Paterson et al. demonstrate that, in contrast to CTLA-4 germline knockout mice, conditional deletion on T reg cells during adulthood confers protection from EAE and does not increase resistance to tumors.
Abstract
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is an essential negative regulator of T cell responses. Germline Ctla4 deficiency is lethal, making investigation of the function of CTLA-4 on mature T cells challenging. To elucidate the function of CTLA-4 on mature T cells, we have conditionally ablated Ctla4 in adult mice. We show that, in contrast to germline knockout mice, deletion of Ctla4 during adulthood does not precipitate systemic autoimmunity, but surprisingly confers protection from experimental autoimmune encephalomyelitis (EAE) and does not lead to increased resistance to MC38 tumors. Deletion of Ctla4 during adulthood was accompanied by activation and expansion of both conventional CD4+Foxp3− (T conv) and regulatory Foxp3+ (T reg cells) T cell subsets; however, deletion of CTLA-4 on T reg cells was necessary and sufficient for protection from EAE. CTLA-4 deleted T reg cells remained functionally suppressive. Deletion of Ctla4 on T reg cells alone or on all adult T cells led to major changes in the Ctla4 sufficient T conv cell compartment, including up-regulation of immunoinhibitory molecules IL-10, LAG-3 and PD-1, thereby providing a compensatory immunosuppressive mechanism. Collectively, our findings point to a profound role for CTLA-4 on T reg cells in limiting their peripheral expansion and activation, thereby regulating the phenotype and function of T conv cells.
Although the specificity of T cell activation is determined by the interaction of antigenic peptide–MHC complex and the TCR, the functional outcome of the T cell response is profoundly influenced by co-stimulatory and co-inhibitory signals. The co-inhibitory receptor cytotoxic T lymphocyte antigen-4 (CTLA-4; CD152) is a potent negative regulator of T cell responses (Sharpe and Freeman, 2002; Fife and Bluestone, 2008). CTLA-4 is a structural homologue of the co-stimulatory receptor CD28, but binds with higher affinity to the same ligands, B7-1 (CD80) and B7-2 (CD86), which are primarily expressed by APCs (Freeman et al., 1991, 1993; Harper et al., 1991; Linsley et al., 1991). Whereas CD28 is constitutively expressed on most T cells, CTLA-4 is constitutively expressed on CD4+Foxp3+ regulatory T (T reg) cells (Metzler et al., 1999; Read et al., 2000; Takahashi et al., 2000), and appears on CD4+Foxp3− conventional T (T conv) cells after activation (Freeman et al., 1992; Linsley et al., 1992; Walunas et al., 1996). Germline Ctla4-deficient mice develop a fatal lymphoproliferative disorder within the first month of life (Tivol et al., 1995; Waterhouse et al., 1995). Ctla4 has been implicated as a susceptibility gene in human autoimmune diseases, with several disease-associated polymorphisms reported (Ueda et al., 2003; Gough et al., 2005; Scalapino and Daikh, 2008). Furthermore, anti–CTLA-4 antibodies have demonstrated efficacy in enhancing antitumor immune responses in cancer patients (Hodi et al., 2010; Robert et al., 2011), and an anti–CTLA-4 monoclonal antibody is now approved by the United States Food and Drug Administration (FDA).
Despite the striking phenotype of the Ctla4-deficient mouse, the mechanisms by which CTLA-4 restrains T cell activation remain controversial. Initial studies pointed to a cell-intrinsic role; a tyrosine-containing motif in the cytoplasmic tail of CTLA-4 is responsible for recruitment of phosphatases SHP-2 and PP2A, which leads to inhibition of signaling downstream of TCR (Marengère et al., 1996; Cilio et al., 1998; Lee et al., 1998; Chuang et al., 1999, 2000). CTLA-4 signaling, as well as exclusion of CD28 from the immunological synapse due to preferential binding of B7 molecules by CTLA-4, results in less activation of NF-κB, NFAT, and AP-1, and inhibition of proliferation and IL-2 secretion (Krummel and Allison, 1996; Fraser et al., 1999; Olsson et al., 1999; Greenwald et al., 2002). The cytoplasmic domain of CTLA-4 is also necessary for TCR hyposignaling in T reg cells (Tai et al., 2012). The kinase PKC-η binds to the CTLA-4 cytoplasmic domain, is recruited to the immunological synapse in T reg cells, and is an important mediator of CTLA-4 function in T reg cells (Kong et al., 2014).
Although several studies support cell-intrinsic mechanisms of CTLA-4 function (Ise et al., 2010; Jain et al., 2010), there are also data supporting cell-extrinsic mechanisms: mixed BM chimeras of wild-type and CTLA-4–deficient BM are healthy, whereas chimeras reconstituted with CTLA-4–deficient BM stem cells alone develop a fatal autoimmune phenotype similar to that of germline CTLA-4–deficient mice (Bachmann et al., 1999). Cell-extrinsic mechanisms of CTLA-4 function are most likely achieved by interaction of a CTLA-4–expressing cell with a B7-expressing cell. CTLA-4 has been suggested to down-regulate B7-1 or B7-2 on APCs, either by indirect suppression of the APC, signaling through B7, or removal of B7 from the cell surface by trans-endocytosis (Fallarino et al., 2003; Onishi et al., 2008; Wing et al., 2008; Qureshi et al., 2011). Foxp3+ T reg cells are known to act via a dominant, trans-acting function, and the Ctla4 gene is a transcriptional target of Foxp3 (Wu et al., 2006; Zheng et al., 2007). Mice specifically lacking CTLA-4 on T reg cells (throughout development) die of an autoimmune syndrome similar to that seen in CTLA-4–deficient mice, albeit with delayed kinetics (Wing et al., 2008). In addition, the health of mixed blastocyst and bone marrow chimeras has been shown to depend on the ongoing presence of CTLA-4–sufficient Foxp3+ T reg cells (Friedline et al., 2009).
T reg cells are present (in fact, expanded) in CTLA-4–deficient mice, suggesting that this molecule is not required for T reg cell development and proliferation (Tang et al., 2004; Schmidt et al., 2009). However, there is controversy over whether CTLA-4 is essential for T reg cell suppressive function (Walker, 2013). Multiple studies of antibody-mediated CTLA-4 blockade suggest a role for CTLA-4 in T reg cell suppressor function (Read et al., 2000, 2006; Takahashi et al., 2000; Liu et al., 2001). However, CTLA-4–deficient T reg cells are capable of suppressing disease in colitis and EAE models (Read et al., 2006; Verhagen et al., 2009), although not in an adoptive transfer model of diabetes (Schmidt et al., 2009).
The role of CTLA-4 in thymic development has been controversial, as well. Some studies have not revealed a role (Chambers et al., 1997; Schmidt et al., 2009), whereas others have shown that CTLA-4 plays a role in negative selection (Wagner et al., 1996; Cilio et al., 1998; Buhlmann et al., 2003; Takahashi et al., 2005), modulating the TCR repertoire and inhibiting natural T reg cell generation (Verhagen et al., 2009, 2013). CTLA-4 likely opposes the critical role of CD28 in promoting negative selection and thymic T reg cell differentiation (Punt et al., 1994, 1997; Salomon et al., 2000; Tang et al., 2003; Tai et al., 2005). The lack of a murine model in which CTLA-4 can be deleted on mature T cells in adult mice has led to uncertainty on the role of CTLA-4 in T reg cell function and in peripheral tolerance.
To dissect the function of CTLA-4 during adulthood, we have developed a model in which conditional ablation of Ctla4 can be pharmacologically induced by tamoxifen administration. Using this approach, we demonstrate that adult mice lacking CTLA-4 not only fail to develop spontaneous autoimmunity, but are also protected from development of experimental autoimmune encephalomyelitis (EAE) and do not show enhanced antitumor immunity. Protection from EAE is accompanied by marked expansion of functional, CTLA-4–deficient T reg cells. Inducible deletion of CTLA-4 only on T reg cells in adult mice recapitulates both EAE protection and its underlying cellular phenotypes, indicating that loss of CTLA-4 function on T reg cells is responsible for protection from disease. CTLA-4–deficient T reg cells and T conv cells show up-regulation of several immunosuppressive molecules that may contribute to EAE protection. Collectively, these studies demonstrate that CTLA-4 has a T reg cell–intrinsic role in limiting peripheral T reg cell expansion and activation, and in their capacity to control T conv cells.
RESULTS
Deletion of Ctla4 in adult mice
Due to the limitations in using the germline Ctla4 knockout mouse to study CTLA-4 function, we generated a novel C57BL/6 strain harboring a conditional Ctla4 allele (Fig. 1 A). To validate this mouse strain, we crossed Ctla4fl/fl to LckCre+ mice to generate LckCre+Ctla4fl/fl mice, which would delete Ctla4 in T cells expressing Lck during thymic selection. These mice displayed a very similar phenotype to germline Ctla4 knockout mice, developing massive lymphadenopathy and dying by 28 d of age (unpublished data).
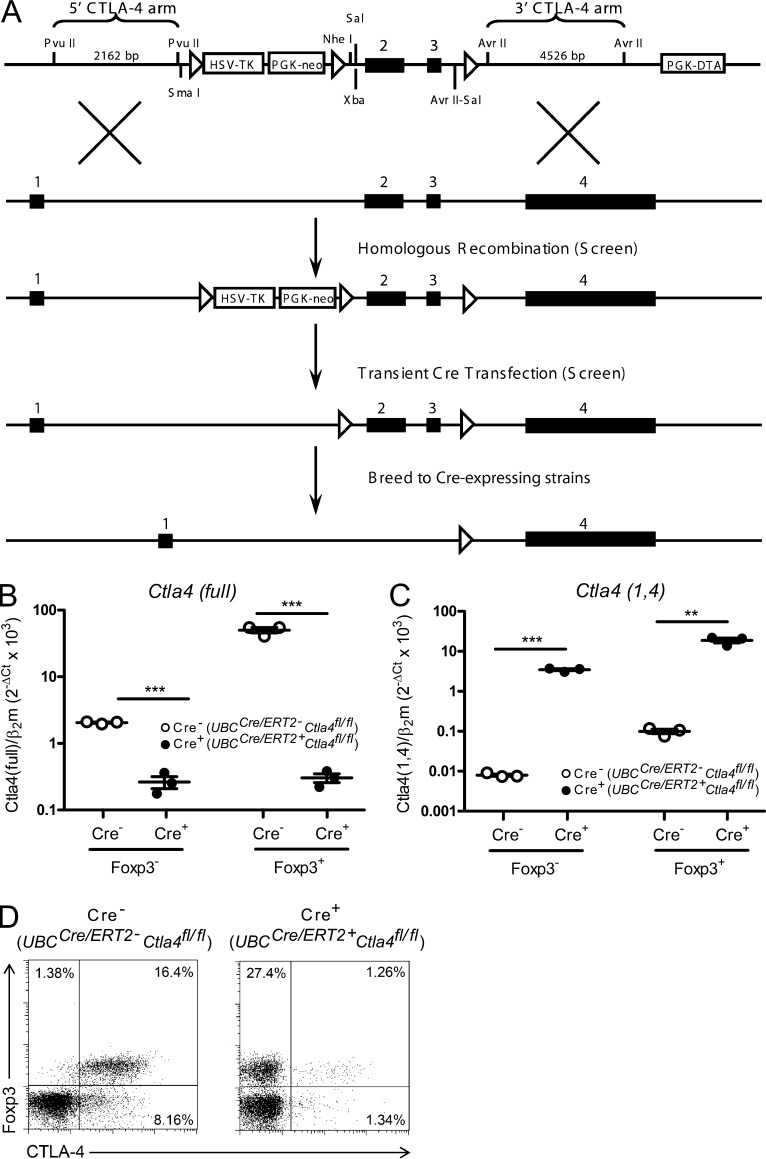
Figure 1.
Conditional deletion of Ctla4 in adult mice. (A) Targeting strategy and map of construct used to generate CTLA-4 conditional knockout mouse. Exons of the Ctla4 gene are depicted by filled, numbered boxes. Exon 1, leader; Exon 2, IgV; Exon 3, transmembrane; Exon 4, cytoplasmic. Arrows indicate loxP sites. (B and C) Ctla4fl/fl mice that were either UBCCre/ERT2- (Cre−) or UBCCre/ERT2+ (Cre+) were treated with tamoxifen to induce CTLA-4 deletion. RNA was isolated from sorted populations of Foxp3− T conv cells and Foxp3+ T reg cells, and analyzed by quantitative RT-PCR for expression of full-length CTLA-4 (B) and the 1,4 isoform of CTLA-4 (C). Data are representative of two independent experiments. Expression of β2-microglobulin (β2m) was used as the normalization standard. n = 3 samples per group (each sample derived from 1–2 mice). Ct, cycle threshold. (D) Ctla4fl/fl mice that were either UBCCre/ERT2- (Cre−) or UBCCre/ERT2+ (Cre+) were treated with tamoxifen and assessed for CTLA-4 expression by intracellular staining before immunization. Data are representative of at least three independent experiments. **, P < 0.01; ***, P < 0.001.
