ABSTRACT
The life cycle of herpes simplex virus (HSV) has the potential to be further manipulated to yield novel, more effective therapeutic treatments. Recent research has demonstrated that HSV-1 can increase telomerase activity and that expression of the catalytic component of telomerase, telomerase reverse transcriptase (TERT), alters sensitivity to HSV-dependent apoptosis. Telomerase is a cellular enzyme that synthesizes nucleotide repeats at the ends of chromosomes (telomeres), which prevents shortening of the 3′ ends of DNA with each cell division. Once telomeres reach a critical length, cells undergo senescence and apoptosis. Here, we used a cell-permeable, reversible inhibitor of the telomerase enzyme, MST-312, to investigate telomerase activity during HSV infection. Human mammary epithelial cells immortalized through TERT expression and human carcinoma HEp-2 cells were infected with the KOS1.1 strain of HSV-1 in the presence of MST-312. MST-312 treatment reduced the number of cells displaying a cytopathic effect and the accumulation of immediate early and late viral proteins. Moreover, the presence of 20 μM to 100 μM MST-312 during infection led to a 2.5- to 5.5-log10 decrease in viral titers. MST-312 also inhibited the replication of HSV-2 and a recent clinical isolate of HSV-1. Additionally, we determined that MST-312 has the largest impact on viral events that take place prior to 5 h postinfection (hpi). Furthermore, MST-312 treatment inhibited virus replication, as measured by adsorption assays and quantification of genome replication. Together, these findings demonstrate that MST-312 interferes with the HSV life cycle. Further investigation into the mechanism for MST-312 is warranted and may provide novel targets for HSV therapies.
IMPORTANCE Herpes simplex virus (HSV) infections can lead to cold sores, blindness, and brain damage. Identification of host factors that are important for the virus life cycle may provide novel targets for HSV antivirals. One such factor, telomerase, is the cellular enzyme that synthesizes DNA repeats at the ends of chromosomes during replication to prevent DNA shortening. In this study, we investigate role of telomerase in HSV infection. The data demonstrate that the telomerase inhibitor MST-312 suppressed HSV replication at multiple steps of viral infection.
INTRODUCTION
Herpes simplex virus (HSV) is well known as the causative agent of cold sores and genital herpes (reviewed in reference 1). When the virus infects tissues other than the oral or genital mucosa, such as ocular and brain tissues, much more serious diseases occur. Initial HSV-1 infections generally occur in toddlers and young children, and over two-thirds of the U.S. population is infected by adulthood (2). Primary infections may be asymptomatic or lead to the formation of characteristic blistering lesions (e.g., on or around the oral mucosa). The host immune system is usually able to clear the virus from infected epithelial tissues. However, HSV-1 establishes a latent infection in neuronal ganglia servicing the site of initial infection and persists there for the lifetime of the infected individual. HSV-1 may then reactivate from its latent state, which leads to recurrent viral replication in the epithelium and the formation of new lesions. Although currently available antiviral agents that act against HSV polymerase (Pol) can limit lytic replication in epithelial tissues, they are unable to eliminate latent HSV infections. Improvement of treatment for HSV disease relies heavily on a better understanding of cellular factors controlling HSV-1 replication in general.
One such cellular factor is telomerase. Increased telomerase activity has been detected in cells infected with a variety of herpesviruses (3–11). There is evidence that HSV can mediate elevated telomerase activity during infection. Telomerase activity was found to be between 4 and 8 times greater in cells infected with a replication-defective mutant of HSV than in uninfected counterparts (3). This increase in activity was accompanied by enhanced telomerase reverse transcriptase (TERT) promoter activity.
Previously, we determined that the levels of TERT could determine the sensitivity to HSV-dependent apoptosis (HDAP). Specifically, when tumor suppressor pathways were restored in HeLa cells, expression of TERT sensitized the cells to HDAP (12). This finding suggested that telomerase activation was sufficient to confer sensitivity to HDAP in HeLa cells. The initial goal of this study was to determine whether telomerase was necessary for HDAP. To do this, we utilized a small-molecule inhibitor of the telomerase enzyme, MST-312 (13). Further experiments demonstrated the inhibition of HSV replication by MST-312 treatment.
MATERIALS AND METHODS
Cell lines and viruses.
hTERT-HME1 cells (Clontech) originated from primary human mammary epithelial cells and have been made to express the human catalytic portion of telomerase, hTERT. Vero (14) and HEp-2 (15, 16) cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum (FBS). hTERT-HME1 cells were grown in mammary epithelial cell growth medium (MEGM) (Lonza).
Wild-type strains of HSV-1 used in these experiments include KOS1.1, F, 17syn+, and the recent clinical isolate TC (17). The wild-type HSV-2 strain used in these experiments is strain G. The ICP27-null, proapoptotic recombinant virus vBSΔ27 (18) was used to investigate HSV-dependent apoptosis in this study. Viruses were generously provided by John A. Blaho (CUNY, New York, NY).
TRAP assay.
To measure the enzymatic activity of telomerase within cells, a quantitative PCR (qPCR)-based telomeric repeat amplification protocol (TRAP) assay was performed essentially as reported previously (19). In this assay, the telomerase from the cell lysates binds to a synthetic substrate (TS primer AAT CCG TCG AGC AGA GGT) and synthesizes telomeric repeats. The TRAP assay uses a SYBR green PCR master mix with the addition of TS and ACX [GCG CGC (CTT ACC)3 CTA AAC] primers to amplify the synthesized products (20).
Briefly, a confluent plate of cells was washed with phosphate-buffered saline (PBS), trypsinized, and collected by centrifugation. The supernatant was removed, the cell pellet was resuspended in PBS, and the number of cells in the suspension was determined via a hemacytometer. A suspension volume equivalent to 200,000 cells was removed, and cells were collected by centrifugation. The cells were lysed in 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) buffer. For all samples, the cell lysate equivalent to 1,000 cells was mixed with the TS primer (Integrated DNA Technologies), the ACX primer (Integrated DNA Technologies), SYBR green PCR core reagents (Applied Biosystems), and a single-stranded binding protein, T4 gene 32 protein (NEB), and the reaction mixture was incubated for 30 min at room temperature to allow telomerase to synthesize telomere repeats. Subsequently, the reaction mixtures were subjected to qPCR on a MiniOpticon PCR machine (95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min). All TRAP assays were run in quadruplicate. The relative telomerase activity was determined by comparison to a standard curve generated by TRAP assays performed by using 5-fold dilutions (5,000, 1,000, 200, 40, and 8 cell equivalents) of uninhibited hTERT-HME1 cell lysates.
Telomerase inhibitors.
