Abstract
The Epstein-Barr virus (EBV) BNLF2a gene product provides immune evasion properties to infected cells through inhibition of transporter associated with antigen processing (TAP)-mediated transport of antigen peptides. Although BNLF2a is considered to be a lytic gene, we demonstrate that it is expressed in nearly half of the EBV-associated gastric carcinomas analyzed. Further, we show that BNLF2a expression is dissociated from lytic gene expression. BNLF2a is therefore expressed in this latency setting, potentially helping protect the infected tumor cells from immunosurveillance.
TEXT
The early lytic gene BNLF2a plays an integral role in evading immune recognition of Epstein-Barr virus (EBV)-infected cells undergoing lytic replication, a setting where substantial numbers of viral antigens are expressed (1–7). BNLF2a functions through inhibition of peptide loading onto major histocompatibility complex (MHC) class I molecules, thereby blocking antigen presentation to cytotoxic T lymphocytes (2–7). In contrast to the lytic phase of the EBV infection cycle, latent infection typically results in a highly restricted pattern of gene expression where primarily noncoding RNAs and a minimal number of viral protein-coding genes are expressed, presumably lessening the dependence on overt adaptive immune inhibitory mechanisms.
We previously found one of the four EBV-positive gastric carcinoma (GC) biopsy specimens from an early The Cancer Genome Atlas (TCGA) gastric adenocarcinoma cohort that unexpectedly showed expression of the viral lytic immune evasion gene BNLF2a (1). We raised the possibility that though the finding of only a single BNLF2a-positive sample may be incidental, its expression in gastric cancer could be a means of providing antitumor and/or antivirus immune responses (1).
To investigate this issue further, we first performed a global virome analysis on the expanded cohort of patient GC RNA-seq (n = 285) and whole-exome sequence (WXS) (n = 352) data sets from TCGA (8), using a directed virome analysis approach that we have reported previously (9, 10). For this analysis, all RNA-seq and WXS data sets were aligned to an index containing the human genome (Genome Reference Consortium GRCh37) plus 740 mammalian viral genomes, using the transcript aligner STAR (Spliced Transcripts Alignment to a Reference) (11) run with default options plus the clip5pNbases 6 and outFilterMultimapNmax 1000 command options (removes the first 6 bases of each read and filters out any reads that map to more than 1,000 regions of the genome, respectively). As a way to help gauge true tumor virus association from systemic viral infection, we analyzed 33 RNA-seq TCGA data sets from matched normal gastric tissues. We also performed a virome analysis on 23 RNA-seq gastric cancer cell line data sets from the Cancer Cell Line Encyclopedia (CCLE) project (12).
No substantial viral reads were detected in any of the normal gastric RNA-seq data sets (Fig. 1A; see also Table S1 in the supplemental material). In line with TCGA marker paper (8), we identified 25 GC biopsy specimens with substantial EBV reads in both the RNA-seq and the WXS data (Fig. 1A), although lower EBV read numbers were observed in some additional samples (14 RNA-seq data sets and 11 WXS data sets) that possibly represent low-level infections or the presence of EBV-positive infiltrating B cells (see Tables S1 and S2 in the supplemental material). We also detected human cytomegalovirus (HCMV) reads in 19 RNA-seq and 6 WXS samples (Fig. 1A; see also Tables S1 and S2 in the supplemental material). Although HCMV read numbers were low in most cases, they were detected in both the RNA-seq data and the WGS data in two cases, raising the possibility of low-level infections. Of the GC cell line RNA-seq data sets analyzed, the SNU719 cell line was the only one in which EBV was detected (Fig. 1B). Notably, very high murine leukemia virus (MuLV) read levels (1.5% of human mapped reads) were detected in the LMSU cell line (Fig. 1B; see also Table S3 in the supplemental material), which likely reflects incidental laboratory infection of this cell line, similar to previous MuLV virome findings from our lab for other cell lines (9, 13).
FIG 1.
