Abstract
Epstein-Barr virus (EBV) is a gammaherpesvirus, associated with infectious mononucleosis and various types of malignancy. We focused here on the BDLF4 gene of EBV and identified it as a lytic gene, expressed with early kinetics. Viral late gene expression of the BDLF4 knockout strain was severely restricted; this could be restored by an exogenous supply of BDLF4. These results indicate that BDLF4 is important for the EBV lytic replication cycle, especially in late gene expression.
TEXT
Epstein-Barr virus (EBV) is a human gammaherpesvirus that is present in >90% the world's population. EBV is associated with infectious mononucleosis and neoplasms such as Burkitt lymphoma, Hodgkin's lymphoma, posttransplant lymphoproliferative disease, nasopharyngeal carcinoma, and gastric carcinoma (1, 2).
The EBV BDLF4 gene is an open reading frame (ORF), located between BGLF1 and BDLF3.5. Sequence similarities suggest that it is conserved among the members of the betaherpesvirus and gammaherpesvirus subfamilies (UL92 of human cytomegalovirus [HCMV] and ORF31 of murine gammaherpesvirus 68 [MHV-68] and Kaposi's sarcoma-associated herpesvirus [KSHV]) but that it is not present in the alphaherpesviruses. It has been reported that KSHV ORF31, MHV-68 ORF31, HCMV UL92, and MCMV M92 are required for the efficient transcription of viral true late genes (3–7), but the EBV BDLF4 has not been characterized well.
BDLF4 expression in B95-8 cells with early kinetics.
We first prepared antiserum against EBV BDLF4 by immunizing rabbits with a glutathione S-transferase (GST)-fused BDLF4 protein expressed in bacteria. This anti-BDLF4 antiserum reacted with a protein of about ∼22 kDa in the lysate from B95-8 cells, treated with tetradecanoyl phorbol acetate (TPA), A23187, and sodium butyrate (T/A/B) for 48 h (Fig. 1A). The BDLF4 protein could be detected readily by 24 h, and expression was increased at 48 h (Fig. 1B). While BALF4 mRNA expression was clearly blocked by phosphonoacetic acid (PAA), the amount of BDLF4 was decreased only slightly by the inhibitor (Fig. 1C), suggesting that the BDLF4 gene is expressed with E kinetics.
FIG 1.
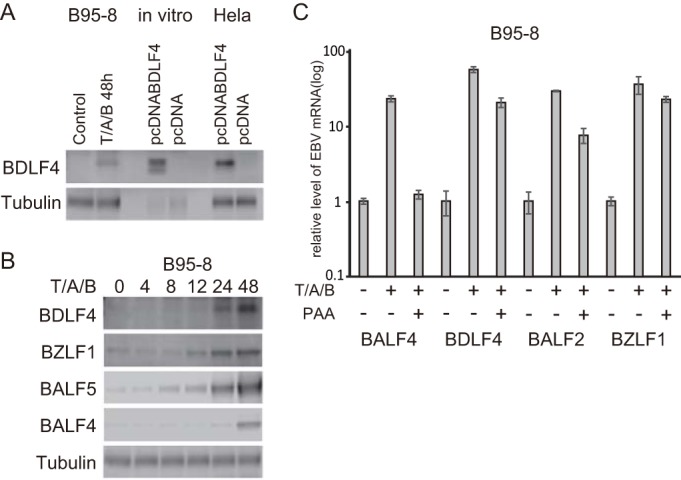
Identification of BDLF4 protein. (A) B95-8 cell lysate, BDLF4 protein synthesized in vitro, and BDLF4 expressed in HeLa cells were subjected to Western blotting. B95-8 cells were incubated with TPA (200 ng/ml), A23187 (0.5 μM), and sodium butyrate (5 mM) (T/A/B) to induce the lytic cycle. (B) Expression levels of BDLF4, BZLF1, BALF5 (Pol), and BALF4 (gB) in B95-8 cells were assessed by Western blotting at the indicated hours after lytic induction. (C) B95-8 cells were treated with PAA to examine the kinetics of BDLF4 expression. Lytic induction was carried out for 48 h (T/A/B) in the presence or absence of 400 μg/ml of PAA. Cells lysates were subjected to RT-PCR using BALF4 (gB), BDLF4, BALF2, and BZLF1 primers as described previously (24).