MST-312 (EMD Millipore Chemicals) was diluted in dimethyl sulfoxide (DMSO) to a 10 mM concentration and stored at −20°C. Unless otherwise noted, DMSO (vehicle control) treatment groups were administered a volume of DMSO equivalent to the volume of the MST-312 stock used for the 100 μM MST-312 treatment group.
Microscopic imaging.
Microscopic images were obtained to observe cell morphology. Hoechst dye was added to the dishes (10 μg/ml) 1 h prior to harvest. Hoechst dye binds to chromatin and is visible under fluorescent light (4′,6-diamidino-2-phenylindole [DAPI] filter). Next, the cells were photographed by using a digital camera attached to an Axioskop Plus inverted fluorescence microscope (10× objective) under phase-contrast and/or fluorescent light.
Immunoblotting.
The cells were scraped into the medium by using a rubber policeman and collected by centrifugation. The cells were then lysed in radioimmunoprecipitation assay (RIPA) buffer, and the protein concentration was quantified via the Bradford assay, as previously reported (21). Twenty micrograms of each protein sample was separated by using SDS-polyacrylamide gel electrophoresis (PAGE). The gel was then transferred onto a nitrocellulose membrane and immunoblotted with antibodies specific to particular viral proteins (ICP4, ICP27, and VP22) or the apoptotic marker (poly(ADP-ribose) polymerase [PARP]), as previously described (21, 22). Previous studies demonstrated that antibodies to ICP27 and VP22 detect both the HSV-1 and HSV-2 versions of these proteins (23). Membranes were also immunoblotted with antibodies specific for cellular actin or tubulin proteins as loading controls. A Chemidoc XRS+ imaging system and Image Lab software version 5.2 (Bio-Rad) were used to scan the blots and quantify the density of bands. Viral protein band densities were first normalized to that of the loading control. Subsequently, the values for the bands in the lanes with HSV plus DMSO were set to 1.0, and the relative densities (RDs) of all other bands are represented relative to this value.
Titer determinations.
Unless otherwise specified, viral titers were determined via methods described previously (24). Briefly, infected-cell monolayers were scraped off the dish into the medium. Sterile milk was added to each virus sample to reach a final concentration of 50%. Virus was released from the samples by using a sonicator. The infectious-virus levels were measured by using plaque assays. Briefly, the samples were diluted in DMEM at dilutions ranging from 10−1 to 10−7. The diluted virus samples were then added to confluent dishes of Vero cells and incubated for 2 h at 37°C before removal and replacement with a medium containing human immunoglobulin (2.5 μg/ml). The dishes were then incubated for 48 to 72 h at 37°C. The medium from the dishes was removed, and the dishes were rinsed twice with PBS. Methanol was added, and the cells were incubated for 5 min to fix the cells. Next, Giemsa stain (10%) was added to each dish. After 20 min, dishes were rinsed with tap water. After the dishes dried, the plaques were counted by visual inspection. Dilutions yielding between 20 and 200 plaques per dish were used for quantification. All titer assays were performed in triplicate.
Viral RNA levels.
Total RNA from triplicate infections performed with 24-well dishes was harvested at 3 to 6 h postinfection (hpi) by using the Absolutely RNA Microprep kit (Agilent), according to the manufacturer's instructions, with the following modifications. The amount of lysis buffer used was doubled in order to avoid viscosity issues. RNA samples were quantified by using a NanoDrop 8000 instrument (Thermo Scientific). Purified RNA was stored at −80°C until the time of cDNA synthesis. Equivalent amounts of RNA (500 to 750 ng) were reverse transcribed to cDNA by using an Affinity Script QPCR cDNA synthesis kit (Agilent) and the pT primer, according to the manufacturer's instruction. Duplicate qPCRs were performed on each replicate by using a SYBR green PCR core kit (Applied Biosystems) and actin, ICP0, ICP27, or Pol primers essentially as previously described (25). Serial dilutions of a cDNA sample from HSV-1-infected cells were used as a standard curve. qPCR was also performed on no-RT reaction mixtures for each sample as a control for contaminating viral DNA. Quantitative PCR was performed on a MiniOpticon PCR machine (Bio-Rad), using the following protocol: 95°C for 2 min and 44 cycles of 95°C for 15 s, 55°C for 25 s, and 72°C for 35 s, followed by a melt curve from 65°C to 95°C. Values for all samples were normalized to values for cellular actin expression by dividing the relative viral RNA level by the actin RNA level for each replicate. The highest normalized value was used as a calibrator, and all other values are expressed as a fraction of the value for the calibrator sample.
Adsorption assay.
An adsorption assay was carried out essentially as described previously by MacLean (26). Briefly, HEp-2, hTERT-HME1, or Vero cells were seeded into 6-well plates at 900,000 cells per well. The following day, 100 PFU of KOS1.1 was added to monolayers, and cells were incubated for 0 to 240 min at 37°C (HEp-2 or hTERT-HME1 cells) or 4°C (Vero cells) in the presence or absence of the inhibitor. At the appropriate time points, the virus solution was removed from the monolayers, and cells were washed extensively with sterile PBS. Subsequently, PBS was replaced with sterile medium containing 2.5 μg/ml pooled human immunoglobulin. After 48 to 72 h (depending on the cell line), monolayers were fixed with methanol and stained with Giemsa stain. Plaques were counted via light microscopy. These assays were performed in triplicate.
Penetration assay.
A penetration assay was carried out essentially as described previously by MacLean (26). Briefly, Vero cells were seeded into 6-well plates at 1 × 106 cells per well. The following day, 260 PFU of KOS1.1 was added to monolayers, and cells were incubated for 60 min at 4°C. The virus solution was removed, and monolayers were washed twice with cold PBS. Prewarmed medium containing 100 μM MST-312 or an equivalent volume of DMSO was added to the monolayers, and the monolayers were incubated at 37°C for 0, 15, 30, 45, 60, or 90 min. Subsequently, the drug-containing medium was removed, and cells were treated with citric acid buffer at pH 3 for 3 min at room temperature to inactivate the virus that bound but had not penetrated the cell membrane. Controls were similarly treated with PBS. Next, buffers were removed, and monolayers were washed twice with PBS. Growth medium containing 2.5 μg/ml pooled human immunoglobulin was added to cultures, and plates were placed into a 37°C incubator. After 72 h, monolayers were fixed with methanol and stained with Giemsa stain. Plaques were counted via light microscopy. These assays were performed in triplicate.
Viral genome replication assay.
