Tang et al. show that cytotoxic effector cells produce and respond to cysteinyl leukotrienes to allow target cell killing dependent on NKG2D and IL-15. They further demonstrate a role for cysteinyl leukotrienes in celiac disease pathogenesis.
Abstract
Eicosanoids are inflammatory mediators that play a key but incompletely understood role in linking the innate and adaptive immune systems. Here, we show that cytotoxic effector T cells (CTLs) are capable of both producing and responding to cysteinyl leukotrienes (CystLTs), allowing for the killing of target cells in a T cell receptor–independent manner. This process is dependent on the natural killer receptor NKG2D and exposure to IL-15, a cytokine induced in distressed tissues. IL-15 and NKG2D signaling drives the up-regulation of key enzymes implicated in the synthesis of CystLTs, as well as the expression of CystLT receptors, suggesting a positive feedback loop. Finally, although the CystLT pathway has been previously linked to various allergic disorders, we provide unexpected evidence for its involvement in the pathogenesis of celiac disease (CD), a T helper 1 cell–mediated enteropathy induced by gluten. These findings provide new insights into the cytolytic signaling pathway of NKG2D and the pathogenesis of organ-specific immune disorders. Furthermore, they suggest that the blockade of CystLT receptors may represent a potent therapeutic target for CD or potentially other autoimmune disorders in which NKG2D has been implicated.
Celiac disease (CD) is a complex T helper 1 (TH1) cell–mediated immune disorder induced by dietary gluten that shares many common features with organ-specific autoimmune disorders, in particular type 1 diabetes and rheumatoid arthritis (Sollid and Jabri, 2013). IL-15 (Abadie and Jabri, 2014) and the activating natural killer receptor NKG2D have been implicated in these three organ-specific immune disorders. A key function played by NKG2D and IL-15 is to reduce the TCR activation threshold (Bauer et al., 1999; Wu et al., 1999; Groh et al., 2001; Roberts et al., 2001) and promote lymphokine killer activity in cytotoxic effector T cells (CTLs; Meresse et al., 2004). More specifically in patients with active CD, NKG2D has been shown to be up-regulated in intraepithelial CTLs (IE-CTLs; Meresse et al., 2004), allowing for the killing of intestinal epithelial cells (IECs) expressing the stress-inducible molecule MICA (Hüe et al., 2004; Meresse et al., 2004). In contrast to other activating NK receptors that signal through the immunoreceptor tyrosine activation motif (ITAM)–containing adapter DAP12, NKG2D exclusively associates with DAP10 in humans, which lacks ITAM sequences (Bauer et al., 1999; Wu et al., 1999; Rosen et al., 2004). Consequently, NKG2D cannot activate Zap70, and cytolysis through this receptor has thus prompted extensive work to elucidate the signaling pathway involved. Work by Leibson and colleagues has shown that, in addition to phosphoinositide 3-kinase (PI3K; Wu et al., 1999), Vav, growth factor receptor–bound protein 2 (Grb2), and phospholipase C γ (PLCγ; Billadeau et al., 2003; Upshaw and Leibson, 2006; Upshaw et al., 2006; Segovis et al., 2009) are critically involved in NKG2D-mediated cytolysis. Our group has further dissected the downstream signaling events and shown that, in contrast to the TCR, NKG2D requires extracellular signal-regulated kinase (ERK), JNK, and type IV cytosolic phospholipase A2 (cPLA2) activation to mediate cytolysis (Meresse et al., 2004; Tang et al., 2009).
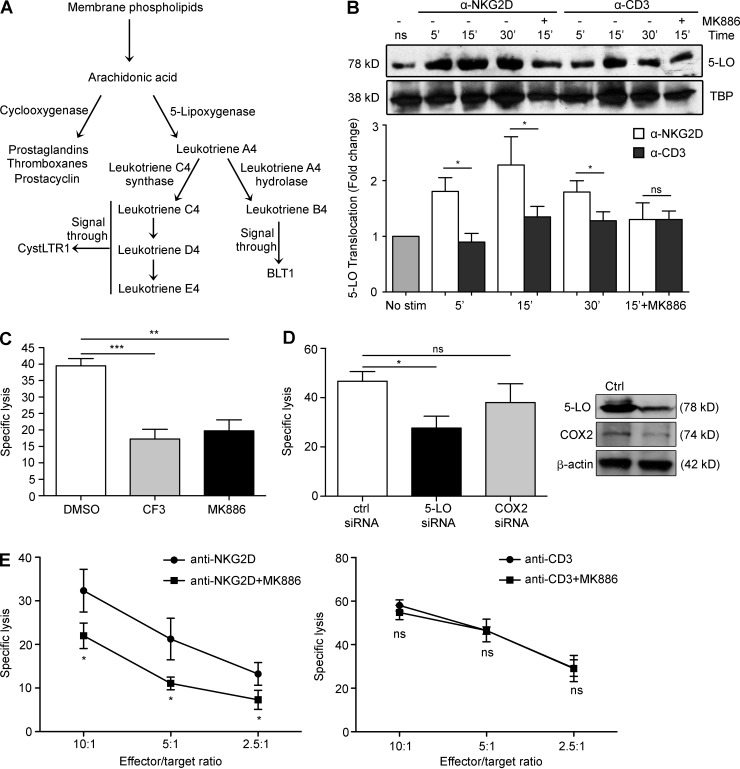
Because cPLA2 plays a key role in the synthesis of eicosanoids by catalyzing the release of arachidonic acid (AA) from membrane phospholipids (Funk, 2001; Peters-Golden and Henderson, 2007), we wanted to know which, if any, eicosanoids were involved in NKG2D-mediated cytolysis and CD pathogenesis. Eicosanoids are signaling molecules that are involved in multiple pathophysiological processes, including inflammation and immunity (Funk, 2001; Peters-Golden and Henderson, 2007). cPLA2 plays a key role in the synthesis of eicosanoids by catalyzing the release of AA from membrane phospholipids. AA serves as substrate for cyclooxygenase-2 (COX2) and 5-lipoxygenase (5-LO), enzymes that process AA into prostaglandins and leukotrienes, respectively (Funk, 2001; Peters-Golden and Henderson, 2007). The overproduction of leukotrienes is a major cause of inflammatory disorders (Samuelsson, 1983; Peters-Golden and Henderson, 2007; Funk, 2011). They are broadly divided into two categories: the cysteinyl leukotrienes (CystLTs), which require the enzyme leukotriene C4 (LTC4) synthase (LTC4S) for their synthesis and are involved in the pathogenesis of allergic disorders such as asthma and allergic rhinitis (Funk, 2011; Kanaoka and Boyce, 2014), and leukotriene B4 (LTB4), which requires the enzyme leukotriene A4 (LTA4) hydrolase (LTA4H) and is involved in the pathogenesis of organ-specific autoimmune disorders such as rheumatoid arthritis and psoriasis (Fig. 1 A; Peters-Golden and Henderson, 2007; Yokomizo, 2015).
