ABSTRACT
Epidemiological and functional studies implicate NK cells in HIV control. However, there is little information available on which NK cell populations, as defined by the inhibitory NK cell receptors (iNKRs) they express, respond to autologous HIV-infected CD4+ (iCD4) T cells. NK cells acquire antiviral functions through education, which requires signals received from iNKRs, such as NKG2A and KIR3DL1 (here, 3DL1), engaging their ligands. NKG2A interacts with HLA-E, and 3DL1 interacts with HLA-A/B antigens expressing the Bw4 epitope. HIV-infected cells downregulate HLA-A/B, which should interrupt negative signaling through 3DL1, leading to NK cell activation, provided there is sufficient engagement of activating NKRs. We examined the functionality of NK cells expressing or not NKG2A and 3DL1 stimulated by HLA-null and autologous iCD4 cells. Flow cytometry was used to gate on each NKG2A+/NKG2A− 3DL1+/3DL1− (NKG2A+/− 3DL1+/−) population and to measure the frequency of all possible combinations of CD107a expression and gamma interferon (IFN-γ) and CCL4 secretion. The highest frequency of functional NK cells responding to HLA-null cell stimulation was the NKG2A+ 3DL1+ NK cell population. The highest frequencies of functional NK cells responding to autologous iCD4 cells were those expressing NKG2A; coexpression of 3DL1 did not further modulate responsiveness. This was the case for the functional subsets characterized by the sum of all functions tested (total responsiveness), as well as by the trifunctional CD107a+ IFN-γ+ CCL4+, CD107a+ IFN-γ+, total CD107a+, and total IFN-γ+ functional subsets. These results indicate that the NKG2A receptor has a role in NK cell-mediated anti-HIV responses.
IMPORTANCE HIV-infected CD4 (iCD4) cells activate NK cells, which then control HIV replication. However, little is known regarding which NK cell populations iCD4 cells stimulate to develop antiviral activity. Here, we examine the frequency of NK cell populations, defined by the presence/absence of the NK cell receptors (NKRs) NKG2A and 3DL1, that respond to iCD4 cells. NKG2A and 3DL1 are involved in priming NK cells for antiviral functions upon encountering virus-infected cells. A higher frequency of NKG2A+ than NKG2A− NK cells responded to iCD4 cells by developing antiviral functions such as CD107a expression, which correlates with NK cell killing, and secretion of gamma interferon and CCL4. Coexpression of 3DL1 on the NKG2A+ and NKG2A− NK cells did not modulate responses to iCD4 cells. Understanding the mechanisms underlying the interaction of NK cells with iCD4 cells that lead to HIV control may contribute to developing strategies that harness NK cells for preventing or controlling HIV infection.
INTRODUCTION
Natural killer (NK) cells are a subset of lymphocytes that mediate immune responses against virally infected and transformed cells (1). They contribute to innate immune defenses directly by eliciting functions such as cytotoxicity and the secretion of cytokines and chemokines. They also contribute to shaping adaptive immune responses through their interactions with dendritic cells (2). NK cell activation can occur without prior sensitization before T cell-mediated immune responses can be induced (3). The timing of NK cell responses suggests that they may have a role in initial viral control. This is supported by studies that implicate NK cells in resistance to human immunodeficiency virus (HIV) (4–6). NK cells also appear to play a role in several viral infections (HIV, human cytomegalovirus [HCMV], hepatitis B virus [HBV], hepatitis C virus [HCV], and influenza virus) (7–11). The importance of NK cell function in the context of HIV infection is highlighted by the development of HIV sequence polymorphisms that allow the virus to evade NK cell antiviral pressure (12).
The state of activation of NK cells is determined by the integration of signals received from stochastically expressed germ line-encoded cell surface receptors upon interaction with ligands on target cells. NK cells acquire functional competence through an ontogenic process known as education, which requires the interaction of inhibitory NK receptors (iNKRs) with their cognate HLA ligands on neighboring cells (13, 14). Education is not an on/off switch as functionality can be tuned by the number of iNKRs engaged, the strength of interactions between NKRs and their ligands, and the absence or presence of activating NK cell receptor (aNKR) engagement (15, 16). NK cells lacking iNKRs for self-HLA ligands remain uneducated and hyporesponsive (14). Educated NK cells are tolerant of normal healthy cells but have the potential to respond to target cells that upregulate ligands for aNKRs and have reduced levels of cell surface HLA ligands for iNKRs, as often occurs in viral infection and tumor transformation.
The killer immunoglobulin-like receptor (KIR) 3DL1 and NKG2A surface receptors are both examples of iNKRs. NKG2A, a C-type lectin receptor expressed in all people, forms a heterodimer with CD94 and interacts with nonclassical major histocompatibility complex (MHC) class I HLA-E molecules that present leader peptides from many HLA class I (HLA-I) proteins (17, 18). Thus, NKG2A+ NK cells can survey autologous cells for overall HLA levels. KIR3DL1 (henceforth, 3DL1) interacts with a subset of HLA-A and -B antigens that belong to the Bw4 subset (19, 20). HLA variants belonging to the Bw4 group differ from the remaining Bw6 HLA-B variants at amino acids 77 to 83 of the HLA heavy chain (21). Bw6 isoforms do not interact with any 3DL1 receptors. Unlike 3DL1+ NK cells from individuals homozygous for Bw6 (with no Bw4 alleles at the HLA-A locus), those from Bw4+ subjects are educated through 3DL1. NK cell activation can occur when inhibitory signals through iNKRs are interrupted due to loss of ligands for iNKRs on target cells or if signaling through aNKRs overcomes negative signals originating through iNKR engagement (13, 22).
At present there is little known about the impact of coexpression of NKG2A and 3DL1 iNKRs on NK functionality in the context of HIV infection. We hypothesized that NK cells expressing both iNKRs would have higher functional potential than NK cells expressing one or none of these iNKRs, as assessed by responsiveness to HLA-null cells (23), and that this principle would also apply to NK responses to stimulation by autologous HIV-infected CD4 (iCD4) T cells. To test this hypothesis, we first characterized the functional potential of NK cell populations expressing the four possible combinations of NKG2A and 3DL1 expression following stimulation with the HLA-null cell lines, K562 and 721.221 (henceforth, 721), which are commonly used to assess NK functionality. We found that while NK cells coexpressing NKG2A and 3DL1 had a higher functional potential in response to HLA-null cells, NKG2A+ NK cells responded more robustly than NKG2A− NK cells to autologous iCD4 cells, irrespective of whether they coexpressed the 3DL1 iNKR. This is the first time a study has examined the impact of these iNKRs on multifunctional responses to iCD4 cells.
MATERIALS AND METHODS
Ethics statement.
This study was conducted in accordance with the principles expressed in the Declaration of Helsinki. It was approved by the Institutional Review Boards of the Comité d'Éthique de la Recheche du Centre Hospitalier de l'Université de Montréal and the Research Ethics Committee of the McGill University Health Centre-Montreal General Hospital. All subjects provided written informed consent for the collection of samples and subsequent analysis.
Study population.
Twenty-six HIV-1-uninfected individuals who were homozygous for 3DL1 were studied, including 16 with at least one Bw4 allele and 10 who were homozygous for Bw6 and had no Bw4 alleles at the HLA-A locus. The HLA and 3DL1 allotypes of each of the study participants are listed in Table 1.
TABLE 1.