To study the role of CTLA-4 during adulthood, we crossed the Ctla4fl/fl strain to a tamoxifen-inducible Cre recombinase-expressing strain, UBCCre/ERT2. The resulting UBCCre/ERT2+Ctla4fl/fl mice were treated with tamoxifen for 5 consecutive days beginning at 7 wk of age. Within 7 d of initial tamoxifen administration, CTLA-4 mRNA expression in splenocytes from UBCCre/ERT2+Ctla4fl/fl mice was substantially reduced compared with UBCCre/ERT2-Ctla4fl/fl controls as assessed by quantitative RT-PCR; CTLA-4 expression was reduced ∼10-fold in splenic Foxp3− conventional T cells (T conv cells) and nearly 200-fold in in splenic Foxp3+ regulatory T cells (T reg cells), in Cre+ mice as compared with Cre− controls (Fig. 1 B). Although germline Ctla4 knockout mice lack expression of full-length CTLA-4, they have increased mRNA expression of the 1/4CTLA-4 isoform, which lacks exons 2 and 3 (Liu et al., 2012). We therefore measured expression of this isoform in splenocytes from UBCCre/ERT2+Ctla4fl/fl mice after treatment with tamoxifen and found it to be increased compared with controls, similar to germline Ctla4 knockout mice (Fig. 1 C). Deletion of CTLA-4 at the protein level was measured by co-staining with antibodies against Foxp3 and CTLA-4 11 d after initiation of tamoxifen treatment. Splenic Foxp3+ T reg cells, which are normally 91.24 ± 2.09% positive for CTLA-4 in UBCCre/ERT2-Ctla4fl/fl mice were reduced to 7.00 ± 3.34% positive in UBCCre/ERT2+Ctla4fl/fl mice (Fig. 1 D). Whereas Foxp3− T conv cells normally express lower levels of CTLA-4 than T reg cells, a reduction in CTLA-4 staining on this population was also observed. Deletion on this non-T reg cells subset was confirmed by staining cells after in vitro stimulation with anti-CD3 (unpublished data). We monitored adult mice treated with tamoxifen during the course of all experiments and observed no overt disease in UBCCre/ERT2+Ctla4fl/fl mice (unpublished data). Thus, in contrast to germline Ctla4 knockout mice, inducible deletion of Ctla4 in adult mice does not result in a spontaneous phenotype of lethal inflammation.
Deletion of Ctla4 in adult mice induces resistance to EAE
We investigated the consequences of inducing autoimmunity in adult UBCCre/ERT2+Ctla4fl/fl mice treated with tamoxifen by immunization with the self-peptide MOG35-55 to induce EAE. In contrast to UBCCre/ERT2-Ctla4fl/fl littermate controls, which developed disease, UBCCre/ERT2+Ctla4fl/fl mice were markedly resistant to EAE (Fig. 2 A and Table 1). Consistent with clinical disease score, UBCCre/ERT2+Ctla4fl/fl mice had ∼10-fold fewer inflammatory foci in the brains and spinal cords than their littermate controls (Fig. 2 C and Table 1).
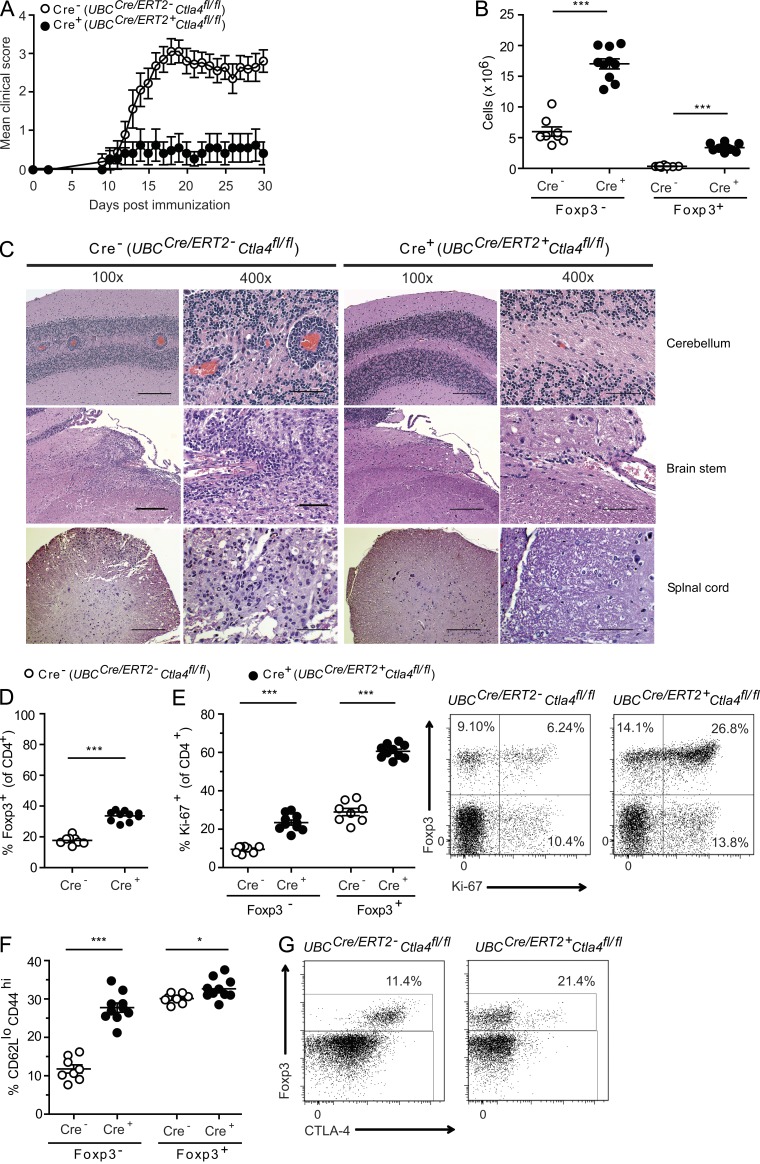
Figure 2.
Deletion of Ctla4 leads to EAE resistance and expanded, apparently activated, T cell populations. Ctla4fl/fl mice that were either UBCCre/ERT2- (Cre−) or UBCCre/ERT2+ (Cre+) were treated with tamoxifen and immunized with MOG35-55 to induce EAE. Clinical scores are shown in A and representative CNS pathology is shown in C. CD4+ cells from cervical LNs of mice immunized with MOG35-55 to induce EAE were analyzed at the peak of disease for (B) the number of CD4+Foxp3− and CD4+Foxp3+ cells, (D) the percentage of Foxp3+ cells, (E) Ki-67 expression by intracellular staining, and (F) CD62L and CD44 expression by surface staining within the cervical LN. (E, right) Representative dot plots gated on CD4+, for which mean data are plotted on the left. (G) Brains and spinal cords from MOG35-55 immunized mice were analyzed at the onset of disease for percentage of Foxp3+ cells and CTLA-4 expression. Data are gated on CD4+ T cells. Data in each panel are representative of at least two independent experiments (mean and SEM for at least 7 mice per group). Bars: 200 µm (100× magnification); 50 µm (400× magnification). *, P < 0.05; ***, P < 0.001.
Table 1.
EAE in UBC-CreERT2 and Foxp3-CreERT2 mice with induced deletion of CTLA-4
| Clinical EAE | Histological EAE | ||||||
| Parameters | Incidence | Day of onset | Mean maximal score | Incidence | Meningeal foci | Parenchyma foci | Total foci |
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |||
| UBC−Cre− (all) | 41/49 (83.7%) | 13.9 ± 0.4 | 2.2 ± 0.4 | 11/11 (100%) | 55.5 ± 13.9 | 69.5 ± 23.5 | 125.2 ± 36.9 |
| UBC-Cre+ (sunflower oil) | 5/5 (100%) | 13.0 ± 0.7 | 3.5 ± 0.2 | 4/4 (100%) | 106 ± 11.8 | 99.8 ± 12.8 | 205.8 ± 23.2 |
| UBC−Cre+ (tamoxifen) | 16/44 (36.4%) | 14.1 ± 1.0 | 0.4 ± 0.3 (P < 0.0001)a | 12/13 (92.3%) | 10.2 ± 3.8 (P = 0.003) | 5.5 ± 2.3 (P = 0.007) | 15.6 ± 5.7 (P = 0.004) |
| Foxp3−Cre− CTLA-4fl/fl | 7/8 (87.5%) | 11.9 ± 0.5 | 2.9 ± 0.5 | ||||
| Foxp3−Cre+ CTLA-4+/+ | 2/2 (100%) | 10.5 ± 0.5 | 3.3 ± 0.8 | ||||
| Foxp3−Cre+ CTLA-4fl/+ | 12/13 (92.3%) | 12.0 ± 1.1 | 2.7 ± 0.3 | ||||
| Foxp3−Cre+ CTLA-4fl/fl | 2/16 (12.5%) | 14.0 ± 4.0 | 0.3 ± 0.2 (P < 0.0001) | ||||
Data in Clinical EAE are pooled from seven independent experiments (four with UBC−CreERT2 mice and three with Foxp3−CreERT2 mice) in which EAE was induced by immunization with 150 µg MOG35-55 subcutaneously, and administration of 250 ng pertussis toxin intraperitoneally on the day of immunization and two days later. Mice with no disease were included in calculations of maximal score, but excluded from calculations of day of disease onset.
All p-values are compared with Cre− control.
We next compared the phenotype of T cells in the cervical LNs (cLNs; draining the CNS) of control and UBCCre/ERT2+Ctla4fl/fl mice 14 d after immunization with MOG35-55. Within the CD4+ T cell subset, both Foxp3+ T reg cells and Foxp3− T conv cells were expanded in UBCCre/ERT2+Ctla4fl/fl mice when compared with UBCCre/ERT2-Ctla4fl/fl mice, with the proportional increase of T reg being greater (Fig. 2, B and D). Intracellular Ki-67 staining was increased in both subsets, suggesting increased peripheral expansion of these cells (Fig. 2 E). Analysis of CD62L and CD44 surface staining revealed T conv cells, and to a lesser extent T reg cells, from the UBCCre/ERT2+Ctla4fl/fl mice to be more activated than those from control mice (Fig. 2 F). In the CNS, at the onset of disease, we found a similar percentage of total CD4+ T cells in both groups (not depicted) and an increase in the frequency of T reg cells in UBCCre/ERT2+Ctla4fl/fl mice (Fig. 2 G), similar to that observed in the periphery. T reg cells in the CNS of UBCCre/ERT2+Ctla4fl/fl mice were profoundly deleted for CTLA-4, as in the periphery. Thus, deletion of CTLA-4 in adult mice is accompanied by resistance to autoimmunity and is characterized by expansion and increased activation of T conv cells. This is counterbalanced by a proportionally greater expansion of T reg cells both in the periphery and in the target tissue.
Resistance to autoimmunity is dependent on CTLA-4 deletion on T reg cells
To determine if resistance to EAE is caused by the absence of Ctla4 during thymic selection, we adoptively transferred mature CD4+ T cells from either UBCCre/ERT2-Ctla4fl/fl or UBCCre/ERT2+Ctla4fl/fl adult mice into TCRα−/− recipients, which were then treated with tamoxifen and immunized with MOG35-55 to induce EAE. This approach deletes CTLA-4 in mature T cells and eliminates possible effects of CTLA-4 deficiency in the thymus. Recipients of UBCCre/ERT2+Ctla4fl/fl T cells were protected from EAE, recapitulating the protection seen in tamoxifen-treated, intact UBCCre/ERT2+Ctla4fl/fl mice (Fig. 3 A). In this setting, we again observed expansion of both T reg cells and T conv cells when Ctla4 was deleted (unpublished data). Thus, deletion of Ctla4 on mature CD4+ T cells can lead to EAE resistance.
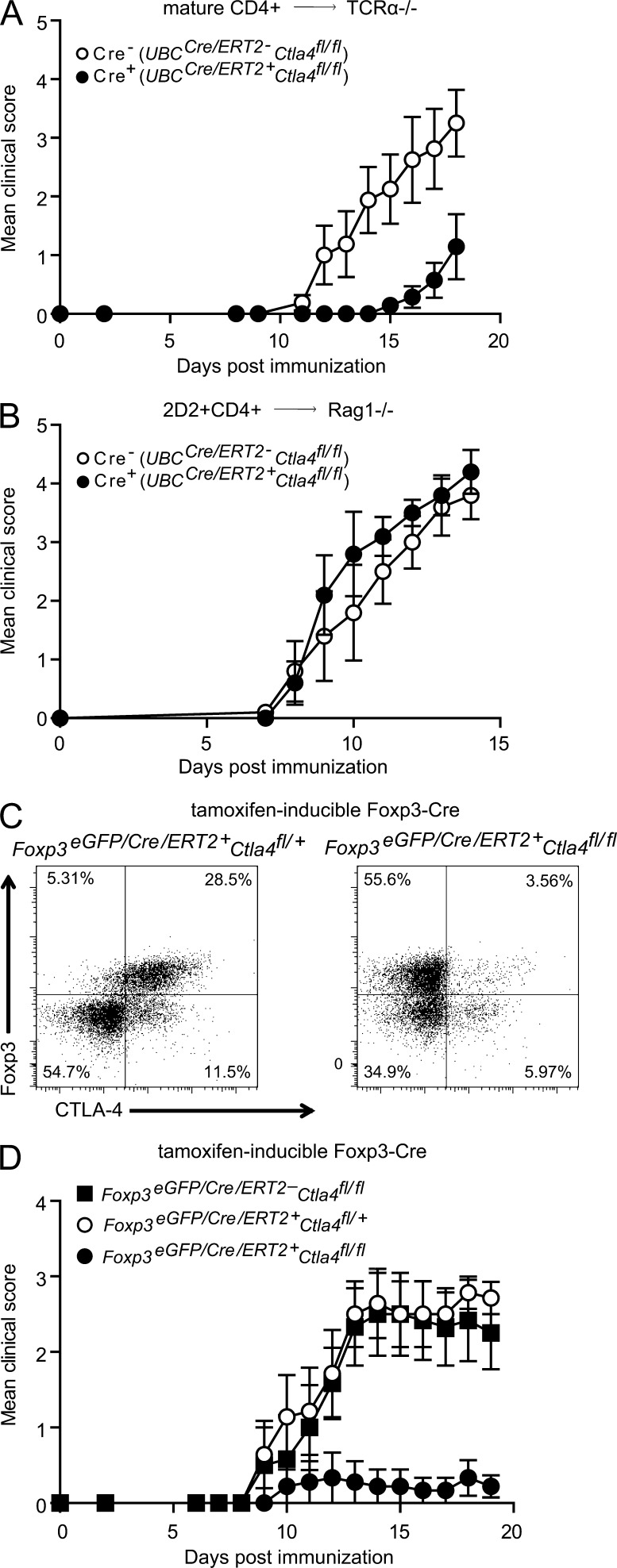
Figure 3.