HEp-2 cells were grown in triplicate and infected with HSV-1 at a multiplicity of infection (MOI) of 5. At 1 hpi, virus and medium were removed, and fresh medium containing 20 to 100 μM MST-312 or a volume of DMSO equivalent to 100 μM MST-312 was added to cell cultures. At 18 hpi, cells were harvested from monolayers via trypsinization and combined with cells collected from the culture medium. DNA was isolated from infected cells by using a QIAamp DNA Blood minikit (Qiagen), according to the manufacturer's instructions. DNA levels in samples were quantified by using a NanoDrop 8000 instrument (Thermo Scientific) to ensure DNA isolation efficiency. Equivalent volumes of DNA (5 μl) were analyzed via qPCR in duplicate reactions using a SYBR green PCR core kit (Applied Biosystems) and UL44 primers (sense primer GGGTATAAATTCCGGAAGGGG and antisense primer CTGCGAGGGATCGGCTAGCG) or α4 primers (sense primer GCCCGGGCGCTGCTTGTTCTCC and antisense primer CGTCCGCCGTCGCAGCCGTATC) (27). qPCR was performed with a MiniOpticon PCR machine (Bio-Rad), using the following protocol: 95°C for 10 s and 44 cycles of 95°C for 30 s and 60°C for 1 min, followed by a melt curve from 65°C to 95°C. The relative starting quantity for each sample was determined by comparison to a standard curve generated by qPCR assays performed using 10-fold serial dilutions (1, 10−1, 10−2, 10−3, 10−4, and 10−5) of untreated infected-cell lysates. The average value for the DMSO-treated groups was set to 1, and all values are represented relative to this value.
Statistical analysis.
Statistical analysis was performed by using Microsoft Excel 2010. Two-tailed, equal-variance Student t tests were performed. P values of ≤0.05 were considered significant.
RESULTS
MST-312 is a synthetic compound with moieties related to epigallocatechin gallate (EGCG). EGCG was previously reported to specifically inhibit the telomerase enzyme (28, 29). Previous studies demonstrated that MST-312 can suppress telomerase activity in in vitro telomere repeat amplification protocol (TRAP) assays (30). In addition, long-term treatment (80 population doublings) of U937 human leukemia cells caused a shortening of telomere length and a reduced growth rate, indicating that it has activity on cells in culture. Furthermore, primary ependymoma cancer cells treated for 72 h with MST-312 displayed reduced cell viability and a decreased proliferative index (13). We set out to use MST-312 to investigate the role of telomerase in the HSV-1 life cycle.
In our first series of experiments, we sought to determine whether MST-312 would be a potent suppressor of telomerase activity in our system. To accomplish this, MST-312 was added to the lysate of hTERT-HME1 cells. This cell line was generated by expressing hTERT in primary mammary epithelial cells and has been shown to support the HSV-1 life cycle. The telomerase activity in the hTERT-HME1 cell lysates with or without MST-312 was quantified by using a qPCR-based TRAP assay (31). DMSO was used as a vehicle control. Treatment with 20 and 40 μM MST-312 reduced telomerase activity by >97% (Fig. 1). Treatment with 70 and 100 μM MST-312 reduced telomerase activity to levels below the threshold of detection. At these concentrations, there appears to be a biphasic response to the drug rather than a linear dose-dependent effect. Nevertheless, we conclude that MST-312 is a potent inhibitor of telomerase activity in our experimental system.
FIG 1.

MST-312 inhibits telomerase activity in hTERT-HME1 cell lysates. Telomerase activity was measured by using a qPCR-based TRAP assay. Between 20 and 100 μM MST-312 or DMSO was added to hTERT-HME1 lysates at the time of assay. Relative quantitation was accomplished by comparing the threshold cycle values of samples to those of standards consisting of serial dilutions of hTERT-HME1 lysates (1,000, 200, 40, 8, and 0 cell equivalents). All assays with samples and standards were performed in quadruplicate, and SYBR green dye was used for detection. # indicates levels below the threshold of detection. Error bars represent standard deviations.
Our next goal was to determine whether MST-312 would be toxic to cells in our experimental system. Although MST-312 was previously shown to induce toxicity in other cell lines, this required exposure periods of 72 h (13). In our experiments, the maximum time of MST-312 exposure was 24 h. To test the cellular toxicity of this exposure, HEp-2 and hTERT-HME1 cells were exposed to concentrations of between 2 and 100 μM MST-312. DMSO was used as a vehicle control. Twenty-four hours following drug addition, the cells were harvested by trypsinization, and cell death was measured by a trypan blue exclusion assay. The percent cell death was <6% for all treatment groups for HEp-2 cells (Fig. 2A and B). The percent cell death was <23% for all hTERT-HME1 treatments (Fig. 2C and D). These numbers were similar to those for the DMSO controls. Therefore, MST-312 appeared to have a modest influence on cell survival.
FIG 2.
Levels of cell death in MST-312-treated cells are similar to those in DMSO-treated control cells. MST-312 was added to the medium of HEp-2 (A and B) or hTERT-HME1 (C and D) cells at concentrations of between 2 and 10 μM (A and C) or between 20 and 100 μM (B and D). DMSO was added to control cells at a volume equivalent to 10 μM (A and C) or 100 μM (B and D) MST-312. After 18 h of drug exposure, the cells were collected by trypsinization, and percent cell death was assessed by trypan blue staining. Bars represent the averages of data for triplicate samples. Error bars denote the standard deviations for samples.
MST-312 inhibits viral cytopathic effects and protein accumulation.
