Abstract
Acute necrotising ulcerative gingivitis is an acute onset disease characterised by ulceration, necrosis, pain and bleeding in gingival surfaces. It is predominantly seen in severely malnourished children and young adults with advanced HIV infection. We present a unique presentation in a young adult with high-grade osteogenic sarcoma.
Background
Acute necrotising ulcerative gingivitis (ANUG) is a type of periodontal disease, characterised by a painful ulceration of gingival surfaces, that may result in progressive destruction of the gingivae and, if left untreated, can eventually lead to cancrum oris, a severe form with gangrenous orofacial lesions.1 Also known as ‘trench mouth’, ANUG was classically seen in epidemic proportions in the military population during World War I, presumably due to multiple risk factors including malnutrition, poor oral hygiene and intense psychological stress.1 It has appeared at much lower rates in the general population, since then. Much of the literature on civilian populations has focused on patients with HIV and severely malnourished children in developing countries. Few cases of ANUG in oncology patients have been reported, and no cases have been reported in patients with bone tumours or sarcoma. We present what we believe to be the first report of ANUG in a young adult with high-grade osteogenic sarcoma undergoing chemotherapy.
Case presentation
A 24-year-old man, 5 months into intensive chemotherapy for localised high-grade osteogenic sarcoma of the tibia, with 2 days of fever and mouth pain, presented to our hospital. His initial chemotherapy regimen consisted of the standard 12 weeks of cisplatin, doxorubicin and high-dose methotrexate, followed by surgical resection of the tumour. Based on the poor histological response to therapy, the patient received experimental therapy with the addition of ifosfamide and etoposide while on the Children's Oncology Group protocol AOST0331.2 His last chemotherapy began 14 days prior to admission, with standard dose ifosfamide and etoposide (week 16 of the protocol). He received granulocyte colony-stimulating factor prophylaxis following this and all previous myelosuppressive cycles of chemotherapy.
Two days prior to admission, the patient developed rapid onset gingival pain accompanied by progressive black discolouration on the anterior and posterior lower gingiva with subsequent spread to the upper gingiva. These friable lesions bled with tooth brushing. The patient also reported subjective fevers, malaise, chills and pain on mastication. He had no other significant medical history or known allergies. He denied cigarette smoking, illicit drugs, unsafe sex practices or narcotic use. He reported occasional, non-binge alcohol use at a frequency of less than once per week. He had not received any dental care in 4 years; the patient also reported that he had not brushed his teeth for several days prior to onset of infection.
Physical examination revealed a thin, alert, afebrile, non-toxic appearing man. He had mild tachycardia; otherwise, his vital signs were within normal limits. Oral examination revealed halitosis and necrotic, sloughing, gingival tissue with pseudomembrane formation, moderate erythaema and multiple ulcerations prominent in the interdental papilla of the lower canines, central and lateral incisors, as well as the upper central incisors (figure 1). There was moderate tenderness to palpation over the gingiva. There was no tenderness of the jaw or on the floor of the mouth. Submandibular lymphadenopathy was noted. The patient's port-a-cath was without evidence of infection.
Figure 1.
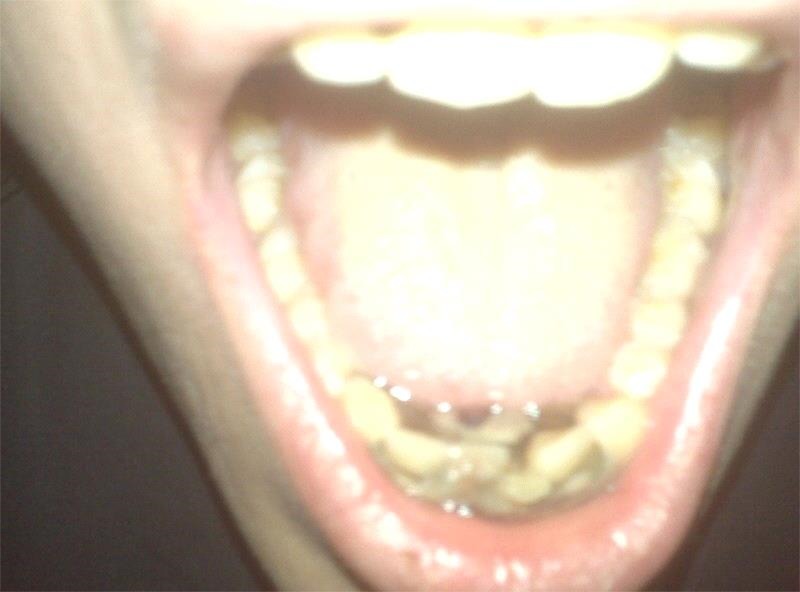
Necrotising changes of the lower gingiva with pseudomembrane formation.