Study population HLA and KIR3DL1 allotypes
| Subject no. | Allele category | HLA allotype |
3DL1 allotype |
Stimulus |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A |
B |
C |
Allele 1 | Allele 2 | Cell linea | HIV | |||||
| Allele 1 | Allele 2 | Allele 1 | Allele 2 | Allele 1 | Allele 2 | ||||||
| 1 | Bw4 | 01:01 | 03:01 | 14:02 | 57:01 | 06:02 | 08:02 | 004 | 015 | ✓ | |
| 2 | Bw4 | 01:01 | 02:01 | 15:01 | 57:01 | 05 | 06:02 | 001 | 001 | ✓ | |
| 3 | Bw4 | 02:02 | 30:02 | 53:01 | 57:03 | 04:01 | 18 | 001 | 001 | ✓ | |
| 4 | Bw4 | 02:01 | 24:02 | 44:02 | 51:01 | 05 | 08 | 002 | 005 | ✓ | |
| 5 | Bw4 | 02:01 | 25:01 | 18:01 | 55:01 | 03:03 | 12:03 | 004 | 005 | ✓ | ✓ |
| 6 | Bw4 | 01:01 | 02:01 | 38:01 | 57:01 | 06:02 | 12:03 | 004 | 002 | ✓ | ✓ |
| 7 | Bw4 | 01:01 | 26:01 | 38:01 | 57:01 | 06:02 | 12:03 | 001 | 001 | ✓ | ✓ |
| 8 | Bw4 | 02:01 | 02:01 | 07:02 | 57:01 | 05 | 06:02 | 004 | 005 | ✓ | |
| 9 | Bw4 | 24:02 | 26:01 | 15:01 | 57:01 | 05 | 06:02 | 005 | 005 | ✓ | |
| 10 | Bw4 | 02:01 | 03:01 | 07:02 | 27:05 | 01:02 | 07:02 | 005 | 005 | ✓ | |
| 11 | Bw6 | 02:01 | 02:01 | 07:02 | 08:01 | 07 | 07 | 001 | 015 | ✓ | |
| 12 | Bw6 | 02:01 | 33:03 | 15:01 | 35:08 | 03:03 | 04:01 | 002 | 005 | ✓ | ✓ |
| 13 | Bw6 | 03:01 | 11:01 | 07:02 | 35:01 | 04:01 | 07:02 | 001 | 001 | ✓ | ✓ |
| 14 | Bw6 | 02:01 | 03:01 | 35:01 | 40:01 | 03:04 | 04:01 | 002 | 004 | ✓ | |
| 15 | Bw4 | 01:01 | 03:01 | 44:03 | 49:01 | 07:01 | 16:01 | 001 | 002 | ✓ | |
| 16 | Bw4 | 29:01 | 36:01 | 07:02 | 53:01 | 04:01 | 07:02 | 015 | 015 | ✓ | ✓ |
| 17 | Bw6 | 01:01 | 11:01 | 18:01 | 55:01 | 03:04 | 12:03 | 004 | 005 | ✓ | |
| 18 | Bw4 | 01:01 | 23:01 | 14:01 | 38:05 | 08:02 | 12:03 | 001 | 008 | ✓ | ✓ |
| 19 | Bw6 | 02:01 | 30:02 | 07:02 | 35:01 | 04:01 | 07:02 | 002 | 005 | ✓ | ✓ |
| 20 | Bw4 | 01:01 | 31:01 | 49:01 | 49:01 | 07:01 | 07:01 | 004 | 001 | ✓ | ✓ |
| 21 | Bw4 | 02:01 | 31:01 | 27:01 | 40:01 | 03:02 | 15:02 | 004 | 008 | ✓ | ✓ |
| 22 | Bw4 | 01:01 | 24:02 | 18:01 | 35:03 | 04:01 | 07:01 | 001 | 001 | ✓ | ✓ |
| 23 | Bw6 | 02:01 | 03:01 | 07:02 | 40:01 | 03:02 | 07:10 | 004 | 001 | ✓ | |
| 24 | Bw6 | 02:01 | 01:01 | 08:01 | 40:01 | 03:02 | 07:01 | 007 | 007 | ✓ | |
| 25 | Bw6 | 02:01 | 11:01 | 07:02 | 35:01 | 04:01 | 07:02 | 004 | 008 | ✓ | |
| 26 | Bw6 | 02:01 | 03:01 | 07:02 | 08:01 | 07:01 | 16:01 | 004 | 015 | ✓ | |
Cell lines 721 and K562.
Genotyping.
Genomic DNA was extracted from peripheral blood mononuclear cells (PBMCs) or Epstein-Barr virus (EBV)-transformed cells using a QIAamp DNA blood kit (Qiagen). MHC class I alleles were typed by sequencing using commercial reagents (Atria Genetics Inc.). Assign 3.5+ software was used to interpret sequence information for allele assignment (Conexio Genetics). 3DL1 genotyping was performed by PCR as previously described (6). 3DL1 allotypes were identified as described previously (5).
Cells.
PBMCs were isolated by density gradient centrifugation (lymphocyte separation medium; Wisent) and cryopreserved in 10% dimethyl sulfoxide (DMSO; Sigma-Aldrich) and 90% fetal bovine serum (FBS; Wisent). 721 cells were a kind gift from Galit Alter (Ragon Institute, Harvard University). The K562 cell line was obtained from the American Type Culture Collection, (Manassas, VA). 721 and K562 cells were cultured in complete RPMI medium consisting of RPMI 1640 medium, 10% FBS, 2 mM l-glutamine, 50 IU/ml penicillin, 50 mg/ml streptomycin (R10 medium) (all from Wisent). CD4 and NK cells were isolated from PBMCs by positive- and negative-selection kits, respectively (Stemcell Technologies, Inc.). Purity was verified by flow cytometry and was an average of 95.3% and 97.2% for CD4 and NK cells, respectively.
Viral infection.
Autologous CD4 cells were infected with the HIV-1JR-CSF clone as previously described (24). In brief, purified CD4 cells (106/ml) were stimulated overnight with 1 μg/ml phytohemagglutinin (PHA-P; MP Biomedicals) and 100 IU/ml of recombinant human interleukin-2 (rhIL-2; Chiron Corp.) in R10 medium at 37°C in a 5% CO2 humidified incubator. Stimulated CD4 cells were then washed and cultured in R10 medium with 100 IU/ml rhIL-2 for 3 days. On day 4, CD4 cells were infected at a multiplicity of infection of 0.01 with HIV-1JR-CSF for 4 h. Cells were washed and cultured for 7 days in R10 medium with rhIL-2. Uninfected CD4 (CD4) cells were cultured in parallel to iCD4 cells for 7 days in R10 medium with rhIL-2. On day 7, iCD4 and CD4 cells were used to stimulate NK cells. Day 7 iCD4 and CD4 cells were stained with a UV Live/Dead fixable dead cell stain kit (Invitrogen), CD3-BV785 (OKT3), and CD4-BV421 (OTK4) (both from Biolegend). The percentage of HIV-infected cells was measured intracellularly (intracellular staining [ICS] assay) with anti-p24-fluorescein isothiocyanate (FITC; KC57) (Beckman Coulter). The ICS background value for p24 was set on uninfected CD4 controls.
NK cell stimulation and staining.
Cryopreserved PBMCs were thawed and cocultured with HLA-null cells at a 5:1 ratio in a 96-well plate for 6 h at 37°C in a humidified 5% CO2 incubator. CD107a-phycoerythrin (PE)-CF594 (BD) was added at the start of the stimulation; 5 mg/ml brefeldin A (Sigma-Aldrich) and 6 mg/ml monensin (GolgiStop; BD) were added 30 min after the initiation of the coculture. PBMCs were also stimulated with 1.25 μg/ml of phorbol myristate acetate (PMA)–0.25 g/ml ionomycin (Sigma-Aldrich), and all cells used in this study were responsive to this positive control. Viability was assessed using a UV Live/Dead Fixable dead cell staining kit (Invitrogen). Nonspecific interactions of Fc receptors (FcRs) on effectors with the antibody (Ab) panel were minimized using TruStain FcX reagent (BioLegend), as per the manufacturer's instructions. Cells were stained with antibodies to the following cell surface markers: CD3-BV785 (OKT3), CD56-BV711 (NCAM), 3DL1-BV421 (DX9) (all from BioLegend), and NKG2A-PECy7 (Beckman Coulter). After cells were washed, they were fixed and permeabilized using a Fix and Perm kit (Invitrogen) and stained by ICS with CCL4-allophycocyanin (APC) (24006; R&D) and IFN-γ–BV605 (B27; BD). After the cells were stained, they were washed twice, fixed with 1% paraformaldehyde (Santa Cruz), and acquired within 24 h.
Stimulation of purified NK cells with autologous HIV iCD4 cells was done as previously described (24). In brief, 1 × 106 NK cells were cocultured with iCD4 or CD4 cells at a ratio of 10:1 in a 96-well plate in R10 medium with 100 IU/ml rhIL-2 for 24 h. Brefeldin A (6 μg/ml) and monensin (5 μg/ml; GolgiStop) were added 5 h before the end of the culture period. Viability, FcR blocking, and surface staining were done as described above for PBMCs stimulated with HLA-null cells. Samples were washed, fixed, and permeabilized and stained for ICS using CCL4-FITC (24006; R&D) and IFN-γ–Alexa 700 (B27; BD). Samples were washed, fixed with 1% paraformaldehyde, and acquired within 24 h.
For certain experiments purified NK cells were cocultured with iCD4 cells or uninfected CD4 controls for 24 h in the presence or absence of blocking antibodies to NKG2A (clone 131411; R&D), HLA-E (clone MEM-E02; Abcam), or both. Antibodies were added at 10 μg/ml at the initiation of the coculture.