Dependence on deletion of CTLA-4 on T reg cells for resistance to EAE. (A) CD4+ cells or (B) 2D2+CD4+ cells from UBCCre/ERT2- (Cre−) or UBCCre/ERT2+ (Cre+) donors were adoptively transferred to (A) TCRα−/− or (B) Rag1−/− hosts, which were treated with tamoxifen and immunized with MOG35-55 to induce EAE. (C and D) Mice that were either Foxp3eGFP/Cre/ERT2- (Cre−) or Foxp3eGFP/Cre/ERT2+ (Cre+) and that had either one (Ctla4fl/+) or two (Ctla4fl/fl) conditional knockout Ctla4 alleles were treated with tamoxifen and immunized with MOG35-55 to induce EAE. Cervical LN CD4+ T cells were analyzed by intracellular staining for CTLA-4 protein (C) and mice were monitored for clinical disease (D). Data in each panel are representative of at least two independent experiments (n ≥ 5 mice per group).
To investigate the impact of Ctla4 deletion on T conv cells versus T reg cells, we first assessed whether an antigen-specific T conv cell population could transfer disease when Ctla4 is deleted. We bred UBCCre/ERT2+Ctla4fl/fl mice to mice bearing the 2D2 transgenic TCR, specific for MOG35-55, and transferred 2D2+CD4+ T cells from UBCCre/ERT2-Ctla4fl/fl or UBCCre/ERT2+Ctla4fl/fl mice to Rag1−/− recipients, and then immunized with MOG35-55 concurrently with tamoxifen treatment. Recipients of 2D2+UBCCre/ERT2+Ctla4fl/fl and 2D2+ UBCCre/ERT2-Ctla4fl/fl T cells developed EAE with similar onset and severity (Fig. 3 B). Additionally, we sorted CD4+Foxp3−GFP− T cells from non-TCR transgenic UBCCre/ERT2+Ctla4fl/fl and UBCCre/ERT2-Ctla4fl/fl mice and transferred these to TCRα−/− recipients, which were then treated with tamoxifen and immunized with MOG35-55. UBCCre/ERT2+Ctla4fl/fl and UBCCre/ERT2-Ctla4fl/fl CD4+Foxp3-GFP− T cells induced EAE with similar disease courses (unpublished data). Furthermore, IL-17FCre+Ctla4fl/fl mice, which were deleted for Ctla4 on a subset of CD4+ effector T cells, displayed identical disease susceptibility to their littermate controls (unpublished data). Thus, effector CD4+ T cells that lack Ctla4 can become pathogenic and do not appear to be responsible for the EAE resistance of mice in which Ctla4 is deleted during adulthood.
We next asked whether deletion of Ctla4 only in the T reg cell compartment would recapitulate resistance to EAE. We bred the Ctla4fl/fl mouse to a Foxp3eGFP/Cre/ERT2 mouse (Rubtsov et al., 2010) to enable tamoxifen-inducible deletion of Ctla4 specifically on T reg cells. Tamoxifen treatment of adult mice resulted in deletion of CTLA-4 on Foxp3+ T reg cells to a similar degree to that achieved in the UBCCre/ERT2+Ctla4fl/fl mice, whereas CTLA-4 expression on CD4+Foxp3− cells was unchanged (Fig. 3 C). After tamoxifen treatment, mice were immunized with MOG35-55 to induce EAE. EAE developed in control mice (either non-Cre–expressing Ctla4fl/fl, Foxp3eGFP/Cre/ERT2Ctla4fl/+, or Foxp3eGFP/Cre/ERT2Ctla+/+ mice), but not in Foxp3eGFP/Cre/ERT2Ctla4fl/fl mice (Fig. 3 D and Table 1). The degree of protection was profound and comparable to that observed in UBCCre/ERT2+Ctla4fl/fl mice. Thus, deletion of CTLA-4 specifically on T reg cells, during adulthood, leads to protection from induced autoimmunity.
Inducible Ctla4 deletion leads to cell intrinsic changes in T conv cells
Because CTLA-4 deletion on T conv cells alone failed to confer protection from EAE, we questioned whether there might be a functional role for CTLA-4 deletion on T conv cells in a different setting. We targeted deletion of CTLA-4 specifically to T conv cells by transferring T conv cells from either UBCCre/ERT2+Ctla4fl/fl or UBCCre/ERT2+Ctla+/+ mice, together with T reg cells from UBCCre/ERT2− mice into TCRα−/− recipients that were subsequently treated with tamoxifen. Input T conv cells were distinguished from T reg cells by the expression of a Cre reporter (ROSA26-tdTomato). We found that targeted deletion of CTLA-4 on T conv cells led to expansion of T conv but not T reg cells (Fig. 4 A), suggesting that CTLA-4 expression on T conv cells, while insufficient to confer disease protection, does restrain the homeostatic expansion of T conv cells.
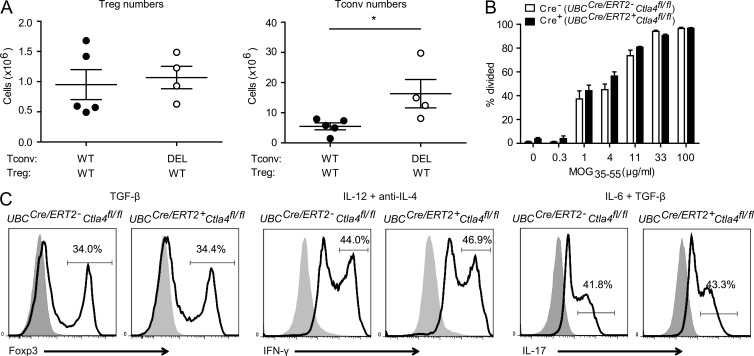
Figure 4.
T conv cell–intrinsic effects of inducible deletion of Ctla4. (A) CD4+Foxp3-GFP+ cells from UBCCre/ERT2-Ctla4fl/fl mice were transferred with CD4+Foxp3-GFP− cells from either UBCCre/ERT2+Ctla4+ROSA26loxP-STOP-loxP-tdTomato (WT) or UBCCre/ERT2+Ctla4fl/flROSA26loxP-STOP-loxP-tdTomato (DEL) mice to TCRα−/− recipients, which were subsequently treated with tamoxifen and immunized with MOG35-55. T reg and T conv cells were quantified in the spleen 26 d after immunization. n ≥ 4 mice per group. (B) Sorted CD4+Foxp3-GFP− cells from tamoxifen-treated TCR transgenic 2D2+UBCCre/ERT2+Ctla4fl/fl (Cre+) or 2D2+UBCCre/ERT2−Ctla4fl/fl (Cre−) mice were cultured with splenocytes from TCRα−/− mice, 4-hydroxy tamoxifen and a dose titration of MOG35-55 peptide for 72 h. Proliferation was assessed by quantitation of CellTrace Violet dilution. n = 3 mice per group. (C) Naive, CD4+CD62LhiFoxp3-GFP− splenocytes were sorted from UBCCre/ERT2-Ctla4fl/fl (Cre−) or UBCCre/ERT2+Ctla4fl/fl (Cre+) mice that had been treated with tamoxifen on days −4 and −3. Cells were cultured in the presence of irradiated TCRα−/− splenocytes, soluble anti-CD3, 4-hydroxy tamoxifen, and the indicated cytokines for 72 h. Cells were then analyzed by flow cytometry for expression of Foxp3-GFP or intracellular cytokines. For analysis of cytokines, cells were stimulated first with PMA/Ionomycin and GolgiStop for 4 h. Gray histograms represent data from cells grown without addition of cytokine for TGF-β condition and cells stimulated with only GolgiStop (no PMA/Ionomycin) for intracellular cytokine stains. n ≥ 2 mice/group. Data in all panels are representative of at least two independent experiments. *, P < 0.05.
We also compared the proliferation response of sorted CD4+Foxp3-GFP− cells from tamoxifen-treated TCR transgenic 2D2+UBCCre/ERT2+Ctla4fl/fl or 2D2+UBCCre/ERT2-Ctla4fl/fl mice to a range of MOG35-55 peptide concentrations in vitro and observed similar proliferation profiles between the two groups (Fig. 4 B). Similarly, we found that non-TCR transgenic cells with inducible loss of CTLA-4 showed equal proliferation to controls in response to an anti-CD3 stimulus (unpublished data).
Furthermore, we analyzed the propensity of T conv cells that do or do not express CTLA-4 to differentiate into various T helper (Th) subsets, independently of T reg cells. We sorted naive, CD4+Foxp3− T cells from UBCCre/ERT2-Ctla4fl/fl or UBCCre/ERT2+Ctla4fl/fl mice that had undergone a truncated in vivo tamoxifen treatment regimen (days −4 and −3 only; to minimize the indirect effects of Ctla4 deletion on T reg cells) and cultured them under various Th differentiation conditions in the presence of 4-hydroxy tamoxifen, to ensure complete deletion. We found no differences in the percentages of either Foxp3-GFP–, IFN-γ–, or IL-17A–expressing populations under conditions of T reg, Th1, or Th17 cell differentiation, respectively (Fig. 4 C). In summary, although we observed a T conv cell–intrinsic role for CTLA-4 in regulating in vivo homeostatic expansion, in vitro measures of proliferation and differentiation were equal between CTLA-4–expressing versus –deleted T conv cells.
T reg cells deleted for CTLA-4 during adulthood are functional
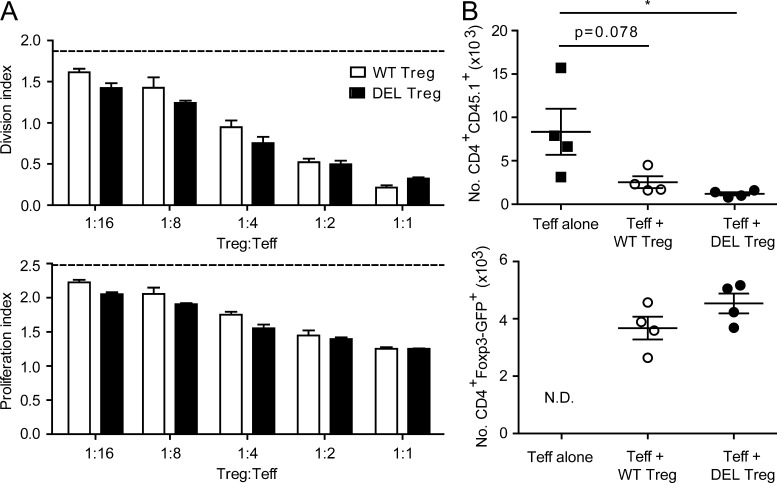
We next assessed the function of T reg cells in which Ctla4 was inducibly deleted in adult mice using in vitro and in vivo assays. We first co-cultured wild-type versus CTLA-4–deleted T reg cells (sorted from tamoxifen-treated UBCCre/ERT2+Ctla4+/+ or UBCCre/ERT2+Ctla4fl/fl mice) in the presence of wild-type T conv cells and APC (live dendritic cells), and stimulated with anti-CD3. In this assay, T reg cells lacking CTLA-4 were able to suppress T conv cell proliferation in vitro equally as well as T reg cells expressing CTLA-4 (Fig. 5 A).
Figure 5.
T reg cells lacking CTLA-4 are functional in vitro and in vivo. (A) Wild-type sorted CD4+CD62LhiCD25− T cells from CD45.1+ congenic splenocytes (T conv cells) were labeled with CellTrace Violet and co-cultured with sorted CD4+Foxp3−GFP+tdTomato+ T reg cells from tamoxifen-treated UBCCre/ERT2+Ctla4fl/flFoxp3−GFPKIROSA26loxP-STOP-loxP-tdTomato (DEL T reg cells) or UBCCre/ERT+Ctla4+/+Foxp3−GFPKIROSA26loxP-STOP-loxP-tdTomato (WT T reg cells) mice at the indicated ratios. Cells were cultured in the presence of wild-type CD11c+, splenic DCs, and soluble anti-CD3 for 3 d. Division index (top) and proliferation index (bottom) are shown (n = 3 mice per group). (B) CD45.1 T conv cells (CD4+CD62LhiCD25−) were transferred to Rag1−/− recipients with or without cotransfer of T reg cells from UBCCre/ERT2+Ctla4fl/fl or UBCCre/ERT2+Ctla4+/+ mice, and then treated with tamoxifen. Spleens were harvested on day 10 after transfer and the number of T conv cells (top) and T reg cells (bottom) were quantitated (n = 4 mice per group). Data in each panel are representative of at least two independent experiments. *, P < 0.05.
To assess T reg cell function in vivo, we developed an assay for suppression of homeostatic proliferation in lymphopenic mice. Wild-type T conv cells were transferred to Rag1−/− hosts in the presence or absence of cotransferred T reg cells from UBCCre/ERT2+Ctla4fl/fl or UBCCre/ERT2+Ctla4+/+ mice. Recipient mice were treated with tamoxifen to induce CTLA-4 deletion in T reg cells, and homeostatic proliferation of the transferred T conv cells was quantitated 10 d after initial tamoxifen administration (on the day of transfer). We found that CTLA-4–deleted T reg cells were equivalent, if not better, than control T reg cells at suppressing homeostatic proliferation in vivo (Fig. 5 B). The number of T reg cells obtained at the end of the assay was not significantly different between groups, suggesting that, on a per cell basis, CTLA-4–deficient T reg cells have suppressive capacity equal to that of control T reg cells. Collectively, these data indicate that T reg cells lacking CTLA-4 maintain their ability to suppress T cell proliferation in vitro and in vivo.