Generation of BDLF4 knockout virus by frameshifting.
To study the biological role of the BDLF4 gene in EBV lytic replication, we constructed a BDLF4-deficient recombinant virus and its revertant, as depicted in Fig. 2A. The integrity of the viral genomes was examined by restriction enzyme analysis, followed by agarose electrophoresis (Fig. 2B), and sequencing confirmed the intended one-nucleotide (nt) deletion.
FIG 2.
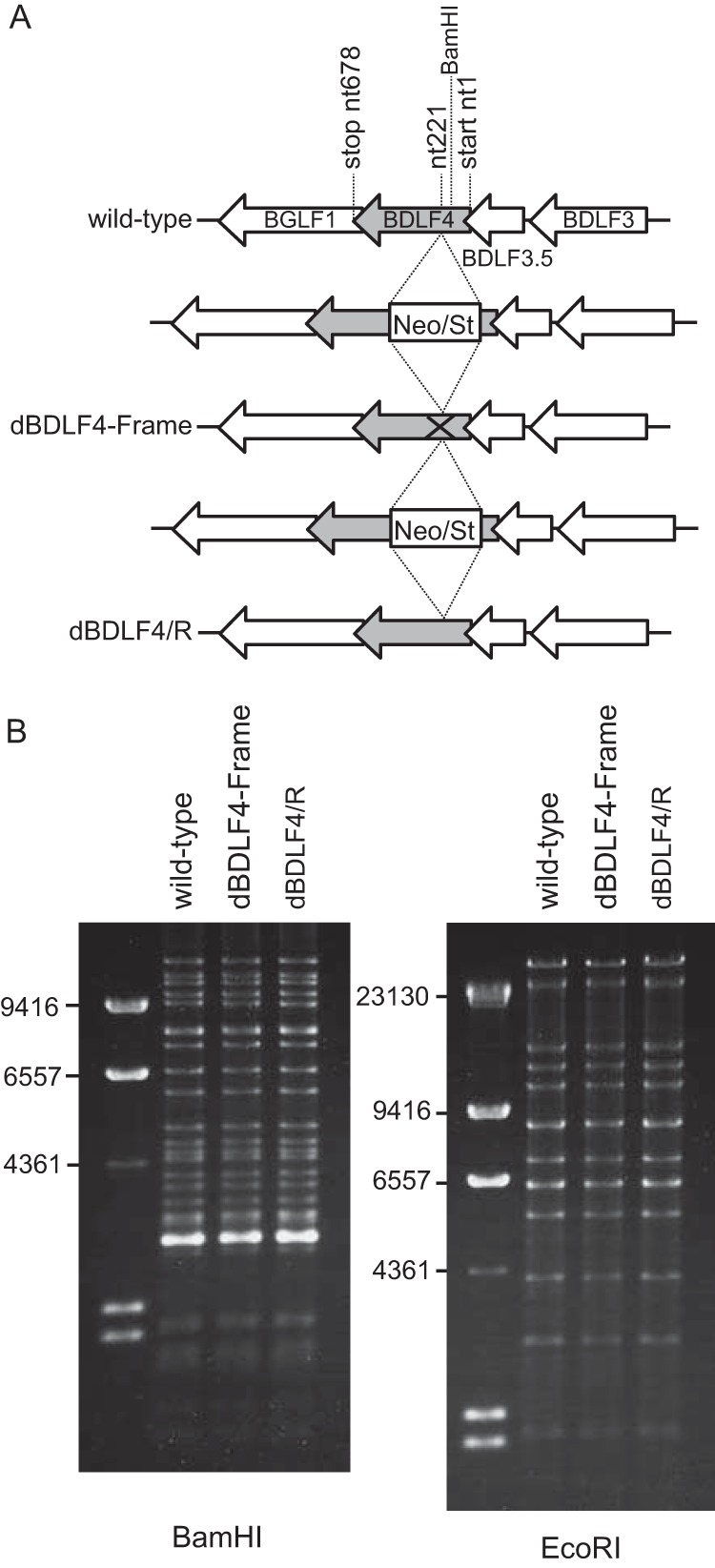
Construction of a point mutant of EBV BDLF4. (A) BDLF4 knockout EBV and its revertant virus were generated using a BAC system in Escherichia coli (25, 26). The neomycin resistance and streptomycin sensitivity genes (Neo/St) were inserted between nucleotides 220 (nt220) and nt221 of the BDLF4 gene. The Neo/St cassette was then replaced by a BDLF4 sequence with a one-nucleotide deletion at T (nt222) (dBDLF4-Frame). This one nucleotide excision caused frame shifting in the BDLF4 gene at residue 74 and creation of a new termination signal after synthesizing 12 nonsense, unrelated amino acids. The Neo/St cassette was again inserted and replaced with a wild-type BDLF4 sequence to make the revertant virus dBDLF4/R. (B) Electrophoresis of the recombinant viruses. EBV BAC DNAs were digested with BamHI or EcoRI and separated in an agarose gel.
