ABSTRACT
Hepatitis B virus (HBV)-specific cytotoxic T lymphocytes (CTLs) are critical in eliminating infection. We developed an animal model in which HBV-infected human hepatocytes are targeted by HBV-specific CTLs. After HBV inoculation in human hepatocyte-transplanted herpes simplex virus type-1 thymidine kinase-NOG mice, human peripheral blood mononuclear cells (PBMCs) were administered, and albumin, HBV DNA, alanine aminotransferase (ALT), and cytokine levels were analyzed. Histopathological and flow-cytometric analysis of infiltrating human immune cells were performed, and the efficacy of CTL-associated antigen-4 immunoglobulin (CTLA4Ig) against liver damage was evaluated. PBMC treatment resulted in massive hepatocyte damage with elevation of ALT, granzyme A, and gamma interferon and decrease in albumin and HBV DNA. The number of liver-infiltrating human lymphocytes and CD8-positive cells was significantly higher in HBV-infected mice. HBV-specific CTLs were detected by core and polymerase peptide-major histocompatibility complex-tetramer, and the population of regulatory T cells was significantly decreased in HBV-infected mice. Serum hepatitis B surface (HBs) antigen became negative, and HBs antibody appeared. CTLA4Ig treatment strongly inhibited infiltration of mononuclear cells. CTLA4Ig treatment will be used to treat patients who develop severe acute hepatitis B to prevent liver transplantation or lethality. This animal model is useful for virological and immunological analysis of HBV infection and to develop new therapies for severe acute hepatitis B.
IMPORTANCE Without liver transplantation, some HBV-infected patients will die from severe liver damage due to acute overreaction of the immune system. No effective treatment exists, due in part to the lack of a suitable animal model. An animal model is necessary to investigate the mechanism of hepatitis and to develop therapeutic strategies to prevent acute liver failure in HBV infection. We developed an animal model in which HBV-infected human hepatocytes are targeted by human HBV-specific CTLs. In this model, HBV-infected human hepatocytes were transplanted into severely immunodeficient NOG mice in order to reconstruct elements of the human immune system. Using this model, we found that CTL-associated antigen-4 immunoglobulin was able to suppress damage to HBV-infected hepatocytes, suggesting an approach to treatment. This animal model is useful for virological and immunological analysis of HBV infection and to develop new therapies for severe acute hepatitis B.
INTRODUCTION
About 2 billion people have been exposed to the hepatitis B virus (HBV) (1). The majority of HBV-infected immunocompetent adults clear the infection spontaneously, but a small number of patients develop fulminant hepatitis due to acute massive hepatocyte degeneration, which leads to liver failure and hepatic encephalopathy. Liver transplantation often is necessary to save the lives of patients who experience potentially fatal liver failure due to the lack of effective therapy for this severe clinical condition.
Fatal severe acute hepatitis also sometimes develops in HBV-positive or -exposed patients who have received strong immunosuppressive therapy known to reactivate HBV or who encounter de novo hepatitis B infection. Although preventive nucleoside analogue treatment has been attempted in patients who developed fulminant hepatitis, the condition often is fatal without liver transplantation (2). The number of patients who can receive liver transplantation is limited due to the lack of donors. Furthermore, patients who receive transplantation require lifelong immunosuppressive therapy to prevent rejection of the transplanted liver. Accordingly, there is an urgent need to develop treatment options to reduce acute massive liver damage due to activation of cytotoxic T lymphocytes against HBV-infected hepatocytes.
To develop therapies against acute hepatitis, an appropriate animal model is necessary. We previously developed a mouse model using urokinase-type plasminogen activator (uPA)-SCID mice transplanted with human hepatocytes and human peripheral mononuclear cells (PBMCs) (3). However, hepatitis in this mouse model was mediated largely by natural killer (NK) cells, and we were unable to detect HBV-specific cytotoxic T cells (CTLs), which have been reported to play a primary role in the elimination of infected liver cells in hepatitis B.
Recently, human hepatocytes were successfully transplanted into severely immunodeficient NOG mice with the herpes simplex virus type-1 thymidine kinase (HSVtk) expressed in mouse hepatocytes (TK-NOG) (4). In this mouse model, liver cells expressing HSVtk were ablated after a brief exposure to ganciclovir (GCV), and transplanted human hepatocytes were stably maintained in the mouse liver without subsequent exogenous drug administration (4). Using this mouse, we developed a model of persistent HBV infection (5). In this study, we developed a new hepatitis mouse model using the TK-NOG mouse and subsequent transplantation of human PBMCs, in which CTLs play a main role in eliminating infected hepatocytes.
CTL-associated antigen-4 immunoglobulin (CTLA4Ig) is a soluble fusion protein with a link between the CTLA4 extracellular domain and the Fc region of the IgG molecule (6–9). CTLA4 is the T cell transmembrane receptor, and the CTLA4 pathway is a key regulator of T cell activation (10–12). CTLA4Ig inhibits T cell activation and proliferation (6, 7) and can prevent the development of autoimmunity and inhibit graft rejection in animal models (8, 9). It is also used in the clinical treatment of refractory rheumatoid arthritis (13–15). We attempted in this study to reduce CTL activity against HBV-infected hepatocytes by utilizing the strong CTL suppressive effect of CTLA4Ig.
MATERIALS AND METHODS
Ethics statement.
All animal protocols described in this study were performed in accordance with the Guide for the Care and Use of Laboratory Animals (16) and the local committee for animal experiments, and the experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of the Graduate School of Biomedical Sciences, Hiroshima University (protocol number 12-93). Human serum samples were obtained from a patient with chronic hepatitis who provided written informed consent using a form that was made in accordance with the Declaration of Helsinki and was approved by the ethical committee of Hiroshima University.
Generation of human hepatocyte chimeric mice.
TK-NOG mice were purchased from the Central Institute for Experimental Animals (CIEA; Kawasaki, Japan). The transplantation of human hepatocytes with HLA-A24 (Phoenix Bio, Hiroshima, Japan) was performed as described previously (4, 5). Infection, extraction of serum samples, and euthanasia were performed under ether anesthesia. The concentration of human serum albumin, which is correlated with the repopulation index (4), was measured as described previously (17). Eight weeks after hepatocyte transplantation, chimeric mice were injected intravenously with 5 × 105 copies of HBV-positive human serum.
Human serum samples.
Human serum samples containing high titers of HBeAg-positive genotype C HBV DNA (10.2 × 106 copies/ml) were obtained from a patient with chronic hepatitis. Serum samples were divided into aliquots and stored in liquid nitrogen until use.
Quantitation of HBV.
Serum HBV DNA levels were measured quantitatively by a TaqMan-based assay using the protocol provided by the manufacturer (COBAS AmpliPre/COBAS TaqMan HBV test, v2.0; Roche Diagnostics, Tokyo, Japan). The lower detection limit of real-time PCR for HBV DNA was 4.4 log copies/ml (18, 19).