T reg cells lacking CTLA-4 impose an overabundance of IL-10 and increased expression of co-inhibitory receptors
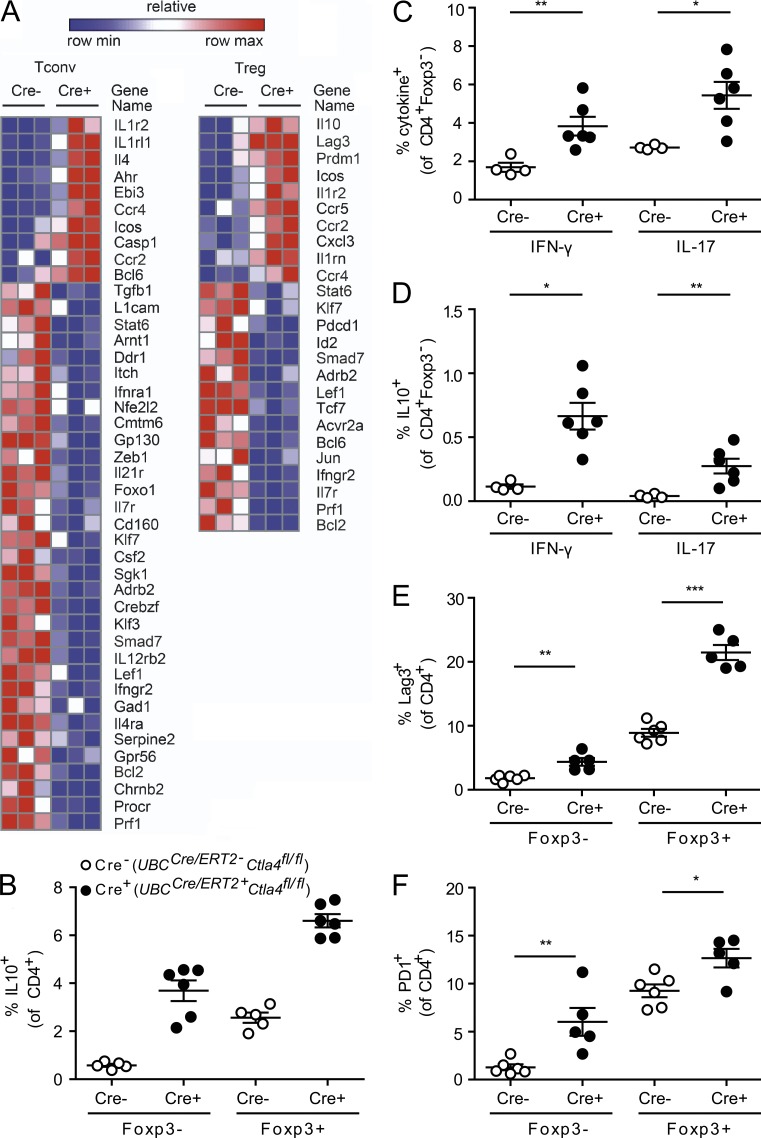
Collectively, our data suggest that whereas deletion of CTLA-4 in mature T cells leads to increased activation of T conv cells, these cells are rendered functionally impotent by the presence of T reg cells lacking CTLA-4, resulting in protection from EAE. To test this hypothesis, we further characterized the phenotype of T conv and T reg cells from UBCCre/ERT2+Ctla4fl/fl mice by performing a focused transcriptional analysis of CTLA-4–deleted T conv and T reg cells using a pathogenic Th17 NanoString codeset (Lee et al., 2012; Yosef et al., 2013). UBCCre/ERT2+Ctla4fl/fl and UBCCre/ERT2-Ctla4fl/fl mice were treated with tamoxifen and immunized with MOG35-55. At the peak of disease, RNA was isolated from T conv cells and T reg cells that had been sorted from spleen and cLNs. We found that both T reg and T conv cell populations adopt a gene expression signature associated with IL-10 production, which has been shown to have a protective role in EAE (Bettelli et al., 1998; Fig. 6 A). Among the most up-regulated genes in UBCCre/ERT2+Ctla4fl/fl relative to UBCCre/ERT2-Ctla4fl/fl T conv cells were Ahr (aryl-hydrocarbon receptor), EBi3 (shared IL27/IL35 subunit), and Icos; each of these genes is involved in promoting the biology of IL-10 production and of type 1 regulatory-1 cells, an IL-10–producing, Foxp3-negative regulatory T cell population. When T reg cells were compared in a similar fashion, Il10, Prdm1 (Blimp-1), and Icos were up-regulated in UBCCre/ERT2+Ctla4fl/fl UBCCre/ERT2-Ctla4fl/fl samples, also suggesting that IL-10 production plays a significant functional role in conferring protection from EAE upon CTLA-4 deletion. Consistent with this hypothesis, we observed increased production of IL-10 by T conv cells and T reg cells from CTLA-4–deleted mice, relative to control mice, during EAE (Fig. 6 B). Although UBCCre/ERT2+Ctla4fl/fl mice had a higher frequency of CD4+ T cells from the cLN that produced the pathogenic cytokines IFN-γ and IL-17 compared with littermate controls (Fig. 6 C), a significantly larger proportion of each of these subsets additionally produced IL-10, relative to the corresponding cells from control mice (Fig. 6 D). CD4+ T cells lacking CTLA-4 also had increased surface expression of the co-inhibitory receptors Lag3 and PD-1 on both T conv cells and T reg cells in the cLN (Fig. 6, E and F), as well as in the spleen (not depicted). Collectively, these data indicate that although CTLA-4 inhibits expansion of both T conv cells and T reg cells, it also acts to inhibit IL-10 production and the expression of co-inhibitory receptors.
Figure 6.
Deletion of CTLA-4 during adulthood leads to increased IL-10, PD-1, and Lag3 expression. (A) Ctla4fl/fl mice that were either UBCCre/ERT2- (Cre−) or UBCCre/ERT2+ (Cre+) were treated with tamoxifen and immunized with MOG35-55 to induce EAE. At the peak of disease, mice were sacrificed and T conv cells and T reg cells were isolated from cervical LNs by cell sorting. RNA was isolated as described, and gene expression quantified by NanoString analysis. Each column represents an individual mouse (3 mice per group). (B–F) CD4+ cells from UBCCre/ERT2+Ctla4fl/fl or UBCCre/ERT2-Ctla4fl/fl mice were immunized with MOG35-55 to induce EAE and analyzed at the peak of disease for cytokine production by intracellular staining. (B) Quantitation of IL-10 expression by T conv cells (Foxp3−) and T reg cells (Foxp3+) splenocytes; (C) Quantitation of IFN-γ and IL-17 production by T conv cells; (D) Quantitation of IL-10 production among IFN-γ– and IL-17–producing T conv cells (i.e., “double positive” cells); (E and F) Quantitation of cell surface expression of Lag3 (E) and PD-1 (F) in cLN. Data are gated on CD4+ T cells (mean and SEM for 4–6 mice per group). Data in each panel are representative of at least two independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
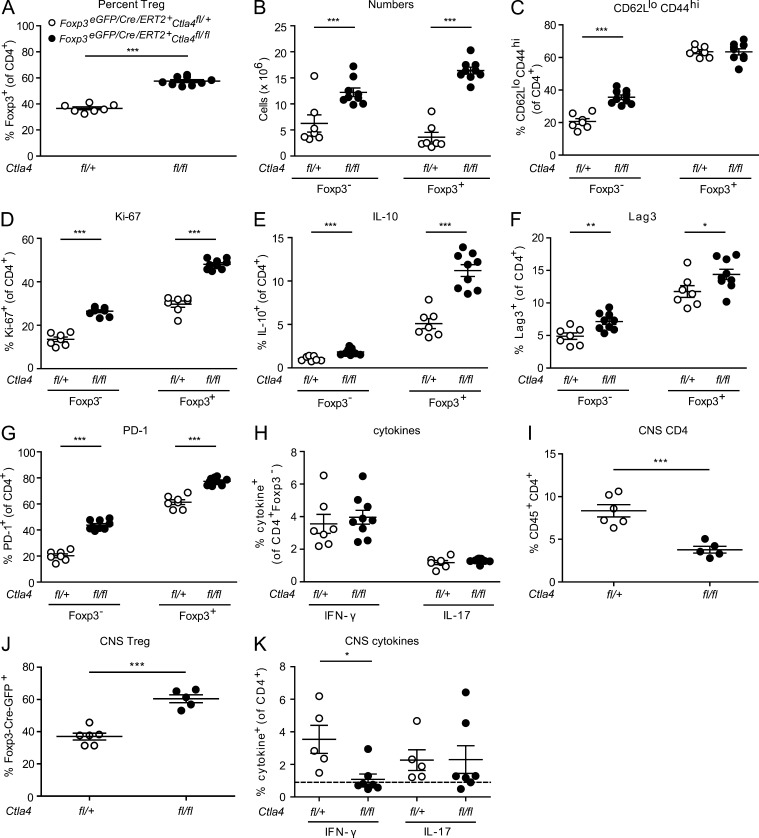
Next, we asked whether loss of CTLA-4 on T reg cells alone was sufficient to induce the phenotypic changes in T conv cells observed in UBCCre/ERT2+Ctla4fl/fl mice. T reg cells from Foxp3eGFP/Cre/ERT2Ctla4fl/fl mice treated with tamoxifen and immunized with MOG35-55 showed strikingly similar cellular phenotypes to those observed in UBCCre/ERT2+Ctla4fl/fl mice. At time points at which control mice had ongoing disease, the CD4+ population of the cLN of Foxp3eGFP/Cre/ERT2Ctla4fl/fl mice was as high as 60% Foxp3+ (Fig. 7 A). Moreover, both T reg cells and T conv cells were increased in number in these mice, although T reg cells were proportionately more so (approximately twofold increase in T conv cells vs. fivefold in T reg cells; Fig. 7 B). The CTLA-4–sufficient T conv cell population from Foxp3eGFP/Cre/ERT2Ctla4fl/fl mice displayed increased activation, as measured by the percentage of CD62LloCD44hi cells (Fig. 7 C), whereas proliferation as assessed by Ki-67 staining was increased in both T conv cells and T reg cells (Fig. 7 D). Furthermore, expression of IL-10, Lag3, and PD-1 was increased in both T cell subsets (Fig. 7, E–G). These findings confirm that the phenotype of T conv cell activation accompanied by increased expression of inhibitory molecules that we observe in UBCCre/ERT2+Ctla4fl/fl mice, is driven by deletion of CTLA-4 specifically on T reg cells and is independent of CTLA-4 expressed by T conv cells.
Figure 7.
Cellular phenotypes of mice lacking CTLA-4 specifically on T reg cells. Foxp3eGFP/Cre/ERT2Ctla4fl/fl (fl/fl) and Foxp3eGFP/Cre/ERT2Ctla4fl/+ (fl/+) littermates were treated with tamoxifen and immunized with MOG35-55 to induce EAE. Cervical LNs were analyzed at peak of disease for (A) the percentage of Foxp3+ cells, (B) the number of Foxp3− and Foxp3+ cells, (C) surface staining for CD62L and CD44, (D) intracellular staining for Ki-67, (E) intracellular staining for IL-10, (F) surface staining for PD-1, (G) surface staining for Lag-3, and (H) intracellular staining for IFN-γ and IL-17A. On day 12 after immunization (onset of disease in controls) leukocytes were isolated from CNS for frequency of (I) CD45+CD4+ cells, (J) Foxp3+ cells (of CD4+ population), or (K) IFN-γ– and IL-17A–producing CD4+ cells. For cytokine analyses, cells were stimulated with PMA/Ionomycin/GolgiStop and analyzed for intracellular expression of IL-10, IFN-γ, or IL-17A. Data in each panel are representative of at least two independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
There were some differences, however, among T conv cells from UBCCre/ERT2+Ctla4fl/fl compared with Foxp3eGFP/Cre/ERT2Ctla4fl/fl mice after tamoxifen treatment and EAE induction with MOG35-55. In contrast to UBCCre/ERT2Ctla4fl/fl mice (Fig. 6 C), mice with specific, inducible T reg cell deletion of CTLA-4 did not display increased production of IFN-γ and IL-17 among peripheral T conv cells (Fig. 7 H). This is an interesting difference between these two models, as it suggests that control of T conv cell production of these inflammatory cytokines is intrinsic to the T conv cells themselves and not an indirect effect of CTLA-4 deletion on T reg cells.
Within the CNS, on day 12 after immunization (time of disease onset in controls), we observed significantly fewer CD45+CD4+ T cells in Foxp3Cre/eGFP/ERT2Ctla4fl/fl mice, compared with Foxp3Cre/eGFP/ERT2Ctla4fl/+ controls (Fig. 7 I). However, the proportion of these cells that were Foxp3+ is higher (Fig. 7 J), consistent with what we have observed in the periphery. We also observed significantly fewer IFN-γ–producing CD4+ cells within the CNS of T reg cell–specific CTLA-4–deleted mice, whereas there are no significant differences in the production of IL-17 (Fig. 7 K).