Knockout of BDLF4 decreased viral L gene expression and progeny production.
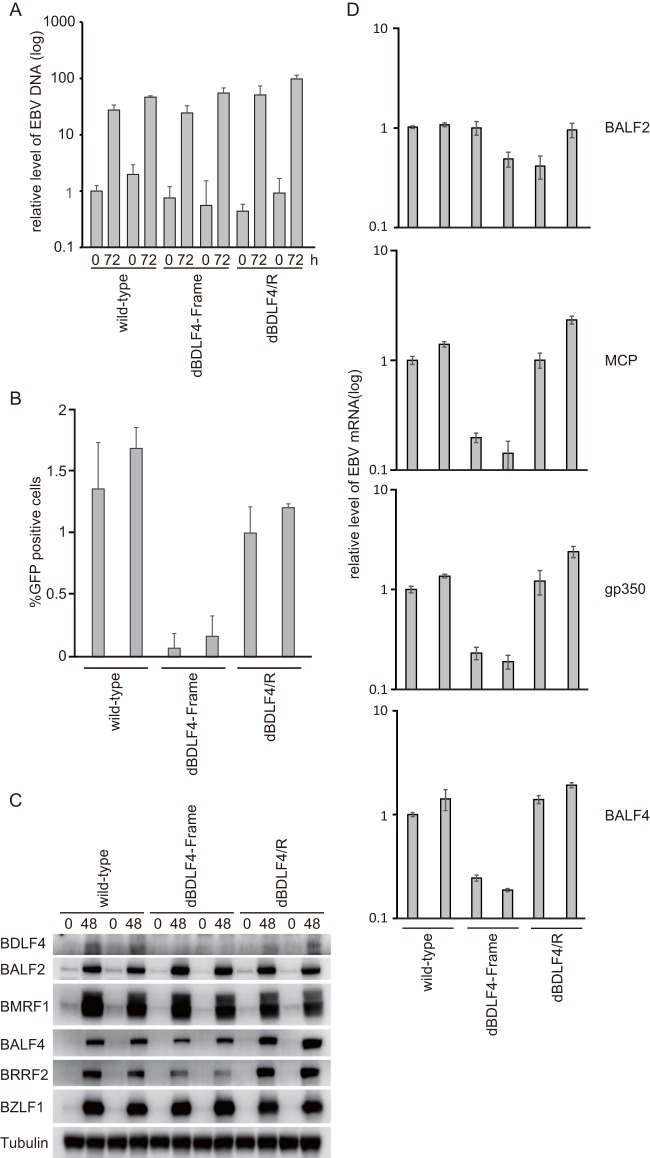
The recombinant EBV-bacterial artificial chromosome (BAC) DNAs were transfected into HEK293 cells, and cells stably and latently containing EBV DNAs were cloned by hygromycin selection. We found that viral DNA synthesis levels of the wild-type, frameshift mutant, and revertant viruses were comparable (Fig. 3A). Disruption of BDLF4 decreased virus yield to approximately 10% to 20% compared with levels seen with the wild-type and revertant viruses (Fig. 3B). BDLF4 protein was not detected in the cell lysate of the frameshift mutant (Fig. 3C). Wild-type, mutant, and revertant viruses exhibited comparable levels of expression of E genes (BALF2 and BMRF1), while the protein expression level of L genes (BALF4 [gB] and BRRF2 [8]) in the knockout virus-infected cells was slightly, but appreciably, lower. Additionally, expression of L genes was monitored by reverse transcription-PCR (RT-PCR). As expected, the mutation in BDLF4 markedly reduced expression of MCP (BcLF1), gp350 (BLLF1), and BALF4 (gB), whereas the E gene, BALF2, was not affected (Fig. 3D), suggesting that BDLF4 may be involved in L gene expression.