Figure 1.
5-LO is activated and translocates to the nucleus in human IELs, a process that is critical for NKG2D-mediated cytotoxicity. (A) Schematic of the various eicosanoid biosynthetic pathways. Upon liberation from membrane phospholipids by cPLA2, AA can be used to synthesize the various eicosanoids. Our previous work established a role for cPLA2 and AA in the NKG2D cytolytic pathway and CD pathogenesis (Tang et al., 2009). This work focuses on the pathways downstream of cPLA2 and, in particular, on the role of eicosanoids in NKG2D-mediated cytolysis and CD. (B) Three human IE-CTL lines were pretreated with vehicle control or 5-LO inhibitor MK886 for 30 min before stimulation with anti-NKG2D or anti-CD3 mAbs for the indicated time points. Translocation was determined by immunoblot analysis of nuclear extracts using an anti–5-LO antibody, with equal loading being assessed using an antibody directed against the nuclear marker TBP. Scanning densitometry was used to determine fold change with respect to unstimulated controls. A representative blot is shown at the top, with fold change ± standard deviation from three independent experiments compiled below. (C) Effector cells were pretreated for 30 min with vehicle control, 10 µM CF3, or 10 µM MK886 before the assay, which was performed against 51Cr-labeled EL4-MICA cells at an effector/target ratio of 18:1. Data are means ± standard deviation of four independent experiments using three different cell lines. (D) TALL cells were transfected with siRNAs against 5-LO, COX2, or a scrambled control siRNA by electroporation and allowed to recover for 24 h. Efficacy of knockdown was determined by Western blot (representative blots shown on the right). NKG2D-mediated cytotoxic capacity was assessed using 51Cr-labeled MICA-EL4 target cells at an effector/target ratio of 5:1. Data are means ± standard deviation of four independent experiments. (E) TALL-104 cells were pretreated with 10 µM MK886 or vehicle control for 30 min before incubation with 51Cr-labeled FcγR+ P815 target cells at the indicated effector/target ratios in the presence of antibodies against NKG2D (left) or CD3 (right). Data are means ± standard deviation of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In this study we investigated which pathway downstream of cPLA2 participated in NKG2D-mediated cytolysis and CD pathogenesis. Surprisingly, we uncovered a novel function for CystLTs as inflammatory mediators that promote NKG2D lymphokine killer activity in IE-CTLs and are up-regulated in the CD mucosa, suggesting that inhibitors of CystLTs used to treat asthma, such as montelukast, might be efficacious in the treatment of CD.
RESULTS AND DISCUSSION
5-LO plays a critical role in NKG2D-mediated cytolysis
Our previous work demonstrated that the function of NKG2D is critically regulated by the release of AA mediated by cPLA2 (Tang et al., 2009). To assess the potential role of leukotrienes in NKG2D-mediated cytolysis, we first sought to determine whether 5-LO was activated upon NKG2D stimulation. When active, 5-LO is found in the nucleus where it oxidizes AA released from phospholipids by cPLA2 into leukotrienes (Fig. 1 A; Peters-Golden and Henderson, 2007). IE-CTLs were stimulated with monoclonal antibodies against NKG2D or CD3, and nuclear fractions were separated and analyzed by Western blot using an anti–5-LO antibody (Fig. 1 B). We noted that stimulation through NKG2D but not CD3 resulted in rapid and significant increases of 5-LO within the nuclear fraction, suggesting that 5-LO is made active and is in close proximity to its substrate. In agreement, pretreatment of cells with MK886, a pharmacological inhibitor of 5-LO, blocked perinuclear increase of 5-LO in response to NKG2D. This suggests that 5-LO plays a specific role in transducing signals through NKG2D but not CD3 in IE-CTLs.
We next assessed whether 5-LO translocation was correlated with NKG2D-mediated cytolysis. IE-CTLs were incubated with the 5-LO inhibitor MK886, and their capacity to lyse MICA-expressing EL4 target cells (EL4-MICA) was determined. Inhibition of 5-LO resulted in significantly reduced cytolysis through NKG2D (Fig. 1 C). As we previously demonstrated the requirement for cPLA2 in NKG2D-mediated killing (Tang et al., 2009), we also used the pharmacological inhibitor of cPLA2 AACOCF3 (CF3) as a control, noting that the use of MK886 had a comparable effect on cytotoxicity. To further demonstrate a role for 5-LO in NKG2D-mediated cytolysis, we took advantage of the TALL-104 T cell line that is amenable to transfection. Using siRNA, we knocked down 5-LO or COX2, an enzyme that processes AA into prostaglandins but is dispensable for leukotriene synthesis (Fig. 1 A). After confirming knockdown by Western blot (not depicted), we performed cytotoxicity assays against EL4-MICA target cells. Ablation of 5-LO resulted in a significant decrease in NKG2D-mediated killing against MICA-expressing target cells compared with a scrambled control siRNA, whereas no significance was observed upon reduction of COX2 (Fig. 1 D).
Finally, we wanted to evaluate whether inhibition of 5-LO also inhibited TCR-mediated cytolysis in CTLs. To test this, we again pretreated IE-CTLs with MK886 or vehicle control and then assessed their cytotoxic capacity using an antibody-redirected lysis assay against target cells not expressing MICA. Upon cross-linking with an anti-NKG2D mAb, there was a significant decline in killing when 5-LO was inhibited (Fig. 1 E, left). However, and in accordance with previous findings showing no role for cPLA2 in TCR-mediated cytolysis (Tang et al., 2009), MK886 did not affect TCR-mediated lysis, even when baseline rates of cytotoxicity were roughly the same (Fig. 1 E, right). Overall, these data suggest that 5-LO is not only active in IE-CTLs, but that it drives NKG2D-mediated lysis of target cells specifically and does not play a role in TCR-mediated cytotoxicity.