Flow cytometry analysis.
Between 4 × 105 and 1.5 × 106 total events were acquired for each sample using an LSRFortessa flow cytometer (BD) calibrated using eight-peak color rainbow beads (Spherotech, Inc.). The Ab panel was standardized and validated, and single-stain control beads (CompBead Plus; BD) were used in every experiment to calculate compensation. Boolean gating was used to identify the frequency of NK cells with each of the seven possible functional profiles, i.e., trifunctional (CD107a+ IFN-γ+ CCL4+), bifunctional (any combination of two functions), and monofunctional (CD107a+, IFN-γ+, or CCL4+) profiles, within the NKG2A− 3DL1−, NKG2A− 3DL1+, NKG2A+ 3DL1−, and NKG2A+ 3DL1+ (or NKG2A+/− 3DL1+/−) NK cell populations. Total responsiveness was defined as the sum of the frequencies of tri-, bi-, and monofunctional NK cell populations. Flow cytometry analysis for NK cell activation after stimulation was performed using FlowJo software, version 9.8 (TreeStar). The data obtained were background corrected by subtracting values for unstimulated cells in the case of HLA-null cell stimulation or for uninfected CD4 cells in the case of iCD4 cell stimulation.
Statistical analysis.
Analysis was performed using GraphPad Prism, version 6. Friedman tests were used to determine significant differences between the four NKG2A+/− 3DL1+/− populations. Where multiple comparisons were performed, such as for the seven possible functional subsets, Bonferroni corrections were applied. A Wilcoxon test was used to assess the significance of comparisons for two within-subject matched data sets for functional NKG2A+/− 3DL1+/− populations. Spearman's correlation tests were used to assess the relationship between the frequencies of NK responses to iCD4 cells and the frequency of intracellular p24+ cells in the iCD4 cell stimuli. P values of <0.05 were considered significant.
RESULTS
Surface expression analysis of NKG2A and 3DL1 following stimulation with HLA-null cell lines.
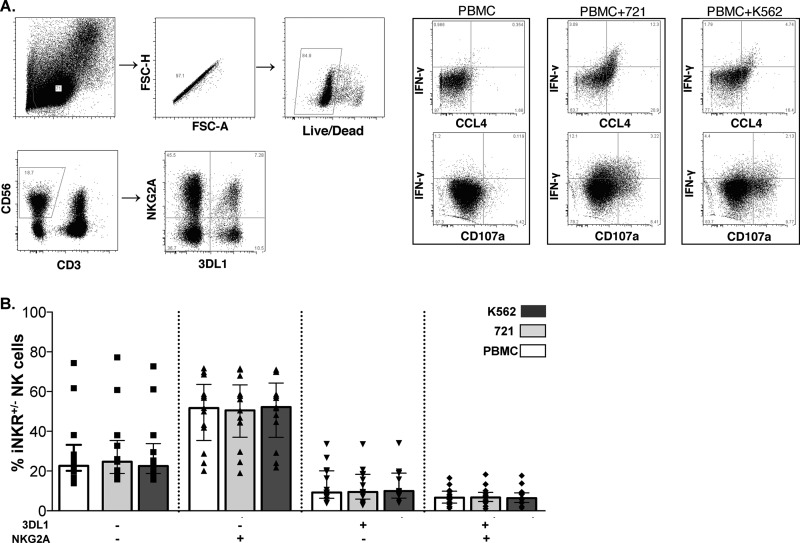
Figure 1A shows the gating strategy used to identify the expression of NKG2A and 3DL1 on CD3− CD56+ NK cells. Boolean gating was then used to identify the frequency of NK cells expressing the four possible combinations of NKG2A and 3DL1 expression. Figure 1B shows the frequency of NKG2A+/− 3DL1+/− populations defined by the surface expression of these iNKRs from 13 HIV-uninfected individuals following stimulation with K562 and 721 cells and without stimulation (PBMCs alone). For unstimulated PBMCs, 6.6% (median; interquartile range [IQR], 3.8, 9.9) of NK cells expressed both iNKRs, 51.7% (IQR, 35.4, 63.7) expressed NKG2A alone, 9.2% (IQR, 6.3, 20.1) expressed 3DL1 alone, and 22.6% (IQR, 20.2, 33.2) lacked expression of both iNKRs. Neither K562 nor 721 stimulation had a significant effect, compared to results with no stimulation, on the frequency of NKG2A and 3DL1 expression on these NK cell populations with these phenotypes (P > 0.05, Friedman).
FIG 1.
Gating strategy for identification of NK cell subsets and functional responses. (A) The live lymphocytic singlet population was used to gate on NK cells, which were defined as CD3− CD56+. The NKG2A+/− 3DL1+/− populations were derived from the NK cell gate. Functional gates for CD107, IFN-γ, and CCL4 were set on gated NK cells in unstimulated PBMCs. (B) The frequency of NKG2A+/− 3DL1+/− populations, as defined by surface expression of these two inhibitory NK receptors, following stimulation with HLA-null cells (K562 or 721) compared to background stimulation with PBMCs alone (PBMC). Data from 13 individuals tested in duplicate were included in this analysis. Bar height represents the median, and error bars represent the IQR for the group. A Friedman test was used determine significance between group comparisons (PFriedman > 0.05; data not shown). FSC-A, forward scatter area; FSC-H, forward scatter height.
721 cells induced a higher frequency of double-iNKR+ NK cells characterized by total, trifunctional, CD107a+ IFN-γ+, IFN-γ+ CCL4+, and IFN-γ+ response profiles, while NKG2A+ 3DL1− cells had the highest CD107a response.
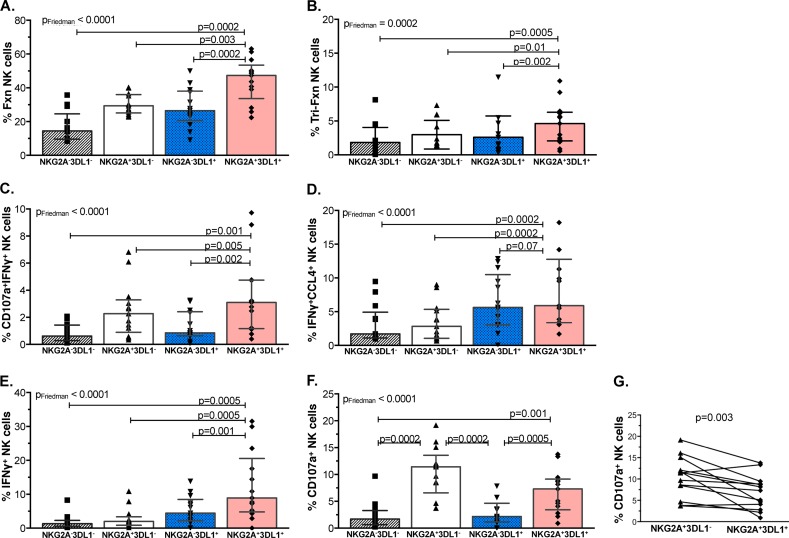
The functional potential of each NKG2A+/− 3DL1+/− NK cell population was measured following stimulation with 721 cells (Fig. 2A) and K562 (see Fig. S1 in the supplemental material). Both 721 and K562 cell lines stimulated different frequencies of total responsiveness among NKG2A+/− 3DL1+/− NK cell populations (P < 0.0001, Friedman; adjusted P value < 0.0007). The frequency of functional NKG2A+ 3DL1+ (double-iNKR+) NK cells was significantly higher than the frequencies of the other three populations (Fig. 2A; see also Fig. S1B to D) (P ≤ 0.003 for all comparisons). The frequency of functional NK cells stimulated with PMA-ionomycin did not differ significantly among the populations identified by NKG2A and/or 3DL1 expression. This suggests that differential levels of stimulation by HLA-null cells is not an innate property of these NK cell populations since they are activated to similar levels by PMA-ionomycin (see Fig. S2 in the supplemental material). Given that K562 and 721 cells induced similar patterns of functional responsiveness among the four phenotypic NK cell populations, subsequent results will show results only for 721 stimulation.
FIG 2.
The NKG2A+ 3DL1+ NK cell population was significantly more responsive to stimulation by HLA-null cell lines in all examined functions except degranulation. Shown on the y axes are the frequencies of the four NKG2A+/− 3DL1+/− NK cell populations that responded to 721 HLA-null cell stimulation with responses characterized by total responsiveness (A), trifunctional CD107a+ IFN-γ+ CCL4+ (B), bifunctional CD107a+ IFN-γ+ (C) and IFN-γ+ CCL4+ (D), and monofunctional IFN-γ+ (E) and CD107a (F and G) functional profiles. Bar height represents the median, and error bars represent the IQR for the data set. Thirteen individuals were analyzed in duplicate. Friedman (PFriedman) and Wilcoxon (P) tests were used determine significance between group differences. P values for between-group comparisons are shown over lines linking the two groups being compared. Fxn, functional.