Preparation of human PBMCs and transplantation into human hepatocyte chimeric mice.
PBMCs were isolated using Ficoll-Hypaque density gradient centrifugation from a patient with HLA-A24 who recovered from an episode of acute severe hepatitis B. After recovery, the level of HBs antibody (HBsAb) was around 1,000 mIU/ml in this serum, and HBV DNA and HBs antigen (HBsAg) were no longer detected. Eight to 10 weeks after HBV inoculation, 5 × 106 human PBMCs were transplanted into human hepatocyte chimeric mice. To assess the effect of depletion of human CD8-positive T cells from administered PBMCs on hepatitis formation, the human CD8 MicroBeads kit (Milteny Biotec, CA) was used.
Treatment of mice with CTLA4 Ig.
To analyze the effect of CTLA4Ig, mice received intraperitoneal injections with 1.5 mg of CTLA4Ig (Bristol-Meyers Squibb, New York, NY) either 1 day before and 7 days after human PBMC transplantation or only 7 days after human PBMC transplantation.
Flow cytometry.
We collected mouse liver-infiltrating cells flowing through the portal vein after hepatectomy (20). Reconstructed human PBMCs in mice were analyzed by flow cytometry with the following MAbs used for surface and intracellular staining: allophycocyanin (APC)-H7 anti-human cluster of differentiation 3 (CD3) (clone SK7), APC-conjugated anti-CD4 (clone SK), BD Horizon V450 anti-human CD8 (clone RPA-T8), HU HRZN V500 monoclonal antibody (MAb)-conjugated anti-human CD45 (clone H130), Alexa Fluor 488-conjugated anti-human CD56 (clone B159), peridinin chlorophyll protein (PerCP)-Cy5.5 anti-human FoxP3 (clone 236a), phycoerythrin (PE)-conjugated anti-human CD25 (clone M-A251), and PE-Cy7-conjugated anti-mouse CD45 (clone 30-F11). Each of the above-described MAbs was purchased from BD Biosciences. PE-conjugated HBV core and polymerase-derived immunodominant CTL epitope (21, 22) was purchased from Medical & Biological Laboratories (Nagoya, Japan) (see Fig. S1 in the supplemental material). Dead cells identified by light scatter and propidium iodide staining were excluded from the analysis. For intracellular staining, cells were permeabilized and fixed after surface staining using the BD Cytofix/Cytoperm kit (BD Bioscience, Heidelberg, Germany). Flow cytometry was performed using a FACSAria II flow cytometer (BD Bioscience), and results were analyzed with FlowJo (TreeStar). Regulatory T (Treg) and human B cells were defined as human CD45+ CD4+ CD25+ FoxP3+ and CD45+ CD3− CD19+ cells, respectively.
Histochemical analysis of mouse liver.
Histochemical analysis and immunohistochemical staining using antibodies against human albumin (Bethyl Laboratories Inc., Montgomery, TX) and hepatitis B core antigen (HBcAg) (Dako Diagnostika, Hamburg, Germany) were performed as described previously (3, 5, 23). Immunoreactive materials were visualized using a streptavidin-biotin staining kit (Histofine SABPO kit; Nichirei, Tokyo, Japan) and diaminobenzidine.
Serological measurements.
Quantification of HBsAg and HBsAb was performed using Abbott Architect platforms (Abbott, Ireland, Diagnostic Division) as recommended by the manufacturer. Mice serum alanine aminotransferase (ALT) levels were measured using Fuji DRI-CHEM (Fuji Film, Tokyo, Japan) according to the instructions provided by the manufacturer.
Cytokine assay.
The concentrations of human gamma interferon (IFN-γ), granzyme A, granzyme B, and interleukin-2 (IL-2), IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, and IL-17A were quantitatively determined by chemokine cytometric bead array (CBA) kits (BD Biosciences, Heidelberg, Germany) in accordance with the manufacturer's instructions. The minimum and maximum detection limits were 10 and 2,500 pg/ml, respectively.
Statistical analysis.
Changes in mouse serum human albumin and HBV DNA levels and the frequencies of human PBMCs in mouse livers were compared by Mann-Whitney U and unpaired t tests. P values of less than 0.05 were considered statistically significant.
RESULTS
Establishment of a hepatitis model using HBV-infected human hepatocyte chimeric TK-NOG mice and human PBMC transplantation.
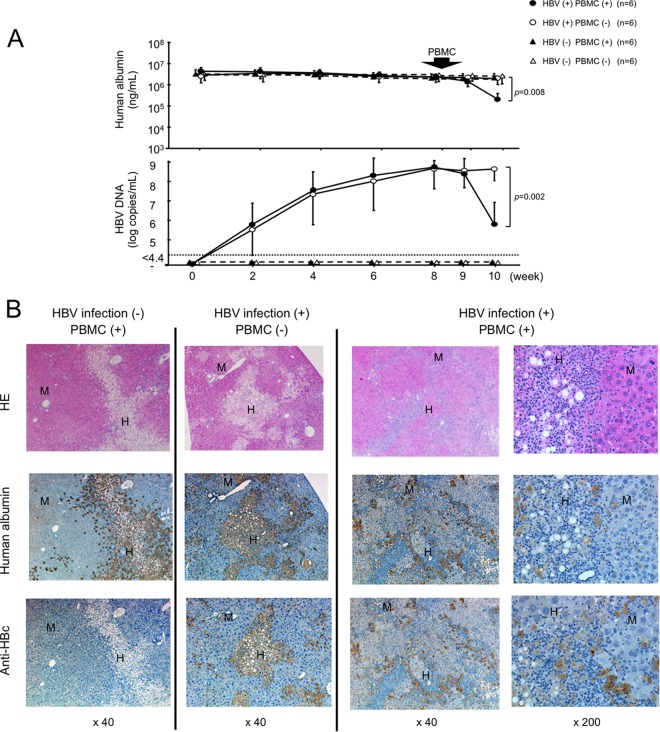
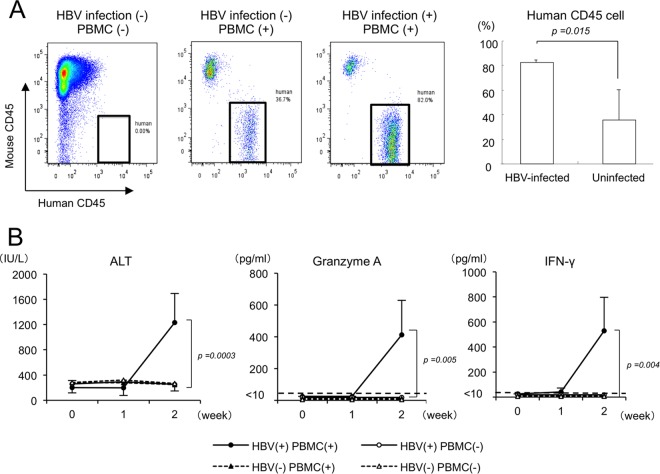