Increased B7 and MHC class II expression on peripheral, but not CNS, APCs of CTLA-4–deleted mice
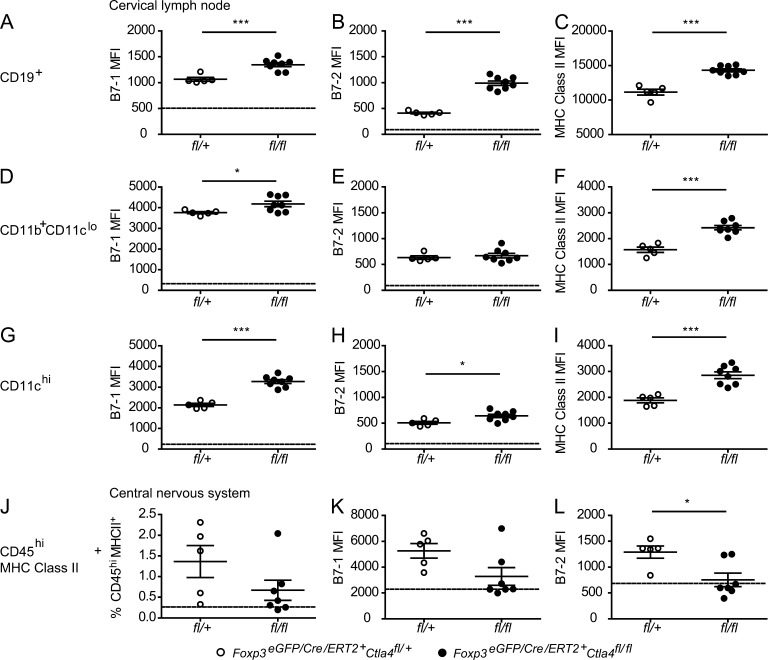
Because CTLA-4 may exert some of its effects by modulating B7 expression, we compared B7-1 and B7-2 expression on APCs in the periphery and CNS of mice that are inducibly deleted for CTLA-4 on T reg cells with those from control mice. Our group has previously shown that survival of T cells within the CNS during EAE is dependent on B7 expression (Chang et al., 2003). We observed increases in B7-1 and B7-2 expression on APC from mice that are inducibly deleted for CTLA-4 specifically on T reg cells (Fig. 8). In particular, B cells and DCs appeared to have appreciably increased levels of B7-1 and B7-2 expression (Fig. 8, A, B, G, and H). However, we also observed highly significant increases in the level of MHC class II expression on APC populations (Fig. 8, C, F, and I), indicating that their overall maturation/activation level is enhanced. Therefore, it is unclear whether the changes in B7 expression are caused by the direct effect of absence of CTLA-4–B7 interactions that lead to their down-regulation (either by transendocytosis or back-signaling) or by indirect effects of other cellular changes. Importantly, we did not find increased B7 levels on the CD45hiMHC class II+ APCs in the CNS of mice immunized for EAE (Fig. 8, J–L), although we did observe increased percentages of T reg cells in the CNS of CTLA-4–deleted mice (Fig. 7 J). These findings suggest that CTLA-4–deficient T reg cells are not causing loss of control of B7 expression on CNS APCs.
Figure 8.
Increased B7 and MHC class II expression on peripheral, but not CNS APC of CTLA-4–deleted mice. Tamoxifen-treated Foxp3eGFP/Cre/ERT2Ctla4fl/fl (fl/fl) and Foxp3eGFP/Cre/ERT2Ctla4fl/+ (fl/+) littermates were immunized for EAE. On day 12 after immunization, when the first control mice were exhibiting onset of disease, cervical LNs (A–I) and brains and spinal cords (J–L) were dissected. Surface staining and flow cytometry were performed to assess expression of the indicated molecules on CD19+ (A–C), CD11b+CD11clo (D–F), CD11chi (G–I), and CD45hiMHC class II+ (J–L) cells. n ≥ 5 mice per group. Data in each panel are representative of two independent experiments. *, P < 0.05; ***, P < 0.001.
Conditional deletion of Ctla4 does not impede tumor growth
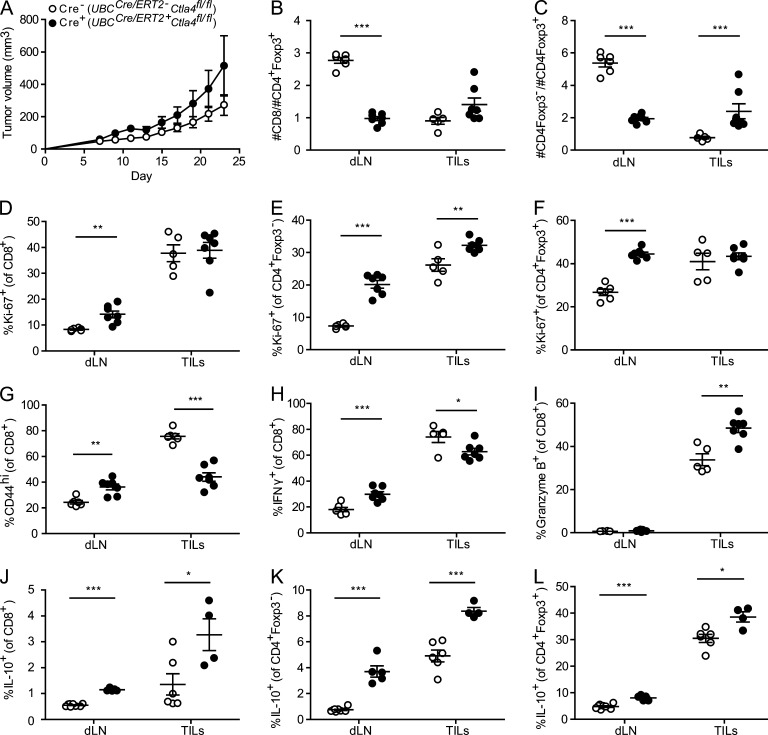
We next examined the consequences of Ctla4 deletion in adult mice on responses to an implanted tumor. We used the subcutaneous tumor model, MC38 colon adenocarcinoma because anti–CTLA-4 mAb leads to modestly enhanced antitumor immunity in this model (Kocak et al., 2006; Allard et al., 2013). We administered tamoxifen to induce deletion of Ctla4 in UBCCre/ERT2 mice before tumor implantation. CTLA-4 deletion did not slow or diminish tumor growth curves. Rather, UBCCre/ERT2+Ctla4fl/fl mice had slightly larger tumors compared with UBCCre/ERT2-Ctla4fl/fl littermate controls (Fig. 9 A). Both the CD8/T reg cells and CD4+ T conv cells/T reg cells ratios were dramatically decreased in the draining (inguinal, proximal to tumor; Fig. 9, B and C) and nondraining (cervical) LNs (not depicted). In contrast to the periphery, the CD8/T reg cells ratio within the tumor was not significantly different between deleted and control mice and the CD4+ T conv/T reg cell ratio was higher in deleted versus control tumors (Fig. 9, B and C). Ki-67 expression is consistent with this finding; CD8+ T cell, CD4+ T conv cell, and T reg cell Ki-67 expression is increased in the dLN of UBCCre/ERT2+Ctla4fl/fl mice relative to controls, whereas among tumor-infiltrating lymphocytes (TILs), only CD4+ T conv cells, but not CD8+ T cells or T reg cells, have increased Ki-67 upon CTLA-4 deletion (Fig. 9, D–F). Thus, there is massive peripheral expansion of T reg cells upon Ctla4 deletion, similar to our findings in EAE; however, this expansion is not seen at the site of the tumor.
Figure 9.
Tumor growth and antitumor immune responses in mice with inducible deletion of Ctla4. UBCCre/ERT2-Ctla4fl/fl (Cre−; open circles) or UBCCre/ERT2+Ctla4fl/fl (Cre+; closed circles) mice were treated with tamoxifen, and then MC38 tumor cells were implanted subcutaneously. (A) Tumor growth was measured every second day until day 23, at which point mice were harvested for analysis. T cells from the tumor (TIL) and draining inguinal LN (dLN) were isolated as described in Materials and methods. Absolute numbers of CD8+ T cells (B) and CD4+ T cells (C) were enumerated and graphed as a ratio against the number of CD4+Foxp3+ T reg cells. The proportion of cells positive for intracellular Ki-67 was quantitated among CD8+ (D), CD4+Foxp3− (E), and CD4+Foxp3+ (F) populations. CD8+ cells were characterized for their expression of surface CD44 (G), their production of IFN-γ in response to PMA/Ionmycin stimulation (H) or expression of intracellular Granzyme B (I). IL-10 production after PMA/Ionomycin stimulation was quantified among CD8+ (J), CD4+Foxp3− (K), and CD4+Foxp3+ (L) populations. Data in each panel are representative of two independent experiments; n ≥ 4 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The function of CD8+ TILs in this model appeared to be altered by Ctla4 deletion. Fewer CD8+ TILs from CTLA-4–deleted mice were CD44hi and produced IFN-γ upon restimulation (Fig. 9, G and H), but more CD8+ TILs produced Granzyme B in CTLA-4 deleted mice than controls (Fig. 9 I). In the dLN, however, there were more CD8+ T cells that were CD44hi and produced IFN-γ. Based on our previous findings of increased IL-10 production in both T conv cells and T reg cells after Ctla4 deletion during EAE, we examined IL-10 production in the dLN and tumor. We found that in both sites, higher percentages of cells in all subsets (CD8+, CD4+ T conv cells, and T reg cells) produced IL-10 (Fig. 9, J–L). This increase in IL-10 may predominate and dampen antitumor immunity in the CTLA-4 deleted mice.
DISCUSSION
The dramatic, lethal phenotype of the germline Ctla4 knockout mouse demonstrates the potency of this molecule as an inhibitory receptor (Tivol et al., 1995; Waterhouse et al., 1995). Because CTLA-4 is largely T cell specific and constitutively expressed on T reg cells, but is also inducibly expressed on T conv cells upon activation, the question of the relative contribution of CTLA-4 expression on T reg cells versus T conv cells has been of great interest. Here, we have developed genetic approaches to address this question, uncoupling the role of CTLA-4 during development and in the adult immune system by using inducible Cre systems that delete CTLA-4 either ubiquitously or specifically on Foxp3+ T reg cells. By deleting CTLA-4 during adulthood, either on all cells or specifically on T reg cells, we have uncovered a surprising phenotype of resistance to induced autoimmunity. The underlying phenotype of T cells in these mice is one of apparent activation, coupled with T reg cell expansion and increased production of anti-inflammatory molecules. The distinct phenotype seen in our model, in contrast to that seen with germline deletion of Ctla4, may reflect necessary roles for CTLA-4 during the neonatal period, at which time T reg cells develop in and exit from the thymus (Asano et al., 1996). Consistent with this, when we crossed this conditional CTLA-4 knockout allele to either LckCre or noninducible Foxp3Cre mice the lethal phenotype of germline Ctla4−/− mice was recapitulated (unpublished data). This indicates that there is a critical window of time between when Lck or Foxp3 genes are turned on in the thymus and adulthood, during which CTLA-4 is needed to prevent systemic autoimmunity. The perinatal period, in which T reg cells seed the periphery, is appreciated to be critical for the establishment of peripheral tolerance (Asano et al., 1996; Dujardin et al., 2004; Samy et al., 2008). It has recently been shown that T reg cells that emerge from the thymus during this period are dependent on Aire for their generation and have a distinct transcriptional profile from T reg cells generated in adult mice (Yang et al., 2015). CTLA-4 (whose expression in T reg cells is regulated by Foxp3) may be essential for selecting the T reg cell repertoire in the thymus and protecting them from thymic deletion during development. However, once selected and seeded to the peripheral immune compartment, CTLA-4 may serve to restrain T reg cell expansion (as it does for T conv cells). In addition, the relationship between preservation of T reg cell function and control of expansion may be very different in the relatively lymphopenic neonatal environment compared with that of the adult; in the neonate, loss of control on T reg cell proliferation may lead to abolished T reg cells suppressive function.
The EAE resistance of adult mice in which CTLA-4 is inducibly deleted ubiquitously or selectively on Foxp3+ T reg cells contrasts with published studies showing that anti–CTLA-4 administration exacerbated EAE in various non-B6 models (Karandikar et al., 1996; Perrin et al., 1996). Karandikar et al. reported worsening of relapsing/remitting PLP139-151-induced EAE on an SJL background upon anti–CTLA-4 administration, and Perrin et al. reported exacerbation of EAE induced by MBP protein in (PL x SJL)F1 mice. To determine if there are differences in the effects of CTLA-4 in the C57BL/6 MOG-induced EAE and other models, we immunized wild-type C57BL/6 mice with MOG35-55 to induce EAE and administered anti–CTLA-4 mAb either before or after immunization. We observed a slight worsening of disease (unpublished data). Recent studies indicate that anti–CTLA-4 treatment in cancer models can lead to T reg cell depletion by FcR-dependent mechanisms (Bulliard et al., 2013; Selby et al., 2013; Simpson et al., 2013), suggesting that effects other than pure blockade may explain the difference in EAE phenotypes in anti–CTLA-4–treated mice and inducible CTLA-4–deficient mice.
Several groups have demonstrated that mice chimeric for wild-type and CTLA-4–deleted cells, either through adoptive transfer or bone marrow chimerism, are spared the lethal phenotype of germline CTLA-4 knockout mice (Bachmann et al., 1999; Friedline et al., 2009). In our system, although CTLA-4 is deleted on the vast majority of cells, a small percentage of CTLA-4–expressing cells persists. We treated UBCCre-ERT2+Ctla4fl/fl mice with anti–CTLA-4 mAb after tamoxifen treatment in an effort to neutralize the effect of any remaining CTLA-4+ cells, and did not observe development of spontaneous autoimmunity or abrogation of EAE protection in these experiments (unpublished data). Furthermore, we observed similar frequencies of CTLA-4–deleted T cells in lymphoid organs and in the CNS during EAE progression (Figs. 1 D and 2 G), arguing against a competitive advantage for CTLA-4–sufficient cells in accessing or accumulating in the target organ.
We find that T reg cells from mice in which CTLA-4 was deleted in adulthood are able to suppress T conv cell proliferation both in vitro and in vivo. We observed massive accumulation of T reg cells in the dLN of CTLA-4–deleted mice, and an increased proportion of T reg cells in the CNS at early time points of EAE. To explain the observed resistance to EAE in this setting, we propose that CTLA-4 on peripheral T reg cells acts as a brake to inhibit T reg cell proliferation. When CTLA-4 is absent, this constraint on T reg cell expansion is removed, and T reg cells accumulate dramatically, while remaining functionally competent. It has been shown that CD28 regulates peripheral homeostasis of T reg cells (Tang et al., 2003), and that T reg cell–specific deletion of CD28 leads to the generation of peripheral T reg cells that are unable to prevent autoimmunity (Zhang et al., 2013). Our findings likely reflect the converse situation; that is, loss of CTLA-4 leads to unrestrained CD28 signaling in T reg cells, resulting in T reg cell expansion and ultimately in EAE resistance.