FIG 3.
DNA synthesis (A), progeny production (B), viral protein expression (C), and viral mRNA levels (D) in wild-type, BDLF4 mutant, and revertant viruses. (A) Viral DNA was prepared from HEK293 cells that carry the indicated recombinant EBV-BAC genomes at 0 or 72 h after transfection of the BZLF1 expression vector and was analyzed by quantitative real-time PCR. (B) Supernatants were collected at 72 h after transfection of pcDNA-BZLF1. Virus titers in 1 ml of supernatants were determined by examining the levels of green fluorescent protein (GFP) expression in Akata(-) cells after 2 days by fluorescence-activated cell sorter (FACS) analysis. (C) HEK293 cells were induced lytically by transfection of pcDNA-BZLF1. Whole-cell lysates were prepared at 0 and 48 h and analyzed by Western blotting using anti-BDLF4, anti-BALF2, anti-BMRF1, anti-BALF4 (gB), anti-BRRF2, anti-BZLF1, and anti-tubulin antibodies. (D) Real-time RT-PCR was carried out to determine expression of viral late genes (MCP [major capsid protein], gP350, and BALF4 [gB]) and an early gene. HEK293 cells carrying the indicated EBV genomes were transfected with pcDNA-BZLF1 and harvested after 72 h. As shown in this figure, two independent cell clones were tested for wild-type, dBDLF4-Frame, and dBDLF4/R. Bars represent the means and standard deviations of results from three independent experiments.
BDLF4 is responsible for viral L gene expression.
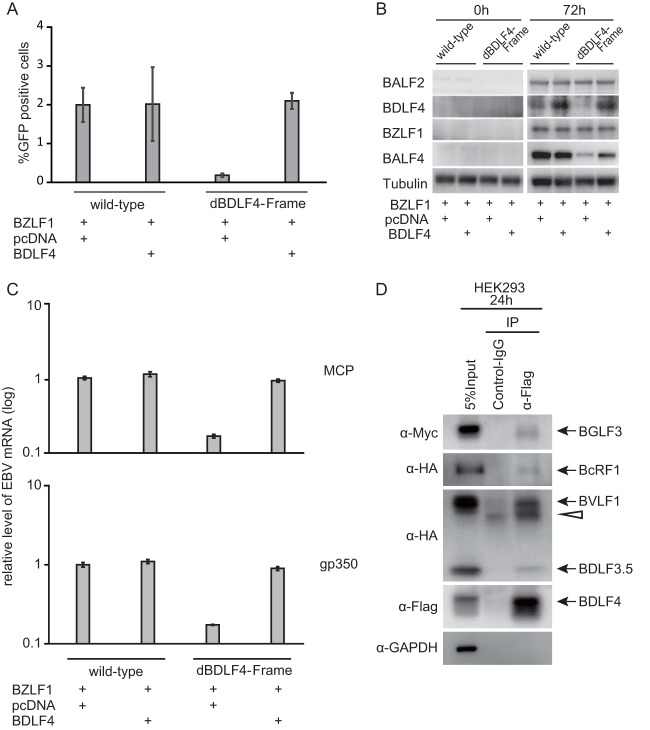
As shown in Fig. 4, we tested if exogenous supply of BDLF4 in trans could restore the reduced virus yield and L gene expression in BDLF4 mutant. In the point-mutated EBV, BDLF4 expression completely complemented the titer (Fig. 4A). The BALF4 protein level was also recovered (Fig. 4B, BALF4 rightmost lane). Other L gene mRNA levels (MCP, gp350) could also be restored by exogenous expression of BDLF4 (Fig. 4C).