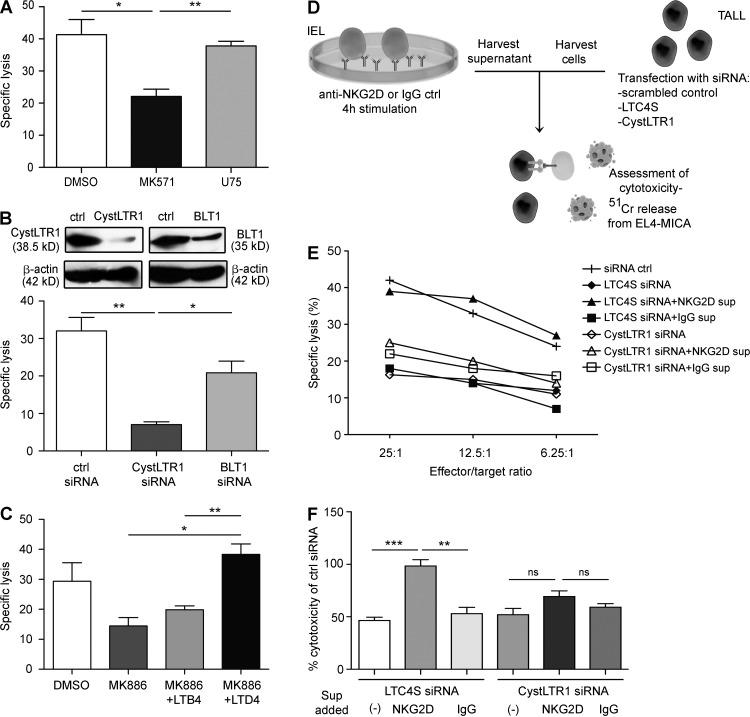
CystLT synthesis and signaling in an autocrine manner plays a role in NKG2D-mediated cytotoxicity in IE-CTLs
Upon liberation of AA from the nuclear membrane by cPLA2, 5-LO catalyzes its oxidation to produce 5-hydroperoxyeicosatetraenoic acid (5-HPETE). It then converts 5-HPETE into LTA4, which can be metabolized into LTB4 by the enzyme LTA4H or the CystLTs LTC4, LTD4, and LTE4 by the enzyme LTC4S (Peters-Golden and Henderson, 2007; Kanaoka and Boyce, 2014; Yokomizo, 2015). These two pathways are distinct, as these two classes of leukotrienes bind to different receptors and have independent effects (Fig. 1 A).
We wanted to determine whether LTB4 or the CystLTs were required for NKG2D-mediated killing or whether 5-LO was involved in a different pathway. To test this, we incubated IE-CTLs with pharmacological inhibitors against LTA4H (U75) or LTC4S (MK571) and then assessed their ability to kill MICA-expressing target cells. Although inhibition of LTA4H had no effect on cytotoxicity, blockade of LTC4S resulted in significant loss of cytolytic capacity (Fig. 2 A). We then transfected TALL-104 cells with siRNA to knockdown BLT1 and CystLT receptor 1 (CystLTR1), receptors for LTB4 and the CystLTs, respectively (Fig. 2 B). Ablation of CystLTR1 resulted in significant loss of NKG2D-mediated killing compared with control siRNA and with BLT1 knockdown (Fig. 2 B).
Figure 2.
CystLT synthesis and signaling in an autocrine manner plays a role in NKG2D-mediated cytotoxicity in IE-CTLs. (A) IE-CTLs were pretreated for 30 min with pharmacological antagonists of CystLTR1 or BLT1 at 10 µM (MK571 and U75, respectively) or vehicle control (DMSO) before the cytolysis assay performed against 51Cr-labeled EL4-MICA target cells at an effector/target ratio of 18:1. Data are means ± standard deviation of three independent experiments using three different CTL lines. (B) TALL-104 cells were transfected with siRNA against CystLTR1 or BLT1 or with a scrambled control siRNA by electroporation. Efficacy of knockdown is shown by a representative Western blot at the top. Cells were allowed to recover for 24 h before incubation with 51Cr-labeled targets at an effector/target ratio of 3:1. Data are means ± standard deviation of five independent experiments. (C) Human IE-CTLs were pretreated for 30 min with 10 µM MK886 or vehicle control, and rescue experiments were performed by adding 1 µM LTD4 or LTB4 after 1 h of co-culture of effector cells with EL4-MICA targets at an effector/target ratio of 18:1. The cytolytic assay was performed after an additional 3 h of incubation. Data are means ± standard deviation of three independent experiments using two different cell lines. (D and E) Schematic diagram of experimental design. Human IE-CTLs were stimulated for 4 h with plate-bound antibodies against NKG2D or an IgG control. Supernatants were collected and used to stimulate TALL-104 cells transfected with siRNA against LTC4S or CystLTR1 or a scrambled control siRNA in the presence of 51Cr-labeled EL4-MICA targets at the indicated effector/target ratios. Two different human IE-CTL lines were used. Data are representative of three independent experiments. (F) Experimental design is as in E, but here the effector/target ratio is 12.5:1 only. Data are normalized to the cytotoxic capacity measured in TALL cells that were transfected with control siRNA and are presented as means ± standard deviation of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To further establish a crucial role for the CystLTs specifically, we tested whether their addition could rescue cytotoxicity after pharmacological inhibition of 5-LO. We administered LTD4 or LTB4 to IE-CTLs pretreated with MK886 and found that the addition of LTD4 but not LTB4 resulted in total restoration of cytotoxicity (Fig. 2 C). Finally, we determined whether LTD4 alone was sufficient to increase NKG2D-mediated cytolysis. Treatment with LTD4 marginally increased NKG2D-mediated cytolysis, albeit not significantly (not depicted). Overall, these data demonstrate an essential role for the CystLT–CystLTR1 but not LTB4–BLT1 pathway in CTL killing mediated by NKG2D.
We then wanted to know whether CystLTs produced through 5-LO activation were capable of driving cytotoxicity through NKG2D in cis in IE-CTLs. We designed an experiment to test this hypothesis (Fig. 2, D–F). Human IE-CTLs were stimulated with plate-bound anti-NKG2D or control IgG antibodies for 4 h. Supernatants were harvested and used in a cytotoxicity assay featuring TALL-104 effector cells that had been subjected to siRNA knockdown of LTC4S or CystLTR1. As shown previously, siRNA knockdown of either LTC4S or CystLTR1 efficiently suppressed killing of MICA-expressing target cells (Fig. 2 E). Transfer of the supernatant from IE-CTLs stimulated with IgG isotype control antibodies had no effect on cytotoxicity and could not rescue killing after knockdown (Fig. 2 E). However, supernatant from IE-CTLs stimulated with anti-NKG2D mAbs was able to completely restore cytolytic capacity to cells lacking LTC4S but crucially had no effect on cells that received the siRNA against CystLTR1 (Fig. 2 E). A summary of the results normalized to siRNA control is shown in Fig. 2 F. These data demonstrate that NKG2D induces CystLT production in CTLs that can act in an autocrine manner to promote NKG2D-mediated cytolysis. CystLTs have been demonstrated to function in the activation of granulocytes, smooth muscle, and some subsets of macrophages (Kanaoka and Boyce, 2014). In adaptive immunity, although CystLTs have been linked to T cell migration (Peters-Golden and Henderson, 2007), a role in T cell effector function and, in particular, cytolysis has never been documented. Although unanticipated, this finding is consistent with the discovery that cPLA2 and IL-15 are involved in NKG2D-mediated cytolysis in CTLs and CD pathogenesis (Tang et al., 2009). The mechanism by which CysLTR1 promotes cytolysis remains elusive. One possibility may involve the ability of CystLTR1 to drive activation and expression of integrins (Massoumi et al., 2003; Meliton et al., 2007; Boehmler et al., 2009).