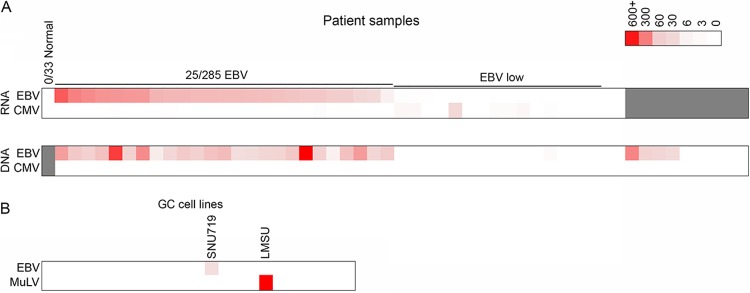
Heat map showing the number of viral reads per million human mapped reads for the TCGA GC patient biopsy specimens (A) and for the 23 GC cell lines (B). Viruses included in this display are those showing at least 2 viral reads per million human mapped reads in at least one RNA-seq sample.
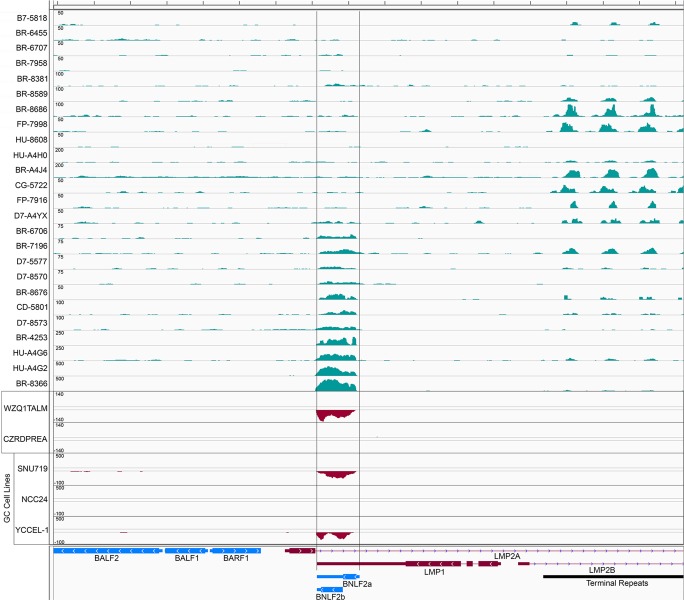
Analysis of read coverage across the EBV genome for all EBV-positive TCGA GC samples with high EBV read numbers showed the expected high-level expression of the noncoding RPMS1 transcript region (see Fig. S1 in the supplemental material) (8), low-level expression of EBNA1, and low-level expression of the immediate early and early genes BZLF1, BSLF2/BMLF1, and BMRF1, which were inconsistently expressed across samples (data not shown). Also consistent with previous studies (1, 8), expression of the latent membrane protein 2 (LMP2) gene was detected in most samples (see Fig. S2 in the supplemental material). In addition to these regions, however, we observed good read coverage across the BNLF2a locus in about half of the samples (Fig. 2), suggesting that BNLF2a is, in fact, expressed in a substantial proportion of EBV-associated GCs.
FIG 2.
Genome browser view of the BNLF2a locus for 25 EBV-positive TCGA GC biopsy specimens, two additional EBV-positive GC tumor biopsy specimens sequenced using the stranded TruSeq library preparation protocol, and three EBV-positive GC cell lines sequenced using the stranded TruSeq library preparation protocol. Values on the y axis represent the number of reads spanning each genomic position. For the stranded sequencing reactions, sense strand coverage is indicated by positive y axis values and antisense strand coverage is indicated by negative y axis values.