Importantly, the proposed role for CTLA-4 in inhibiting peripheral T reg cell expansion does not preclude it also functioning as a mediator of T reg cells suppression, as has been demonstrated by many groups. Our data suggest that CTLA-4 is not required for the ability of T reg cells to suppress T conv cell proliferation and that the net result of CTLA-4 loss in vivo leads to protection from EAE. It may be that increased proliferation of T reg cells, coupled with their increased production of antiinflammatory cytokines, may compensate for a per-cell T reg cells dysfunction that would become apparent in other settings. CNS inflammation and antitumor immunity may be suppressible through CTLA-4–independent T reg cell mechanisms, such as IL-10 production, when T reg cells are present in sufficient numbers, which could explain why CTLA-4–deleted T reg cells are competent to suppress EAE and prevent tumor regression. However, in other models, the trans-acting function of CTLA-4 in modulating B7-expressing cells may be more important. Indeed, our data suggest that this trans-acting role is important in regulating B7 expression in the periphery, but that it may not be critical in the CNS (Fig. 8). Thus, we propose that there are opposing roles for CTLA-4 on T reg cells: CTLA-4 has an intrinsic function that acts as an inhibitor of T reg cell proliferation, which, by virtue of the suppressive nature of T reg cells, counters its extrinsic function as a modulator of B7-expressing cells. Consistent with this, we have recently shown that CTLA-4 has dual roles on T follicular regulatory (Tfr) cells, an effector T reg cell subset that specifically suppresses B cell responses in the germinal center (GC) of the LN. We found that CTLA-4 inhibited differentiation and expansion of Tfr cells and that it was required for their suppressive capacity (Sage et al., 2014). Differences between the dependence on CTLA-4 for suppressive function of Tfr cells in the GC versus effector T reg cells in the context of EAE could be a result of different suppressive mechanisms. Effector T reg cells in the CNS may have multiple, redundant suppressive mechanisms, and any defect in suppression by loss of CTLA-4 may be compensated for by other mechanisms (e.g., IL-10 production) as well as by expansion of T reg cells. Tfr cells, however, may have fewer redundant mechanisms to compensate for the lack of CTLA-4, resulting in severely reduced suppression even with expansion of cells.
Our work does not reveal a necessary role for Ctla4 deletion on T conv cells for the EAE resistance that we have observed; rather, loss of CTLA-4 on T reg cells alone, is sufficient. We do, however, observe profound changes in the T conv cell compartment when Ctla4 is specifically deleted on T reg cells (Fig. 7), consistent with the idea that the expanded CTLA-4–deficient T reg cells are impinging on T conv cells due to the extrinsic nature of T reg cell function. In addition, we do observe a T conv cell-intrinsic role for CTLA-4 in preventing T cell expansion in our adoptive transfer experiments, in which we target Ctla4 deletion only to Foxp3− populations in a lymphopenic environment (Fig. 4 A). We did not, however, find an in vitro role for CTLA-4 in T conv cell proliferation or T helper cell differentiation (Fig. 4, B and C). Because Ctla4 deletion on T cells is likely to have its earliest effects on T reg cells (which constitutively express CTLA-4), the timing of tamoxifen administration relative to disease onset may be relevant.
Given the importance of CTLA-4 as an immunotherapeutic target in cancer, we also investigated the consequences of CTLA-4 deletion in adult mice on antitumor immunity. We used a tumor model (MC38 colon adenocarcinoma) in which antibody targeting of CTLA-4 promotes antitumor immunity (Kocak et al., 2006; Allard et al., 2013), albeit likely due to depletion of T reg cells (Selby et al., 2013). In adult mice deficient for Ctla4, MC38 tumors grew slightly faster than in control mice, with indications of diminished antitumor immunity (Fig. 9 A). These findings show that CTLA-4 is not required for induction of T cell dysfunction in tumors. There are several parallels between these findings and our findings in EAE. In both cases, Ctla4 deficiency leads to a massive peripheral expansion of T reg cells. Similarly to the CLN of mice with EAE, in the dLN of tumor-bearing mice, there was increased proliferation of both effector cells (CD8+ T cells and CD4+ T conv cells) and T reg cells in deleted mice; however, the ratios greatly favor T reg cells. In contrast to EAE, at the effector site in the tumor model (the tumor itself), the ratio of effector cells to T reg cells did not shift to favor T reg cells in deleted mice. In the tumor, CD8+ TIL (the dominant antitumor effector cells) in CTLA-4–deleted mice appeared to be incompletely activated based on lower surface expression of CD44 and less production of IFN-γ, similar to the CD4+ T conv cells in the CNS of deleted mice with EAE, and a greater percentage of these cells produced Granzyme B, a key cytotoxic molecule in antitumor immunity, in deleted mice. The phenotype of these CD8+ TILs is reminiscent of terminally differentiated exhausted CD8+ T cells seen during chronic LCMV infection (Odorizzi et al., 2015). Recent work has shown that there is a critical proliferative hierarchy between Tbethi and Eomeshi subsets of exhausted CD8+ T cells that is needed to maintain exhausted T cells long-term. Analogous to recent studies in the chronic LCMV model in which the absence of PD-1 led to the accumulation of more cytotoxic but terminally differentiated exhausted CD8+ T cells, CTLA-4 inhibitory signals may be needed to prevent overstimulation, excessive proliferation and terminal differentiation of exhausted T cells. The modest effects on tumor growth may reflect effects of Ctla4 deletion on development of dysfunctional CD8+ TILs, as well as on T reg cells. Increased IL-10 in TILs in CTLA-deleted mice also may dampen antitumor immunity and promote effector T cell dysfunction.
Our data point broadly toward the concept that co-inhibitory receptors expressed by T reg cells have inhibitory effects on the T reg cells themselves, with complex and, occasionally, paradoxical impact on the immune response in certain settings. The use of anti–CTLA-4 mAb to unleash an effective immune response against cancer is already an FDA-approved approach. However, the mechanisms by which these antibodies promote antitumor immunity are not yet fully understood, and some data suggest that the therapeutic effects of anti–CTLA-4 are being achieved through depletion of T reg cells (Bulliard et al., 2013; Simpson et al., 2013). Our data suggest that the use of a nondepleting anti–CTLA-4 antibody may, in fact, have undesired effects of promoting T reg cell proliferation and production of antiinflammatory molecules. Such considerations will become increasingly important with use of anti–CTLA-4 in combination with other cancer therapies.
MATERIALS AND METHODS
Mice.
Mice 6–10 wk of age were used for all experiments. Wild-type C57BL/6 mice, B6.Cg-Tg(UBC-cre/ERT2)1Ejb/J (UBCCre/ERT2), B6.129S7-Rag1tm1Mom/J, B6.SJL-Ptprca Pepcb/BoyJ, B6.129S2-Tcratm1Mom/J, and Foxp3tm9(EGFP/cre/ERT2)Ayr/J mice were purchased from The Jackson Laboratory. 2D2 TCR transgenic mice (Bettelli et al., 2003) and Foxp3-GFP reporter mice (Bettelli et al., 2006) were generated as previously described. The UBCCre/ERT2 transgene was maintained in a hemizygous x wild-type breeding strategy, such that UBCCre/ERT2- littermates could be used as controls. All experimental mice were housed in specific pathogen–free conditions and used in accordance with animal care guidelines from the Harvard Medical School Standing Committee on Animals and the National Institutes of Health. Animal protocols were approved by the Harvard Medical School Standing Committee on Animals.
Generation of conditional CTLA-4 knockout mouse.
A conditional knockout targeting vector for the Ctla4 gene was generated to contain three loxP sites: The first and second loxP sites flank a selection cassette consisting of a neomycin resistance gene (neo), under the control of the phosphoglycerate kinase (PGK) promoter and thymidine kinase under the control of the herpes simplex virus (HSV) promoter. This construct also contains diphtheria toxin under the control of the PGK promoter, which allows for selection against nonhomologous recombination events. Exons 2 and 3 of Ctla4 were inserted into the vector downstream of the selection cassette and upstream of the third loxP site using a PCR-based approach, whereas the flanking regions of the Ctla4 gene were cloned from a Ctla4-containing BAC using standard techniques. The targeting vector was introduced into C57BL/6 ES cells by electroporation, and the resulting neomycin-resistant ES cells were screened for homologous recombination. ES cells carrying the desired homologous recombination event were subjected to a transient transfection with a Cre recombinase-expressing plasmid to remove the selection cassette. ES cells containing the selection cassette were selected against using 1-(2-deoxy-2-fluoro-b-d-arabinofuranosyl)-5-iodouridine and ES cells retaining the two loxP sites flanking exons 2 and 3 were identified using a PCR-based approach and confirmed by genomic sequencing. Positive clones were micro-injected into albino-B6 blastocysts and implanted into pseudopregnant females to generate chimeras. Germ-line transmission of the floxed Ctla4 allele was achieved, and heterozygous mice were then bred to homozygosity. Breeding of Ctla4fl/fl mice to B6.Cg-Tg(UBC-cre/ERT2)1Ejb/J was performed to obtain tamoxifen-inducible deletion of Ctla4 and to Foxp3EGFP/Cre/ERT2 mice to obtain inducible deletion on T reg cells only.
Gene expression quantitation by real-time quantitative PCR.
Total RNA was isolated using the RNEasy Mini Plus kit (QIAGEN) and quantitated using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific). cDNA was synthesized by reverse transcription with random hexamer primers using the High Capacity cDNA Synthesis kit (Applied Biosystems). Real-time qPCR was performed either using SYBR Green chemistry (Roche) on a LightCycler 480 instrument or TaqMan chemistry (Applied Biosystems) on an ABI 7500 instrument, as indicated. All samples were run at least in triplicate, and no-template control samples were included for each gene to exclude DNA contamination. Primer sequences for the CTLA-4(1,4) isoform were as in Liu et al. (2012; forward: 5′-TGCCTTCTAGGACTTGGCCTT-3′; reverse: 5′-GAGGACTTCTTTTCTTTAGCTTCAGAGA-3′; probe: 5′-AGCCCTGCTCACTCTTCTTTTCATCCCA-3′). All other primer sequences (CTLA-4 forward: 5′-AGAACCATGCCCGGATTCTG-3′, CTLA-4; reverse: 5′-CATCTTGCTCAAAGAAACAGCAG-3′; B2m forward: 5′-GCTCGGTGACCCTGGTCTTT-3′; B2m reverse: 5′-TGTTCGGCTTCCCATTCTCC-3′) were obtained from PrimerBank (Wang et al., 2012).
Tamoxifen-induced conditional deletion in UBCCre/ERT2 mice.
To prepare tamoxifen solution for injection, tamoxifen (Sigma-Aldrich) was dissolved in ethanol, diluted 1:20 in sunflower oil, and sonicated for 5 min at 37°C. Mice were injected daily with 1 mg tamoxifen intraperitoneally for 5 consecutive days, then rested for a minimum of 72 h before any experimental manipulation.
EAE.
MOG35-55 peptide (MEVGWYRSPFSRVVHLYRNGK) was synthesized at the Biopolymer Laboratory (David Geffen School of Medicine, University of California, La Jolla, CA; purity (>85%) was verified by HPLC. Amounts used were corrected for purity. To induce EAE, groups of 8–12-wk-old mice were immunized with 150 µg of MOG35-55 emulsified 1:1 in CFA with 400 µg of Mycobacterium tuberculosis H37RA (Difco Laboratories) in the two flanks subcutaneously. 250 ng of pertussis toxin (List Biological Laboratories) was injected intraperitoneally on the day of immunization and 2 d later. Mice were observed daily for clinical signs of EAE up to 30 d after immunization, and scored on a scale of 0–5: 0, no disease; 1, limp tail; 2, hind limb weakness; 3, hind limb paralysis; 4, hind and fore limb paralysis; and 5, moribund state. Mean clinical score was calculated by averaging the scores of all mice in each group, including animals that did not develop EAE.
Histopathology.
Tissues were fixed in 10% formalin, and paraffin-embedded sections were stained with hematoxylin and eosin (H&E) or Luxol fast blue/periodic acid-Schiff for light microscopic analysis. CNS pathology was scored blindly by a neuropathologist. Inflammatory foci in meninges and parenchyma were counted as described previously (Sobel et al., 1990). Photomicrographs were acquired on an Olympus BH-2 light microscope at the indicated magnifications using an Olympus DP71 camera and software provided by the manufacturer. Horizontal bars represent 200 µm for 100x images and 50 µm for 400x images.
Analysis of CNS-infiltrating mononuclear cells.
Before dissection, mice were perfused through the left ventricle with cold PBS. The brain and the spinal cord isolated, and CNS tissue was minced with a sharp razor blade and digested for 20 min at 37°C with collagenase D (2.5 mg/ml; Roche) and DNaseI (1 mg/ml; Sigma-Aldrich). Mononuclear cells were isolated by passage of the tissue through a cell strainer (70 µm), followed by centrifugation through a Percoll gradient (30% and 70%). Mononuclear cells in the interface were removed, washed, and resuspended in culture medium for analysis.
Intracellular cytokine staining.
Cells from lymphoid organs were isolated and restimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) and 500 ng/ml ionomycin (both from Sigma-Aldrich) in the presence of GolgiStop (BD) for 4–5 h, then processed for flow cytometric analysis as described below.
Antibodies and flow cytometry.