Investigations
Laboratory tests showed haemoglobin of 8.6×1012 cells/L, a white cell count (WCC) of 7.3×109 cells/L, with 83% neutrophils, 8% lymphocytes and 9% monocytes, absolute neutrophil count (ANC) of 6.1×109 cells/L and a platelet count of 26×109/L. Gingival cultures prior to antibiotics grew α haemolytic streptococci, Micrococcus species, Neisseria species, Prevotella and Porphyromonas species, consistent with normal oral flora. Herpes simplex virus (HSV) type 1 and 2 PCR of lesions was negative. An HIV antibody was negative, a rapid plasma reagin was non-reactive, and blood and urine cultures obtained prior to antibiotics were sterile.
Differential diagnosis
The initial primary differential diagnosis for our patient included: chemotherapy-induced mucositis, neutropenic mucositis, HSV, gingivostomatitis, HIV-associated periodontitis and invasive fungal disease. Since there were no characteristic vesicles (as one would expect in HSV) and there was no involvement of other mouth structures such as the tonsils and tongue (as we typically see in invasive fungal infection) and no known HIV risk factors, this made HSV, HIV and fungal disease less likely. Similarly, WCC and ANC had recovered by day 14 postchemotherapy when the lesions were progressing, which made chemotherapy or neutropenia-associated mucositis unlikely, since count recovery and spontaneous healing is expected in these cases. Thus ANUG was felt to be the best clinical fit for the patient's condition.
The broader differential diagnosis, but not a good clinical fit given the physical findings, laboratory studies and history included: severe malnutrition or liver disease, other infectious causes (rubeola, primary or recurrent varicella-zoster virus, cytomegalovirus (CMV), coxsackie A, candida, diphtheria, syphilis, group A strep), Vincent's angina, vitamin C deficiency ‘scurvy’, systemic diseases such as diabetes, hypophosphatasia, Behçet Disease, leucaemia, aplastic anaemia or systemic lupus, immune deficiency states such as histiocytosis, severe combined immunodeficiency or agranulocytosis, and illicit drug-related gingival disease, so called ‘Meth-mouth’.1 3–7
Treatment
Empiric antibiotics on admission were metronidazole, gentamicin, clindamycin and piperacillin/tazobactam, which were changed to ampicillin/sulbactam, metronidazole and chlorhexidine oral rinse on day one of hospitalisation. The patient underwent urgent gingival debridement by the oromaxillofacial surgery team, where the necrotic tissue was widely excised. On hospital day 4, the patient denied gingival pain and was discharged home on oral amoxicillin/clavulanate, metronidazole, fluconazole and chlorhexidine oral rinse. Intensive chemotherapy was delayed only 1 week and continued at the prescribed dose intensity for the remaining 21 weeks without further delay or complications. The mouth and gum lesions had completely healed 6 weeks after surgical intervention (figures 2 and 3)
Figure 2.
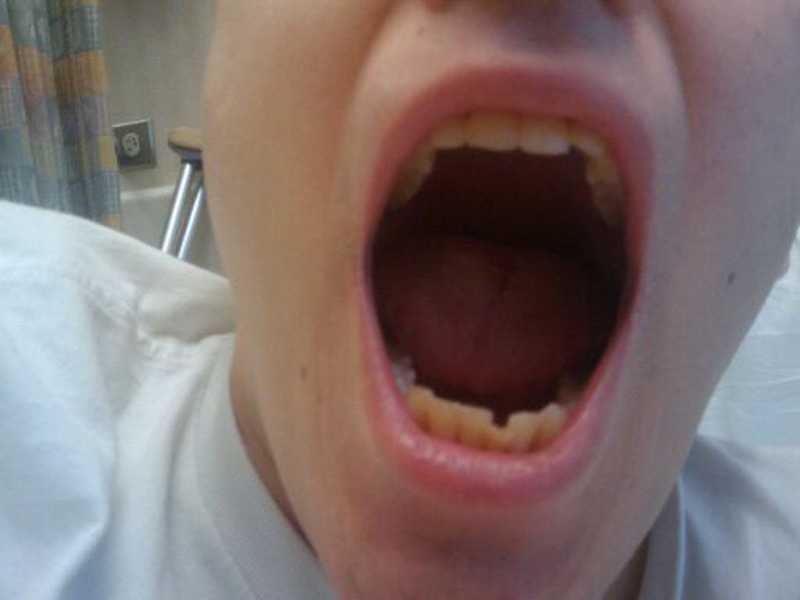
Open-mouth view: complete resolution 6 weeks after debridement. Patient continued on intensive chemotherapy during this period.