Since the TCGA RNA-seq data were generated using library preparation protocols that do not retain source strand information, we sought to investigate the orientation of read coverage at the BNLF2a region in GC biopsy specimens. We analyzed RNAs from 18 independent gastric cancer biopsy specimens (Bioserve) for the presence of EBV through quantitative reverse transcriptase PCR (qRT-PCR) analysis for EBER1 and RPMS1 expression. Two samples were found to be EBV positive (1). RNAs from these samples were subjected to ribodepletion (Ribo-Zero, catalog no. MRZH11124; Epicentre) and the generation of strand-specific TruSeq (catalog no. RS-930-2001; Illumina) sequencing libraries. The libraries were sequenced on an Illumina HiSeq 2000 machine and aligned to the Akata EBV genome (14). One of these samples, WZQ1TALM, showed good coverage at the BNLF2a locus, and reads were found to derive from leftward transcripts (Fig. 2), consistent with the expression of the leftward-oriented BNLF2a gene.
To confirm that BNLF2a can be expressed from tumor cells rather than potentially from EBV-infected stromal components, we performed strand-specific sequencing of three known EBV-positive gastric carcinoma cell lines, SNU719, NCC24, and YCCEL-1 (15–17). SNU719 and YCCEL-1 showed good leftward coverage at the BNLF2a locus (Fig. 2), demonstrating the expression of BNLF2a in GC tumor cells. Further, both SNU719 and YCCEL-1, but not NCC24 or EBV-negative AGS cells, were found to express BNLF2a protein at levels comparable to that observed in the Burkitt's lymphoma cell line Akata, which was induced to undergo reactivation (Fig. 3).
FIG 3.
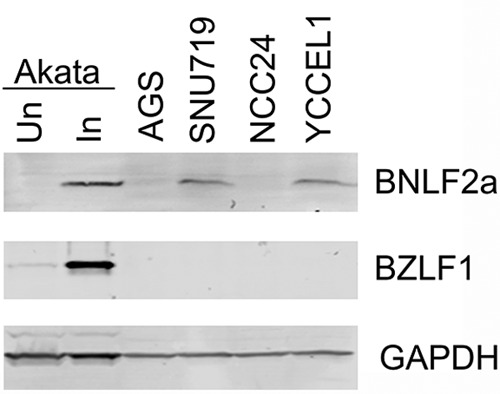
Western blot of BNLF2a expression in untreated Akata cells (Un), Akata cells treated with anti-human IgG for 24 h (In), the EBV-negative gastric cancer cell line AGS, and the EBV-positive gastric cell lines SNU719, NCC24, and YCCEL1. BNLF2a, BZLF1, and GAPDH were detected using the rat anti-BNLF2a monoclonal antibody 5F8 (a generous gift from Elizabeth Kremmer), a mouse anti-ZEBRA monoclonal antibody (catalog no. sc-53904; Santa Cruz Biotechnology), and a goat anti-GAPDH polyclonal antibody (catalog no. GTX100118; GeneTex), respectively.
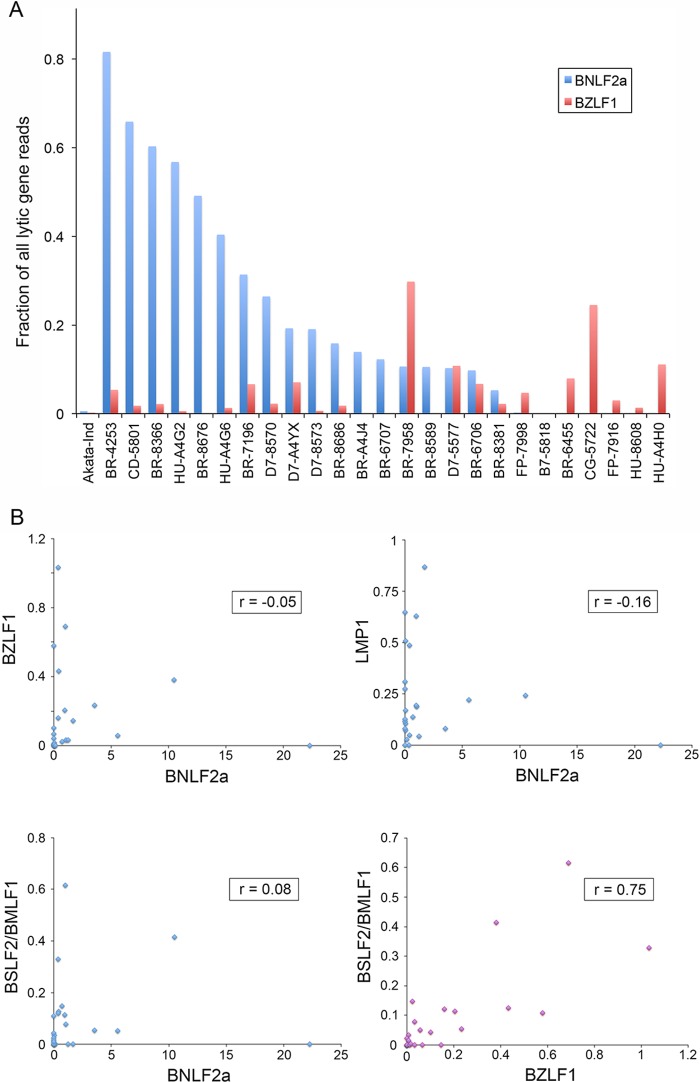
Since BNLF2a is an early lytic gene, we next assessed whether BNLF2a expression was linked to EBV reactivation, due either to full lytic replication in a fraction of cells or to cells exhibiting abortive lytic replication. First, for each of the 25 EBV-positive TCGA GC samples, we calculated the ratio of BNLF2a read numbers to the total number of reads derived from all lytic genes (excluding reads corresponding to lytic genes that overlap with the RPMS1 gene and reads mapping to the BHLF1 locus, whose expression can be decoupled from reactivation in some settings). First, it