Next, we examined the individual 721-induced functional subsets that comprised the total responsiveness of NKG2A+/− 3DL1+/− NK cell populations. 721 cells induced differential levels of trifunctional (Fig. 2B), bifunctional CD107a+ IFN-γ+ and IFN-γ+ CCL4+ (Fig. 2C and D, respectively), and monofunctional IFN-γ+ (Fig. 2E) responses in the four NKG2A+/− 3DL1+/− populations (P ≤ 0.0002, Friedman; adjusted P value, ≤0.0014). The frequencies of the 721-stimulated double-iNKR+ population with the above-mentioned functions were higher than those of the other three populations. Between-population comparisons were significant for all but the IFN-γ+ CCL4+ functional subset, for which the differences between the double-iNKR+ and single-iNKR+ 3DL1+ populations did not achieve significance (Fig. 2D) (P = 0.07, Wilcoxon).
The four NKG2A+/− 3DL1+/− populations also differed from each other in terms of the frequency of 721-triggered CD107a-only expression (Fig. 2F) (Friedman adjusted P, <0.0007). Paired comparisons showed that 721 stimulated a higher frequency of CD107a+ double-iNKR+ than double-iNKR− (NKG2A− 3DL1−) and single-iNKR+ 3DL1+ NK cells (Fig. 2F) (P ≤ 0.001, Wilcoxon). In contrast to what was observed for several other functional subsets, 721 induced a higher frequency of CD107a expression in the single-NKG2A+ than in the double-iNKR+ NK cell population (Fig. 2G) (P = 0.003, Wilcoxon). Therefore, while the double-iNKR+ population had the highest functional potential of the four NKG2A+/− 3DL1+/− groups for total, trifunctional, CD107a+ IFN-γ+, IFN-γ+ CCL4+, and IFN-γ+ responses, the single-iNKR+ NKG2A+ population had the highest functional potential for degranulation.
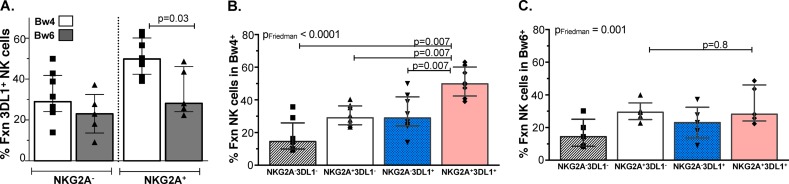
The higher functional potential of NKG2A+ 3DL1+ cells was limited to NK cells originating from individuals carrying a Bw4 allele.
To address whether education via the 3DL1 receptor contributed to the observed results pattern, we extended our analysis based on whether the NK cells originating from the subjects carried the Bw4 allele or not (Bw6 controls). The total responsiveness of 3DL1+ cells following stimulation with 721 cells was significantly greater in Bw4- than Bw6-positive individuals that coexpressed NKG2A (Fig. 3A) (P = 0.03, Mann-Whitney). Furthermore, the double-iNKR+ population from Bw4 carriers was significantly more responsive to HLA-null cells than the other three NKG2A+/− 3DL1+/− populations (Fig. 3B) (P = 0.007 for all comparisons). In contrast, for NK cells from Bw6 homozygotes, this trend was not observed, and the single-iNKR+ NKG2A+ population had the same level of total responsiveness as the double-iNKR+ cells (Fig. 3C) (P = 0.8). Together, these results suggest that having two iNKRs provides a functional advantage to NK cells responding to 721 cells only for NK cells from Bw4+ individuals able to educate their NK cells though both 3DL1 and NKG2A.
FIG 3.
A higher frequency of NKG2A+ 3DL1+ NK cells from carriers of a Bw4 allele respond to 721 cells than those from Bw6+ individuals. The frequency of all functional 721-stimulated NKG2A+/− 3DL1+ NK cells from individuals belonging to the Bw4 and Bw6 groups was plotted on the y axis (A). A Mann-Whitney test was used to determine significance of between-group differences. The frequency of all functional 721-stimulated NKG2A+/− 3DL1+/− populations from Bw4 (B) and Bw6 (C) individuals was plotted on the y axis. Friedman (PFriedman) and Wilcoxon (P) tests were used determine significance of between-group differences. Bar height represents the median, and error bars represent the IQR for 13 individuals analyzed in duplicate.
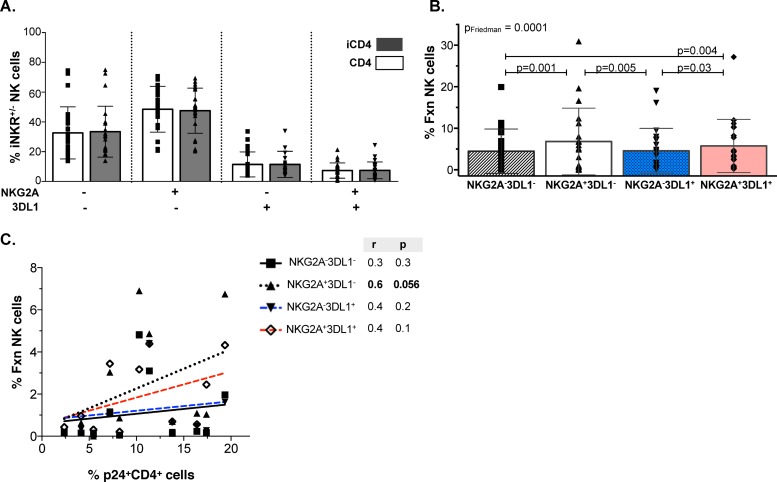
Higher total responsiveness of the NKG2A+ than NKG2A− populations following stimulation with autologous iCD4 cells.
Currently, little is known regarding whether iCD4 cells stimulate NK cell populations differentially and, if so, which populations and functional subsets they activate. Autologous iCD4 cell stimulation of NK cells for 24 h had no effect on the frequency of the four NKG2A+/− 3DL1+/− populations compared to stimulation with stimulation by the uninfected CD4 controls (Fig. 4A) (P > 0.05, Wilcoxon). As seen for HLA-null cell stimulation, iCD4 cells induced differential levels of total responsiveness among the four NK cell populations studied (P = 0.0001, Friedman; adjusted P, 0.0007). Infected CD4 cells triggered frequencies of functional NKG2A+ 3DL1+ populations that were significantly higher than those of NKG2A− 3DL1+ and NKG2A− 3DL1− populations but that were not higher than those of NKG2A+ 3DL1− NK cells (Fig. 4B) (P = 0.03 and P = 0.004, respectively). Furthermore, iCD4 cells also induced a higher frequency of single-iNKR+ NKG2A+ cells than single-iNKR+ 3DL1+ and double iNKR− NK cells (Fig. 4B) (P = 0.005 and P = 0.001, respectively). To assess whether the frequency of p24+ cells within the iCD4 cell stimuli correlated differentially with the frequency of functional responses among the four NK cell populations, we performed a correlation analysis between these parameters. We observed a near-significant correlation between the frequency of p24+ iCD4 cells used for stimulation and the total responsiveness of the single-iNKR+ NKG2A+ NK population (Fig. 4C) (P = 0.056). The trend observed for the correlation of p24+ iCD4 cells and the total responsiveness of the other three NK cell populations was not significant. In summary, unlike HLA-null stimulation, autologous iCD4 cells induced NKG2A+ NK cells more robustly than NKG2A− NK cells, irrespective of 3DL1 coexpression.
FIG 4.
Total responsiveness of NK populations to autologous HIV-iCD4 cells. (A) Frequency of iNKR-bearing CD56+ NKG2A+/− 3DL1+/− populations following stimulation with autologous iCD4 cells and uninfected controls. (B) Frequency of total responsiveness (y axis) of NKG2A+/− 3DL1+/− populations following stimulation with iCD4 cells. Bar height represents the mean ± standard deviation for the group, and Friedman (PFriedman) and Wilcoxon tests (P) were used to determine significance of between-group differences. Data from 24 individuals analyzed in duplicate were used to generate results. (C) Correlation between percentage of p24+ iCD4 cells used for stimulation (x axis) and total responsiveness (y axis) of each NKG2A+/− 3DL1+/− population. Data from 12 individuals analyzed in duplicate were used to generate these plots. Spearman's correlation test (r and P) were used to assess the significance of the association between these two measures.
