atrial fibrillation, hypertension, diabetes, and obesity) has been shown to be efficient. This observation suggests that investigators in future trials should specify the indication of hospitalization for HF and may request to verify the details of patients’ admissions.
However, dual inhibition of the RAS and neprilysin by the agent LCZ696 represents a novel promising therapeutic target for treating patients with HF. LCZ696 in the PARAMOUNT trial is the first agent to show both reductions in NT-proBNP levels and LA size in HFpEF patients, each strong predictors of outcome in HF. The favorable effects of LCZ696 seen in patients with HFpEF in the PARAMOUNT trial are encouraging. Further testing of dual of RAS and neprilysin inhibition in the HFpEF population is warranted.
Footnotes
Conflict-of-interest statement: The authors have no relationships with industry to report and have no conflict of interest including financial, personal, political interest in this study.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 18, 2015
First decision: May 13, 2015
Article in press: August 3, 2015
P- Reviewer: Das UN, Farand P, Ng TMH, Said SAM S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
References
- 1.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 2.Dhingra A, Garg A, Kaur S, Chopra S, Batra JS, Pandey A, Chaanine AH, Agarwal SK. Epidemiology of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2014;11:354–365. doi: 10.1007/s11897-014-0223-7. [DOI] [PubMed] [Google Scholar]
- 3.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson C, Vasan RS. Epidemiology of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:377–388. doi: 10.1016/j.hfc.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becher PM, Lindner D, Fluschnik N, Blankenberg S, Westermann D. Diagnosing heart failure with preserved ejection fraction. Expert Opin Med Diagn. 2013;7:463–474. doi: 10.1517/17530059.2013.825246. [DOI] [PubMed] [Google Scholar]
- 6.Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL. Importance of heart failure with preserved systolic function in patients & gt; or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Becher PM, Blankenberg S, Westermann D. Current treatment of heart failure with preserved ejection fraction: should we add life to the remaining years or add years to the remaining life? Cardiol Res Pract. 2013;2013:130724. doi: 10.1155/2013/130724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA. 2008;300:431–433. doi: 10.1001/jama.300.4.431. [DOI] [PubMed] [Google Scholar]
- 10.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 11.Cleland JG, Pellicori P, Dierckx R. Clinical trials in patients with heart failure and preserved left ventricular ejection fraction. Heart Fail Clin. 2014;10:511–523. doi: 10.1016/j.hfc.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Shah SJ. Matchmaking for the optimization of clinical trials of heart failure with preserved ejection fraction: no laughing matter. J Am Coll Cardiol. 2013;62:1339–1342. doi: 10.1016/j.jacc.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogg K, McMurray J. The treatment of heart failure with preserved ejection fraction (“diastolic heart failure”) Heart Fail Rev. 2006;11:141–146. doi: 10.1007/s10741-006-9488-6. [DOI] [PubMed] [Google Scholar]
- 14.Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 15.Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, Lip GY, Coats AJ, Andersson B, Kirchhof P, et al. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet. 2014;384:2235–2243. doi: 10.1016/S0140-6736(14)61373-8. [DOI] [PubMed] [Google Scholar]
- 16.Ghio S, Magrini G, Serio A, Klersy C, Fucili A, Ronaszèki A, Karpati P, Mordenti G, Capriati A, Poole-Wilson PA, et al. Effects of nebivolol in elderly heart failure patients with or without systolic left ventricular dysfunction: results of the SENIORS echocardiographic substudy. Eur Heart J. 2006;27:562–568. doi: 10.1093/eurheartj/ehi735. [DOI] [PubMed] [Google Scholar]
- 17.Lund LH, Benson L, Dahlström U, Edner M, Friberg L. Association between use of β-blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA. 2014;312:2008–2018. doi: 10.1001/jama.2014.15241. [DOI] [PubMed] [Google Scholar]
- 18.Little WC, Brucks S. Therapy for diastolic heart failure. Prog Cardiovasc Dis. 2005;47:380–388. doi: 10.1016/j.pcad.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 20.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–89. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 23.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 24.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 25.Mottram PM, Haluska B, Leano R, Cowley D, Stowasser M, Marwick TH. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;110:558–565. doi: 10.1161/01.CIR.0000138680.89536.A9. [DOI] [PubMed] [Google Scholar]
- 26.Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the Randomized Aldosterone Antagonism in Heart Failure with Preserved Ejection Fraction trial (RAAM-PEF) J Card Fail. 2011;17:634–642. doi: 10.1016/j.cardfail.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Löffler M, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 28.Lijnen P, Petrov V. Induction of cardiac fibrosis by aldosterone. J Mol Cell Cardiol. 2000;32:865–879. doi: 10.1006/jmcc.2000.1129. [DOI] [PubMed] [Google Scholar]