Figure 3.
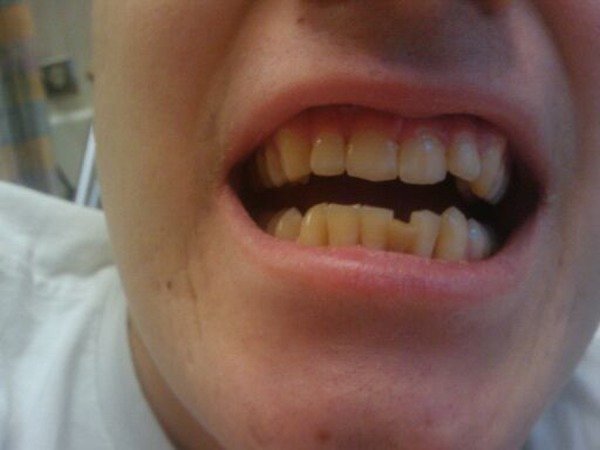
Closed-mouth view: gums healed and teeth unaffected.
Outcome and follow-up
At follow-up a year later, the patient continued to have issues with mild gingival bleeding, which was treated with multivitamins, and he had no recurrence of oral symptoms or infection. To date, he has remained in clinical remission for 5 years from diagnosis and is likely cured of his cancer.
Discussion
Our patient was diagnosed with ANUG, from the less severe part of the spectrum of NUG: the term ‘necrotising ulcerative periodontitis’ is used when the attachments of the teeth (periodontal and alveolar ligaments) are disrupted; ‘necrotising stomatitis’ is the term for when the necrosis has progressed beyond the mucogingival junction; and the term ‘cancrum oris,’ or ‘noma’, is used when there is gangrene of the mouth and face.1 3 ANUG is caused by infection of the gingiva, classically seen in military personnel, but at present most frequently occurring in the setting of systemic disease or severe malnutrition.3–5 ANUG occurs most frequently in young children in developing countries, and in adolescents and young adults in industrialised nations.1 3 Epidemiological studies suggest increased prevalence in two populations: children from low socioeconomic backgrounds in developing countries, such as those in sub-Saharan Africa, and in India; and in HIV-infected patients, in whom prevalence of ANUG varies 4.3–16%.5 Children from developing countries are thought to develop ANUG primarily due to malnutrition, which impairs their innate and adaptive immune systems, associated with an alteration of oral flora and damage to the integrity of tissue.5 HIV-infected patients in an advanced immunocompromised state are thought to be more susceptible to ANUG; it is 20.8 times more likely in patients with CD4 counts less than 0.2×109 cells/L.5
Many aetiologic agents have been implicated in ANUG. In a 2012 study of bacterial causes of ANUG in malnourished children, Prevotella intermedia and Peptostreptococcus species were the most commonly isolated organisms from oral flora, followed by Spirochetes, Fusobacteria and Porphyromonas species.6 In a review of periodontal infection in patients with cancer treated with high-dose chemotherapy, these bacteria were also frequently reported, in addition to streptococci and Pseudomonas species.7 Fungal causes implicated in ANUG include Candida albicans and Aspergillus species.7 Viral aetiologies have also been associated with ANUG and include CMV, human herpesvirus type 6 and Epstein-Barr virus.5 Our patient grew streptococci, Porphyromonas, Prevotella and C. albicans, all of which have been implicated in ANUG.6 Patients with haematological and/or oncological conditions are much less frequently reported to have ANUG. We reviewed the literature for all cases of ANUG occurring in haematology and/or oncology patients, and found that the majority of cases occurred in patients with diagnoses of leucaemia and lymphoma (table 1).