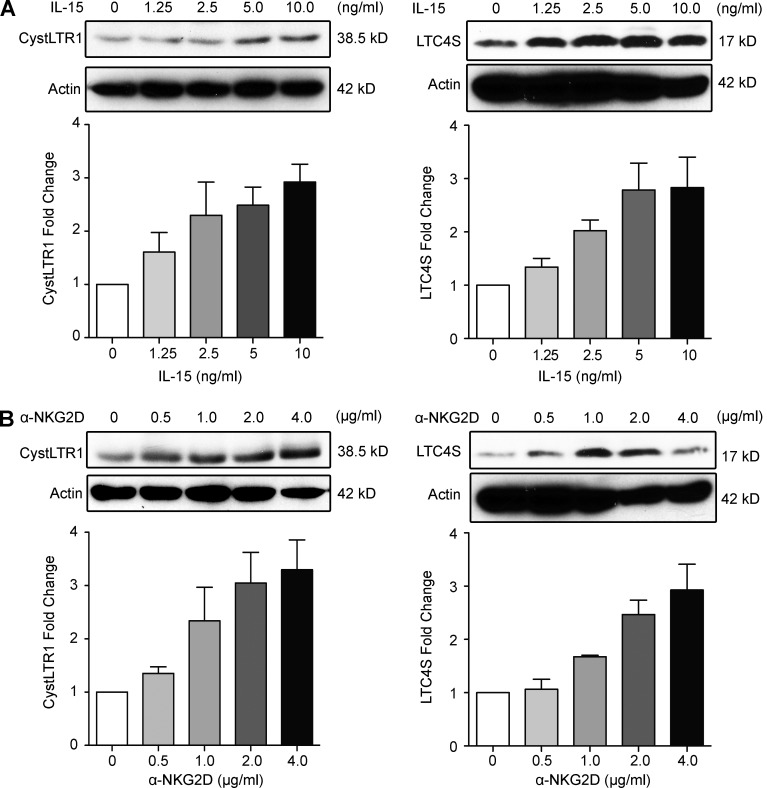
CystLTR1 and LTC4S are up-regulated by IL-15 and NKG2D
IL-15 is a proinflammatory cytokine up-regulated in various tissues under conditions of stress and inflammation (Abadie and Jabri, 2014), including in IECs of CD patients (Jabri et al., 2000; Mention et al., 2003). It is thought to play an important role in tissue immunity by directing immune responses toward TH1 immunity and promoting CTL responses (Abadie and Jabri, 2014). Our previous work has shown that IL-15 acts on multiple levels within the NKG2D–DAP10 signaling pathway, priming IE-CTLs to mediate cytolysis and release AA upon binding of NKG2D to stress ligands (Meresse et al., 2004; Tang et al., 2009). We wanted to determine whether IL-15 also directly promoted the CystLT pathway. Additionally, we wanted to test whether signaling through NKG2D would amplify CystLT production and/or responsiveness. Western blot analysis was used to test expression of CystLTR1 and LTC4S at the protein level after stimulation of IE-CTLs with the indicated doses of IL-15 or a cross-linking anti-NKG2D mAb (Fig. 3). We noted robust up-regulation of both CystLTR1 (Fig. 3, left) and LTC4S (Fig. 3, right) after incubation with either IL-15 (Fig. 3 A) or the NKG2D mAb (Fig. 3 B). In conjunction with our finding that NKG2D cannot mediate cytolysis in IE-CTLs deficient in LTC4S and that supernatants from NKG2D-stimulated cells can promote cytolysis in IE-CTLs expressing CystLTR1 (Fig. 2 E), these data support the notion that CystLTs can act in an autocrine manner in IE-CTLs upon NKG2D stimulation. However, we do not exclude the possibility that CystLTs produced by other cell types may also play a role in driving IE-CTL activation. Furthermore, these data suggest that IL-15, a cytokine associated with TH1 immunity (Abadie and Jabri, 2014), can up-regulate LTC4S and CystLTR1 in an analogous manner to TH2 cytokines (Jiang et al., 2006). Notably, this finding suggests that CystLTs may have deleterious roles in TH1-mediated autoimmune diseases, in particular disorders where IL-15 and NKG2D have been shown to contribute to pathogenesis.
Figure 3.
CystLTR1 and LTC4S are up-regulated by IL-15 and NKG2D in a dose-dependent manner. (A) Fresh IE-CTLs were cultured overnight and stimulated with the indicated concentration of IL-15 for 3 h. Cell lysates were immunoblotted with antibodies against CystLTR1 or LTC4S. Blots are representative of four independent experiments. Bottom panels show mean fold change ± standard deviation of four independent experiments. (B) Fresh IE-CTLs were cultured as in A but were stimulated with an antibody against NKG2D for 3 h in lieu of IL-15. Lysates were isolated and immunoblotted as in A. β-Actin is shown as a loading control. Blots are representative of four independent experiments, and the bottom panels present means ± standard deviation of four independent experiments.