HBV-infected and uninfected mice were treated with 5 × 106 human PBMCs obtained from a donor who recovered from severe acute hepatitis. The treatment resulted in a rapid and significant decline of serum human albumin concentrations (P = 0.008) and HBV DNA levels (P = 0.002) only in HBV-infected and PBMC-treated mice (Fig. 1A). Declines of human albumin concentrations and HBV DNA levels were observed in neither uninfected mice nor HBV-infected mice without PBMC treatment. Two weeks after PBMC treatment, histological examination of livers showed an extensive loss of human liver cells and a massive infiltration of mononuclear cells in the human hepatocyte area only in HBV-infected and PBMC-treated mice (Fig. 1B). Flow cytometry analysis showed that there was no difference in human CD45-positive mononuclear cell chimerism between uninfected and HBV-infected mice 1 week after treatment (see Fig. S2 in the supplemental material). However, treatment with PBMCs resulted in significantly greater human CD45-positive mononuclear cell chimerism in HBV-infected mice than in uninfected mice 2 weeks after treatment (83.8% ± 1.8% versus 33.1% ± 13.5%; P = 0.015) (Fig. 2A). Serum ALT levels in mice increased significantly (P = 0.0003) only in HBV-infected and PBMC-injected mice, reflecting the occurrence of hepatitis (Fig. 2B). Cytokine assays 2 weeks after PBMC treatment demonstrated that serum granzyme A (P = 0.005) and IFN-γ levels (P = 0.004) increased significantly only in HBV-infected and PBMC-treated mice (Fig. 2B). In contrast, no significant difference in FasL-positive cells was observed between uninfected and HBV-infected mice (see Fig. S3A in the supplemental material). No decrease in serum human albumin concentrations was observed following injection of PBMCs obtained from two healthy volunteers and six chronic HBV-infected patients (see Fig. S4 and Table S1 in the supplemental material). HBV DNA reduction was only apparent in mice injected with HLA-matched PBMCs (see Fig. S4 in the supplemental material).
FIG 1.
Establishment of acute hepatitis B model in human hepatocyte chimeric TK-NOG mice. HBV-infected and uninfected human hepatocyte chimeric TK-NOG mice were injected with 5 × 106 human PBMCs. Control HBV-infected mice did not receive PBMC injection. (A) Time course of human albumin concentration (upper) and HBV DNA titer (lower) in mouse serum. Data are represented as the means ± standard deviations (SD) from 6 mice. (B) Histological analysis of liver samples obtained from mice. Liver samples at 2 weeks after injection of human PBMCs were stained with hematoxylin-eosin (HE), anti-human albumin antibody, or anti-hepatitis B core (HBc) antibody. Labeled regions indicate human (H) and mouse (M) hepatocytes, respectively (original magnification, ×40 or ×200).
FIG 2.
Analysis of liver-infiltrating cells and ALT and cytokine levels in human hepatocyte chimeric TK-NOG mice injected with human PBMCs. Three groups of mice were treated as described in the legend to Fig. 1. (A) Liver mononuclear cells isolated from mice 2 weeks after human PBMC treatment were stained with antibodies against human CD45 and mouse CD45 and analyzed by flow cytometry (left). Percentages of human mononuclear cells in PBMC-treated mice (right). (B) Time courses of serum ALT, granzyme A, and IFN-γ in mice. Data are represented as the means ± SD from 6 mice.
Analysis of liver-infiltrating human lymphocytes in mice.
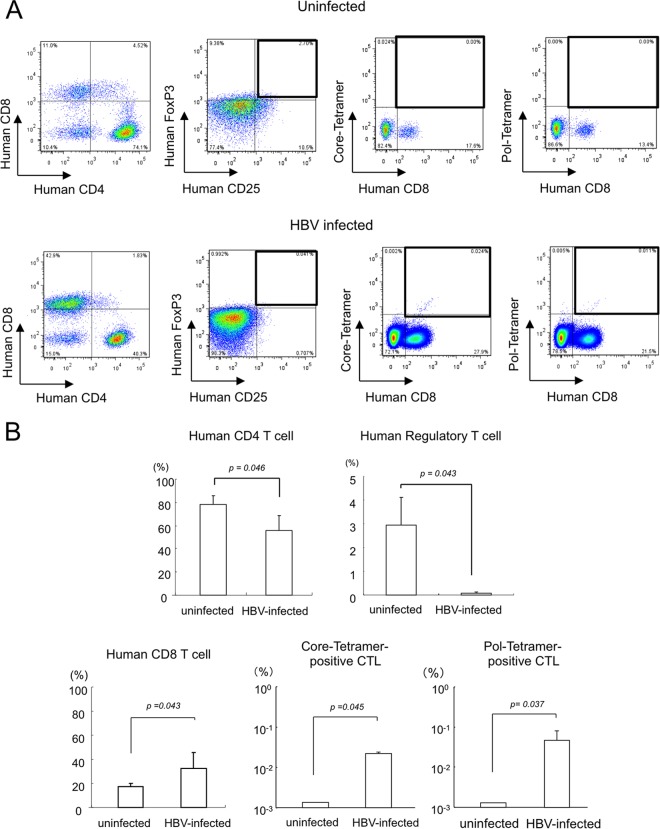
We analyzed liver-infiltrating cells for human cell surface markers in mice injected with PBMCs. Two weeks after human PBMC injection, frequencies of CD4+ CD8− cells decreased significantly in HBV-infected mice (Fig. 3A and B). Among CD4+ CD8− cells, numbers of CD25+ FoxP3+ regulatory T cells were significantly lower in HBV-infected mice (Fig. 3B), consistent with acute liver inflammation (24). Furthermore, frequencies of these cells in uninfected mice were similar to those of donor PBMCs (Fig. 3A; also see Fig. S5 in the supplemental material). In contrast, frequencies of CD4− CD8+ cells (P = 0.043), core-tetramer-positive HBV-specific CTLs (P = 0.045), and polymerase-tetramer-positive HBV-specific CTLs (P = 0.037) increased significantly in HBV-infected mice (Fig. 3B). The fraction of CD4− CD8+ cells was similar to that of donor PBMCs, and HBV-specific CTLs were not detected either (Fig. 3A; see Fig. S5 in the supplemental material).
FIG 3.
Analysis of mononuclear cells isolated from chimeric mice. Liver-infiltrating cells in HBV-infected and uninfected mice were analyzed by flow cytometry. (A) Liver mononuclear cells isolated from uninfected (upper) and HBV-infected (lower) mice 2 weeks after human PBMC treatment were separated with antibodies for human CD4 and CD8 (first image), human CD25 and FoxP3 (second image), human CD8 and core-tetramer (third image), and human CD8 and polymerase (pol)-tetramer (fourth image) and analyzed by flow cytometry. (B) Statistical analysis of percentages of human mononuclear cells in PBMC-treated mice. The means ± SD from 6 mice are presented.