is notable that BNLF2a reads make up the majority of the analyzed lytic reads in four samples and generally comprise a substantial proportion of lytic reads in more than half of the samples (Fig. 4A). This is in contrast to the ratio of BNLF2a reads observed during reactivation of Akata cells, where the proportion of BNLF2a reads is 0.006 (Fig. 4A). Second, the BNLF2a read numbers do not appear to correspond to the proportion of lytic reads that map to the immediate early BZLF1 gene (Fig. 4A).
FIG 4.
Expression of BNLF2a is uncoupled from lytic gene expression. (A) Fraction of lytic gene reads (sans BHLF1 and lytic genes overlapping the RPMS1 latency gene) that map to the BNLF2a and BZLF1 genes for the 25 EBV-positive TCGA GC patient biopsy specimens and reactivated Akata cells (Akata-Ind) as a comparator. (B) Scatter plots and correlation coefficients calculated using normalized read counts for each of the indicated genes.
To more accurately assess the relationship between BNLF2a and BZLF1 expression across samples, we determined the correlation coefficient between BNLF2a and BZLF1 expression. For this analysis, we calculated the number of BNLF2a and BZLF1 reads per million reads mapping to the human or EBV genome for each sample. These values were then plotted in a scatter plot, and the correlation coefficient was calculated. This analysis showed no correlation between BNLF2a expression and BZLF1 (Fig. 4B). Similarly, no correlation was observed between BNLF2a and the early gene BSLF2/BMLF1 (Fig. 4B). In contrast, the correlation coefficient between BZLF1 and BSLF2/BMLF1 was found to be 0.75 (Fig. 4B). Although there are only very low read numbers mapping to the LMP1 gene outside of the region that overlaps with BNLF2a, we assessed whether there was any correlation between the levels of BNLF2a and LMP1. Little correlation was observed between BNLF2a and LMP1 (Fig. 4B). Taken together, these results indicate that BNLF2a expression is decoupled from reactivation in GCs, indicating that its expression is instead controlled by potential tissue-specific cellular factors and/or epigenetic marks in this setting.
Since the lytic gene BHLF1 has similarly been shown to exhibit lytic cycle-independent expression in some cases, we calculated the correlation coefficients between BHLF1 and BNLF2a, between BHLF1 and BZLF1, and between BHLF1 and BSLF2/BMLF1. The correlation coefficients for these relationships were found to be 0.46, 0.3, and 0.6, respectively. While some samples showed expression of both BHLF1 and BNLF2a, the correlation between BHLF1 and BNLF2a expression is low, and expression of BNLF2a is clearly observed in the absence of BHLF1. This indicates that although we cannot rule out some level of regulatory overlap with BHLF1, BNLF2a-specific mechanisms exist to drive its expression.