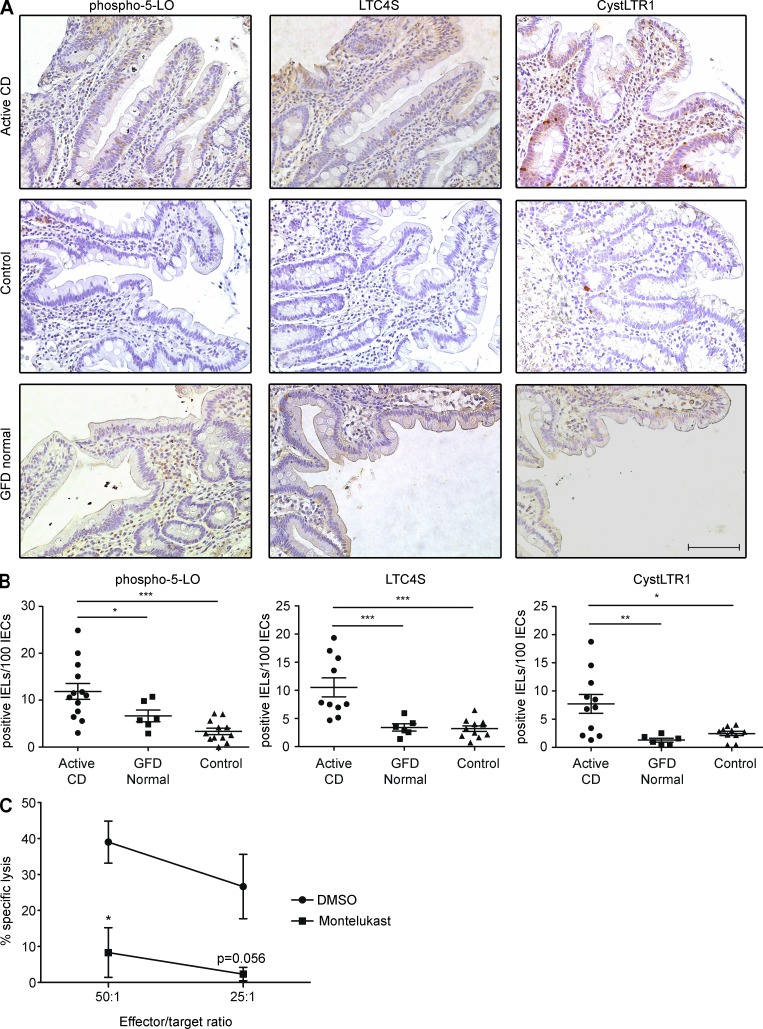
CystLTs are dysregulated in active CD patients
Knowing that NKG2D and IL-15 both induce the CystLT pathway and are up-regulated in CD, we hypothesized that CystLTs and their receptors were up-regulated in CD. To test this hypothesis, we stained intestinal sections of active CD, gluten-free diet (GFD), and control patients undergoing biopsies for unrelated intestinal disorders by immunohistochemistry with antibodies for phospho–5-LO, LTC4S, or CystLTR1 (Fig. 4 A). We noted that there was a significant increase in the number (Fig. 4 B) and percentage (not depicted) of IE-CTLs expressing phospho–5-LO, LTC4S, and CystLTR1 in active CD patients. However, in CD patients on a GFD the CystLT pathway was not up-regulated, in agreement with the reported decrease in the expression of NKG2D and its ligand MICA/B (Hüe et al., 2004; Meresse et al., 2004). This result also indicates a positive correlation between CystLT production and signaling and villous atrophy in CD. Consistent with a role for IL-15 signaling in IECs, we found that the CystLT pathway was also actively induced in these cells (not depicted). CystLTs produced by IE-CTLs and IECs may also activate neutrophils, eosinophils, and macrophages in the intestinal environment of CD patients. How CystLT signaling in IECs and granulocytes may contribute to CD pathogenesis remains to be determined. Overall and in conjunction with the finding that CystLTs are critical mediators in NKG2D-mediated lymphokine killer activity in CTLs (Fig. 2), these data suggest that CystLTs play a role in the pathogenesis of CD.
Figure 4.
The CystLT pathway is dysregulated in active CD patients. (A) Immunohistochemical staining was performed on duodenal paraffin-embedded intestinal sections for the following markers: phospho–5-LO, LTC4S, and CystLTR1. The top panels are representative images from stainings of sections from patients with active CD, defined as those with elevated levels of serum anti–tissue transglutaminase antibodies, partial or total duodenal villous atrophy, crypt hyperplasia, and increased presence of IELs. The middle panels are representative images from control biopsies, defined as patients undergoing upper endoscopy for unrelated disorders who did not meet the criteria for CD. The bottom panels are representative images from patients with CD on a GFD for a mean of 3 yr who lacked villous atrophy. Bar, 100 µM. (B) The number of IELs stained positive for phospho–5-LO, LTC4S, and CystLTR1 per 100 IECs were counted and plotted. Slides from 15 active CD patients, 6 GFD patients, and 12 control patients were stained with each antibody. Data are presented as means ± standard deviation. P-values were determined by Wilcoxon rank sum test. (C) After pretreatment for 30 min with 10 µM montelukast, IELs were incubated with 51Cr-labeled effector cells expressing MICA at the indicated effector to target ratio, and specific lysis was measured. Data are presented as means ± standard deviation of three independent experiments using two different cell lines. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The CystLT pathway might represent a novel therapeutic target for treatment of CD and TH1-mediated autoimmune disorders
Although CystLTs have previously been implicated in allergic diseases, in particular asthma (Kanaoka and Boyce, 2014), here we demonstrate an unexpected role for these molecules in CD, a TH1-mediated disorder. Given that NKG2D plays an important role in IEC destruction and the development of villous atrophy in CD, we believe that drugs targeting the CystLT pathway would constitute a very attractive therapeutic avenue because they would disrupt the end effector response responsible for tissue damage. It is possible to specifically target this pathway using drugs such as montelukast, which is FDA approved for the treatment of asthma and allergic rhinitis (Kanaoka and Boyce, 2014). Indeed, we were able to assess the potential of montelukast to significantly suppress cytotoxicity through NKG2D in human IE-CTL lines in vitro (Fig. 4 C). Such a drug might be able to accelerate mucosal healing after introduction of a GFD in active CD patients. Indeed, in a significant subset of adult CD patients, it is known that the GFD is not sufficient to fully promote normal mucosal healing (Rubio-Tapia et al., 2010). Intriguingly, there have been case reports of patients suffering from both asthma and CD who were prescribed CystLT inhibitors for the treatment of their lung disease for whom an unexpected positive additive effect was the resolution of their CD symptoms poorly controlled on a GFD (Fee, 2002). Conversely, our findings also suggest that cyclooxygenase inhibitors may exacerbate the disease by enhancing the production of leukotrienes. More generally, it is possible that the CystLT pathway could also be a therapeutic target in organ-specific autoimmune disorders where IL-15 and/or NKG2D play a role, such as rheumatoid arthritis (Groh et al., 2003) and type-1 diabetes (Ogasawara et al., 2004; Chen et al., 2013). It has become increasingly appreciated that the functions exerted by CystLTs and their receptors are much more complex than previously thought (Bäck et al., 2011; Kanaoka and Boyce, 2014). Further uncovering how these receptors are regulated and what physiopathological functions they mediate will yield further therapeutic insights and contribute to our understanding of how effector T cells are regulated in tissues.
