Abstract
A 17-year-old girl presented to the A&E department with significant neck swelling with associated chest, neck and throat pain. She reported recreational inhalation of nitrous oxide and ingestion of MDMA (3,4-methylenedioxy-methamphetamine) in the preceding hours. There was no history of trauma or vomiting. Clinical examination revealed extensive subcutaneous emphysema. There was no airway compromise. A chest X-ray suggested the presence of a pneumomediastinum. Subsequent CT of the thorax confirmed an anterior pneumothorax and a pneumopericardium. The patient was admitted for observation and intravenous antibiotics. Further investigations ruled out an oesophageal perforation. The patient was discharged following a period of clinical stability and has since made an uneventful recovery. MDMA ingestion has been cited as a rare cause of spontaneous pneumomediastinum in a series of case reports. In this case, it is likely that the inhalation of nitrous oxide contributed to the development and expansion of a pneumomediastinum.
Background
Free air in the mediastinum or pneumomediastinum is a rare complication of recreational drug use including MDMA (3,4-methylenedioxy-methamphetamine).1 2 There is also a long established association between nitrous oxide inhalation, as used in the induction of dissociative anaesthesia, and air in the pleural space and mediastinum.4 With Home Office figures for 2013/2014 suggesting that 470 000 people in Britain have used nitrous oxide for recreational purposes, including 7.6% of young people aged 16–24 years, this case study highlights the potential for harm posed by abuse of this substance, particularly when combined with other illicit drugs.
Case presentation
A 17-year-old girl with chest discomfort, shortness of breath and swelling extending from the left side of her neck to her chest, presented to the A&E department. The symptoms were preceded by illicit drug and alcohol use. During this period, she admitted to ingestion of MDMA. She also inhaled significant volumes of nitrous oxide gas from prefilled balloons. There was no history of trauma. She had no medical history; she had a contraceptive implant in situ and was a social smoker.
On examination, she appeared comfortable at rest. Oxygen saturations were 99% on room air. There were no signs of airway compromise, despite the clearly evident surgical emphysema around her neck and anterior chest wall. Air entry was good and equal on both sides of her chest. She was neurologically intact.
Investigations
On admission, an ECG revealed sinus rhythm, with no other abnormalities. The patient's blood tests were unremarkable aside from slightly raised inflammatory markers, with white cell count 12.7/L (3.9–11×109/L) and C reactive protein of 26 mg/L (0–5 mg/L). Chest X-ray revealed a double left-sided cardiac border, suggestive of a pneumomediastinum, with no obvious pneumothorax (figure 1). CT of the chest revealed extensive surgical emphysema in the neck and chest, with a pneumomediastinum and small anterior pneumothorax. The rest of the lung parenchyma appeared normal with no definite point of airway rupture/perforation (figures 2–4).
Figure 1.
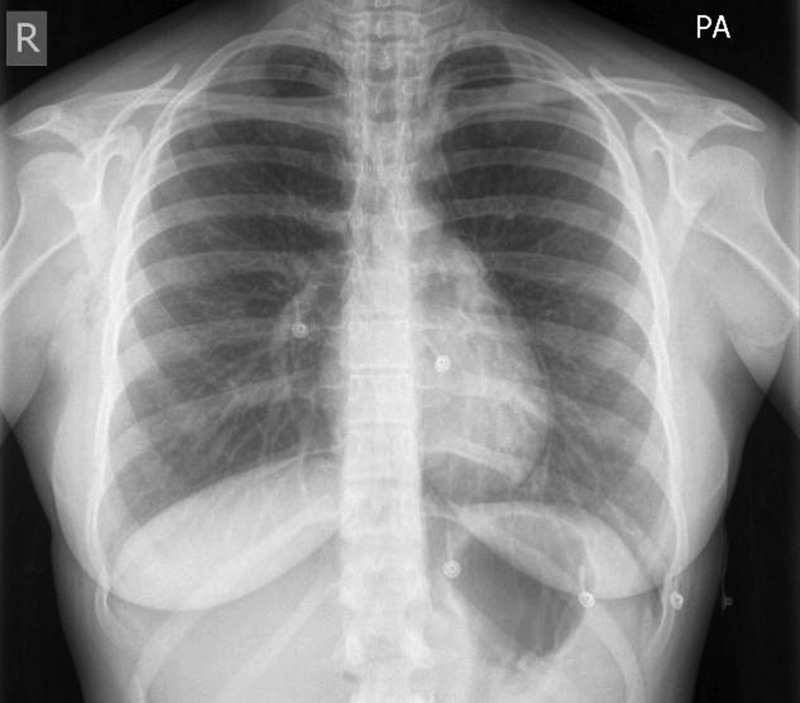
Chest radiograph revealing a double left-sided cardiac border indicating the presence of air in the mediastinum.