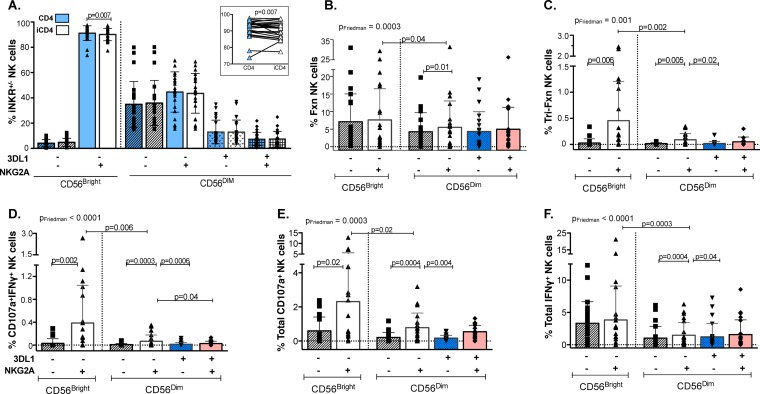
Infected CD4 cell stimulation induced a higher frequency of functional subsets in the NKG2A+ populations, particularly in CD56Bright cells.
The emergence of NKG2A+ NK cells as mediators of antiviral responses prompted us to examine separately the contribution of NK cell compartments expressing CD56 at high and low levels (CD56Bright and CD56Dim, respectively) to the iCD4 cell-induced responses of the NKG2A+/− 3DL1+/− populations (using the modified gating strategy shown in Fig. S3A in the supplemental material). First, we measured the frequency of each of the NKG2A+/− 3DL1+/− populations that was CD56Bright and CD56Dim following a 24-h stimulation with either iCD4 or uninfected CD4 cells. Previous studies reported that CD56Bright NK cells are largely negative for KIRs, including 3DL1 (25, 26); thus, we excluded 3DL1 from the analysis of the cell surface staining results shown in Fig. 5A. We observed a significantly lower percentage of NKG2A+ cells in the CD56Bright compartment following stimulation with iCD4 cells than in those stimulated with uninfected CD4 controls (P = 0.007, Wilcoxon). No other significant differences were seen for the frequency of the other NK cell populations following stimulation with iCD4 cells versus that with uninfected CD4 cells.
FIG 5.
iCD4 cell stimulation of functional subsets contributing to the total responsiveness of 3DL1+/− NKG2A+/− NK cell populations. (A) The frequency of CD56Bright and CD56Dim iNKR-bearing NKG2A+/− 3DL1+/− populations following stimulation with autologous iCD4 cells and uninfected CD4 controls. The inset at the top right is an expanded view of the frequency of the CD56Bright NKG2A+ population in iCD4 cells versus uninfected CD4 conditions. The frequencies of iCD4 cell-stimulated NK cell populations characterized by total responsiveness (B), trifunctional (C), bifunctional CD107a+ IFN-γ+ (D), and total CD107a (E) and total IFN-γ (F) response profiles are shown on the y axis for each CD56Bright NKG2A+/− and CD56Dim NKG2A+/− 3DL1+/− population. Bar height represents the mean ± standard deviation for each group. Data from 24 individuals analyzed in duplicate were used to generate these results. Friedman (PFriedman) and Wilcoxon (P) tests were used to determine significance between data sets. P values for between-group comparisons are shown over lines linking the two groups being compared.
Autologous iCD4 cells induced differential frequencies of total, trifunctional, CD107a+ IFN-γ+, total CD107a+, and total IFN-γ+ NK functional subsets among the NKG2A+/− 3DL1+/− populations (Fig. 5B to F) (P = 0.0003, P = 0.001, P < 0.0001, P = 0.0003, and P < 0.0001, respectively; Friedman). For the CD56Bright compartment, iCD4 cells induced higher frequencies of functional NKG2A+ than NKG2A− NK cells among the trifunctional, CD107a+ IFN-γ+, and total CD107a+ functional subsets (Fig. 5C to E) (P ≤ 0.02, Wilcoxon). Analysis of the CD56Dim compartment revealed a trend similar to the one observed for the CD56+ analysis of total responsiveness (Fig. 4B), where no significant differences were observed in the frequency of iCD4 cell-induced functional cells between the NKG2A+ populations that expressed 3DL1 or not, with the exception of the CD107a+ IFN-γ+ subset (Fig. 5D) (P = 0.04, Wilcoxon). In addition, CD56Dim NKG2A+ 3DL1− cells were more responsive to iCD4 cells than double-iNKR− and NKG2A− 3DL1+ populations (Fig. 5C to F) (P ≤ 0.04, Wilcoxon). Comparisons of iCD4 cell-induced CD56Bright versus CD56Dim single-iNKR+ NKG2A+ functional cells revealed that CD56Bright NK cells were consistently more responsive to autologous iCD4 cells than CD56Dim NK cells (P < 0.04 for all comparisons, Wilcoxon). Infected CD4 cells did not induce significant differences in the frequencies of bifunctional CD107a+ CCL4+, CCL4+ IFN-γ+, or total CCL4+ responses between the CD56Bright and CD56Dim NKG2A+/− 3DL1+/− populations (P = 0.1, P = 0.1, and P = 0.5, respectively; unadjusted Friedman) (data not shown). Together, these results indicate that NKG2A+ NK cell populations, particularly CD56Bright NKG2A+, were activated by iCD4 cells to higher levels than NKG2A− populations and that 3DL1 coexpression did not have an impact on modulating functionality to iCD4 cells.
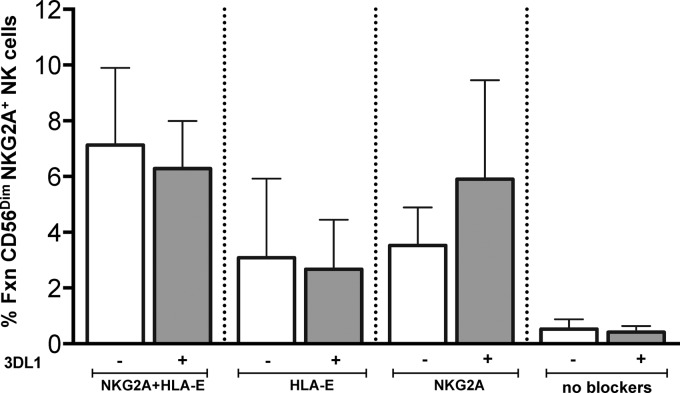
Infected CD4 cells maintain expression of HLA-E (27), which should inhibit NK cells expressing the iNKR NKG2A. The enhanced activity of NKG2A+ cells in response to iCD4 cells prompted us to verify whether NKG2A was behaving as an iNKR. We addressed this by including blocking antibodies specific for NKG2A or HLA-E or both in the 24-h coculture of NK cells with iCD4 cells. The gating strategy used to analyze the results of this experiment is shown in Fig. S3A in the supplemental material. The presence of blocking antibodies enhanced the total responsiveness of CD56Dim NK cells to iCD4 cells (Fig. 6). Blocking both NKG2A and HLA-E together resulted in an even higher percentage of functional CD56Dim NKG2A+ NK cells than blocking either the receptor or ligand separately (Fig. 6). Similar results were observed for NK cells secreting IFN-γ and CCL4 (see Fig. S3B). Blocking the interaction of HLA-E and NKG2A produced a more modest increase in the frequency of NK cells expressing CD107a (see Fig. S3C). Overall, these results confirm that NKG2A maintains its behavior as an inhibitory NK receptor.
FIG 6.
Blocking NKG2A and HLA-E enhances antiviral functional responses of CD56Dim NKG2A+ cells. Purified NK cells were cocultured with autologous iCD4 cells or uninfected CD4 controls for 24 h. Blocking antibodies to NKG2A, HLA-E, or both were added to the corresponding cocultures overnight. These cocultures were processed as per the section describing NK cell stimulation and staining in Materials and Methods. We focused on CD56Dim NKG2A+ cells and measured total responsiveness (y axis). Data for four individuals are shown, and bars represent the mean ± standard deviation.