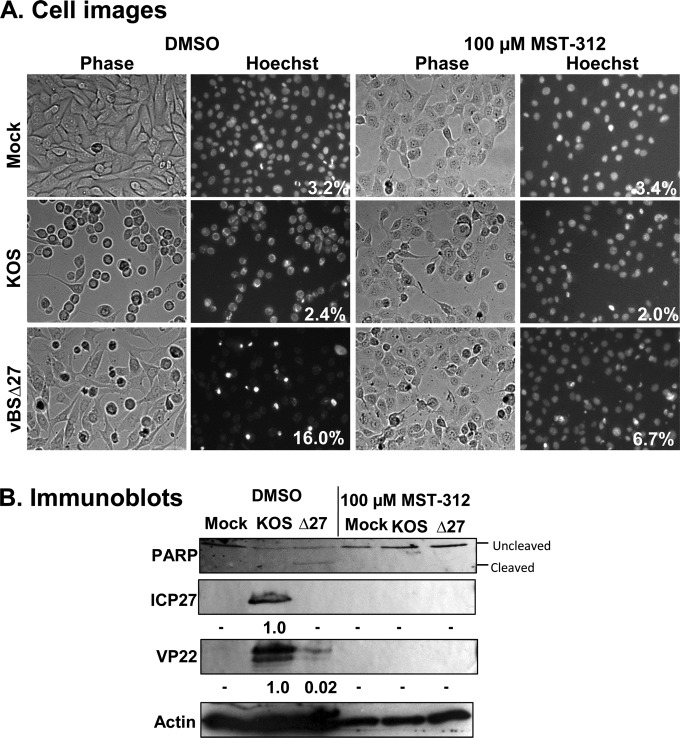
Our previous study determined that hTERT overexpression sensitized epithelial cells to HSV-dependent apoptosis, indicating that it was sufficient for this effect (12). We initially set out to use MST-312 to determine whether telomerase activity was required for sensitization to HSV-dependent apoptosis. To accomplish this, hTERT-HME1 cells were infected with wild-type HSV-1 strain KOS1.1 and the proapoptotic ICP27-null virus vBSΔ27 at an MOI of 10. One hour prior to infection, hTERT-HME1 cells were treated with MST-312 (100 μM) or the DMSO vehicle control, and this inhibitor concentration was maintained until harvest. At 18 hpi, microscopic images were captured, and the cells were harvested for immunoblotting for the caspase substrate PARP. Additionally, immunoblot analysis was used to detect the presence of specific viral proteins. This was done by using antibodies specific for the immediate early protein ICP27 and the late protein VP22.
KOS1.1-infected cells displayed a rounding of cell membranes and chromatin marginalization, which are classic features of a cytopathic effect (CPE). In contrast, vBSΔ27-infected cells displayed membrane blebbing and chromatin condensation, which are indicators of apoptosis (32). MST-312 treatment reduced the percentage of vBSΔ27-infected cells displaying apoptotic morphologies (16% to 6.7%) (Fig. 3A). With the addition of MST-312, vBSΔ27-infected cells also displayed decreased PARP cleavage, demonstrating decreased levels of caspase activation (Fig. 3B). Therefore, MST-312 inhibited apoptosis in HSV-1-infected cells. Unexpectedly, the inhibitor also decreased the number of KOS1.1-infected cells displaying a cytopathic effect. In addition, the accumulation of the viral proteins ICP27 and VP22 was decreased to nondetectable levels, demonstrating inhibition of representative immediate early and late viral proteins. Together, these results suggested that this compound may suppress HSV-1 replication. Therefore, the suppression of HSV-1-dependent apoptosis by MST-312 may be explained by an effect on HSV-1 replication in general rather than an apoptosis-specific event. Nevertheless, the dramatic alteration of virus infection by MST-312 led us to further explore its antiviral activities and the mechanisms behind these effects.
FIG 3.
MST-312 inhibits HSV-1-dependent cytopathic effects, apoptosis, and viral protein accumulation. hTERT-HME1 cells were infected with HSV-1 KOS1.1 (KOS) or vBSΔ27 (Δ27) at an MOI of 10 with the addition of 100 μM MST-312 or DMSO. At 18 hpi, Hoechst dye (10 μg/ml) was added, and photographs were taken under phase (Phase) and fluorescent (Hoechst) light. These cells were then harvested, and samples were subjected to immunoblot analysis. (A) Microscopic images showing cytopathic effects and apoptotic morphologies in infected cells. Cytopathic effects and apoptosis were determined by observation of the plated cells under a fluorescence microscope. Apoptosis was calculated as a percentage and is displayed in the bottom right corners of the Hoechst panels. (B) Immunoblotting of cell lysates using antibodies specific for ICP27, VP22, and PARP. The migration of the full-length form (uncleaved) and the product of caspase-dependent PARP cleavage (cleaved) are indicated. Numbers below viral protein blots represent normalized protein band densities relative to the band density for KOS1.1-infected DMSO-treated control cells. − indicates density values that are below the threshold of detection.
Next, we set out to determine whether there was a dose-dependent impact of MST-312 treatment on HSV-1 replication. For these experiments, hTERT-HME1 cells were infected with wild-type HSV-1 strain KOS1.1 at an MOI of 10. Concentrations of between 20 and 100 μM MST-312 were added to the medium of hTERT-HME1 cells 1 h prior to infection and maintained until the time of harvest. At 18 hpi, microscopic images were captured to assess CPE. As expected, the majority of the KOS1.1-infected cells without MST-312 treatment displayed a rounded and refractile appearance and chromatin marginalization and had lost their attachment to surrounding cells, indicative of cells undergoing HSV-1-induced CPE (Fig. 4A). In contrast, the proportion of the cells displaying CPE was reduced when infection was performed in the presence of 20 or 40 μM MST-312. Cells infected in the presence of 100 μM MST-312 displayed morphologies indistinguishable from those of mock-infected, MST-312-treated cells. The cells were lysed, and the lysates were immunoblotted for the HSV-1 immediate early proteins ICP4 and ICP27 and the late protein VP22 (Fig. 4B). A modest reduction of ICP27 protein accumulation (∼23 to 44%) was observed for all MST-312 treatment groups. Additionally, VP22 levels were decreased between 55 and 62% when cells were treated with 70 and 100 μM MST-312. In contrast, ICP4 protein accumulation was not reduced upon MST-312 treatment in these cells.
FIG 4.
MST-312 reduces HSV-1 protein accumulation and cytopathic effects in hTERT-HME1 cells in a dose-dependent manner. hTERT-HME1 cells were infected with HSV-1 KOS1.1 (KOS), at an MOI of 10, in the presence MST-312 at the indicated concentrations or DMSO. At 18 hpi, Hoechst dye was added to the medium (10 μg/ml), and cells were photographed and harvested for immunoblot assays. (A) Cytopathic effect determined by observation by phase (Phase) and fluorescence (Hoechst) microscopy. (B) Immunoblot analysis of viral protein accumulation using antibodies specific for ICP4, ICP27, and VP22. Numbers below viral protein blots represent normalized protein band densities relative to the band density for KOS1.1-infected DMSO-treated control cells. − indicates density values that are below the threshold of detection.
To determine whether this inhibition would be observed in cells more typically used for HSV experiments, we performed a similar experiment using HEp-2 cells. Specifically, HEp-2 cells were infected in the presence or absence of 100 μM MST-312. At 18 hpi, cells were assessed for morphological changes and viral protein accumulation. There did not appear to be an obvious change in CPE in the presence of MST-312 (Fig. 5A). However, this may be difficult to determine given the difficulty in visualizing CPE in this cell line with phase-contrast and nonconfocal UV microscopy. Additionally, ICP27 and VP22 protein accumulation was reduced by MST-312 treatment. ICP4 levels remained unaffected. These results led us to conclude that MST-312 is able to perturb the HSV-1 life cycle in multiple cell types.