Figure 2.
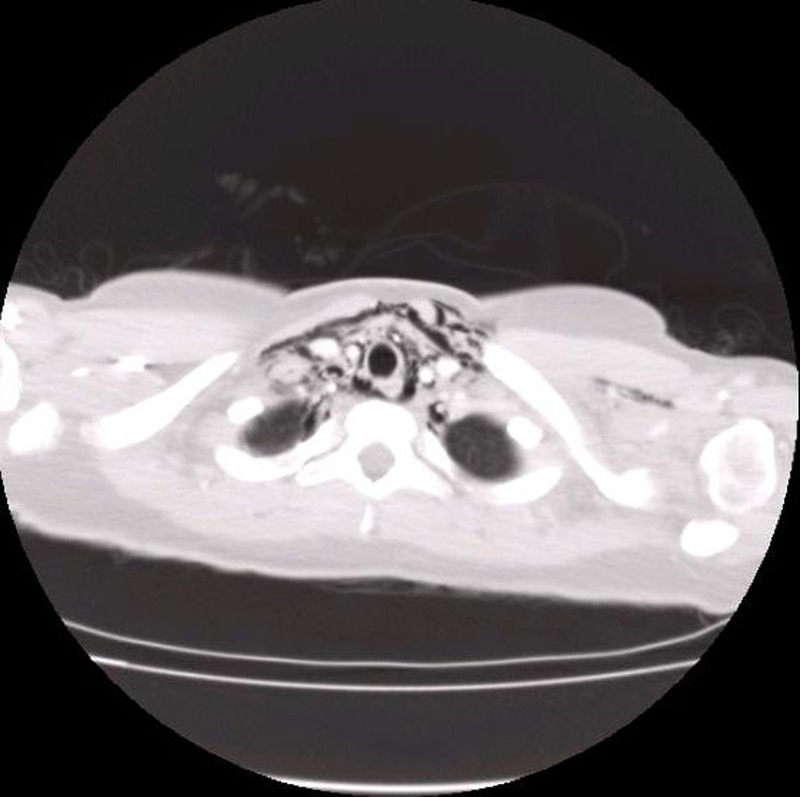
CT of the thorax demonstrating subcutaneous emphysema and air dissecting the fascial planes.
Figure 3.
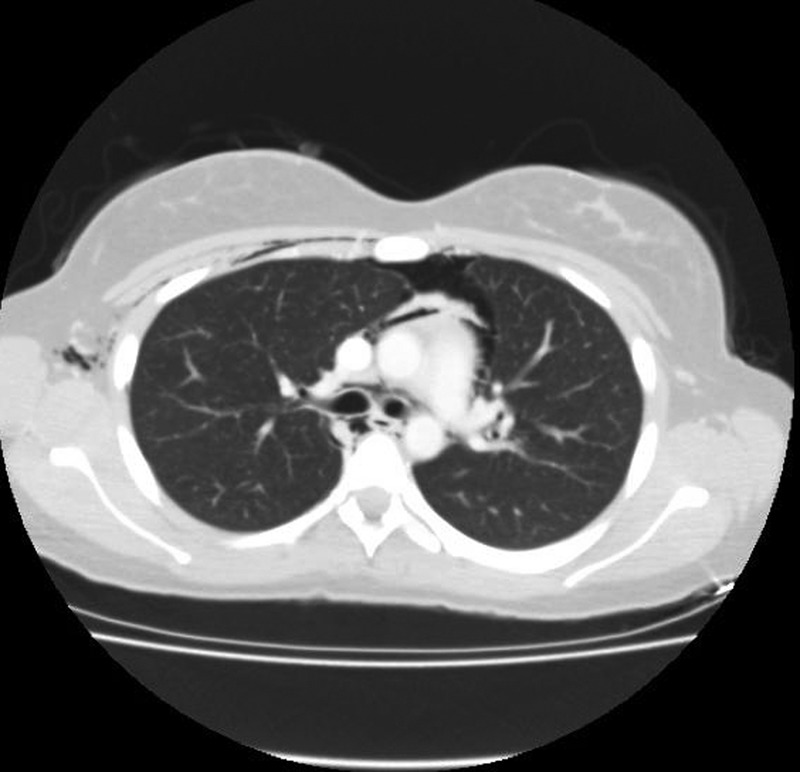
CT of the thorax confirming the presence of an anterior pneumothorax and a pneumopericardium.