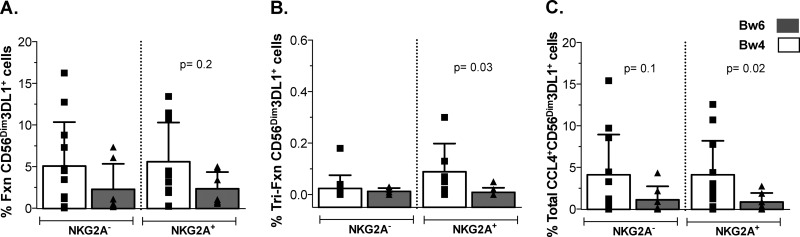
NK cells from Bw4+ individuals have higher frequencies of trifunctional and total CCL4+ functional CD56Dim NKG2A+ 3DL1+ cells than Bw6 homozygotes.
Epidemiological studies have shown that certain 3DL1-Bw4 combinations are associated with slow time to AIDS and protection from HIV infection. For example, carriage of the high-expression 3DL1 genotype (*h/*y) with its ligand HLA-B*57 (*h/*y+B*57) has a stronger effect on slow time to AIDS and HIV load control than that of a Bw6 homozygous genotype, and its carriage is associated with protection from HIV infection (5, 28, 29).The impact of carriage of *h/*y+B*57 on NK cell education and function has been documented (24, 30, 31). NK cells from *h/*y+B*57 carriers are superior to those from Bw6 homozygotes in inhibiting HIV replication through mechanisms that involve secretion of CC-chemokines (24). Furthermore, 3DL1+ NK cells from *h/*y+B*57 carriers secrete more CC-chemokines than 3DL1− NK cells (24). We questioned whether comparing iCD4 cell-stimulated NK cell functional profiles from carriers of a Bw4 allele versus Bw6 homozygotes would reveal evidence of 3DL1-Bw4 interactions with antiviral responses to autologous iCD4 cells. Although the total responsiveness of CD56Dim 3DL1+ cells following stimulation with iCD4 cells was higher among NK cells from Bw4 carriers than that from Bw6 homozygotes, differences did not achieve statistical significance (Fig. 7A) (P = 0.2, Mann-Whitney). Analysis of the individual functional subsets that contributed to the total responsiveness revealed significant differences between Bw4 and Bw6 groups for trifunctional and total CCL4+ responses, where CD56Dim 3DL1+ cells that coexpressed NKG2A from Bw4 individuals were more functional than those cells from Bw6 individuals (Fig. 7B and C) (P = 0.03 and P = 0.02, respectively). Collectively, our data suggest that responses to iCD4 cells of NK cell populations coexpressing 3DL1 and NKG2A were comparable to those of single-iNKR+ CD56Dim NKG2A+ cells. However, CD56Dim 3DL1+ NK cells from individuals educated via 3DL1-Bw4 interactions that also coexpress NKG2A respond more robustly to iCD4 cells than do these cells from Bw6 homozygotes by secreting CCL4, either alone or in a trifunctional combination with IFN-γ and CD107a.
FIG 7.
iCD4 cell-stimulated CD56Dim NKG2A+ 3DL1+ cells from Bw4+ individuals have greater trifunctional and total CCL4 responses than those from Bw6 homozygotes. The frequencies of iCD4 cell-stimulated CD56Dim NKG2A+/− 3DL1+ NK cells from carriers of a Bw4 allele versus Bw6 homozygotes characterized by total responsiveness (A), trifunctional response (B), and total CCL4 responses profiles are shown on the y axis. Bar height represents the mean ± standard deviation for each group. Data from 24 individuals analyzed in duplicate are plotted. A Mann-Whitney test was used to determine significance of between-group differences.
DISCUSSION
In this report we investigated the functional responses of four NK cell populations defined by expression of the iNKRs NKG2A and/or 3DL1 to stimulation with HLA-null and autologous HIV iCD4 cells. The frequency of HLA-null-stimulated functional cells was significantly higher for the NKG2A+ 3DL1+ population than for the two single-iNKR+ and double-iNKR− populations. This observation was limited to individuals carrying the 3DL1-Bw4 genotype combination. In contrast, iCD4 cell stimulation induced a higher frequency of NKG2A+ than NKG2A− NK cells, irrespective of whether 3DL1 was coexpressed. The functional subsets that contributed to the higher functionality of iCD4 cell-stimulated CD56Bright/Dim NKG2A+ NK cells included the trifunctional, CD107a+ IFN-γ+, total CD107a+, and total IFN-γ+ subsets. Higher frequencies of these functional subsets were seen in the CD56Bright than in the CD56Dim compartment. Given that NK cells have been implicated in HIV control (7, 24, 28, 32), these results further elucidate the NK cell populations and antiviral functions induced by autologous iCD4 cells that may play a role in HIV pathogenesis.
The term “functional potential” refers to NK cell responsiveness induced by HLA-null cell stimulation. The HLA-null 721 and K562 cell lines are commonly used to activate NK cells educated through the interaction of iNKRs with HLAs. Their lack of cell surface HLA abrogates negative signaling through iNKRs, which inhibits NK cell function. NK cell functional potential depends on how potently NK cells were educated, which in turn depends on the iNKRs expressed by an NK cell, the HLA type of the NK cell donor, the number of iNKRs to self-ligands an NK cell expresses, and the potency of particular iNKR-HLA ligand combinations (30, 31, 33, 34). NK cell activation also requires the engagement of aNKRs with their ligands present on HLA-null cells. We originally hypothesized that NK cells expressing both iNKRs would demonstrate a greater functional potential than NK cells expressing one or neither of the iNKRs investigated here (23). The higher response frequency of NKG2A+ 3DL1+ cells to HLA-null cells than any of the other NKG2A− 3DL1+/− populations is in line with this hypothesis. However, this higher functionality should be limited to NK cells from Bw4+ individuals with the ligand for 3DL1 (i.e., with at least two iNKRs to self-HLA). Most NKG2A+ cells should be educated through this iNKR since its ligand, HLA-E, is expressed on most human cells (35–37). In response to stimulation with 721 cells, NKG2A+ 3DL1+ cells from Bw4+ individuals (educated via 3DL1) were significantly more functional than double-iNKR+ cells from Bw6 controls (not educated via 3DL1). Furthermore, these educated cells were significantly more functional than the other three NKG2A+/− 3DL1+/− populations in the Bw4+ group, which was not observed for the Bw6 group (Fig. 3B and C). Thus, the higher frequency of HLA-null-induced NKG2A+ 3DL1+ NK cells than of the other NK populations studied is limited to NK cells from individuals expressing the HLA ligand for 3DL1. It is likely that the NK cells in these NKG2A+/− 3DL1+/− populations were also educated through other iNKRs depending on the KIR/HLA genotype of the individual they originated from. However, any influence of education through iNKRs other than NKG2A and 3DL1 is likely to be similar among the NK cell populations defined by NKG2A and 3DL1 expression (i.e., that developed in the same iNKR-self HLA environment).
NK cell functionality can also vary according to the state of NK cell maturation/differentiation and to the stimuli used to induce function (25, 38). The expression levels of CD56, NKG2A, and KIRs can distinguish five subsets of peripheral blood NK cells that range from least to most differentiated. As the NK cell matures, NKG2A is lost, and inhibitory KIRs (iKIRs) are sequentially acquired and can be phenotypically categorized from the least to most differentiated as follows: CD56Bright NKG2A+ KIR− < CD56Dim NKG2A+ KIR− < CD56Dim NKG2A+ KIR+ < CD56Dim NKG2A− KIR+ and CD56Dim NKG2A− KIR−. The last population is considered to be hyporesponsive due to a failure to acquire iKIRs before the loss of NKG2A (25, 39–42). Consistent with this scheme, we found that the CD56+/Dim NKG2A− 3DL1− population, which would include hyporesponsive NK cells negative for both iKIRs to self-HLA, was poorly responsive to HLA-null and iCD4 cells. 721 cells stimulated the highest functionality for most of the functional subsets examined, except degranulation, in the less mature NKG2A+ 3DL1+ (i.e., double-iNKR+) population rather than in the more mature NKG2A− 3DL1+ (i.e., single-iNKR+) population (Fig. 2). In a previous report, Fauriat et al. showed that CD56Bright NK cells were poorly stimulated by HLA-null cells; thus, results for total CD56+ cells would be expected to reflect what is occurring in the CD56Dim subset (43). In an extended analysis of functional results for the 721 stimulation, we reached similar findings (data not shown). Therefore, for stimulation with HLA-null cells, we show functional results for CD56+ NK cells that encompass the functional contributions of both the low-responding CD56Bright and high-responding CD56Dim subsets (Fig. 2). Other studies have shown no significant differences in the frequency of cells responding to HLA-null stimuli (CD107a or IFN-γ responses) in the CD56Dim compartment between the more mature CD57+ and the less mature CD57− subsets (25, 38). This implies that within the CD56Dim compartment, differentiation stage does not play a primary role in determining responses to HLA-null cells. On the other hand, education via iNKR self-HLA was found to be important in the acquisition of functional potential to HLA-null cell stimuli as the double-iNKR+ NK cells had higher total responses than the other three NKG2A+/− 3DL1+/− populations in individuals who were carriers of a Bw4 allele than in the Bw6 controls (Fig. 3B and C).