CD8+ cells were almost completely depleted in the mice injected with CD8-depleted human mononuclear cells (see Fig. S6 in the supplemental material), and HBV DNA and human albumin levels were not reduced in these mice (Fig. 4A). Additionally, histological examination of livers showed no indication of hepatitis (Fig. 4B).
FIG 4.
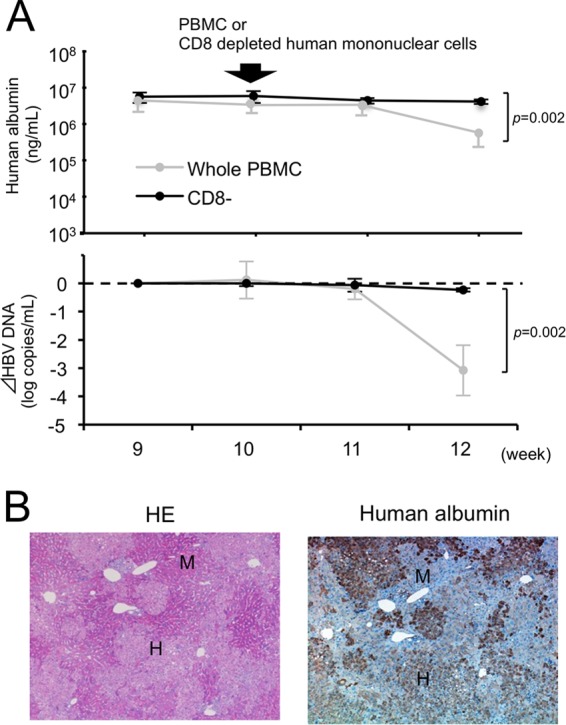
Analysis of mice injected with CD8-depleted human mononuclear cells. CD8-positive cells were depleted from PBMCs, and the CD8-negative fraction was injected intraperitoneally into HBV-infected mice (n = 3). (A) Time course of human albumin concentration (upper) and the reduction in HBV DNA titer (lower) in mice injected with CD8-depleted human mononuclear cells. The mice injected with whole PBMCs (as shown in Fig. 2B) are shown for comparison (shaded closed circle). (B) Histological analysis of livers of mice injected with CD8-depleted human mononuclear cells. Liver samples were stained with HE and anti-human albumin antibody. Labeled regions indicate human (H) and mouse (M) hepatocytes, respectively (original magnification, ×40).
In these human hepatocyte chimeric mice, HBs antigen levels were as high as those in patients with HBV infection (Fig. 5A). Two weeks after human PBMC treatment, serum HBs values decreased to undetectable levels. In contrast, HBsAb became positive in mouse serum 2 weeks after injection of PBMCs. Reconstitution of B cells was seen in uninfected and HBV-infected mice 2 weeks after PBMC transplantation (Fig. 5B).
FIG 5.
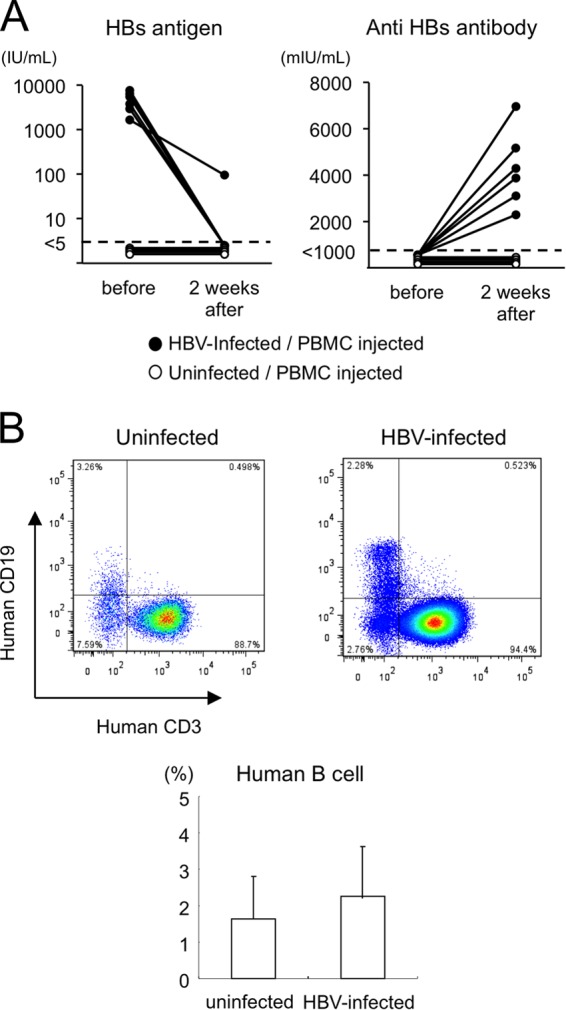
HBs antigen anti-HBs seroconversion observed in human hepatocyte chimeric TK-NOG mice. (A) Serum HBsAg and HBsAb levels in HBV-infected mice (closed circles) and uninfected mice (open circles) before and 2 weeks after human PBMC transplantation. The detection limit of HBsAg was 5 IU/ml, and the detection limit of HBsAb was 1,000 mIU/ml. (B) Flow cytometry analysis of liver mononuclear cells. After defining human PBMCs, mouse liver mononuclear cells were separated with antibodies against anti-human CD3 and CD19. Human B cells were defined as CD3− CD19+ cells.
CTLA4Ig suppressed HBV-infected human hepatocyte damage by human PBMCs.