Figure 4.
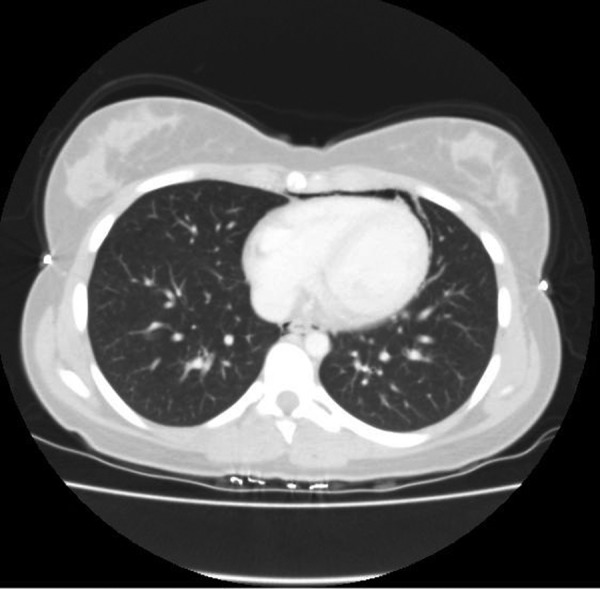
CT of the thorax confirming the presence of an anterior pneumothorax and a pneumopericardium.
Differential diagnosis
Boerhaave syndrome.
Treatment
The patient's case was discussed with the cardiothoracic surgical team, which advised conservative management, as the patient remained stable. She was admitted for close monitoring given the high risk of mediastinitis. Further tests were performed to exclude Boerhaave syndrome. An oesophago-gastro-duodenoscopy (OGD) revealed normal appearance of the oesophagus and gastro-oesophageal junction with no perforation. A gastrograffin swallow excluded leakage of contrast from the oesophagus and stomach. The patient was administered high flow oxygen and started on prophylactic oral antibiotics to cover for potential mediastinitis.
Outcome and follow-up
The patient remained stable on the general medical ward after a 3-day admission. A repeat chest X-ray showed improvement in the pneumomediastinum. She was discharged home with the advice to stop smoking and to rest for 2 weeks. The risks of future illicit drug use were outlined. She has had no further admissions.
Discussion
Spontaneous pneumomediastinum (SPM) is rare, with a reported incidence in the region of 1 in 30 000 hospital admissions.1 Common presenting symptoms include chest pain, dyspnoea, neck pain and hypernasal speech or rhinolalia.5 6
There is usually no recognisable cause for SPM; however, there may be a preceding factor that contributes, such as vigorous coughing (especially seen in asthmatic men), sneezing or exertion. The mechanism of SPM is poorly understood. It is believed to occur as a result of increased intra-alveolar pressure as a large pressure gradient is created against a closed glottis.6 This so-called ‘Macklin effect’ can lead to alveolar rupture with free air dissecting the bronchovascular planes to reach the mediastinum. Air then tracks through the superficial fascial layers of the neck and chest, causing surgical emphysema. The free air can travel through to the pleura (pneumothorax), pericardium (pneumopericardium) and even the abdomen (pneumoperitoneum). The presence of subpleural blebs may play a role in the pathogenesis of this condition, as a result of rupture.