FIG 5.
MST-312 inhibits HSV-1 protein accumulation in HEp-2 cells. HEp-2 cells were infected with HSV-1 KOS1.1, at an MOI of 10, in the presence of 100 μM MST-312 or DMSO. At 18 hpi, Hoechst dye was added to the medium (10 μg/ml), and cells were photographed and harvested for immunoblot analyses. (A) Cytopathic effects in KOS1.1-infected cells determined by observation of cells with a fluorescence microscope. (B) Immunoblot analysis of the cell lysates using antibodies specific for ICP4, ICP27, and VP22. Numbers below the viral protein blots represent normalized protein band densities relative to the band density for KOS1.1-infected DMSO-treated control cells. − indicates density values that are below the threshold of detection.
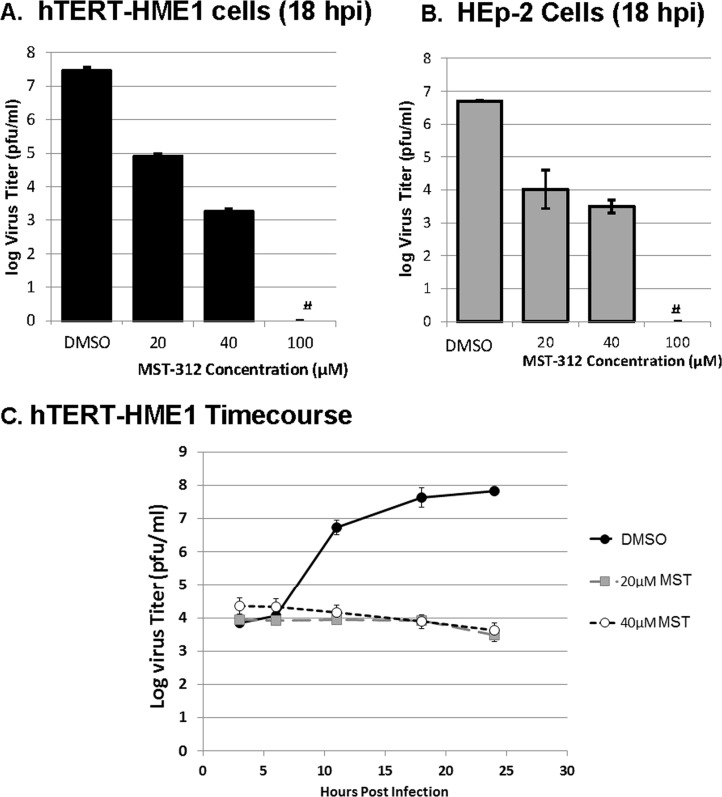
The next series of experiments was performed to determine the effect of MST-312 on the production of HSV-1 infectious progeny. To accomplish this, hTERT-HME1 (Fig. 6A) or HEp-2 (Fig. 6B) cells were infected with KOS1.1 at an MOI of 10 in the presence or absence of 20 to 100 μM MST-312. At 18 hpi, the cells were harvested, virus was released by sonication, and the levels of infectious virus for each treatment were quantified by using a plaque assay. MST-312 conferred a dose-dependent inhibition of the production of HSV-1 infectious progeny. The 100 μM MST-312 dose suppressed virus production to levels below the threshold of detection (≤10 PFU/ml). Additionally, a single-cycle growth experiment was used to evaluate the growth kinetics of infections performed in the presence of MST-312. hTERT-HME1 cells were infected with HSV-1 KOS1.1 in the presence of the DMSO control or 20 μM or 40 μM MST-312. At time points between 3 and 24 hpi, cells were lysed, and the levels of infectious virus were determined by plaque assays. Infections performed in the presence of 20 μM or 40 μM MST-312 displayed reductions in infectious-virus production at all times at or after 12 hpi (Fig. 6C). Together, these results demonstrate that MST-312 inhibits the life cycle of the KOS1.1 strain of HSV-1.
FIG 6.
MST-312 inhibits virus replication in a dose-dependent manner. (A and B) HSV-1 titers in hTERT-HME1 (A) and HEp-2 (B) cells in the presence or absence of MST-312 at the indicated concentrations. hTERT-HME1 cells and HEp-2 cells were pretreated with different concentrations of the inhibitor or the appropriate volume of the vehicle control (DMSO) for 1 h and then infected with HSV-1 KOS1.1 (KOS) in the presence of MST-312 or DMSO. Samples were harvested at 18 hpi by scraping cells from the dish. The cells were combined with medium, and the mixture was sonicated to free virus particles from the cells. The level of infectious virus was quantified by a plaque assay on Vero cells. The graph depicts the averages ± standard deviations of data from triplicate samples. # indicates titers that were at or below the threshold of detection. (C) Single-cycle growth curves of hTERT-HME1 cells in the presence or absence of 20 or 40 μM MST-312. hTERT-HME1 cells were treated with the inhibitor or the appropriate volume of the vehicle control (DMSO) at the time of infection. The cells were then infected with HSV-1 KOS1.1 in the presence of MST-312 or DMSO. Samples were harvested at 3, 6, 11, 18, and 24 hpi by scraping cells from the dish. The cells were combined with medium, and the mixture was sonicated to free virus particles from the cells. The level of infectious virus was quantified via a plaque assay on Vero cells. The graph depicts averages ± standard deviations of data from triplicate samples.
Our next goal was to investigate whether the effect of MST-312 was a virus strain-dependent phenomenon. Therefore, we performed similar experiments using multiple strains of HSV-1, including the recent clinical isolate TC (17), and one strain of HSV-2 (Fig. 7 and 8). HEp-2 cells were infected with HSV-1 strain KOS1.1, F, 17syn+, or TC or HSV-2 strain G at an MOI of 10 in the presence or absence of 40 μM (Fig. 7A and 8A) or 100 μM (Fig. 7B and 8B) MST-312. At 18 hpi, the cells were harvested and analyzed for virus replication or viral protein accumulation. In all cases, the presence of 40 μM MST-312 led to a >4-log10 reduction in HSV-1 titers. Additionally, treatment with 100 μM reduced the accumulation of VP22. For HSV-2, MST-312-treated cells produced virus at a 3-log10-lower level than the vehicle controls. Reductions in level of the late protein VP22 were also observed with MST-312 treatment. The data shown in Fig. 3 to 8 led us to conclude that MST-312 inhibits HSV replication and that this is applicable to multiple cell types and HSV strains.
FIG 7.