FIG 4.
Loss of BDLF4 is responsible for the inhibition of late gene expression (A) HEK293 cells latently infected with EBV were transfected with the indicated expression vector and harvested after 96 h. After freeze-thawing and centrifugation, the supernatants were cultured with Akata(-) cells. FACS analysis was performed to count GFP-positive cells. (B) Levels of viral proteins. HEK293 cells with recombinant EBV were transfected with the indicated vector. Whole-cell lysates were prepared at 0 and 72 h and analyzed by Western blotting with anti-BALF2, anti-BDLF4, anti-BZLF1, anti-BALF4 (gB), and anti-tubulin. (C) Levels of viral late mRNAs. HEK293 cells with recombinant EBVs were transfected with the indicated vector. The mRNAs were prepared at 72 h and analyzed by real-time RT-PCR. (D) Association of BDLF4 with other late gene regulators. HEK293 cells were transfected with expression vectors for Flag-BDLF4, Myc-BGLF3, hemagglutinin-BcRF1 (HA-BcRF1), HA-BVLF1, and HA-BDLF3.5. Immunoprecipitation (IP) was carried out using anti-Flag antibody, and the results were detected by Western blotting using anti-Myc, anti-HA, and anti-Flag antibodies. Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a negative control. The arrowhead indicates immunoglobulin.
Because KSHV, MHV-68, and HCMV homologs of BVLF1, BcRF1, BDLF3.5, BDLF4, and BGLF3 have been reported to act together in L gene regulation (4–7, 9–18), we tested if BDLF4 interacted with these factors by immunoprecipitation (Fig. 4D). We confirmed the BGLF3, BcRF1, BVLF1, and BDLF3.5 proteins were coprecipitated with BDLF4 (Fig. 4D). These results strongly indicate the involvement of BDLF4 protein in the regulation of L gene expression in the lytic phase of EBV infection.
Currently, it is known that BVLF1, BcRF1, BDLF3.5, BDLF4, BGLF3, and BFRF2 gene products of EBV are essential for efficient L gene transcription as monitored by luciferase assays (19). Among these, knockout viruses of only BcRF1 and BFRF2 have been prepared and characterized (12, 19). In this study, we newly prepared a BDLF4 mutant and provided evidence that BDLF4 plays an important role in L gene transcription.
Historically, Serio and others found that EBV L genes carry a noncanonical TATA box, TATTAAA, which can function only after de novo viral DNA replication (20). Recently, in MHV-68, the DNA sequence required for the L gene promoter activation was mapped to a core element containing the TATT sequence and its neighboring sequences (21). The noncanonical TATA box is crucial for HCMV L gene expression, too (22).
The mechanisms regulating this noncanonical TATA box in betaherpesviruses and gammaherpesviruses have remained elusive, but accumulating evidence indicates that BcRF1 (or its homologs) binds to the noncanonical TATA box (10, 12, 23), providing specificity to the L promoters. Some other components of the complex are reported to interact with and recruit RNA polymerase II (Pol II) to enhance the transcriptional start of L genes (17, 19). It is also reported that HCMV UL79 protein also interacts with Pol II and enhances the elongation rate (15).
Because disruption of either of the trans-acting factors can prevent L gene expression, such an attenuated virus may be a suitable candidate strain for a therapeutic live vaccine (13). Instead, if a small-molecule inhibitor of the complex could be developed, such an inhibitor might be a useful candidate antiviral agent not only for EBV but also for KSHV, HCMV, and HHV-6/7.