Table 1.
Cases of ANUG in haematology/oncology patients reported in the medical literature
| Author, Year (# Cases), [Source] | Demo-graphics | Disease | Presentation | Infectious agents | Other risk factors | Treatment and outcome |
|---|---|---|---|---|---|---|
| Miyairi et al, 2005 (n=1)8 | 8 years Female | Relapsed ALL |
Developed port infection and then ANUG | HSV, Steno-trophomonas maltophilia, rare Entero-coccus faecalis | FLU, AraC | Amikacin and ticarcillin/clavulanic acid, and debridement. Died 2 months later due to progression of leucaemia |
| Khoury et al, 2003 (n=1)9 | 26 years Female Asian | AML | 4 days after Induction Chemo-therapy |
Aspergillus terreus; HSV-1 |
AraC, DAUN | Debridement and oral itraconazole, improvement within 72 h. Remission 16 months from diagnosis |
| Ryan et al, 1983 (n=18)10 | Ages 15 months to 23 years. Median 9.5 years |
7 ALL, 5 AML, 3 HD, 2 NHL, 1 HIST | NA | NA | VCR 12, MTX 11, PRED 7, CYM 7, LASP 6, DAUN 6 | 6/18 (33%) of cases had persistent infection despite therapy. Five of 15 patients died within 1 month of ANUG diagnosis |
| Srirangarajan et al, 2011 (n=1)11 | 17 years Female Indian | Fanconi’s Anaemia | ANUG then chronic recurrent gum bleeding | NA | NA | Debridement, amoxicillin, metronidazole and hydrogen peroxide rinse. Died 3 months later due to bone marrow failure |
| Feller et al, 2006 (n=1)12 | 34 years Male South African | KS, Pulm TB; Possible HIV but refused testing | ANUG super-imposed on 2 months chronic periodontitis | NA | TOB, Alcohol, Poor Oral Hygiene | Metronidazole, amoxicillin, chlorhexidine digluconate mouthwash; lost to follow-up. Died 2 months later, cause of death unknown |
ALL, acute lymphocytic leukemia; AML, acute myelocytic leucaemia; AraC, cytarabine CYM, cyclophosphamide; DAUN, daunomycin; FLU, fludarabine; HD, Hodgkin’s disease; HIST, histiocytosis; KS, Karposi Sarcoma; LASP, L-Asparinginase; MTX, methotrexate; NA, not applicable; NHL, non-Hodgkin's lymphoma; PRED, prednisone; Pulm TB, pulmonary tuberculosis; TOB, tobacco smoking; VCR, vincristine.
In this report, we present a case of ANUG in the setting of intensive chemotherapy for high-grade osteogenic sarcoma, which, to the best of our knowledge, has not been previously reported. Additionally, our patient's onset of ANUG (day 14 postchemotherapy) occurred during the WCC and ANC recovery period. Most reports of ANUG occur in patients with neutropenia or significant depression in WCC function.1 It is plausible that our patient had white cell dysfunction from chemotherapy despite appropriate numbers.
There is sparse literature on specific chemotherapies associated with ANUG; however, methotrexate, anthracyclines, cytarabine and vincristine have been reported.8–10 Our patient last had doxorubicin and methotrexate at weeks 12 and 15, but developed ANUG 14 days after his week 16 doses of ifosfamide and etoposide, drugs not previously associated with ANUG.
Several other risk factors are thought to contribute to the development of ANUG. Smoking is frequently mentioned as a predisposing factor to ANUG, likely due to catecholamines released in response to nicotine, which could cause a reduction in gingival papillary flow and thus lead to papillary necrosis.5 Poor oral hygiene has also been implicated as a predisposing factor, although the recent literature has questioned its relative contribution.5 In adolescent populations, diabetes has also been correlated with development of ANUG, presumably due to multiple aspects of the diabetic state including microangiopathy, delayed wound healing, impaired function of neutrophils and disturbances in collagen formation due to glycation.13 Psychological stress is also believed to contribute to ANUG by causing an elevation in adrenocortical secretion.5 Our patient demonstrated some of these risk factors in combination with his immunocompromised state; he was in his early 20s, had not seen a dentist in 4 years, and he suffered from psychological stress from his recent cancer diagnosis.
Patient's perspective.