The use of MDMA has been linked to SPM in a number of case reports, and this is thought to reflect an increase in transpulmonary pressures produced by Valsalva manoeuver and extreme physical activity rather than a direct pharmacological effect of the drug.
Nitrous oxide was discovered in 1772 by a British scientist named Joseph Priestley. When the chemist Humphrey Davy subsequently described its euphoric properties, nitrous oxide, or ‘laughing gas’, gained popularity as the recreational drug of choice among the British upper class. Its role in anaesthesia was outlined 40 years later by the dentist Horace Wells.
A common source of nitrous oxide is whippets, small canisters that contain the gas in pressurised form. Using a device designed for dispensing whipped cream, the nitrous oxide can then be inhaled directly from the canisters. More commonly, however, the gas is released into a balloon and is inhaled with a peak onset of effect occurring 10–20 s later.
Owing to its high relative solubility, the inhalation of nitrous oxide causes expansion of closed, gas-filled cavities in the body, as small volumes of nitrogen from the cavity are exchanged for much larger volumes of nitrous oxide from the blood. The potential to expand a pneumothorax or pneumomediastinum has been a well-recognised complication of nitrous oxide anaesthesia, since it was described by Hunter et al in 1955.3
This case highlights the potential for serious harm associated with combined recreational abuse of MDMA and nitrous oxide. The effects of MDMA and the transpulmonary pressure gradient associated with deep inhalation of nitrous oxide led to alveolar rupture and dissection of gas along the mediastinal planes. The rapid diffusion of nitrous oxide into this closed, gas space, likely led to further expansion of the pneumomediastinum such that on presentation to hospital the patient had free air in the pleural space bilaterally, in her pericardium and extending into the tissues of her neck.
SPM is a serious condition and, rarely, requires surgical intervention. However, a period of clinical observation is advised in order to ensure the airway is not compromised. Recreational nitrous oxide abuse and use of other drugs may be implicated in the development of this condition. As the popularity of nitrous oxide increases steadily, it is important that physicians take a clear drug history in all patients presenting with SPM.
Learning points.
Drug intake is a rare cause of spontaneous pneumomediastinum and thus a drug history is vital at the time of presentation.
Nitrous oxide is now the second most common recreational drug among individuals aged 16–24 years in the UK.
Nitrous oxide may result in rapid expansion of free air in the mediastinum.
In most cases, pneumomediastinum is a self-limiting condition with no surgical requirement.
Footnotes
Contributors: RMcD is the guarantor and contributed to conception, design and drafting of the report, and approved the manuscript before submission and is accountable for its integrity. KT contributed to conception, design and drafting of the report, and approved the manuscript before submission and is accountable for integrity. MB contributed to conception, design and drafting of the report, and approved the manuscript before submission and is accountable for integrity. NH assisted in compiling the images and gathering the clinical data.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Rezvani K, Kurbaan AS, Brenton D. Ecstasy induced pneumomediastinum. Thorax 1996;51:960–1. 10.1136/thx.51.9.960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marasco SF, Lim HK. Ecstasy-associated pneumomediastinum. Ann R Coll Surg Engl 2007;89:389–93. 10.1308/003588407X183373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter AR. Problems of anaesthesia in artificial pneumothorax. Proc R Soc Med 1955;48:765–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Newcombe A, Clarke P. Spontaneous pneumomediastinum. Chest 2005;128: 3298–302. 10.1378/chest.128.5.3298 [DOI] [PubMed] [Google Scholar]
- 5.Al-Mufarrej F, Badar J, Gharagozloo F et al. Spontaneous pneumomediastinum: diagnostic and therapeutic interventions. J Cardiothorac Surg 2008;3:59 10.1186/1749-8090-3-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macklin MT, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: an interpretation of the clinical literature in the light of laboratory experiment. Medicine (Baltimore) 1944;23:281–358. 10.1097/00005792-194412000-00001 [DOI] [Google Scholar]