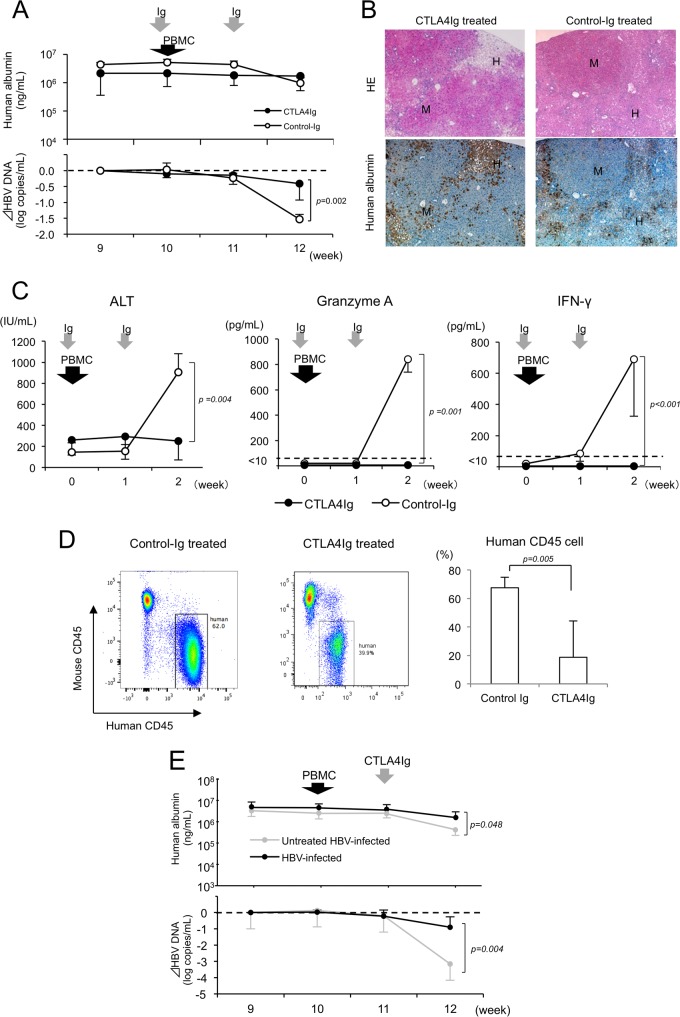
Using this mouse model, we analyzed the effect of CTLA4Ig on liver cell damage and the decline of HBV viremia levels in HBV-infected and PBMC-transplanted mice. CTLA4Ig treatment dramatically inhibited the decline of both serum human albumin concentrations and HBV DNA levels in HBV-infected and human PBMC-treated mice compared to that of control-Ig-treated mice (Fig. 6A). Histological examination also revealed neither invasion of mononuclear cells in the human liver tissue nor human liver cell damage in HBV-infected and CTLA4Ig-treated mice, similar to that of uninfected mice (Fig. 6B; also see Fig. S7 in the supplemental material). While serum ALT levels increased sharply by week 2 in control Ig-treated HBV-infected mice, no increase in ALT levels was observed in HBV-infected mice treated with CTLA4Ig (P = 0.004), consistent with inhibition of hepatitis (Fig. 6C). An increase in serum granzyme A (P = 0.001) and IFN-γ (P < 0.001) levels by human PBMC injection also was observed in control Ig-treated mice, but serum granzyme A and IFN-γ levels became undetectable after CTLA4Ig treatment (Fig. 6C). The frequency of human mononuclear cells in the liver was significantly lower in CTLA4Ig-treated mice than in control Ig-treated mice (P = 0.005) (Fig. 6D).
FIG 6.
Effect of CTLA4Ig in HBV-infected and PBMC-infected hepatitis B model mice. One day before and 7 days after human PBMC transplantation, mice were treated with intraperitoneal injection of CTLA4Ig (closed circles) and control Ig (open circles). (A) Time course of human albumin concentration (upper) and the reduction in HBV DNA titer (lower) in serum of CTLA4Ig-treated (n = 6) and control Ig-treated mice (n = 3). Data are presented as the means ± SD. (B) Histological analysis of livers. Liver samples 2 weeks after PBMC transplantation were stained by hematoxylin-eosin (HE) or anti-human albumin antibody. Regions are shown for human (H) and mouse (M) hepatocytes, respectively (original magnification, ×40). (C) Statistical analysis of mouse serum ALT, granzyme A, and IFN-γ levels 2 weeks after PBMC transplantation. Data are presented as the means ± SD. (D) Percentages of human mononuclear cells 2 weeks after PBMC transplantation in CTLA4Ig-treated and control Ig-treated mice. (E) Time course of human albumin concentration (upper) and the reduction in HBV DNA titer (lower) in mice treated with CTLA4Ig 7 days after human PBMC transplantation. Time courses of HBV-infected mice without CTLA4Ig treatment (Fig. 2B) are shown for comparison (shaded closed circle).
CTLA4Ig treatment after human PBMC transplantation also significantly inhibited the decline in both human albumin (P = 0.048) and HBV DNA (P = 0.004) levels in HBV-infected mice (Fig. 6E).
DISCUSSION
HBV is a noncytopathic DNA virus, and elimination of the virus occurs via destruction of infected cells by host cellular immune responses (25). HBV-related fulminant hepatitis results not only from acute infection but also from chronic infection following alteration of the immunological status of the host (1, 2). To date, no effective measure to suppress immunological elimination of infected hepatocytes exists. Accordingly, mortality of this condition is high without liver transplantation. Consequently, development of new therapeutic strategies and a small-animal model for fulminant hepatitis is urgently needed.
We previously established a mouse model capable of demonstrating massive HBV-infected human hepatocyte degeneration using uPA-SCID mice with human PBMCs (3). Although extensive human hepatocyte damage occurred and rapid decline in HBV DNA and human albumin were observed, the human mononuclear cell chimerism was only 7% in HBV-infected mice, and CTLs and Tregs were not detected. The main immune cells responsible for human liver cell damage in these mice were not the HBV-specific CTLs thought to be crucial in development of fulminant hepatitis (26, 27) but instead were NK cells (3). The poor chimerism and lack of CTLs in the uPA-SCID model might result from the presence of mouse NKs and macrophages in uPA-SCID mice, although we attempted to eliminate them with clodronate and anti-asialo GM1 antibody. Another factor is that induction of CTLs in uPA-SCID mice might be depressed by inhibition of dendritic cell (DC) activation due to pretreatment with clodronate. We observed a very small number of CD3− CD56+ NK cells in mouse livers at 2 weeks after PBMC injection compared to that in the donor's PBMCs (see Fig. S8 in supplemental material). This is consistent with a report (28) showing that NK cells administered to TK-NOG mice disappear rapidly.
NOG mice provide a suitable platform for the in vivo reconstruction of the human immune system. Death between 14 and 30 days due to graft-versus-host disease (GVHD) has been reported for mice inoculated with human PBMCs (29). In this study, we also injected human PBMCs and observed the mice for more than 3 weeks, during which time we observed the development of GVHD, e.g., rapid and severe weight loss, ruffled hair, and reduced mobility, in NOG mice (data not shown). As a result, we concluded that long-term experiments would be impractical due to GVHD and sacrificed the mice 2 weeks after PBMC injection. Additionally, we performed experiments using PBMCs obtained from two healthy volunteers and six patients with chronic HBV infection. Human albumin and HBV DNA levels did not decrease in most mice injected with these PBMCs (see Fig. S4 and Table S1 in the supplemental material). We speculated that the decline of human albumin and HBV DNA levels during the 2 weeks following human PBMC inoculation was due to hepatitis. The proportion of human CD45-positive mononuclear cell chimerism in uninfected mice was 30 to ∼40%, whereas in HBV-infected mice the proportion was almost 90% 2 weeks after injection of human PBMCs (Fig. 2A). This may be due to the more severely immunodeficient nature of NOG mice, which lack the IL-2 receptor gamma chain (30), T cells, B cells (31), NK cells, macrophages, and DCs (32, 33). Interestingly, there was no difference in the chimerism between HBV-infected mice and uninfected mice only 1 week after injection (see Fig. S2 in supplemental material). These results suggest that HBV-activated PBMCs emerged between the first and second week after PBMC injection.