Many of the EBV genes known to be expressed in GCs, including the noncoding BamHI A rightward transcripts, the EBV-encoded microRNAs, the Pol III transcripts, EBER1 and EBER2, and LMP2, likely contribute to oncogenesis through known influences on cell cycle and apoptosis pathways. Nevertheless, escape from immune surveillance is coming to be appreciated as one of the most critical steps in the development of cancer.
The finding that the lytic BNLF2a gene is expressed in gastric cancers independently of reactivation is important because it identifies a new form of type II latency (which we refer to as type IIc, a latency program displaying expression of the protein-coding genes EBNA1, LMP2, and BNLF2a) and shows for the first time that EBV may contribute to GC survival and persistence through an immune evasion mechanism.
Microarray data accession numbers.
Sequencing data is available from the NCBI GEO database under accession numbers GSE45453 and GSE70513.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grants R01AI101046 and R01AI106676 to E.K.F., F30CA177267 to M.J.S., and P20GM103518 to Prescott Deininger, which supported the Tulane Cancer Center Next Generation Sequence Analysis Core, and a Louisiana Clinical and Translational Science Center pilot grant to Z.L.
Next-generation sequencing was performed at the University of Wisconsin Biotechnology Center. Data analysis was carried out in the Tulane Cancer Center Next Generation Sequence Analysis Core using core computational resources. We thank Elizabeth Kremmer for graciously providing the 5F8 anti-BNLF2a antibody.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01110-15.
REFERENCES
- 1.Strong MJ, Xu G, Coco J, Baribault C, Vinay DS, Lacey MR, Strong AL, Lehman TA, Seddon MB, Lin Z, Concha M, Baddoo M, Ferris M, Swan KF, Sullivan DE, Burow ME, Taylor CM, Flemington EK. 2013. Differences in gastric carcinoma microenvironment stratify according to EBV infection intensity: implications for possible immune adjuvant therapy. PLoS Pathog 9:e1003341. doi: 10.1371/journal.ppat.1003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell MJ, Abbott RJ, Croft NP, Hislop AD, Burrows SR. 2009. An HLA-A2-restricted T-cell epitope mapped to the BNLF2a immune evasion protein of Epstein-Barr virus that inhibits TAP. J Virol 83:2783–2788. doi: 10.1128/JVI.01724-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horst D, van Leeuwen D, Croft NP, Garstka MA, Hislop AD, Kremmer E, Rickinson AB, Wiertz EJ, Ressing ME. 2009. Specific targeting of the EBV lytic phase protein BNLF2a to the transporter associated with antigen processing results in impairment of HLA class I-restricted antigen presentation. J Immunol 182:2313–2324. doi: 10.4049/jimmunol.0803218. [DOI] [PubMed] [Google Scholar]
- 4.Croft NP, Shannon-Lowe C, Bell AI, Horst D, Kremmer E, Ressing ME, Wiertz EJ, Middeldorp JM, Rowe M, Rickinson AB, Hislop AD. 2009. Stage-specific inhibition of MHC class I presentation by the Epstein-Barr virus BNLF2a protein during virus lytic cycle. PLoS Pathog 5:e1000490. doi: 10.1371/journal.ppat.1000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horst D, Favaloro V, Vilardi F, van Leeuwen HC, Garstka MA, Hislop AD, Rabu C, Kremmer E, Rickinson AB, High S, Dobberstein B, Ressing ME, Wiertz EJ. 2011. EBV protein BNLF2a exploits host tail-anchored protein integration machinery to inhibit TAP. J Immunol 186:3594–3605. doi: 10.4049/jimmunol.1002656. [DOI] [PubMed] [Google Scholar]