ACKNOWLEDGMENTS
We are grateful to W. Hammerschmidt, H. J. Delecluse, T. Kanda, T. Tsurumi, S. Ohno, and H. Isomura for materials and scientific discussions.
This work was supported by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (to T.M. [30470165] and H.K. [25293109]) and the Ministry of Health, Labor and Welfare (to H.K. [H26-Nanchi-013]) and partly by the Takeda Science Foundation, the Kanae Foundation for Promotion of Medical Science, and the General Assembly of the Japanese Association of Medical Sciences (to T.M.).
REFERENCES
- 1.Young LS, Rickinson AB. 2004. Epstein-Barr virus: 40 years on. Nat Rev Cancer 4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 2.Murata T, Sato Y, Kimura H. 2014. Modes of infection and oncogenesis by the Epstein-Barr virus. Rev Med Virol 24:242–253. doi: 10.1002/rmv.1786. [DOI] [PubMed] [Google Scholar]
- 3.Chapa TJ, Perng YC, French AR, Yu D. 2014. Murine cytomegalovirus protein pM92 is a conserved regulator of viral late gene expression. J Virol 88:131–142. doi: 10.1128/JVI.02684-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia Q, Wu TT, Liao HI, Chernishof V, Sun R. 2004. Murine gammaherpesvirus 68 open reading frame 31 is required for viral replication. J Virol 78:6610–6620. doi: 10.1128/JVI.78.12.6610-6620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omoto S, Mocarski ES. 2014. Transcription of true late (gamma2) cytomegalovirus genes requires UL92 function that is conserved among beta- and gammaherpesviruses. J Virol 88:120–130. doi: 10.1128/JVI.02983-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song MJ, Hwang S, Wong WH, Wu TT, Lee S, Liao HI, Sun R. 2005. Identification of viral genes essential for replication of murine gamma-herpesvirus 68 using signature-tagged mutagenesis. Proc Natl Acad Sci U S A 102:3805–3810. doi: 10.1073/pnas.0404521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brulois K, Wong LY, Lee HR, Sivadas P, Ensser A, Feng P, Gao SJ, Toth Z, Jung JU. 2015. Association of Kaposi's sarcoma-associated herpesvirus ORF31 with ORF34 and ORF24 is Critical for late gene expression. J Virol 89:6148–6154. doi: 10.1128/JVI.00272-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe T, Tsuruoka M, Narita Y, Katsuya R, Goshima F, Kimura H, Murata T. 2015. The Epstein-Barr virus BRRF2 gene product is involved in viral progeny production. Virology 484:33–40. doi: 10.1016/j.virol.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Arumugaswami V, Wu TT, Martinez-Guzman D, Jia Q, Deng H, Reyes N, Sun R. 2006. ORF18 is a transfactor that is essential for late gene transcription of a gammaherpesvirus. J Virol 80:9730–9740. doi: 10.1128/JVI.00246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis ZH, Verschueren E, Jang GM, Kleffman K, Johnson JR, Park J, Von Dollen J, Maher MC, Johnson T, Newton W, Jager S, Shales M, Horner J, Hernandez RD, Krogan NJ, Glaunsinger BA. 2015. Global mapping of herpesvirus-host protein complexes reveals a transcription strategy for late genes. Mol Cell 57:349–360. doi: 10.1016/j.molcel.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong D, Wu NC, Xie Y, Feng J, Tong L, Brulois KF, Luan H, Du Y, Jung JU, Wang CY, Kang MK, Park NH, Sun R, Wu TT. 2014. Kaposi's sarcoma-associated herpesvirus ORF18 and ORF30 are essential for late gene expression during lytic replication. J Virol 88:11369–11382. doi: 10.1128/JVI.00793-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruffat H, Kadjouf F, Mariame B, Manet E. 2012. The Epstein-Barr virus BcRF1 gene product is a TBP-like protein with an essential role in late gene expression. J Virol 86:6023–6032. doi: 10.1128/JVI.00159-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isomura H, Stinski MF. 2013. Coordination of late gene transcription of human cytomegalovirus with viral DNA synthesis: recombinant viruses as potential therapeutic vaccine candidates. Expert Opin Ther Targets 17:157–166. doi: 10.1517/14728222.2013.740460. [DOI] [PubMed] [Google Scholar]