To confirm the utility of this model, we performed experiments using PBMCs obtained from chronic hepatitis patients with HLA-A24. We observed a reduction in HBV DNA in mice infected with HBV and injected the mice with these PBMCs (see Fig. S4 and Table S1 in the supplemental material). On the other hand, we observed apparent hepatitis in mice injected with PBMCs donated from an individual who had recently recovered from severe acute hepatitis. Furthermore, we detected HBV-specific CTLs in liver-infiltrating cells, which are thought to be necessary in acute liver inflammation (34–37), as well as memory T cells (38). As we confirmed that depletion of CD8-positive T cells resulted in no occurrence of hepatitis (Fig. 4), we concluded that this model of inflammation was CTL dependent. The frequency of HBV-specific CD8 cells was reported to be around 0.2 to 0.5% of total CD8 cells (39). The reason for this small number might derive from the fact that we were able to use only two tetramers that were commercially available, and additional CTLs that were not detected by these two tetramers are likely to be present. In contrast, HBV-specific CTLs were not detected in blood from patients who recovered (see Fig. S5 in the supplemental material). This may be due to the presence of only a very small number of HBV-specific CTLs in the patient's blood. Another possibility is that only memory T cells reactive to HBV remained in the blood, and these cells caused the hepatitis seen in the mouse model. As the response of memory T cells against HBV persists several years after clinical recovery from acute hepatitis B (40), we speculate that PBMCs obtained from the recovered patient contained memory T cells against HBV. Failure to establish apparent hepatitis using PBMCs from chronic hepatitis patients might be because their CTLs were functionally impaired or exhausted (41), which would be consistent with the observation that only a small number of HBV-specific CTLs are detectable in patients with chronic hepatitis B (42).
Human mononuclear cell chimerism was significantly more pronounced in HBV-infected mice than in HBV-uninfected mice (Fig. 2A and 3A and B), but the frequency of human regulatory T cells was significantly lower (Fig. 3B). Regulatory T cells play an important role in immune homeostasis and tolerance (43–45). The frequency of regulatory T cells in uninfected mice was similar to that in healthy human blood (46). The decrease in Tregs might contribute to severe acute liver cell damage, although the precise mechanism should be clarified further.
We observed HBs antigen-antibody seroconversion in this model (Fig. 5A). The rapid emergence of HBs antibody might result from memory cells present in PBMCs from a donor who recovered from severe acute hepatitis B infection, and cooperation between T cells and B cells obtained from this donor might lead to rapid B cell activation and production of HBs antigen-specific antibody (47).
CTLA4Ig, an immunoglobulin fusion protein, contains an extracellular CTLA4 domain fused to human immunoglobulin IgG1. CTLA4Ig binds to B7, the ligand for CD28, on antigen-presenting cells (48) and inhibits the activation and proliferation of naive T cells (7), memory T cells (49), and effector T cells (50). Both plasmacytoid and myeloid dendritic cells were detected 2 weeks after the injection of human PBMCs (see Fig. S9 in the supplemental material). We analyzed activation and proliferation markers such as CD11a, CD107a, HLA-DR, CD69, IFN-γ, and cyclin D. Unexpectedly, these markers were similar for mice with and without treatment with CTLA4Ig, and there were no significant phenotypic differences between the two groups of mice due to the reaction of human PBMCs to mouse tissues (data not shown). However, CTLA4Ig treatment resulted in significantly less human CD45-positive mononuclear cell chimerism than in untreated mice 2 weeks after PBMC injection (see Fig. S10 in the supplemental material). In addition, there was no difference in the population of CD8 T cells between CTLA4Ig-treated and untreated mice (data not shown). These findings suggest that the actual number of CD3+ CD8+ cells among the liver-infiltrating cells in CTLA4Ig-treated mice was less than that in untreated mice and that CD8-positive T cells increased rapidly without CTLA4Ig. We hypothesized that the strong suppressive effect of CTLA4Ig can be used to suppress acute liver cell injury. As expected, CTLA4Ig treatment of HBV-infected PBMC-injected mice dramatically suppressed HBV-infected hepatocyte damage and inhibited hepatitis (Fig. 5). These results demonstrate the potential of CTLA4Ig as an investigational therapeutic tool in patients with severe acute hepatitis B.
Although the administration of CTLA4Ig appears to have effectively inhibited infiltration of mononuclear cells and suppressed several markers of hepatitis in PBMC-treated mice, we acknowledge several limitations of the study, including reliance on PBMCs collected from a single acute hepatitis donor, as well as a short window of time before the onset of graft-versus-host disease in these mice. Because of the paucity of commercially available HBV-specific tetramers, the frequency of HBV-specific T cells also is likely to be underestimated. The anticipated lack of a decline in HBV DNA or human albumin when PBMCs from healthy controls and patients with chronic HBV were used suggests that the decline in HBV DNA and human albumin observed in the mice injected with PBMCs from an acute HBV patient can be attributed to hepatitis instead of xeno- and alloreactive T-cell-mediated immune responses; however, this should be confirmed in future studies using additional markers and PBMCs from other compatible acute HBV patients, if available.
In a previous study, we showed that NK cells also may have an important role in acute severe hepatitis B. Recent papers have suggested the importance of NK cells under these conditions (51–53). Accordingly, suppression of both CTLs and NK cells should be considered in the treatment of patients with severe acute hepatitis B. The administration of both CTLA4Ig and anti-FAS-L should be considered.
In summary, we established an animal model of fulminant hepatitis caused by HBV infection using human hepatocyte chimeric TK-NOG mice injected with human PBMCs. The hepatitis in this model caused by HBV-specific CTLs is very similar to human acute hepatitis B, and CTLA4Ig was shown to be effective in suppressing hepatitis. CTLA4Ig as well as other therapeutic options should be investigated further as a potential therapy for patients with severe acute hepatitis B. This model also should be useful for studying immunological reactions and viral clearance in HBV infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Rie Akiyama and Yoko Matsumoto for their expert technical assistance.
This study was supported in part by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Labor, Health and Welfare and MEXT.
Part of this work was carried out at the Analysis Center of Life Science, Natural Science Center for Basic Research and Development, Hiroshima University.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01126-15.