MST-312 interferes with the life cycles of various HSV-1 strains. HEp-2 cells were infected with laboratory-derived HSV-1 strains (KOS1.1, F, and 17syn+) and a recent clinical isolate of HSV-1 (TC) at an MOI of 5 in the presence of 40 μM MST-312 or an equivalent volume of DMSO. (A) Samples were harvested at 18 hpi, and level of infectious virus was quantified via a plaque assay on Vero cells. The graph depicts averages ± standard deviations of data from triplicate samples. (B) HEp-2 cells were infected with HSV-1 strains (KOS, F, 17syn+, and TC) at an MOI of 5 in the absence or presence of 100 μM MST-312. At 18 hpi, cells were harvested, and lysates were immunoblotted for viral proteins. Numbers below viral protein blots represent normalized protein band densities relative to the band density for HSV-infected DMSO-treated control cells for each strain.
FIG 8.
MST-312 interferes with the HSV-2 life cycle. HEp-2 cells were infected with either an HSV-1 (KOS1.1) or HSV-2 (G) strain at an MOI of 5 in the presence of 40 μM MST-312 or an equivalent volume of DMSO. (A) Samples were harvested at 18 hpi, and the level of infectious virus was quantified via a plaque assay on Vero cells. The graph depicts averages ± standard deviations of data from triplicate samples. (B) HEp-2 cells were infected with HSV-2 strain G at an MOI of 5 in the absence or presence of 100 μM MST-312. At 18 hpi, cells were harvested, and lysates were immunoblotted for viral proteins. Numbers below viral protein blots represent normalized protein band densities relative to the band density for the DMSO-treated controls for HSV-1 or HSV-2.
To rule out that the effect on virus titers was due to a carryover of MST-312 during virus harvest and preparation, the effect of MST-312 treatment on cell-associated virus was determined. Specifically, HEp-2 cells were infected with KOS1.1 in the presence of 40 or 100 μM MST-312 or an equivalent volume of DMSO. At 18 hpi, the infected monolayers were removed by scraping the monolayers from the dish. The cells were pelleted by centrifugation, and the medium was removed. Cell pellets were washed twice in saline and resuspended in sterile milk. Virus was harvested from these cells by sonication. Plaque assays were performed on Vero cells to quantify virus levels (Fig. 9). Treatment with 40 μM MST-312 reduced the average levels of HSV-1 by >4 log10 units. Treatment with 100 μM MST-312 reduced the virus titers by 5 log10 units. The magnitude of suppression by MST-312 in this experiment was similar to that observed in experiments in which MST-312-containing medium was combined with cells during stock preparation. Together, these data led us to conclude that the suppressive effect of MST-312 is unlikely due to drug carryover in the viral stocks.
FIG 9.
MST-312 inhibits replication of cell-associated HSV-1. HEp-2 cells were infected with HSV-1 at an MOI of 5 in the presence of 40 or 100 μM MST-312 or an equivalent volume of DMSO. At 18 hpi, the cells were scraped into the medium and collected by centrifugation. The medium was removed and discarded. The cell pellet was washed in cold PBS and resuspended in drug-free medium. Sterile milk was added, and the samples were sonicated to release virus particles. The level of infectious virus was quantified by a plaque assay on Vero cells. The graph depicts averages ± standard deviations of data from triplicate samples.
The HSV-1 life cycle is a highly coordinated process (33, 34). The time frame in which the virus proceeds through each of the steps in its virus life cycle during infections of model cell lines is well established (35). The initial stage of infection takes place at between 0 and 2 hpi. During this time, the virus attaches to and enters the host cell, transfers its viral genome to the nucleus, and initiates expression of the first set of viral genes, the immediate early (IE) genes. The peak expression of these genes occurs at 2 to 4 hpi. At 4 hpi, the next set of viral genes, the early (E) genes, is detected. Peak expression of the early viral genes occurs at between 6 and 12 hpi. The products of many of these genes are responsible for replication of the viral genome. Viral genome replication initiates at 3 hpi, and the accumulation of nascent viral genomes proceeds in a nearly exponential fashion until 15 hpi. Expression of the final set of viral genes, the late (L) genes, peaks between 6 and 8 hpi. A single round of HSV-1 replication in cultured cells is complete by 18 hpi. In our studies, we took advantage of this clearly delineated timeline of viral infection to elucidate initial information on the stage of the life cycle on which MST-312 is acting. For these experiments, 100 μM MST-312 was added to the medium of hTERT-HME1 cells at between 0 and 11 hpi. At 18 hpi, images were captured to detect CPE (Fig. 10A), and the cells were lysed for measurement of progeny virion production (Fig. 10B). In general, reductions in the extent of CPE were evident when MST-312 was added to the infected cells at between 0 and 8 hpi. A portion of the KOS1.1-infected cells treated with MST-312 at 3 hpi appeared to be undergoing CPE. Although the morphology of the infected cells was altered when MST-312 was added at 0 and 1 h postinfection, the morphological changes appeared to be distinct from CPE because these cells possessed a less-rounded appearance than that of DMSO-treated, KOS1.1-infected cells and lacked chromatin marginalization. These changes were similar to the morphology observed for MST-312-treated, mock-infected cells (Fig. 4) and therefore are likely to be independent of virus infection. A 5-log10 suppression of virus replication was observed when MST-312 was added before 6 hpi. A smaller reduction in virus replication was evident when MST-312 was added at 6 and 8 hpi. When MST-312 was added at 11 hpi, no effect on virus replication was apparent.
FIG 10.
MST-312 reduces HSV-1 cytopathic effects and viral replication when added prior to 5 h postinfection. hTERT-HME1 cells were infected with HSV-1 KOS1.1 (KOS) at an MOI of 10 with the addition of 100 μM MST-312 or DMSO at 0, 1, 3, 6, 8, and 11 h postinfection. (A) At 18 hpi, Hoechst dye (10 μg/ml) was added, and photographs were taken under phase (Phase) and fluorescent (Hoechst) light. (B) This experiment was repeated, and these cells were sonicated to free virus particles from the cells. The level of infectious virus was quantified via a plaque assay on Vero cells. (C) hTERT-HME1 cells infected with HSV-1 KOS1.1 at an MOI of 10 with the addition of 100 μM MST-312 or DMSO at 0, 3, 4, 5, and 6 h postinfection. Infected cells were sonicated to free virus particles from the cells, and the level of infectious virus was quantified via a plaque assay on Vero cells. # indicates virus titers that are below the threshold of detection. (D) hTERT-HME1 cells were infected with HSV-1 KOS1.1 at an MOI of 10 in the presence of 100 μM MST-312 or DMSO. At 3 or 6 hpi, the infected cells were harvested, and RNA was extracted. cDNA synthesis was performed, and levels were quantified via qPCR using primers specific to the ICP27, ICP0, or Pol open reading frame. Relative quantities were calculated by comparing the sample values to a standard curve generated from serial dilutions of cDNA from HEp-2 cells infected with HSV-1. Primers for cellular actin cDNA were used for normalization. Graphs depict averages ± standard deviations of data from triplicate samples.