Cells from lymphoid organs, CNS, or in vitro cultures were isolated and resuspended in staining buffer (PBS containing 1% FCS and 2 mM EDTA) and were stained with the following directly labeled antibodies: anti-CD3 (145-2C11), anti-CD4 (RM4-5), anti-CD8α (53–6.7), anti-CD44 (IM7), anti-CD45.1 (A20), anti-CD45.2 (104), anti-CD62L (MEL-14), anti–IL-10 (JES5-16E3), anti–IL-17F (9D3.1C8), anti–PD-1 (RMP1-30), anti-Lag3 (C9B7W), and anti–IFN-γ (XMG1.2, all from BioLegend). 7-aminoactinomycin D (7-AAD; BioLegend) was used for exclusion of nonviable cells. For intracellular staining (anti–CTLA-4 [UC10-4B9, BD]; anti-Foxp3 [FJK-16, eBioscience]; anti-Ki-67 [B56, BD]), cells were fixed and permeabilized using the Foxp3/Transcription Factor Staining kit (eBioscience) after surface staining, and LIVE/DEAD-Violet reagent (Life Technologies) was used for exclusion of nonviable cells. All flow cytometry data were acquired on an LSR II cytometer with standard filter sets using FACSDiva software (BD), and were further analyzed with FlowJo software (Tree Star).
Adoptive transfers.
Bulk CD4+ (isolated from spleens by CD4 MicroBeads; Miltenyi Biotec) or CD4+Foxp3-GFP− (isolated from spleens by fluorescence-activated cell sorting; FACS) were transferred to TCRα−/− or Rag1−/− recipients that were then treated with tamoxifen and immunized with MOG35-55 to induce EAE.
T helper differentiation assays.
CD4+CD62LhiFoxp3-GFP− cells were sorted by fluorescence-activated cell sorting (purity >99%) and placed in culture in 24-well plates with TCRα−/− splenocytes (1:4 T cell/splenocyte ratio), 2 µg/ml anti-CD3 (clone 2C11), 1 µM 4-hydroxytamoxifen and either 5 ng/ml TGF-β (T reg cell conditions), 5 ng/ml TGF-β + 30 ng/ml IL-6 (Th17 conditions), or 10 ng/ml IL-12 + 10 µg/ml anti-IL4 (Th1 conditions; all cytokines were obtained from BioLegend; anti-IL-4 clone 11B11 was purchased from BioXCell). Cells were harvested on day 4 of culture and analyzed by flow cytometry. For Th1 and Th17 conditions, cells were stimulated with PMA and ionomycin, as described above, before analysis.
In vitro T reg cells suppression assay.
Responder T conv cells were purified by sorting of CD4+CD62LhiCD25− T cells from CD45.1+ congenic splenocytes and labeling with CellTrace Violet Cell Proliferation kit (Molecular Probes) according to the manufacturer’s instructions. CD4+Foxp3−GFP+tdTomato+ T reg cells were purified by sorting from splenocytes from tamoxifen-treated UBCCre/ERT2+Ctla4fl/flFoxp3-GFPKIROSA26loxP-STOP-loxP-tdTomato or UBCCre/ERT+Ctla4+/+Foxp3-GFPKIROSA26loxP-STOP-loxP-tdTomato mice. DCs were isolated from collagenase-treated, wild-type C57BL/6 spleens using CD11c MicroBeads and MACS columns (Miltenyi Biotec). T conv and T reg cells were co-cultured at varying ratios in the presence of 10% DCs and 2 µg/ml anti-CD3 for 3 d. Co-cultures were then harvested and analyzed by flow cytometry. Responder T conv cells were gated on CD4 and CD45.1 (7-AAD+ cells were excluded), and dilution of CellTrace Violet was assessed using the proliferation platform of FlowJo.
In vivo T reg cell suppression assay.
This assay was adapted from Workman et al. (2011). CD4+Foxp3-GFP+ cells were purified by sorting from lymphoid organs of Foxp3-GFPKI mice that were either UBCCre/ERT2+Ctla4fl/fl or UBCCre/ERT2+Ctla4+/+. CD4+CD62LhiCD25− T conv cells were purified by sorting from lymphoid organs of CD45.1 congenic mice. T reg and T conv cells were adoptively cotransferred into Rag1−/− hosts, such that T reg cells comprised 25% of the total transferred cell population; a separate group of mice received T conv cells alone (positive control). Recipients were treated with tamoxifen on days 0–4. 10 d after transfer, mice were sacrificed and CD45.1+CD4+ T conv cells and CD45.1−CD4+Foxp3-GFP+ T reg cells were quantitated by cell counting and flow cytometric analysis of splenocytes.
Gene expression quantitation by NanoString analysis.
Gene expression in sorted T conv cells and T reg cell populations was quantified using a custom nCounter probe set containing 301 probe pairs, including additional probes for positive and negative controls and housekeeping genes (NanoString Technologies; Yosef et al., 2013). In brief, total cellular RNA was isolated and quantitated as above, and 100 ng was hybridized with customized Reporter CodeSet and Capture ProbeSet (NanoString Technologies) by overnight incubation in a thermal cycler at 65°C. The next day, flow cell preparation and scanning were performed using an nCounter instrument (NanoString Technologies) according to the manufacturer’s instructions. Raw data were normalized using nSolver software (NanoString Technologies) and exported as raw transcript counts for presentation. To normalize data, average background was subtracted from raw count, and normalization was performed using the geometric mean count from four housekeeping genes (Gapdh, Hprt, Actb, and Tubb5). Heat maps were generated using GENE-E software.
MC38 tumor model.
Mice were treated with tamoxifen on days −7 through −3 as described above. On day 0, 100,000 MC38 adenocarcinoma cells (a gift from D. Vignali, University of Pittsburgh School of Medicine, Pittsburgh, PA) were injected s.c. on the flank. Tumor measurement was performed on day 7 after tumor injection and every other day thereafter. Tumor volume was calculated as 1/2 × D × d2, where D is the major axis and d the minor axis. On the day of harvest, tumors were dissected, disaggregated, and subjected to collagenase type I (400 U/ml; Worthington Biochemical) digestion for 30 min at 37°C, after which they were passed through a cell strainer (70 µm). Mononuclear cells were isolated by centrifugation through a Percoll gradient (30 and 70%). The interface was removed, washed and resuspended in culture medium for analysis.
Statistical analysis.
Prism software (GraphPad) was used for statistical analysis. Student’s two-tailed t test was used for all pairwise comparisons. Differences were considered statistically significant with a p-value of <0.05 (*), <0.01 (**), or <0.001 (***).
Acknowledgments
The authors thank Baolin Chang, Xiaohui He, Vition Mbrica, Milagros Ávalo, and Louise Barias for assistance with mouse genotyping and colony maintenance, and Sarah Hillman for administrative support.
This work was supported by grants from the National Institutes of Health (AI38310 and AI40614 to A.H. Sharpe; P01 AI39671 to A.H. Sharpe and V.K. Kuchroo; PO1 AI56299 to A.H. Sharpe, G.J. Freeman, and V.K. Kuchroo) and the National Multiple Sclerosis Society (FG-1916-A-1 to S.B. Lovitch).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- cLN
- cervical LNs
- CNS
- central nervous system
- CTLA-4
- cytotoxic T lymphocyte antigen-4
- EAE
- experimental autoimmune encephalomyelitis
- T conv
- conventional CD4+Foxp3- T cells
- TILs
- tumor infiltrating lymphocytes
- T reg cells
- regulatory Foxp3+ T cells
References
- Allard B., Pommey S., Smyth M.J., and Stagg J.. 2013. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin. Cancer Res. 19:5626–5635. 10.1158/1078-0432.CCR-13-0545 [DOI] [PubMed] [Google Scholar]
- Asano M., Toda M., Sakaguchi N., and Sakaguchi S.. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184:387–396. 10.1084/jem.184.2.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Köhler G., Ecabert B., Mak T.W., and Kopf M.. 1999. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J. Immunol. 163:1128–1131. [PubMed] [Google Scholar]
- Bettelli E., Das M.P., Howard E.D., Weiner H.L., Sobel R.A., and Kuchroo V.K.. 1998. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J. Immunol. 161:3299–3306. [PubMed] [Google Scholar]
- Bettelli E., Pagany M., Weiner H.L., Linington C., Sobel R.A., and Kuchroo V.K.. 2003. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197:1073–1081. 10.1084/jem.20021603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., and Kuchroo V.K.. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238. 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Buhlmann J.E., Elkin S.K., and Sharpe A.H.. 2003. A role for the B7-1/B7-2:CD28/CTLA-4 pathway during negative selection. J. Immunol. 170:5421–5428. 10.4049/jimmunol.170.11.5421 [DOI] [PubMed] [Google Scholar]
- Bulliard Y., Jolicoeur R., Windman M., Rue S.M., Ettenberg S., Knee D.A., Wilson N.S., Dranoff G., and Brogdon J.L.. 2013. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J. Exp. Med. 210:1685–1693. 10.1084/jem.20130573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C.A., Cado D., Truong T., and Allison J.P.. 1997. Thymocyte development is normal in CTLA-4-deficient mice. Proc. Natl. Acad. Sci. USA. 94:9296–9301. 10.1073/pnas.94.17.9296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.T., Sobel R.A., Wei T., Ransohoff R.M., Kuchroo V.K., and Sharpe A.H.. 2003. Recovery from EAE is associated with decreased survival of encephalitogenic T cells in the CNS of B7-1/B7-2-deficient mice. Eur. J. Immunol. 33:2022–2032. 10.1002/eji.200323180 [DOI] [PubMed] [Google Scholar]
- Chuang E., Lee K.M., Robbins M.D., Duerr J.M., Alegre M.L., Hambor J.E., Neveu M.J., Bluestone J.A., and Thompson C.B.. 1999. Regulation of cytotoxic T lymphocyte-associated molecule-4 by Src kinases. J. Immunol. 162:1270–1277. [PubMed] [Google Scholar]
- Chuang E., Fisher T.S., Morgan R.W., Robbins M.D., Duerr J.M., Vander Heiden M.G., Gardner J.P., Hambor J.E., Neveu M.J., and Thompson C.B.. 2000. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 13:313–322. 10.1016/S1074-7613(00)00031-5 [DOI] [PubMed] [Google Scholar]
- Cilio C.M., Daws M.R., Malashicheva A., Sentman C.L., and Holmberg D.. 1998. Cytotoxic T lymphocyte antigen 4 is induced in the thymus upon in vivo activation and its blockade prevents anti-CD3-mediated depletion of thymocytes. J. Exp. Med. 188:1239–1246. 10.1084/jem.188.7.1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin H.C., Burlen-Defranoux O., Boucontet L., Vieira P., Cumano A., and Bandeira A.. 2004. Regulatory potential and control of Foxp3 expression in newborn CD4+ T cells. Proc. Natl. Acad. Sci. USA. 101:14473–14478. 10.1073/pnas.0403303101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F., Grohmann U., Hwang K.W., Orabona C., Vacca C., Bianchi R., Belladonna M.L., Fioretti M.C., Alegre M.L., and Puccetti P.. 2003. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 4:1206–1212. 10.1038/ni1003 [DOI] [PubMed] [Google Scholar]
- Fife B.T., and Bluestone J.A.. 2008. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 224:166–182. 10.1111/j.1600-065X.2008.00662.x [DOI] [PubMed] [Google Scholar]
- Fraser J.H., Rincón M., McCoy K.D., and Le Gros G.. 1999. CTLA4 ligation attenuates AP-1, NFAT and NF-kappaB activity in activated T cells. Eur. J. Immunol. 29:838–844. [DOI] [PubMed] [Google Scholar]
- Freeman G.J., Gray G.S., Gimmi C.D., Lombard D.B., Zhou L.J., White M., Fingeroth J.D., Gribben J.G., and Nadler L.M.. 1991. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J. Exp. Med. 174:625–631. 10.1084/jem.174.3.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G.J., Lombard D.B., Gimmi C.D., Brod S.A., Lee K., Laning J.C., Hafler D.A., Dorf M.E., Gray G.S., Reiser H., et al. 1992. CTLA-4 and CD28 mRNA are coexpressed in most T cells after activation. Expression of CTLA-4 and CD28 mRNA does not correlate with the pattern of lymphokine production. J. Immunol. 149:3795–3801. [PubMed] [Google Scholar]
- Freeman G.J., Borriello F., Hodes R.J., Reiser H., Gribben J.G., Ng J.W., Kim J., Goldberg J.M., Hathcock K., Laszlo G., et al. 1993. Murine B7-2, an alternative CTLA4 counter-receptor that costimulates T cell proliferation and interleukin 2 production. J. Exp. Med. 178:2185–2192. 10.1084/jem.178.6.2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedline R.H., Brown D.S., Nguyen H., Kornfeld H., Lee J., Zhang Y., Appleby M., Der S.D., Kang J., and Chambers C.A.. 2009. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J. Exp. Med. 206:421–434. 10.1084/jem.20081811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough S.C., Walker L.S., and Sansom D.M.. 2005. CTLA4 gene polymorphism and autoimmunity. Immunol. Rev. 204:102–115. 10.1111/j.0105-2896.2005.00249.x [DOI] [PubMed] [Google Scholar]
- Greenwald R.J., Oosterwegel M.A., van der Woude D., Kubal A., Mandelbrot D.A., Boussiotis V.A., and Sharpe A.H.. 2002. CTLA-4 regulates cell cycle progression during a primary immune response. Eur. J. Immunol. 32:366–373. [DOI] [PubMed] [Google Scholar]
- Harper K., Balzano C., Rouvier E., Mattéi M.G., Luciani M.F., and Golstein P.. 1991. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J. Immunol. 147:1037–1044. [PubMed] [Google Scholar]
- Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363:711–723. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ise W., Kohyama M., Nutsch K.M., Lee H.M., Suri A., Unanue E.R., Murphy T.L., and Murphy K.M.. 2010. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat. Immunol. 11:129–135. 10.1038/ni.1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N., Nguyen H., Chambers C., and Kang J.. 2010. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc. Natl. Acad. Sci. USA. 107:1524–1528. 10.1073/pnas.0910341107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karandikar N.J., Vanderlugt C.L., Walunas T.L., Miller S.D., and Bluestone J.A.. 1996. CTLA-4: a negative regulator of autoimmune disease. J. Exp. Med. 184:783–788. 10.1084/jem.184.2.783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocak E., Lute K., Chang X., May K.F. Jr., Exten K.R., Zhang H., Abdessalam S.F., Lehman A.M., Jarjoura D., Zheng P., and Liu Y.. 2006. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 66:7276–7284. 10.1158/0008-5472.CAN-05-2128 [DOI] [PubMed] [Google Scholar]
- Kong K.F., Fu G., Zhang Y., Yokosuka T., Casas J., Canonigo-Balancio A.J., Becart S., Kim G., Yates J.R. III, Kronenberg M., et al. 2014. Protein kinase C-η controls CTLA-4-mediated regulatory T cell function. Nat. Immunol. 15:465–472. 10.1038/ni.2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel M.F., and Allison J.P.. 1996. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 183:2533–2540. 10.1084/jem.183.6.2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.M., Chuang E., Griffin M., Khattri R., Hong D.K., Zhang W., Straus D., Samelson L.E., Thompson C.B., and Bluestone J.A.. 1998. Molecular basis of T cell inactivation by CTLA-4. Science. 282:2263–2266. 10.1126/science.282.5397.2263 [DOI] [PubMed] [Google Scholar]
- Lee Y., Awasthi A., Yosef N., Quintana F.J., Xiao S., Peters A., Wu C., Kleinewietfeld M., Kunder S., Hafler D.A., et al. 2012. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13:991–999. 10.1038/ni.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P.S., Brady W., Urnes M., Grosmaire L.S., Damle N.K., and Ledbetter J.A.. 1991. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 174:561–569. 10.1084/jem.174.3.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P.S., Greene J.L., Tan P., Bradshaw J., Ledbetter J.A., Anasetti C., and Damle N.K.. 1992. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J. Exp. Med. 176:1595–1604. 10.1084/jem.176.6.1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Geboes K., Hellings P., Maerten P., Heremans H., Vandenberghe P., Boon L., van Kooten P., Rutgeerts P., and Ceuppens J.L.. 2001. B7 interactions with CD28 and CTLA-4 control tolerance or induction of mucosal inflammation in chronic experimental colitis. J. Immunol. 167:1830–1838. 10.4049/jimmunol.167.3.1830 [DOI] [PubMed] [Google Scholar]
- Liu S.M., Sutherland A.P., Zhang Z., Rainbow D.B., Quintana F.J., Paterson A.M., Sharpe A.H., Oukka M., Wicker L.S., and Kuchroo V.K.. 2012. Overexpression of the Ctla-4 isoform lacking exons 2 and 3 causes autoimmunity. J. Immunol. 188:155–162. 10.4049/jimmunol.1102042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengère L.E., Waterhouse P., Duncan G.S., Mittrücker H.W., Feng G.S., and Mak T.W.. 1996. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 272:1170–1173. 10.1126/science.272.5265.1170 [DOI] [PubMed] [Google Scholar]
- Metzler B., Burkhart C., and Wraith D.C.. 1999. Phenotypic analysis of CTLA-4 and CD28 expression during transient peptide-induced T cell activation in vivo. Int. Immunol. 11:667–675. 10.1093/intimm/11.5.667 [DOI] [PubMed] [Google Scholar]
- Odorizzi P.M., Pauken K.E., Paley M.A., Sharpe A., and Wherry E.J.. 2015. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J. Exp. Med. 212:1125–1137. 10.1084/jem.20142237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson C., Riesbeck K., Dohlsten M., and Michaëlsson E.. 1999. CTLA-4 ligation suppresses CD28-induced NF-kappaB and AP-1 activity in mouse T cell blasts. J. Biol. Chem. 274:14400–14405. 10.1074/jbc.274.20.14400 [DOI] [PubMed] [Google Scholar]
- Onishi Y., Fehervari Z., Yamaguchi T., and Sakaguchi S.. 2008. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl. Acad. Sci. USA. 105:10113–10118. 10.1073/pnas.0711106105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin P.J., Maldonado J.H., Davis T.A., June C.H., and Racke M.K.. 1996. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J. Immunol. 157:1333–1336. [PubMed] [Google Scholar]
- Punt J.A., Osborne B.A., Takahama Y., Sharrow S.O., and Singer A.. 1994. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J. Exp. Med. 179:709–713. 10.1084/jem.179.2.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt J.A., Havran W., Abe R., Sarin A., and Singer A.. 1997. T cell receptor (TCR)-induced death of immature CD4+CD8+ thymocytes by two distinct mechanisms differing in their requirement for CD28 costimulation: implications for negative selection in the thymus. J. Exp. Med. 186:1911–1922. 10.1084/jem.186.11.1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi O.S., Zheng Y., Nakamura K., Attridge K., Manzotti C., Schmidt E.M., Baker J., Jeffery L.E., Kaur S., Briggs Z., et al. 2011. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 332:600–603. 10.1126/science.1202947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Malmström V., and Powrie F.. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. 10.1084/jem.192.2.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Greenwald R., Izcue A., Robinson N., Mandelbrot D., Francisco L., Sharpe A.H., and Powrie F.. 2006. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J. Immunol. 177:4376–4383. 10.4049/jimmunol.177.7.4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C., Thomas L., Bondarenko I., O’Day S., Weber J., Garbe C., Lebbe C., Baurain J.F., Testori A., Grob J.J., et al. 2011. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364:2517–2526. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- Rubtsov Y.P., Niec R.E., Josefowicz S., Li L., Darce J., Mathis D., Benoist C., and Rudensky A.Y.. 2010. Stability of the regulatory T cell lineage in vivo. Science. 329:1667–1671. 10.1126/science.1191996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage P.T., Paterson A.M., Lovitch S.B., and Sharpe A.H.. 2014. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 41:1026–1039. 10.1016/j.immuni.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon B., Lenschow D.J., Rhee L., Ashourian N., Singh B., Sharpe A., and Bluestone J.A.. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 12:431–440. 10.1016/S1074-7613(00)80195-8 [DOI] [PubMed] [Google Scholar]
- Samy E.T., Wheeler K.M., Roper R.J., Teuscher C., and Tung K.S.. 2008. Cutting edge: Autoimmune disease in day 3 thymectomized mice is actively controlled by endogenous disease-specific regulatory T cells. J. Immunol. 180:4366–4370. 10.4049/jimmunol.180.7.4366 [DOI] [PubMed] [Google Scholar]
- Scalapino K.J., and Daikh D.I.. 2008. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol. Rev. 223:143–155. 10.1111/j.1600-065X.2008.00639.x [DOI] [PubMed] [Google Scholar]
- Schmidt E.M., Wang C.J., Ryan G.A., Clough L.E., Qureshi O.S., Goodall M., Abbas A.K., Sharpe A.H., Sansom D.M., and Walker L.S.. 2009. Ctla-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J. Immunol. 182:274–282. 10.4049/jimmunol.182.1.274 [DOI] [PubMed] [Google Scholar]
- Selby M.J., Engelhardt J.J., Quigley M., Henning K.A., Chen T., Srinivasan M., and Korman A.J.. 2013. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 1:32–42. 10.1158/2326-6066.CIR-13-0013 [DOI] [PubMed] [Google Scholar]
- Sharpe A.H., and Freeman G.J.. 2002. The B7-CD28 superfamily. Nat. Rev. Immunol. 2:116–126. 10.1038/nri727 [DOI] [PubMed] [Google Scholar]
- Simpson T.R., Li F., Montalvo-Ortiz W., Sepulveda M.A., Bergerhoff K., Arce F., Roddie C., Henry J.Y., Yagita H., Wolchok J.D., et al. 2013. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 210:1695–1710. 10.1084/jem.20130579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel R.A., Tuohy V.K., Lu Z.J., Laursen R.A., and Lees M.B.. 1990. Acute experimental allergic encephalomyelitis in SJL/J mice induced by a synthetic peptide of myelin proteolipid protein. J. Neuropathol. Exp. Neurol. 49:468–479. 10.1097/00005072-199009000-00002 [DOI] [PubMed] [Google Scholar]
- Tai X., Cowan M., Feigenbaum L., and Singer A.. 2005. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat. Immunol. 6:152–162. 10.1038/ni1160 [DOI] [PubMed] [Google Scholar]
- Tai X., Van Laethem F., Pobezinsky L., Guinter T., Sharrow S.O., Adams A., Granger L., Kruhlak M., Lindsten T., Thompson C.B., et al. 2012. Basis of CTLA-4 function in regulatory and conventional CD4+ T cells. Blood. 119:5155–5163. 10.1182/blood-2011-11-388918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T.W., and Sakaguchi S.. 2000. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192:303–310. 10.1084/jem.192.2.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Kataoka H., Hara S., Yokosuka T., Takase K., Yamasaki S., Kobayashi W., Saito Y., and Saito T.. 2005. In vivo overexpression of CTLA-4 suppresses lymphoproliferative diseases and thymic negative selection. Eur. J. Immunol. 35:399–407. 10.1002/eji.200324746 [DOI] [PubMed] [Google Scholar]
- Tang Q., Henriksen K.J., Boden E.K., Tooley A.J., Ye J., Subudhi S.K., Zheng X.X., Strom T.B., and Bluestone J.A.. 2003. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J. Immunol. 171:3348–3352. 10.4049/jimmunol.171.7.3348 [DOI] [PubMed] [Google Scholar]
- Tang Q., Boden E.K., Henriksen K.J., Bour-Jordan H., Bi M., and Bluestone J.A.. 2004. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur. J. Immunol. 34:2996–3005. 10.1002/eji.200425143 [DOI] [PubMed] [Google Scholar]
- Tivol E.A., Borriello F., Schweitzer A.N., Lynch W.P., Bluestone J.A., and Sharpe A.H.. 1995. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 3:541–547. 10.1016/1074-7613(95)90125-6 [DOI] [PubMed] [Google Scholar]
- Ueda H., Howson J.M., Esposito L., Heward J., Snook H., Chamberlain G., Rainbow D.B., Hunter K.M., Smith A.N., Di Genova G., et al. 2003. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 423:506–511. 10.1038/nature01621 [DOI] [PubMed] [Google Scholar]
- Verhagen J., Gabrysová L., Minaee S., Sabatos C.A., Anderson G., Sharpe A.H., and Wraith D.C.. 2009. Enhanced selection of FoxP3+ T-regulatory cells protects CTLA-4-deficient mice from CNS autoimmune disease. Proc. Natl. Acad. Sci. USA. 106:3306–3311. 10.1073/pnas.0803186106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen J., Genolet R., Britton G.J., Stevenson B.J., Sabatos-Peyton C.A., Dyson J., Luescher I.F., and Wraith D.C.. 2013. CTLA-4 controls the thymic development of both conventional and regulatory T cells through modulation of the TCR repertoire. Proc. Natl. Acad. Sci. USA. 110:E221–E230. 10.1073/pnas.1208573110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D.H. Jr., Hagman J., Linsley P.S., Hodsdon W., Freed J.H., and Newell M.K.. 1996. Rescue of thymocytes from glucocorticoid-induced cell death mediated by CD28/CTLA-4 costimulatory interactions with B7-1/B7-2. J. Exp. Med. 184:1631–1638. 10.1084/jem.184.5.1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.S. 2013. Treg and CTLA-4: two intertwining pathways to immune tolerance. J. Autoimmun. 45:49–57. 10.1016/j.jaut.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas T.L., Bakker C.Y., and Bluestone J.A.. 1996. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med. 183:2541–2550. 10.1084/jem.183.6.2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Spandidos A., Wang H., and Seed B.. 2012. PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res. 40(D1):D1144–D1149. 10.1093/nar/gkr1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse P., Penninger J.M., Timms E., Wakeham A., Shahinian A., Lee K.P., Thompson C.B., Griesser H., and Mak T.W.. 1995. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 270:985–988. 10.1126/science.270.5238.985 [DOI] [PubMed] [Google Scholar]
- Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., and Sakaguchi S.. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 322:271–275. 10.1126/science.1160062 [DOI] [PubMed] [Google Scholar]
- Workman C.J., Collison L.W., Bettini M., Pillai M.R., Rehg J.E., and Vignali D.A.. 2011. In vivo Treg suppression assays. Methods Mol. Biol. 707:119–156. 10.1007/978-1-61737-979-6_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Borde M., Heissmeyer V., Feuerer M., Lapan A.D., Stroud J.C., Bates D.L., Guo L., Han A., Ziegler S.F., et al. 2006. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 126:375–387. 10.1016/j.cell.2006.05.042 [DOI] [PubMed] [Google Scholar]
- Yang S., Fujikado N., Kolodin D., Benoist C., and Mathis D.. 2015. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 348:589–594 (Epub ahead of print) 10.1126/science.aaa7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosef N., Shalek A.K., Gaublomme J.T., Jin H., Lee Y., Awasthi A., Wu C., Karwacz K., Xiao S., Jorgolli M., et al. 2013. Dynamic regulatory network controlling TH17 cell differentiation. Nature. 496:461–468. 10.1038/nature11981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Huynh A., Whitcher G., Chang J., Maltzman J.S., and Turka L.A.. 2013. An obligate cell-intrinsic function for CD28 in Tregs. J. Clin. Invest. 123:580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Josefowicz S.Z., Kas A., Chu T.T., Gavin M.A., and Rudensky A.Y.. 2007. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 445:936–940. 10.1038/nature05563 [DOI] [PubMed] [Google Scholar]