REFERENCES
- 1.Tillmann HL, Zachou K, Dalekos GN. 2012. Management of severe acute to fulminant hepatitis B: to treat or not to treat or when to treat? Liver Int 32:544–553. doi: 10.1111/j.1478-3231.2011.02682.x. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, Csako G. 2008. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med 148:519–528. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okazaki A, Hiraga N, Imamura M, Hayes CN, Tsuge M, Takahashi S, Aikata H, Abe H, Miki D, Ochi H, Tateno C, Yoshizato K, Ohdan H, Chayama K. 2012. Severe necroinflammatory reaction caused by natural killer cell-mediated Fas/Fas ligand interaction and dendritic cells in human hepatocyte chimeric mouse. Hepatology 56:555–566. doi: 10.1002/hep.25651. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa M, Kawai K, Mitsui T, Taniguchi K, Monnai M, Wakui M, Ito M, Suematsu M, Peltz G, Nakamura M, Suemizu H. 2011. The reconstituted “humanized liver” in TK-NOG mice is mature and functional. Biochem Biophys Res Commun 405:405–410. doi: 10.1016/j.bbrc.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosaka K, Hiraga N, Imamura M, Yoshimi S, Murakami E, Nakahara T, Honda Y, Ono A, Kawaoka T, Tsuge M, Abe H, Hayes CN, Miki D, Aikata H, Ochi H, Ishida Y, Tateno C, Yoshizato K, Sasaki T, Chayama K. 2013. A novel TK-NOG based humanized mouse model for the study of HBV and HCV infections. Biochem Biophys Res Commun 441:230–235. doi: 10.1016/j.bbrc.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo VA Jr, Lombard LA, Gray GS, Nadler LM. 1993. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science 262:909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 7.Wells AD, Gudmundsdottir H, Turka LA. 1997. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Investig 100:3173–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knoerzer DB, Karr RW, Schwartz BD, Mengle-Gaw LJ. 1995. Collagen-induced arthritis in the BB rat. Prevention of disease by treatment with CTLA-4-Ig. J Clin Investig 96:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olthoff KM, Judge TA, Gelman AE, da Shen X, Hancock WW, Turka LA, Shaked A. 1998. Adenovirus-mediated gene transfer into cold-preserved liver allografts: survival pattern and unresponsiveness following transduction with CTLA4Ig. Nat Med 4:194–200. doi: 10.1038/nm0298-194. [DOI] [PubMed] [Google Scholar]
- 10.Walker LS, Sansom DM. 2011. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol 11:852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 11.Wang CJ, Kenefeck R, Wardzinski L, Attridge K, Manzotti C, Schmidt EM, Qureshi OS, Sansom DM, Walker LS. 2012. Cutting edge: cell-extrinsic immune regulation by CTLA-4 expressed on conventional T cells. J Immunol 189:1118–1122. doi: 10.4049/jimmunol.1200972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corse E, Allison JP. 2012. Cutting edge: CTLA-4 on effector T cells inhibits in trans. J Immunol 189:1123–1127. doi: 10.4049/jimmunol.1200695. [DOI] [PubMed] [Google Scholar]
- 13.Kremer JM, Dougados M, Emery P, Durez P, Sibilia J, Shergy W, Steinfeld S, Tindall E, Becker JC, Li T, Nuamah IF, Aranda R, Moreland LW. 2005. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve-month results of a phase iib, double-blind, randomized, placebo-controlled trial. Arthritis Rheum 52:2263–2271. doi: 10.1002/art.21201. [DOI] [PubMed] [Google Scholar]
- 14.Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, Szechinski J, Li T, Ge Z, Becker JC, Westhovens R. 2006. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med 144:865–876. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 15.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, Li T, Aranda R, Hagerty DT, Dougados M. 2005. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 353:1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 16.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 17.Tateno C, Yoshizane Y, Saito N, Kataoka M, Utoh R, Yamasaki C, Tachibana A, Soeno Y, Asahina K, Hino H, Asahara T, Yokoi T, Furukawa T, Yoshizato K. 2004. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am J Pathol 165:901–912. doi: 10.1016/S0002-9440(10)63352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goedel S, Rullkoetter M, Weisshaar S, Mietag C, Leying H, Boehl F. 2009. Hepatitis B virus (HBV) genotype determination by the COBAS AmpliPrep/COBAS TaqMan HBV Test, v2.0 in serum and plasma matrices. J Clin Virol 45:232–236. doi: 10.1016/j.jcv.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Berger A, Gohl P, Sturmer M, Rabenau HF, Nauck M, Doerr HW. 2010. Detection and quantitation of HBV DNA in miniaturized samples: multi centre study to evaluate the performance of the COBAS AmpliPrep/COBAS TaqMan hepatitis B virus (HBV) test v2.0 by the use of plasma or serum specimens. J Virol Methods 169:404–408. doi: 10.1016/j.jviromet.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Ohdan H, Mizunuma K, Tashiro H, Tokita D, Hara H, Onoe T, Ishiyama K, Shibata S, Mitsuta H, Ochi M, Nakahara H, Itamoto T, Asahara T. 2003. Intraoperative near-infrared spectroscopy for evaluating hepatic venous outflow in living-donor right lobe liver. Transplantation 76:791–797. doi: 10.1097/01.TP.0000074603.36553.BD. [DOI] [PubMed] [Google Scholar]
- 21.Sobao Y, Sugi K, Tomiyama H, Saito S, Fujiyama S, Morimoto M, Hasuike S, Tsubouchi H, Tanaka K, Takiguch M. 2001. Identification of hepatitis B virus-specific CTL epitopes presented by HLA-A*2402, the most common HLA class I allele in East Asia. J Hepatol 34:922–929. doi: 10.1016/S0168-8278(01)00048-4. [DOI] [PubMed] [Google Scholar]
- 22.Sobao Y, Tomiyama H, Sugi K, Tokunaga M, Ueno T, Saito S, Fujiyama S, Morimoto M, Tanaka K, Takiguchi M. 2002. The role of hepatitis B virus-specific memory CD8 T cells in the control of viral replication. J Hepatol 36:105–115. doi: 10.1016/S0168-8278(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 23.Tsuge M, Hiraga N, Takaishi H, Noguchi C, Oga H, Imamura M, Takahashi S, Iwao E, Fujimoto Y, Ochi H, Chayama K, Tateno C, Yoshizato K. 2005. Infection of human hepatocyte chimeric mouse with genetically engineered hepatitis B virus. Hepatology 42:1046–1054. doi: 10.1002/hep.20892. [DOI] [PubMed] [Google Scholar]
- 24.Stross L, Gunther J, Gasteiger G, Asen T, Graf S, Aichler M, Esposito I, Busch DH, Knolle P, Sparwasser T, Protzer U. 2012. Foxp3+ regulatory T cells protect the liver from immune damage and compromise virus control during acute experimental hepatitis B virus infection in mice. Hepatology 56:873–883. doi: 10.1002/hep.25765. [DOI] [PubMed] [Google Scholar]
- 25.Jung MC, Pape GR. 2002. Immunology of hepatitis B infection. Lancet Infect Dis 2:43–50. doi: 10.1016/S1473-3099(01)00172-4. [DOI] [PubMed] [Google Scholar]