- 6.Wycisk AI, Lin J, Loch S, Hobohm K, Funke J, Wieneke R, Koch J, Skach WR, Mayerhofer PU, Tampe R. 2011. Epstein-Barr viral BNLF2a protein hijacks the tail-anchored protein insertion machinery to block antigen processing by the transport complex TAP. J Biol Chem 286:41402–41412. doi: 10.1074/jbc.M111.237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hislop AD, Ressing ME, van Leeuwen D, Pudney VA, Horst D, Koppers-Lalic D, Croft NP, Neefjes JJ, Rickinson AB, Wiertz EJ. 2007. A CD8+ T cell immune evasion protein specific to Epstein-Barr virus and its close relatives in Old World primates. J Exp Med 204:1863–1873. doi: 10.1084/jem.20070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Cancer Genome Atlas Research Network. 2014. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao S, Strong MJ, Wang X, Moss WN, Concha M, Lin Z, O'Grady T, Baddoo M, Fewell C, Renne R, Flemington EK. 2015. High-throughput RNA sequencing-based virome analysis of 50 lymphoma cell lines from the Cancer Cell Line Encyclopedia project. J Virol 89:713–729. doi: 10.1128/JVI.02570-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strong MJ, Baddoo M, Nanbo A, Xu M, Puetter A, Lin Z. 2014. Comprehensive high-throughput RNA sequencing analysis reveals contamination of multiple nasopharyngeal carcinoma cell lines with HeLa cell genomes. J Virol 88:10696–10704. doi: 10.1128/JVI.01457-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, Reddy A, Liu M, Murray L, Berger MF, Monahan JE, Morais P, Meltzer J, Korejwa A, Jane-Valbuena J, Mapa FA, Thibault J, Bric-Furlong E, Raman P, Shipway A, Engels IH, Cheng J, Yu GK, Yu J, Aspesi P, de Silva M, Jagtap K, Jones MD, Wang L, Hatton C, Palescandolo E, Gupta S, Mahan S, Sougnez C, Onofrio RC, Liefeld T, MacConaill L, Winckler W, Reich M, Li N, Mesirov JP, Gabriel SB, Getz G, Ardlie K, Chan V, Myer VE, Weber BL, Porter J, Warmuth M, Finan P, Harris JL, Meyerson M, Golub TR, Morrissey MP, Sellers WR, Schlegel R, Garraway LA. 2012. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Z, Puetter A, Coco J, Xu G, Strong MJ, Wang X, Fewell C, Baddoo M, Taylor C, Flemington EK. 2012. Detection of murine leukemia virus in the Epstein-Barr virus-positive human B-cell line JY, using a computational RNA-seq-based exogenous agent detection pipeline, PARSES. J Virol 86:2970–2977. doi: 10.1128/JVI.06717-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Z, Wang X, Strong MJ, Concha M, Baddoo M, Xu G, Baribault C, Fewell C, Hulme W, Hedges D, Taylor CM, Flemington EK. 2013. Whole-genome sequencing of the Akata and Mutu Epstein-Barr virus strains. J Virol 87:1172–1182. doi: 10.1128/JVI.02517-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DN, Seo MK, Choi H, Kim SY, Shin HJ, Yoon AR, Tao Q, Rha SY, Lee SK. 2013. Characterization of naturally Epstein-Barr virus-infected gastric carcinoma cell line YCCEL1. J Gen Virol 94:497–506. doi: 10.1099/vir.0.045237-0. [DOI] [PubMed] [Google Scholar]
- 16.Ku JL, Kim KH, Choi JS, Kim SH, Shin YK, Chang HJ, Bae JM, Kim YW, Lee JH, Yang HK, Kim WH, Jeong SY, Park JG. 2012. Establishment and characterization of six human gastric carcinoma cell lines, including one naturally infected with Epstein-Barr virus. Cell Oncol 35:127–136. doi: 10.1007/s13402-012-0073-9. [DOI] [PubMed] [Google Scholar]
- 17.Oh ST, Cha JH, Shin DJ, Yoon SK, Lee SK. 2007. Establishment and characterization of an in vivo model for Epstein-Barr virus positive gastric carcinoma. J Med Virol 79:1343–1348. doi: 10.1002/jmv.20876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.