To further delineate the point at which MST-312 is acting, another experiment in which MST-312 was added at between 3 and 6 hpi was performed. Specifically, 100 μM MST-312 was added to the medium of HSV-1-infected hTERT-HME1 cells at 0, 3, 4, 5, and 6 hpi. DMSO was added to one culture at 0 hpi as a control. At 18 hpi, the virus was harvested from the cells and medium and quantified via a plaque assay (Fig. 10C). Similar to the experiments described above (Fig. 10B), when MST-312 was added to infected cells at 3 hpi, an ∼5-log10 suppression of virus replication was observed. The addition of MST-312 at 4 hpi led to a >3-log10 inhibition of virus titers. However, when MST-312 was added at 5 hpi, a smaller reduction in virus replication was evident. This level of suppression was similar to that observed when MST-312 was added at 6 hpi. In this experiment, the addition of MST-312 to the cultures at 0 hpi led to a reduction in virus titers to levels below the threshold of detection. The reason for this difference from the data presented in Fig. 10B is unclear; however, it may be due to an overall lower titer of recovered virus in this experiment.
To calibrate the virus life cycle events occurring during this time frame, levels of representative immediate early and early HSV-1 mRNAs in the presence or absence of 100 μM MST-312 were quantified at 3 and 6 hpi (Fig. 10D). At 3 hpi, ICP27 and ICP0 mRNAs were detected in control infections. MST-312 treatment reduced mRNA accumulation to <0.25 relative mRNA units. At 6 hpi, ICP0 and Pol viral mRNAs were detected. MST-312 treatment reduced the RNA levels of these genes to <0.1 relative units. Thus, an impact of MST-312 can be observed at the early stages of infection. Together, these results demonstrate that MST-312 interferes with HSV-1 life cycle events that occur prior to 11 hpi and that the greatest impact likely occurs prior to 5 hpi.
Early events in the HSV life cycle that correlate with the 0- to 5-hpi time frame most greatly affected by MST-312 include virion attachment and entry. To further investigate this, a series of adsorption assays were performed. In each assay, HEp-2 or hTERT-HME1 cell monolayers were incubated with HSV-1 with or without MST-312 for 0 to 240 min to allow adsorption. The monolayers were then extensively washed to remove unbound virions, and the remaining bound virus was detected by allowing the formation of plaques on the monolayers. Between 15 and 45% less adsorption was observed for hTERT-HME1 and HEp-2 cells treated with 20 μM MST-312 (Fig. 11A and B). Viral adsorption in hTERT-HME1 cells was reduced from 95% with DMSO controls to 20% with 100 μM MST-312 (Fig. 11C). An even greater effect on HEp-2 cells was present, in which viral adsorption was decreased from 90% to <5% (Fig. 11D). This finding is consistent with MST-312 altering the ability of HSV-1 to adsorb to the surface of permissive cells. However, because the assay was performed at 37°C, it is possible that the effect was due to MST-312's inhibition of a very early postattachment step in the life cycle. To omit this possibility, the experiment was repeated by using cold-tolerant Vero cells. This allowed the infection to be performed at 4°C, where virion attachment occurs, but steps beyond this point are not kinetically favorable. Under these conditions, 20 μM MST-312 reduced plaque formation by 63% (Fig. 12A). At 100 μM MST-312, the level of plaque formation was below the threshold of detection (1 PFU; 99% reduction in plaque formation) (Fig. 12B). It is interesting to note that the adsorption assay performed at 4°C led to a lower level of virus in MST-312-treated cells than in the adsorption assay performed at 37°C. As a result of the decreased temperature, the kinetics of the process of viral attachment are likely reduced, which could explain the further decreased viral attachment at reduced temperatures. Alternatively, this difference could be due to differences between cell lines.
FIG 11.
MST-312 inhibits HSV-1 plaque formation in an adsorption assay. hTERT-HME1 (A and C) or HEp-2 (B and D) monolayers were incubated with 100 to 200 PFU of HSV-1 KOS1.1 in the presence of 20 μM (A and B) or 100 μM (C and D) MST-312 at 37°C. Equivalent volumes of DMSO were used for control experiments. At time points between 15 and 240 min post-virus addition, the monolayers were rinsed extensively to remove unbound virus and overlaid with medium containing pooled human immunoglobulin. Forty-eight hours to 72 h later, the monolayers were fixed and stained with Giemsa stain to detect plaque formation. The number of plaques was counted by visual inspection and verified by microscopy. The percent adsorbed virus was calculated as (number of PFU at a given time point/average number of PFU at the peak time point) × 100. Graphs represent averages ± standard deviations of data from triplicate samples.
FIG 12.
MST-312 inhibits HSV-1 plaque formation in an adsorption assay at 4°C. Vero cell monolayers were incubated with 100 PFU of HSV-1 KOS1.1 in the presence of 20 μM (A) or 100 μM (B) MST-312 at 4°C. Equivalent volumes of DMSO were used for control experiments. At time points between 15 and 90 min post-virus addition, the monolayers were rinsed extensively to remove unbound virus and overlaid with medium containing pooled human immunoglobulin. Seventy-two hours later, the monolayers were fixed and stained with Giemsa stain to detect plaque formation. The number of plaques was counted by visual inspection and verified by microscopy. The percent adsorbed virus was calculated as (number of PFU at a given time point/average number of PFU at the peak time point) × 100. Graphs represent averages ± standard deviations of data from triplicate samples.