- 26.Ando K, Moriyama T, Guidotti LG, Wirth S, Schreiber RD, Schlicht HJ, Huang SN, Chisari FV. 1993. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med 178:1541–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cote PJ, Toshkov I, Bellezza C, Ascenzi M, Roneker C, Ann Graham L, Baldwin BH, Gaye K, Nakamura I, Korba BE, Tennant BC, Gerin JL. 2000. Temporal pathogenesis of experimental neonatal woodchuck hepatitis virus infection: increased initial viral load and decreased severity of acute hepatitis during the development of chronic viral infection. Hepatology 32:807–817. doi: 10.1053/jhep.2000.17681. [DOI] [PubMed] [Google Scholar]
- 28.Shiokawa M, Takahashi T, Murakami A, Kita S, Ito M, Sugamura K, Ishii N. 2010. In vivo assay of human NK-dependent ADCC using NOD/SCID/gammac(null) (NOG) mice. Biochem Biophys Res Commun 399:733–737. doi: 10.1016/j.bbrc.2010.07.145. [DOI] [PubMed] [Google Scholar]
- 29.Ito R, Katano I, Kawai K, Hirata H, Ogura T, Kamisako T, Eto T, Ito M. 2009. Highly sensitive model for xenogenic GVHD using severe immunodeficient NOG mice. Transplantation 87:1654–1658. doi: 10.1097/TP.0b013e3181a5cb07. [DOI] [PubMed] [Google Scholar]
- 30.Ohbo K, Suda T, Hashiyama M, Mantani A, Ikebe M, Miyakawa K, Moriyama M, Nakamura M, Katsuki M, Takahashi K, Yamamura K, Sugamura K. 1996. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood 87:956–967. [PubMed] [Google Scholar]
- 31.Koyanagi Y, Tanaka Y, Kira J, Ito M, Hioki K, Misawa N, Kawano Y, Yamasaki K, Tanaka R, Suzuki Y, Ueyama Y, Terada E, Tanaka T, Miyasaka M, Kobayashi T, Kumazawa Y, Yamamoto N. 1997. Primary human immunodeficiency virus type 1 viremia and central nervous system invasion in a novel hu-PBL-immunodeficient mouse strain. J Virol 71:2417–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL. 1995. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol 154:180–191. [PubMed] [Google Scholar]
- 33.Greiner DL, Shultz LD, Yates J, Appel MC, Perdrizet G, Hesselton RM, Schweitzer I, Beamer WG, Shultz KL, Pelsue SC, Leif JH, Rajan TV. 1995. Improved engraftment of human spleen cells in NOD/LtSz-scid/scid mice as compared with C.B-17-scid/scid mice. Am J Pathol 146:888–902. [PMC free article] [PubMed] [Google Scholar]
- 34.Santantonio T, Jung MC, Miska S, Pastore G, Pape GR, Will H. 1991. High prevalence and heterogeneity of HBV preC mutants in anti-HBe-positive carriers with chronic liver disease in southern Italy. J Hepatol 13(Suppl 4):S78–S81. [DOI] [PubMed] [Google Scholar]
- 35.Nayersina R, Fowler P, Guilhot S, Missale G, Cerny A, Schlicht HJ, Vitiello A, Chesnut R, Person JL, Redeker AG, Chisari FV. 1993. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J Immunol 150:4659–4671. [PubMed] [Google Scholar]
- 36.Penna A, Chisari FV, Bertoletti A, Missale G, Fowler P, Giuberti T, Fiaccadori F, Ferrari C. 1991. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J Exp Med 174:1565–1570. doi: 10.1084/jem.174.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rehermann B, Fowler P, Sidney J, Person J, Redeker A, Brown M, Moss B, Sette A, Chisari FV. 1995. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med 181:1047–1058. doi: 10.1084/jem.181.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung MC, Schraut W, Santantonio T, Spengler U, Eichenlaub D, Eisenburg J, Zachoval R, Hoffmann R, Paumgartner G, Pastore G, Will H, Riethmuller G, Ziegler-Heitbrock H-WL. 1993. Increased frequency of CD8+ CD45R0+ memory T lymphocytes in acute hepatitis B virus infection. J Hepatol 18:295–300. doi: 10.1016/S0168-8278(05)80273-9. [DOI] [PubMed] [Google Scholar]
- 39.Reignat S, Webster GJ, Brown D, Ogg GS, King A, Seneviratne SL, Dusheiko G, Williams R, Maini MK, Bertoletti A. 2002. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med 195:1089–1101. doi: 10.1084/jem.20011723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penna A, Artini M, Cavalli A, Levrero M, Bertoletti A, Pilli M, Chisari FV, Rehermann B, Del Prete G, Fiaccadori F, Ferrari C. 1996. Long-lasting memory T cell responses following self-limited acute hepatitis B. J Clin Investig 98:1185–1194. doi: 10.1172/JCI118902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chisari FV, Ferrari C. 1995. Hepatitis B virus immunopathogenesis. Annu Rev Immunol 13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 42.Bertoletti A, Costanzo A, Chisari FV, Levrero M, Artini M, Sette A, Penna A, Giuberti T, Fiaccadori F, Ferrari C. 1994. Cytotoxic T lymphocyte response to a wild type hepatitis B virus epitope in patients chronically infected by variant viruses carrying substitutions within the epitope. J Exp Med 180:933–943. doi: 10.1084/jem.180.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. 2003. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med 197:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang Q, Bluestone JA. 2008. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol 9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. 2010. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 46.Baecher-Allan C, Viglietta V, Hafler DA. 2004. Human CD4+CD25+ regulatory T cells. Semin Immunol 16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Mitchison NA. 2004. T-cell-B-cell cooperation. Nat Rev Immunol 4:308–312. doi: 10.1038/nri1334. [DOI] [PubMed] [Google Scholar]
- 48.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. 1992. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science 257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 49.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, Nadler SG, Farber DL. 2006. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol 177:7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 50.Deppong CM, Bricker TL, Rannals BD, Van Rooijen N, Hsieh CS, Green JM. 2013. CTLA4Ig inhibits effector T cells through regulatory T cells and TGF-beta. J Immunol 191:3082–3089. doi: 10.4049/jimmunol.1300830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang M, Sun R, Wei H, Tian Z. 2013. Simultaneous knockdown of multiple ligands of innate receptor NKG2D prevents natural killer cell-mediated fulminant hepatitis in mice. Hepatology 57:277–288. doi: 10.1002/hep.25959. [DOI] [PubMed] [Google Scholar]
- 52.Bi J, Zhang Q, Liang D, Xiong L, Wei H, Sun R, Tian Z. 2014. T-cell Ig and ITIM domain regulates natural killer cell activation in murine acute viral hepatitis. Hepatology 59:1715–1725. doi: 10.1002/hep.26968. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Cao G, Zheng X, Wang J, Wei H, Tian Z, Sun R. 2013. CRACC-CRACC interaction between Kupffer and NK cells contributes to poly I:C/D-GalN induced hepatitis. PLoS One 8:e76681. doi: 10.1371/journal.pone.0076681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.