With respect to iCD4 cell stimulation, we also observed that the less mature CD56Bright NKG2A+ 3DL1−, CD56Dim NKG2A+ 3DL1−, and CD56Dim NKG2A+ 3DL1+ populations exhibited stronger functional responses than the more mature CD56Dim NKG2A− 3DL1+ counterparts. The CD56Bright NKG2A+ 3DL1− NK population responded to iCD4 cell stimulation with the highest frequency of functional cells (Fig. 5). It is notable that we observed a modest but significantly lower frequency of CD56Bright NKG2A+ NK cells following a 24-h coculture with iCD4 cells than with uninfected CD4 cells (Fig. 5A). Despite the short coculture period, the downregulation of NKG2A expression may be related to the potent stimulation of the CD56Bright NKG2A+ population by autologous iCD4 cells. We also observed a modest impact of education via 3DL1-Bw4 on responses to iCD4 cells since we measured significantly higher trifunctional and total CCL4 responses for CD56Dim NKG2A+ 3DL1+ cells in Bw4+ subjects than in Bw6 controls (Fig. 7). These results further highlight the impact that education via the 3DL1-Bw4 pair has on NK cell functionality in response to HIV-infected targets.
Our results suggest that interactions between NKG2A and HLA-E may influence NK cell responses to iCD4 cells. HIV Nef does not downmodulate HLA-E expression on infected cells. On the contrary, its expression is increased in CD4 cells in vitro infected with R5- and X4-tropic clinical isolates (44). Blocking HLA-E or NKG2A with antibodies increases lysis of in vitro-HIV-infected cells (44–46). These findings are consistent with our observation that iCD4 cell-induced NK cell functionality is increased by blocking the interaction of HLA-E and NKG2A (Fig. 6; see also Fig. S3B and C in the supplemental material). This suggests that NKG2A is acting as an iNKR but that inhibition is incomplete. The aNKRs NKG2C and NKG2E also bind HLA-E though with a lower affinity than NKG2A (36, 47). However, NKG2C and NKG2A are usually expressed on nonoverlapping NK cell populations (48), making it unlikely that coexpression of NKG2C accounts for the triggering of the NKG2A+ NK cells by iCD4 cells. Thus, it remains unclear how NKG2A+ NK cells are being activated by iCD4 cells. A possible explanation for this finding may be HIV infection-driven changes in the proteomic environment that NKG2A+ cells detect through interactions with leader peptides expressed by HLA-E. The cell surface expression of HLA-E depends on binding of highly conserved leader peptides, mostly from HLA-A, -B, -C, and -G (36, 47). The downregulation of HLA-A and -B by HIV may affect the pool of leader peptides available to bind HLA-E, which could have an impact on interactions between HLA-E and NKG2A (49, 50). Viruses have also developed other mechanisms to evade immune recognition by encoding peptide homologues to stabilize expression of MHC class I. HIV-derived Gag peptide p24 consisting of amino acids 14 to 22 has been shown to stabilize and increase HLA-E surface expression (44). Further investigations are needed to understand how autologous iCD4 cells are activating NKG2A+ NK cells.
In conclusion, we investigated the responses of four distinct iNKR-bearing NK cell populations (NKG2A+/− 3DL1+/−) to HLA-null and HIV-infected CD4 cells and characterized the patterns of their functional subsets. To our knowledge, this is the first time a study has examined the impact of these iNKRs on multifunctional responses to HIV-1. Infected CD4 cells stimulated a significantly higher frequency of NKG2A+ cells with several functional profiles and in both the CD56Bright and CD56Dim compartments, and coexpression of 3DL1 did not further modulate the functionality of NKG2A+ NK cells. Further studies are required to elucidate the mechanism of NKG2A+ NK cell recognition of HIV-infected CD4 cells and identify other factors that may enhance the potency of these functional responses. It is important to understand how these parameters are modulated by NK cells in response to iCD4 cells due to the diverse roles that NK cells have in shaping immune responses (2, 51). Our results suggest that NKG2A+ NK cells may have an important role in NK cell responses to HIV-infected cells. Clarifying these mechanisms may shed light on correlates of protection and aid in developing strategies that harness NK cells to prevent or control HIV infection.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Canadian Institutes for Health Research (CIHR) MOP 123800 and the Fonds de la Recherche du Québec-Santé (FRQ-S) AIDS and Infectious Diseases Network. I.L. was supported by a Ph.D. scholarship from FRQ-S and CIHR. G.I was supported by a Post-Doctoral Fellowship from the Canadian HIV Trials Network and CIHR. N.F.B. is a member of the Research Institute of the McGill University Health Centre, an institution funded in part by the FRQ-S.
We acknowledge Pacale Arlotto and Josée Girouard for expert nursing skills in obtaining leukapheresis samples from participants. We also acknowledge the contribution of the study participants. We are grateful to Rachel Bouchard for contacting study participants and coordinating their recruitment to this study. We also acknowledge the expert technical support of Tsoarello Mabanga and Xiaoyan Ni.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01546-15.
REFERENCES
- 1.Trinchieri G. 1989. Biology of natural killer cells. Adv Immunol 47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munz C, Steinman RM, Fujii S. 2005. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med 202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. 2003. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med 198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott-Algara D, Truong LX, Versmisse P, David A, Luong TT, Nguyen NV, Theodorou I, Barre-Sinoussi F, Pancino G. 2003. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J Immunol 171:5663–5667. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 5.Boulet S, Kleyman M, Kim JY, Kamya P, Sharafi S, Simic N, Bruneau J, Routy JP, Tsoukas CM, Bernard NF. 2008. A combined genotype of KIR3DL1 high expressing alleles and HLA-B*57 is associated with a reduced risk of HIV infection. AIDS 22:1487–1491. doi: 10.1097/QAD.0b013e3282ffde7e. [DOI] [PubMed] [Google Scholar]
- 6.Boulet S, Sharafi S, Simic N, Bruneau J, Routy JP, Tsoukas CM, Bernard NF. 2008. Increased proportion of KIR3DS1 homozygotes in HIV-exposed uninfected individuals. AIDS 22:595–599. doi: 10.1097/QAD.0b013e3282f56b23. [DOI] [PubMed] [Google Scholar]
- 7.Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, Trowsdale J, Wilson M, O'Brien SJ, Carrington M. 2002. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet 31:429–434. [DOI] [PubMed] [Google Scholar]
- 8.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, Cox S, Little AM, Alexander GJ, Cramp ME, O'Brien SJ, Rosenberg WM, Thomas DL, Carrington M. 2004. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 9.Ahlenstiel G, Martin MP, Gao X, Carrington M, Rehermann B. 2008. Distinct KIR/HLA compound genotypes affect the kinetics of human antiviral natural killer cell responses. J Clin Invest 118:1017–1026. doi: 10.1172/JCI32400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, Bruno S, Mondelli MU. 2009. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology 137:1151–1160.e7. doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 11.Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, Hoofnagle JH, Liang TJ, Heller T, Rehermann B. 2010. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology 138:325–335.e2. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alter G, Heckerman D, Schneidewind A, Fadda L, Kadie CM, Carlson JM, Oniangue-Ndza C, Martin M, Li B, Khakoo SI, Carrington M, Allen TM, Altfeld M. 2011. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature 476:96–100. doi: 10.1038/nature10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. 2005. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 14.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. 2006. Human NK cell education by inhibitory receptors for MHC class I. Immunity 25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Brodin P, Karre K, Hoglund P. 2009. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol 30:143–149. doi: 10.1016/j.it.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. 2009. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol 182:4572–4580. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braud V, Jones EY, McMichael A. 1997. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur J Immunol 27:1164–1169. doi: 10.1002/eji.1830270517. [DOI] [PubMed] [Google Scholar]