- 29.Pitt D. ACE inhibitor co-therapy in patients with heart failure: rationale for the Randomized Aldactone Evaluation Study (RALES) Eur Heart J. 1995;16 Suppl N:107–110. doi: 10.1093/eurheartj/16.suppl_n.107. [DOI] [PubMed] [Google Scholar]
- 30.Pitt B. Effect of aldosterone blockade in patients with systolic left ventricular dysfunction: implications of the RALES and EPHESUS studies. Mol Cell Endocrinol. 2004;217:53–58. doi: 10.1016/j.mce.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Zannad F, McMurray JJ, Drexler H, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pitt B. Rationale and design of the Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure (EMPHASIS-HF) Eur J Heart Fail. 2010;12:617–622. doi: 10.1093/eurjhf/hfq049. [DOI] [PubMed] [Google Scholar]
- 32.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. [Google Scholar]
- 33.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–972. e10. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 34.McMurray JJ, O’Connor C. Lessons from the TOPCAT trial. N Engl J Med. 2014;370:1453–1454. doi: 10.1056/NEJMe1401231. [DOI] [PubMed] [Google Scholar]
- 35.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF, Gheorghiade M. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maier LS, Layug B, Karwatowska-Prokopczuk E, Belardinelli L, Lee S, Sander J, Lang C, Wachter R, Edelmann F, Hasenfuss G, et al. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study. JACC Heart Fail. 2013;1:115–122. doi: 10.1016/j.jchf.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Ghofrani HA, Rose F, Schermuly RT, Olschewski H, Wiedemann R, Kreckel A, Weissmann N, Ghofrani S, Enke B, Seeger W, et al. Oral sildenafil as long-term adjunct therapy to inhaled iloprost in severe pulmonary arterial hypertension. J Am Coll Cardiol. 2003;42:158–164. doi: 10.1016/s0735-1097(03)00555-2. [DOI] [PubMed] [Google Scholar]
- 39.Michelakis ED, Tymchak W, Noga M, Webster L, Wu XC, Lien D, Wang SH, Modry D, Archer SL. Long-term treatment with oral sildenafil is safe and improves functional capacity and hemodynamics in patients with pulmonary arterial hypertension. Circulation. 2003;108:2066–2069. doi: 10.1161/01.CIR.0000099502.17776.C2. [DOI] [PubMed] [Google Scholar]
- 40.Ghofrani HA, Voswinckel R, Reichenberger F, Olschewski H, Haredza P, Karadaş B, Schermuly RT, Weissmann N, Seeger W, Grimminger F. Differences in hemodynamic and oxygenation responses to three different phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension: a randomized prospective study. J Am Coll Cardiol. 2004;44:1488–1496. doi: 10.1016/j.jacc.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 41.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 42.Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4:8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 43.Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, Deswal A, Hernandez AF, Lee KL, Braunwald E. PhosphdiesteRasE-5 Inhibition to Improve CLinical Status and EXercise Capacity in Diastolic Heart Failure (RELAX) trial: rationale and design. Circ Heart Fail. 2012;5:653–659. doi: 10.1161/CIRCHEARTFAILURE.112.969071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kron J, Aranda JM, Miles WM, Burkart TA, Woo GW, Saxonhouse SJ, Sears SF, Conti JB. Benefit of cardiac resynchronization in elderly patients: results from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) and Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE-ICD) trials. J Interv Card Electrophysiol. 2009;25:91–96. doi: 10.1007/s10840-008-9330-2. [DOI] [PubMed] [Google Scholar]
- 46.O’Brien TM, Schloss EJ, Chung ES. Indications for cardiac resynchronization therapy. Cardiol Clin. 2014;32:293–298. doi: 10.1016/j.ccl.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Chung ES, Katra RP, Ghio S, Bax J, Gerritse B, Hilpisch K, Peterson BJ, Feldman DS, Abraham WT. Cardiac resynchronization therapy may benefit patients with left ventricular ejection fraction & gt; 35%: a PROSPECT trial substudy. Eur J Heart Fail. 2010;12:581–587. doi: 10.1093/eurjhf/hfq009. [DOI] [PubMed] [Google Scholar]
- 48.Kaye D, Shah SJ, Borlaug BA, Gustafsson F, Komtebedde J, Kubo S, Magnin C, Maurer MS, Feldman T, Burkhoff D. Effects of an interatrial shunt on rest and exercise hemodynamics: results of a computer simulation in heart failure. J Card Fail. 2014;20:212–221. doi: 10.1016/j.cardfail.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Loffredo FS, Nikolova AP, Pancoast JR, Lee RT. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res. 2014;115:97–107. doi: 10.1161/CIRCRESAHA.115.302929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 51.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 52.Lang CC, Struthers AD. Targeting the renin-angiotensin-aldosterone system in heart failure. Nat Rev Cardiol. 2013;10:125–134. doi: 10.1038/nrcardio.2012.196. [DOI] [PubMed] [Google Scholar]