I am happy that you are submitting this case because it may help other patients with cancer to know about the importance of mouth care and brushing. Also, I had been told not to go away on the weekend when this happened because my counts were low, but I didn't listen. I'm fine now, except for sensitive gums.
Learning points.
Acute necrotising ulcerative gingivitis (ANUG), usually seen in malnourished children and in advanced HIV infection, can be seen in otherwise healthy patients undergoing intensive chemotherapy.
Oral hygiene is important to help prevent ANUG in patients with mucositis-associated chemotherapy.
Recognition and urgent surgical debridement of ANUG is needed to prevent serious and life-threatening complications.
Footnotes
Acknowledgements: The authors wish to thank the patient in this report for his help and participation, and for his courage and inspiration. Each author listed on the manuscript has significantly contributed and approved the submission of this version of the manuscript, and takes full responsibility for the manuscript. No conflicts of interest exist, to the best of our knowledge, for any author. The authors thank Jessica V Ramirez of Providence Medical Associates in Hawthorne, California, for her help in reviewing this manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Melnick SL, Roseman JM, Engel D et al. . Epidemiology of acute necrotizing ulcerative gingivitis. Epidemiol Rev 1988;10:191–211. [DOI] [PubMed] [Google Scholar]
- 2.Combination chemotherapy, PEG-interferon alfa-2b, and surgery in treating patients with osteosarcoma. 2014. https://clinicaltrials.gov/ct2/show/study/NCT00134030. Updated (accessed 6 Jan 2015).
- 3.Horning GM. Necotizing gingivostomatitis: NUG to noma. Compend Contin Educ Dent 1996;17:951–4, 956, 957-8 passim; quiz 964. PMID 9533316. [PubMed] [Google Scholar]
- 4.Ali AA. Vesiculo-Bullous diseases. Dental college at CoD UoD. http://www.slideshare.net/UDDent/vesiculo-bullous-d1 (accessed May 2015).
- 5.Folayan MO. The epidemiology, etiology, and pathophysiology of acute necrotizing ulcerative gingivitis associated with malnutrition. J Contemp Dent Pract 2004;5:28–41. [PubMed] [Google Scholar]
- 6.Bolivar I, Whiteson K, Stadelmann B et al. . Bacterial diversity in oral samples of children in Niger with acute noma, acute necrotizing gingivitis, and healthy controls. PLoS Negl Trop Dis 2012;6:e1556 10.1371/journal.pntd.0001556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raber-Durlacher JE, Epstein JB, Raber J et al. . Periodontal infection in cancer patients treated with high-dose chemotherapy. Support Care Cancer 2002;10:466–73. 10.1007/s00520-002-0346-3 [DOI] [PubMed] [Google Scholar]
- 8.Miyairi I, Franklin JA, Andreansky M et al. . Acute necrotizing ulcerative gingivitis and bacteremia caused by stenotrophomonas maltophilia in an immunocompromised host. Pediatr Infect Dis J 2002;24:181–3. 10.1097/01.inf.0000151038.82538.de [DOI] [PubMed] [Google Scholar]
- 9.Khoury H, Poh CF, Williams M et al. . Acute myelogenous leukemia complicated by acute necrotizing ulcerative gingivitis due to aspergillus terreus. Leuk Lymphoma 2003;44:709–13. 10.1080/1042819031000060573 [DOI] [PubMed] [Google Scholar]
- 10.Ryan ME, Hopkins K, Wilbur RB. Acute necrotizing ulcerative gingivitis in children with cancer. Am J Dis Child 1983;137:592–4. [DOI] [PubMed] [Google Scholar]
- 11.Srirangarajan S, Shetty S, Prasanna D. Necrotic ulcerative changes in Fanconi's anaemia: a case report. Oral Health Prev Dent 2011;9:91–7. [PubMed] [Google Scholar]
- 12.Feller L, Lemmer J, Wood NH et al. . Necrotizing gingivitis of kaposi sarcoma affected gingivae. SADJ 2006;61:314–17. [PubMed] [Google Scholar]
- 13.Lopez R, Fernandez O, Jara G et al. . Epidemiology of necrotizing ulcerative gingival lesions in adolescents. J Periodontal Res 1983;37:439–44. 10.1034/j.1600-0765.2002.01377.x [DOI] [PubMed] [Google Scholar]