MATERIALS AND METHODS
Human subjects.
For immunohistochemical experiments, the total number of patients (% female) was 15 (66.7%), 6 (83.3%), and 12 (66.7%) for active CD, GFD, and control subjects, respectively. The mean age was 32.1 yr for active CD, 37.5 yr for GFD, and 28.3 yr for control subjects. Additionally, biopsies were obtained from four active CD and three control patients to generate seven NKG2D+TCRαβ+CD8+ IE-CTL cell lines.
Diagnosis of CD was based on the presence of elevated anti-transglutaminase antibodies in serum, the expression of HLA DQ2 or DQ8, the presence of increased intraepithelial lymphocytes (IELs), partial or total duodenal villous atrophy, crypt hyperplasia on duodenal biopsy, and clinical response to a GFD. Villous atrophy was found in 100% of active CD patients, 0% of GFD patients, and 0% of controls. The mean time on a GFD was 3 yr for the GFD group. Control individuals were undergoing endoscopies and biopsies for functional intestinal disorders of nonceliac origin. All subjects gave written informed consent, and all protocols were approved by the University of Chicago Institutional Review Board.
Generation of CTL lines and cell culture.
Intraepithelial and peripheral blood NKG2D+TCR+CD8+ CTL lines and clones were isolated as previously described from biopsies of four active CD and three control patients (Jabri et al., 2000). After isolation, IE-CTLs were stained with anti-NKG2D, anti-TCRαβ, anti-CD8α, and anti-CD103 antibodies, and NKG2D+TCRαβ+CD8+ IE-CTLs were purified using a FACSAria (BD). After sorting, cell lines were generated and cultured as previously described (Jabri et al., 2002). Importantly, NKG2D signaling and NKG2D-mediated cytolysis are similar in control and CD NKG2D+TCRαβ+CD8+ IE-CTL lines, as long as these IE-CTLs are in an effector state and cultured in the presence of IL-15 or high concentrations of IL-2, which mimic the effects of IL-15 (Meresse et al., 2004). These conditions reflect the environment of IE-CTLs in CD (Meresse et al., 2004). Indeed, what differentiates CD and controls in vivo is that both IL-15 and the NKG2D ligand MICA/B are up-regulated in the epithelium of CD patients, thus providing both signals to IE-CTLs that enable NKG2D to mediate direct cytolysis (Meresse et al., 2004).
The TALL-104 line is a CD8+TCRαβ+ cytotoxic cell line established from the blood of a patient with acute lymphoblastic leukemia (ATCC). It was cultured in Iscove’s modified Dulbecco’s medium plus 20% FCS, antibiotics, and 100 U/ml recombinant human IL-2.
MICA-transfected EL4 (EL4-MICA) and control vector EL4 (ATCC TIB-39) are mouse T lymphoma cell lines. They were grown in RPMI 1640 supplemented with 10% FCS, glutamine, and antibiotics, and MICA-expressing cells were maintained using G418. As a target cell for the antibody-redirected lysis experiments, the P815 (ATCC TIB-64TM) mouse mastocytoma cell line was used. It was grown in RPMI 1640 supplemented with 10% FCS, glutamine, and antibiotics.
Reagents, antibodies, and recombinant cytokines.
Human IL-15 and IL-2 were purchased from BD. LTB4, LTD4, BLT1 antagonist U75302, polyclonal antibodies specific for 5-LO, polyclonal antibodies specific for CystLTR1, polyclonal antibodies specific for BLT1, and montelukast (sodium salt) were obtained from Cayman Chemical. Anti–TATA-binding protein (TBP) antibody was obtained from Biodesign International. Anti-CD3 (clone UCHT1, IgG1) and anti-NKG2D (clone 1D11, IgG1) monoclonal antibodies with corresponding IgG1 isotype-matched control were purchased from BD. Polyclonal antibodies against human LTC4S were purchased from Aviva Systems Biology and Cell Signaling Technology. A monoclonal antibody specific for COX2 (clone D5H5) was purchased from Cell Signaling Technology. Anti-phosphotyrosine monoclonal antibodies (clone 4G10) were obtained from EMD Millipore. The chemical antagonists for 5-LO (MK886) and CystLTR1 (MK571), along with anti–β-actin monoclonal antibodies, were purchased from Sigma-Aldrich. F(ab′)2 goat anti–mouse antibodies were obtained from Jackson ImmunoResearch Laboratories, Inc.
siRNA and transfection.
siRNAs specific for human 5-LO, COX2, CystLTR1, BLT1, LTC4S, and a scrambled control siRNA were purchased from Santa Cruz Biotechnology, Inc. TALL-104 cells were electroporated using a Lonza Amaxa Nucleofector in Lonza cell line nucleofection solution V and program T-20. Cells were allowed to recover in culture for 24 h after transfection before use in experiments. Efficiency and specificity of knockdown were assessed at the protein level by Western blot.
Western blot analysis.
Before stimulation with IL-15 and/or NKG2D, human IELs were starved of IL-2 in culture for 20–24 h. To examine CystLTR1 and LTC4S expression, cells were treated with IL-15 at the indicated concentrations for 3 h at 37°C. Immunoreceptor cross-linking was performed by incubating cells for 4 min with the indicated receptor-specific antibody before the addition of F(ab′)2 GAM for 3 h at 37°C. Cells were then harvested and lysed for 20 min in cold lysis buffer containing fresh protease inhibitors (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton-X 100, 1 mM EDTA, and a protease inhibitor cocktail tablet [Thermo Fisher Scientific]). Cellular debris was removed by centrifugation at 15,000 RPM in a table-top centrifuge for 20 min at 4°C. 80 µg lysate protein was subjected to SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad Laboratories). Detection was performed using the indicated primary antibodies, followed by an HRP-conjugated secondary IgG and the LumiGLO chemiluminescent substrate kit (Cell Signaling Technology). Scanning densitometry was performed using ImageJ software (National Institutes of Health).
To investigate perinuclear translocation of 5-LO in response to stimulation, nuclear fractions were extracted using the NE-PER nuclear extraction kit (Thermo Fisher Scientific). Samples were then run on a 10% polyacrylamide gel in the presence of SDS and were subjected to electrophoresis. Separated proteins were transferred to nitrocellulose membranes (Bio-Rad Laboratories) and blocked with 5% nonfat dry milk in TBS-T for 1 h, followed by overnight incubation with primary antibody at 4°C. Membranes were washed and then incubated for 45 min with an HRP-linked goat anti–rabbit or goat anti–mouse antibody at room temperature. Development was performed by enhanced chemiluminescence, and membranes were exposed to films. Equal loading and the nuclear nature of the extract were confirmed using an anti-TBP antibody that recognizes a DNA-binding protein specific for the TATA box.