- 14.Isomura H, Stinski MF, Murata T, Yamashita Y, Kanda T, Toyokuni S, Tsurumi T. 2011. The human cytomegalovirus gene products essential for late viral gene expression assemble into prereplication complexes before viral DNA replication. J Virol 85:6629–6644. doi: 10.1128/JVI.00384-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perng YC, Campbell JA, Lenschow DJ, Yu D. 2014. Human cytomegalovirus pUL79 is an elongation factor of RNA polymerase II for viral gene transcription. PLoS Pathog 10:e1004350. doi: 10.1371/journal.ppat.1004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong E, Wu TT, Reyes N, Deng H, Sun R. 2007. Murine gammaherpesvirus 68 open reading frame 24 is required for late gene expression after DNA replication. J Virol 81:6761–6764. doi: 10.1128/JVI.02726-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu TT, Park T, Kim H, Tran T, Tong L, Martinez-Guzman D, Reyes N, Deng H, Sun R. 2009. ORF30 and ORF34 are essential for expression of late genes in murine gammaherpesvirus 68. J Virol 83:2265–2273. doi: 10.1128/JVI.01785-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Guindy A, Lopez-Giraldez F, Delecluse HJ, McKenzie J, Miller G. 2014. A locus encompassing the Epstein-Barr virus bglf4 kinase regulates expression of genes encoding viral structural proteins. PLoS Pathog 10:e1004307. doi: 10.1371/journal.ppat.1004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aubry V, Mure F, Mariame B, Deschamps T, Wyrwicz LS, Manet E, Gruffat H. 2014. Epstein-Barr virus late gene transcription depends on the assembly of a virus-specific preinitiation complex. J Virol 88:12825–12838. doi: 10.1128/JVI.02139-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serio TR, Cahill N, Prout ME, Miller G. 1998. A functionally distinct TATA box required for late progression through the Epstein-Barr virus life cycle. J Virol 72:8338–8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong-Ho E, Wu TT, Davis ZH, Zhang B, Huang J, Gong H, Deng H, Liu F, Glaunsinger B, Sun R. 2014. Unconventional sequence requirement for viral late gene core promoters of murine gammaherpesvirus 68. J Virol 88:3411–3422. doi: 10.1128/JVI.01374-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isomura H, Stinski MF, Kudoh A, Murata T, Nakayama S, Sato Y, Iwahori S, Tsurumi T. 2008. Noncanonical TATA sequence in the UL44 late promoter of human cytomegalovirus is required for the accumulation of late viral transcripts. J Virol 82:1638–1646. doi: 10.1128/JVI.01917-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wyrwicz LS, Rychlewski L. 2007. Identification of herpes TATT-binding protein. Antiviral Res 75:167–172. doi: 10.1016/j.antiviral.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Murata T, Narita Y, Sugimoto A, Kawashima D, Kanda T, Tsurumi T. 2013. Contribution of myocyte enhancer factor 2 family transcription factors to BZLF1 expression in Epstein-Barr virus reactivation from latency. J Virol 87:10148–10162. doi: 10.1128/JVI.01002-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delecluse HJ, Hilsendegen T, Pich D, Zeidler R, Hammerschmidt W. 1998. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc Natl Acad Sci U S A 95:8245–8250. doi: 10.1073/pnas.95.14.8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murata T, Isomura H, Yamashita Y, Toyama S, Sato Y, Nakayama S, Kudoh A, Iwahori S, Kanda T, Tsurumi T. 2009. Efficient production of infectious viruses requires enzymatic activity of Epstein-Barr virus protein kinase. Virology 389:75–81. doi: 10.1016/j.virol.2009.04.007. [DOI] [PubMed] [Google Scholar]