- 18.Llano M, Lee N, Navarro F, Garcia P, Albar JP, Geraghty DE, Lopez-Botet M. 1998. HLA-E-bound peptides influence recognition by inhibitory and triggering CD94/NKG2 receptors: preferential response to an HLA-G-derived nonamer. Eur J Immunol 28:2854–2863. doi:. [DOI] [PubMed] [Google Scholar]
- 19.Cella M, Longo A, Ferrara GB, Strominger JL, Colonna M. 1994. NK3-specific natural killer cells are selectively inhibited by Bw4-positive HLA alleles with isoleucine 80. J Exp Med 180:1235–1242. doi: 10.1084/jem.180.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gumperz JE, Litwin V, Phillips JH, Lanier LL, Parham P. 1995. The Bw4 public epitope of HLA-B molecules confers reactivity with natural killer cell clones that express NKB1, a putative HLA receptor. J Exp Med 181:1133–1144. doi: 10.1084/jem.181.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan AM, Ennis P, Parham P, Holmes N. 1986. The primary structure of HLA-A32 suggests a region involved in formation of the Bw4/Bw6 epitopes. J Immunol 137:3671–3674. [PubMed] [Google Scholar]
- 22.Sunwoo JB, Kim S, Yang L, Naik T, Higuchi DA, Rubenstein JL, Yokoyama WM. 2008. Distal-less homeobox transcription factors regulate development and maturation of natural killer cells. Proc Natl Acad Sci U S A 105:10877–10882. doi: 10.1073/pnas.0805205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Heller G, Chewning J, Kim S, Yokoyama WM, Hsu KC. 2007. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol 179:5977–5989. doi: 10.4049/jimmunol.179.9.5977. [DOI] [PubMed] [Google Scholar]
- 24.Song R, Lisovsky I, Lebouche B, Routy JP, Bruneau J, Bernard NF. 2014. HIV protective KIR3DL1/S1-HLA-B genotypes influence NK cell-mediated inhibition of HIV replication in autologous CD4 targets. PLoS Pathog 10:e1003867. doi: 10.1371/journal.ppat.1003867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Bjorklund AT, Flodstrom-Tullberg M, Michaelsson J, Rottenberg ME, Guzman CA, Ljunggren HG, Malmberg KJ. 2010. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood 116:3853–3864. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 26.Bashirova AA, Thomas R, Carrington M. 2011. HLA/KIR restraint of HIV: surviving the fittest. Annu Rev Immunol 29:295–317. doi: 10.1146/annurev-immunol-031210-101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661–671. doi: 10.1016/S1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 28.Martin MP, Qi Y, Gao X, Yamada E, Martin JN, Pereyra F, Colombo S, Brown EE, Shupert WL, Phair J, Goedert JJ, Buchbinder S, Kirk GD, Telenti A, Connors M, O'Brien SJ, Walker BD, Parham P, Deeks SG, McVicar DW, Carrington M. 2007. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet 39:733–740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tallon BJ, Bruneau J, Tsoukas CM, Routy JP, Kiani Z, Tan X, Bernard NF. 2014. Time to seroconversion in HIV-exposed subjects carrying protective versus non protective KIR3DS1/L1 and HLA-B genotypes. PLoS One 9:e110480. doi: 10.1371/journal.pone.0110480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boulet S, Song R, Kamya P, Bruneau J, Shoukry NH, Tsoukas CM, Bernard NF. 2010. HIV protective KIR3DL1 and HLA-B genotypes influence NK cell function following stimulation with HLA-devoid cells. J Immunol 184:2057–2064. doi: 10.4049/jimmunol.0902621. [DOI] [PubMed] [Google Scholar]
- 31.Kamya P, Boulet S, Tsoukas CM, Routy JP, Thomas R, Cote P, Boulassel MR, Baril JG, Kovacs C, Migueles SA, Connors M, Suscovich TJ, Brander C, Tremblay CL, Bernard N, Canadian Cohort of HIV Infected Slow Progressors . 2011. Receptor-ligand requirements for increased NK cell polyfunctional potential in slow progressors infected with HIV-1 coexpressing KIR3DL1*h/*y and HLA-B*57. J Virol 85:5949–5960. doi: 10.1128/JVI.02652-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alter G, Martin MP, Teigen N, Carr WH, Suscovich TJ, Schneidewind A, Streeck H, Waring M, Meier A, Brander C, Lifson JD, Allen TM, Carrington M, Altfeld M. 2007. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med 204:3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson S, Johansson M, Rosmaraki E, Vahlne G, Mehr R, Salmon-Divon M, Lemonnier F, Karre K, Hoglund P. 2005. Natural killer cell education in mice with single or multiple major histocompatibility complex class I molecules. J Exp Med 201:1145–1155. doi: 10.1084/jem.20050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. 2009. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood 113:2434–2441. doi: 10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]
- 35.Houlihan JM, Biro PA, Harper HM, Jenkinson HJ, Holmes CH. 1995. The human amnion is a site of MHC class Ib expression: evidence for the expression of HLA-E and HLA-G. J Immunol 154:5665–5674. [PubMed] [Google Scholar]
- 36.Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG. 1998. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med 187:813–818. doi: 10.1084/jem.187.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. 2011. Innate or adaptive immunity? The example of natural killer cells. Science 331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. 2010. CD57 defines a functionally distinct population of mature NK cells in the human CD56dim CD16+ NK-cell subset. Blood 116:3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouyang Q, Baerlocher G, Vulto I, Lansdorp PM. 2007. Telomere length in human natural killer cell subsets. Ann N Y Acad Sci 1106:240–252. doi: 10.1196/annals.1392.001. [DOI] [PubMed] [Google Scholar]
- 40.Romagnani C, Juelke K, Falco M, Morandi B, D'Agostino A, Costa R, Ratto G, Forte G, Carrega P, Lui G, Conte R, Strowig T, Moretta A, Munz C, Thiel A, Moretta L, Ferlazzo G. 2007. CD56bright CD16− killer Ig-like receptor-NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol 178:4947–4955. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 41.Beziat V, Descours B, Parizot C, Debre P, Vieillard V. 2010. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS One 5:e11966. doi: 10.1371/journal.pone.0011966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu J, Mao HC, Wei M, Hughes T, Zhang J, Park IK, Liu S, McClory S, Marcucci G, Trotta R, Caligiuri MA. 2010. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood 115:274–281. doi: 10.1182/blood-2009-04-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. 2010. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 115:2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nattermann J, Nischalke HD, Hofmeister V, Kupfer B, Ahlenstiel G, Feldmann G, Rockstroh J, Weiss EH, Sauerbruch T, Spengler U. 2005. HIV-1 infection leads to increased HLA-E expression resulting in impaired function of natural killer cells. Antivir Ther 10:95–107. [DOI] [PubMed] [Google Scholar]
- 45.Bonaparte MI, Barker E. 2004. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood 104:2087–2094. doi: 10.1182/blood-2004-02-0696. [DOI] [PubMed] [Google Scholar]
- 46.Ward JP, Bonaparte MI, Barker E. 2004. HLA-C and HLA-E reduce antibody-dependent natural killer cell-mediated cytotoxicity of HIV-infected primary T cell blasts. AIDS 18:1769–1779. doi: 10.1097/00002030-200409030-00005. [DOI] [PubMed] [Google Scholar]
- 47.Braud VM, Allan DS, O'Callaghan CA, Soderstrom K, D'Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, Lanier LL, McMichael AJ. 1998. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C Nature 391:795–799. [DOI] [PubMed] [Google Scholar]
- 48.Beziat V, Dalgard O, Asselah T, Halfon P, Bedossa P, Boudifa A, Hervier B, Theodorou I, Martinot M, Debre P, Bjorkstrom NK, Malmberg KJ, Marcellin P, Vieillard V. 2012. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur J Immunol 42:447–457. doi: 10.1002/eji.201141826. [DOI] [PubMed] [Google Scholar]
- 49.Thananchai H, Gillespie G, Martin MP, Bashirova A, Yawata N, Yawata M, Easterbrook P, McVicar DW, Maenaka K, Parham P, Carrington M, Dong T, Rowland-Jones S. 2007. Cutting edge: allele-specific and peptide-dependent interactions between KIR3DL1 and HLA-A and HLA-B. J Immunol 178:33–37. doi: 10.4049/jimmunol.178.1.33. [DOI] [PubMed] [Google Scholar]
- 50.Fadda L, Borhis G, Ahmed P, Cheent K, Pageon SV, Cazaly A, Stathopoulos S, Middleton D, Mulder A, Claas FH, Elliott T, Davis DM, Purbhoo MA, Khakoo SI. 2010. Peptide antagonism as a mechanism for NK cell activation. Proc Natl Acad Sci U S A 107:10160–10165. doi: 10.1073/pnas.0913745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Altfeld M, Fadda L, Frleta D, Bhardwaj N. 2011. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol 11:176–186. doi: 10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.