Cytotoxicity assay.
Chromium release assays were performed as previously described (Tang et al., 2009) In brief, EL4-MICA and control EL4 cells were radiolabeled with 51Cr before incubation with effector cells in duplicate wells at the indicated effector/target ratios. Chromium release into the supernatant was measured using a scintillation counter (Packard). Maximum release was determined by the addition of 10% SDS to target cells, while spontaneous release was also assessed and ranged from 5–10% of the maximum. The percentage of specific cytotoxicity was calculated using the formula 100 × (CPM experimental − CPM spontaneous)/(CPM maximum − CPM spontaneous). Where indicated, effector cells were treated for 30 min before and during the cytotoxic assay using various inhibitors or lipid mediators or, alternatively, equivalent concentrations of either DMSO or ethanol vehicle control. Alternatively, antibody-redirected cytotoxicity assays were performed, wherein effector cells were activated through a specific cross-linking antibody and directed to lyse target cells lacking stress ligands. Finally, cytotoxicity was also assessed after stimulation with conditioned supernatants. In brief, IELs were stimulated with plate-bound NKG2D-specific cross-linking antibodies for 4 min before the addition of GAM for 3 h, and supernatants were harvested and applied to TALL cells 24 h after being transfected with siRNA specific for LTC4S or CystLTR1. Subsequent cytotoxicity of these TALL cells against MICA-expressing targets was then evaluated.
Immunohistochemical staining.
Immunohistochemical staining was performed on paraffin sections after antigen retrieval and peroxidase block (EnVision+ HRP system; Dako). Slides were stained with anti-phospho–5-LO (1:50, L1168; Sigma-Aldrich), anti-CystLTR1 (5 ng/µl, C3491; Sigma-Aldrich), and anti-LTC4S (0.005 ng/µl, ARP49035_T100; Aviva) antibodies, followed by HRP-conjugated anti–rabbit secondary antibody (Dako). Antibody revelation was performed with DAB chromogen (Dako), and counterstain was completed with hematoxylin (Gill 3 Hematoxylin; Thermo Fisher Scientific). Slides were evaluated on a DM2500 microscope (Leica) using an HC Plan Apochromat 20×/0.70 lens (Leica) at 22°C. Images were acquired by a Retiga Exi FAST 1394 camera (QImaging), processed by Image-Pro Plus 7.0 software, and exported in TIF format. Counting of positive IELs was performed by two independent readers in a double-blinded manner.
Statistical analysis.
Wilcoxon rank sum testing was used to assess statistical significance, denoted as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Acknowledgments
We thank CD patients and their family members, as well as the University of Chicago Celiac Disease Center for supporting our research. We thank Luis B. Barreiro for fruitful discussions.
This work was supported by grants from the Digestive Diseases Research Core Center (DK42086) at the University of Chicago and from the US National Institutes of Health (RO1DK67180) to B. Jabri.
The authors declare no competing financial interests.
Author contributions: F. Tang, B. Sally, V. Discepolo, K. Lesko, V. Abadie, and C. Ciszewski performed the experiments. F. Tang, B. Sally, V. Discepolo, K. Lesko, S.S. Kupfer, and B. Jabri analyzed the data. C. Semrad, S. Guandalini, and S.S. Kupfer provided clinical data and patient material. B. Sally and B. Jabri wrote the manuscript. B. Jabri conceived, designed, and supervised the study.
Footnotes
Abbreviations used:
- 5-LO
- 5-lipoxygenase
- AA
- arachidonic acid
- CD
- celiac disease
- CTL
- cytotoxic effector T cell
- CystLT
- cysteinyl leukotriene
- GFD
- gluten-free diet
- IE-CTL
- intraepithelial CTL
- IEC
- intestinal epithelial cell
- IEL
- intraepithelial lymphocyte
- TBP
- TATA-binding protein
References
- Abadie V., and Jabri B.. 2014. IL-15: a central regulator of celiac disease immunopathology. Immunol. Rev. 260:221–234. 10.1111/imr.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäck M., Dahlén S.-E., Drazen J.M., Evans J.F., Serhan C.N., Shimizu T., Yokomizo T., and Rovati G.E.. 2011. International Union of Basic and Clinical Pharmacology. LXXXIV: leukotriene receptor nomenclature, distribution, and pathophysiological functions. Pharmacol. Rev. 63:539–584. 10.1124/pr.110.004184 [DOI] [PubMed] [Google Scholar]
- Bauer S., Groh V., Wu J., Steinle A., Phillips J.H., Lanier L.L., and Spies T.. 1999. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 285:727–729. 10.1126/science.285.5428.727 [DOI] [PubMed] [Google Scholar]
- Billadeau D.D., Upshaw J.L., Schoon R.A., Dick C.J., and Leibson P.J.. 2003. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat. Immunol. 4:557–564. 10.1038/ni929 [DOI] [PubMed] [Google Scholar]
- Boehmler A.M., Drost A., Jaggy L., Seitz G., Wiesner T., Denzlinger C., Kanz L., and Möhle R.. 2009. The CysLT1 ligand leukotriene D4 supports α4β1- and α5β1-mediated adhesion and proliferation of CD34+ hematopoietic progenitor cells. J. Immunol. 182:6789–6798. 10.4049/jimmunol.0801525 [DOI] [PubMed] [Google Scholar]
- Chen J., Feigenbaum L., Awasthi P., Butcher D.O., Anver M.R., Golubeva Y.G., Bamford R., Zhang X., St. Claire M.B., Thomas C.J., et al. 2013. Insulin-dependent diabetes induced by pancreatic beta cell expression of IL-15 and IL-15Rα. Proc. Natl. Acad. Sci. USA. 110:13534–13539. 10.1073/pnas.1312911110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee W.H. 2002. Irritable bowel syndrome helped by montelukast. Chest. 122:1497 10.1378/chest.122.4.1497 [DOI] [PubMed] [Google Scholar]
- Funk C.D. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 294:1871–1875. 10.1126/science.294.5548.1871 [DOI] [PubMed] [Google Scholar]
- Funk C.D. 2011. Leukotriene inflammatory mediators meet their match. Sci. Transl. Med. 3:ps3 10.1126/scitranslmed.3002040 [DOI] [PubMed] [Google Scholar]
- Groh V., Rhinehart R., Randolph-Habecker J., Topp M.S., Riddell S.R., and Spies T.. 2001. Costimulation of CD8αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2:255–260. 10.1038/85321 [DOI] [PubMed] [Google Scholar]
- Groh V., Bruhl A., El-Gabalawy H., Nelson J.L., and Spies T.. 2003. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 100:9452–9457. 10.1073/pnas.1632807100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüe S., Mention J.-J., Monteiro R.C., Zhang S., Cellier C., Schmitz J., Verkarre V., Fodil N., Bahram S., Cerf-Bensussan N., and Caillat-Zucman S.. 2004. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 21:367–377. 10.1016/j.immuni.2004.06.018 [DOI] [PubMed] [Google Scholar]
- Jabri B., de Serre N.P.M., Cellier C., Evans K., Gache C., Carvalho C., Mougenot J.F., Allez M., Jian R., Desreumaux P., et al. 2000. Selective expansion of intraepithelial lymphocytes expressing the HLA-E–specific natural killer receptor CD94 in celiac disease. Gastroenterology. 118:867–879. 10.1016/S0016-5085(00)70173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabri B., Selby J.M., Negulescu H., Lee L., Roberts A.I., Beavis A., Lopez-Botet M., Ebert E.C., and Winchester R.J.. 2002. TCR specificity dictates CD94/NKG2A expression by human CTL. Immunity. 17:487–499. 10.1016/S1074-7613(02)00427-2 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Kanaoka Y., Feng C., Nocka K., Rao S., and Boyce J.A.. 2006. Cutting edge: Interleukin 4-dependent mast cell proliferation requires autocrine/intracrine cysteinyl leukotriene-induced signaling. J. Immunol. 177:2755–2759. 10.4049/jimmunol.177.5.2755 [DOI] [PubMed] [Google Scholar]
- Kanaoka Y., and Boyce J.A.. 2014. Cysteinyl leukotrienes and their receptors; emerging concepts. Allergy Asthma Immunol. Res. 6:288–295. 10.4168/aair.2014.6.4.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoumi R., Nielsen C.K., Azemovic D., and Sjölander A.. 2003. Leukotriene D4-induced adhesion of Caco-2 cells is mediated by prostaglandin E2 and upregulation of α2β1-integrin. Exp. Cell Res. 289:342–351. 10.1016/S0014-4827(03)00285-4 [DOI] [PubMed] [Google Scholar]
- Meliton A.Y., Munoz N.M., and Leff A.R.. 2007. Blockade of avidity and focal clustering of β2-integrin by cysteinyl leukotriene antagonism attenuates eosinophil adhesion. J. Allergy Clin. Immunol. 120:1316–1323. 10.1016/j.jaci.2007.07.038 [DOI] [PubMed] [Google Scholar]
- Mention J.-J., Ben Ahmed M., Bègue B., Barbe U., Verkarre V., Asnafi V., Colombel J.F., Cugnenc P.H., Ruemmele F.M., McIntyre E., et al. 2003. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 125:730–745. 10.1016/S0016-5085(03)01047-3 [DOI] [PubMed] [Google Scholar]
- Meresse B., Chen Z., Ciszewski C., Tretiakova M., Bhagat G., Krausz T.N., Raulet D.H., Lanier L.L., Groh V., Spies T., et al. 2004. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 21:357–366. 10.1016/j.immuni.2004.06.020 [DOI] [PubMed] [Google Scholar]
- Ogasawara K., Hamerman J.A., Ehrlich L.R., Bour-Jordan H., Santamaria P., Bluestone J.A., and Lanier L.L.. 2004. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity. 20:757–767. 10.1016/j.immuni.2004.05.008 [DOI] [PubMed] [Google Scholar]
- Peters-Golden M., and Henderson W.R. Jr. 2007. Leukotrienes. N. Engl. J. Med. 357:1841–1854. 10.1056/NEJMra071371 [DOI] [PubMed] [Google Scholar]
- Roberts A.I., Lee L., Schwarz E., Groh V., Spies T., Ebert E.C., and Jabri B.. 2001. NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J. Immunol. 167:5527–5530. 10.4049/jimmunol.167.10.5527 [DOI] [PubMed] [Google Scholar]
- Rosen D.B., Araki M., Hamerman J.A., Chen T., Yamamura T., and Lanier L.L.. 2004. A Structural basis for the association of DAP12 with mouse, but not human, NKG2D. J. Immunol. 173:2470–2478. 10.4049/jimmunol.173.4.2470 [DOI] [PubMed] [Google Scholar]
- Rubio-Tapia A., Rahim M.W., See J.A., Lahr B.D., Wu T.T., and Murray J.A.. 2010. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am. J. Gastroenterol. 105:1412–1420. 10.1038/ajg.2010.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B. 1983. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 220:568–575. 10.1126/science.6301011 [DOI] [PubMed] [Google Scholar]
- Segovis C.M., Schoon R.A., Dick C.J., Nacusi L.P., Leibson P.J., and Billadeau D.D.. 2009. PI3K links NKG2D signaling to a CrkL pathway involved in natural killer cell adhesion, polarity, and granule secretion. J. Immunol. 182:6933–6942. 10.4049/jimmunol.0803840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid L.M., and Jabri B.. 2013. Triggers and drivers of autoimmunity: lessons from coeliac disease. Nat. Rev. Immunol. 13:294–302. 10.1038/nri3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F., Chen Z., Ciszewski C., Setty M., Solus J., Tretiakova M., Ebert E., Han J., Lin A., Guandalini S., et al. 2009. Cytosolic PLA2 is required for CTL-mediated immunopathology of celiac disease via NKG2D and IL-15. J. Exp. Med. 206:707–719. 10.1084/jem.20071887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upshaw J.L., and Leibson P.J.. 2006. NKG2D-mediated activation of cytotoxic lymphocytes: unique signaling pathways and distinct functional outcomes. Semin. Immunol. 18:167–175. 10.1016/j.smim.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Upshaw J.L., Arneson L.N., Schoon R.A., Dick C.J., Billadeau D.D., and Leibson P.J.. 2006. NKG2D-mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells. Nat. Immunol. 7:524–532. 10.1038/ni1325 [DOI] [PubMed] [Google Scholar]
- Wu J., Song Y., Bakker A.B., Bauer S., Spies T., Lanier L.L., and Phillips J.H.. 1999. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 285:730–732. 10.1126/science.285.5428.730 [DOI] [PubMed] [Google Scholar]
- Yokomizo T. 2015. Two distinct leukotriene B4 receptors, BLT1 and BLT2. J. Biochem. 157:65–71. 10.1093/jb/mvu078 [DOI] [PubMed] [Google Scholar]