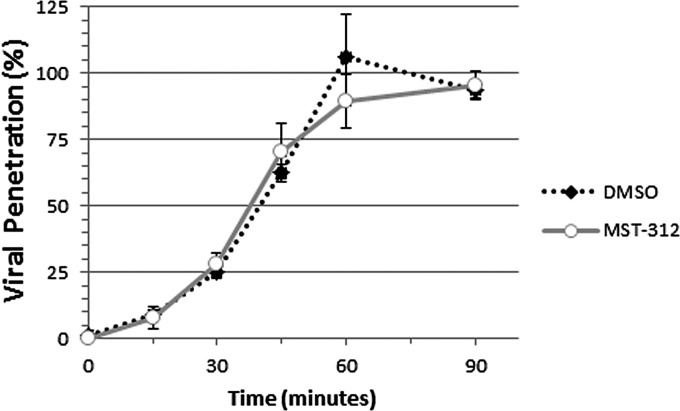
To further determine the point of entry at which MST-312 acts on HSV replication, a penetration assay was performed. Specifically, the virus was allowed to bind to monolayers of Vero cells at 4°C in the absence of MST-312. Subsequently, the cultures were shifted to a temperature of 37°C in the presence of 100 μM MST-312 or an equivalent volume of DMSO and incubated for between 0 and 90 min. The cells were then treated with citric acid buffer to inactivate virions that had attached to but not penetrated the plasma membrane or PBS as a control. The cultures were washed, drug-free medium was added, and virus penetration was quantified by determining plaque formation in the citric acid-treated groups (Fig. 13). The level of virus penetration in MST-312-treated cultures was similar to that of the DMSO-treated groups at all time points studied. These data led us to conclude that MST-312 most likely inhibits the attachment step in the entry process of HSV-1.
FIG 13.
Levels of HSV-1 penetration in MST-312-treated cultures are similar to those in DMSO-treated control cells. Vero cell monolayers were incubated with 260 PFU of HSV-1 KOS1.1 for 60 min at 4°C. Subsequently, the virus solution was removed. Prewarmed medium containing 100 μM MST-312 or an equivalent volume of DMSO was added to the monolayers, and cells were incubated for time points between 0 and 90 min at 37°C. Subsequently, monolayers were treated with citrate buffer (pH 3) or PBS for 3 min at room temperature. (For each time point, three monolayers were treated with citric acid buffer, and three were treated with PBS.) The monolayers were rinsed extensively to remove unbound virus and overlaid with medium containing pooled human immunoglobulin. Seventy-two hours later, the monolayers were fixed and stained with Giemsa stain to detect plaque formation. The number of plaques was counted by visual inspection and verified by microscopy. The percent virus penetration was calculated for each time point as (number of PFU in citric acid-treated monolayers/average number of PFU in PBS-treated monolayers) × 100. Graphs represent averages ± standard deviations of data from triplicate samples.
The data described above provide evidence that MST-312 interferes with the attachment of HSV-1, which inhibits viral mRNA accumulation and, ultimately, viral replication in this manner. At a 100 μM concentration, blocking of attachment alone may explain the effect of MST-312 on virus replication. In contrast, at a concentration of 20 μM MST-312, the 40 to 60% reduction in viral attachment (Fig. 11 and 12) cannot completely explain the 2.5-log10 reduction in virus titers (Fig. 6). These data led us to investigate potential postentry mechanisms of MST-312 virus inhibition.
The effect of MST-312 on DNA replication was investigated to determine whether the compound was playing a role in inhibiting this postentry process. In order to separate the effects of MST-312 on attachment from postentry effects, HEp-2 cell infections were initiated in the absence of any drug treatment (MOI of 10). At 1 hpi, the virus-containing medium was removed from the cells and replaced with fresh medium containing 20 to 100 μM MST-312, phosphonoacetic acid (PAA), or DMSO. At 18 hpi, samples were harvested, and DNA was collected. SYBR green-based qPCR was performed by using primers specific for regions of the genome corresponding to the UL44 gene. The initial qPCR experiments indicated that the DNA levels of mock-infected and PAA-treated, HSV-1-infected cells were <1 × 10−6 relative genome equivalents (data not shown). Significant reductions (200- to 10,000-fold) in relative DNA levels were seen in samples treated with 20 to 100 μM MST-312 compared to the DMSO control, indicating that in the presence of MST-312, HSV-1 DNA replication is impaired (Table 1). However, there did not appear to be a clear trend in the magnitude of the reduction in DNA replication for concentrations of 20 to 100 μM. From these data, we conclude that MST-312 suppresses viral genome replication.
TABLE 1.
HSV-1 genome accumulation in the presence of MST-312a

HEp-2 cells were grown in triplicate and infected with HSV-1 at an MOI of 5. At 1 hpi, virus was removed, and 20 to 100 μM MST-312 or a volume of DMSO equivalent to 100 μM MST-312 was added to cell cultures. At 18 hpi, cells were harvested, and DNA was isolated by using a QIAamp DNA Blood minikit. Equivalent volumes of isolated DNA and primers specific for the UL44 genomic region of HSV-1 were used for duplicate qPCRs (27).
The average value of the DMSO replicates was set to 1, and all other values are represented relative to this average.
Statistically significant value compared to that of the DMSO control (P < 0.0001) based on Student's t test.
Together, the data described above indicate that at a concentration of 20 μM, MST-312 has a modest effect on viral attachment and immediate early gene expression but a dramatic impact on DNA replication and the production of infectious virions (Table 2). At 100 μM concentrations, MST-312 has a stronger effect on viral attachment and gene expression. However, DNA replication inhibition was observed even when 100 μM MST-312 was added at a postattachment stage (1 hpi) (Table 2). This indicates that there are multiple anti-HSV-1 mechanisms utilized by MST-312.
TABLE 2.
Effects of MST-312 on HSV-1 replication
| Assay | % reduction of HSV-1 replication compared to that in untreated infected cells |
|
|---|---|---|
| 20 μM MST-312 | 100 μM MST-312 | |
| Adsorption at 37°C | 41–50 | 90 |
| Adsorption at 4°C | 57 | BTDa |
| Viral titer | >99 | >99 |
| DNA accumulation | >99 | >99 |
BTD, below the threshold of detection (1 PFU; 99% reduction).
DISCUSSION
The results presented here demonstrate that MST-312 inhibits the replication of herpes simplex viruses. This effect of MST-312 on HSV replication was initially discovered during our attempts to analyze the role of telomerase in HSV-dependent apoptosis. Although the telomerase inhibitor MST-312 reduced the level of HSV-dependent apoptosis, it is likely that this is due to a general effect on HSV-1 replication rather than a specific effect on apoptosis processes. Here, we show that MST-312 dramatically suppressed the ability of several strains of HSV-1 and an HSV-2 strain to produce progeny virions. These results indicate that the effect of MST-312 on HSV is broadly applicable. Furthermore, the observation that MST-312 had a similar effect on the replication of a recent clinical isolate of HSV-1 implies biological relevance. The data presented in Fig. 10 indicate that MST-312 must be added to cultured cells prior to 5 hpi to have a maximal effect on virus replication. Furthermore, the addition of the compound at 11 hpi did not alter virus replication. These results point to MST-312 affecting an early step in the virus life cycle. As shown in Fig. 11 and 12, MST-312 can suppress the ability of HSV to form plaques when MST-312 is added during binding. This result could be due to two possible scenarios: (i) MST-312 directly inhibits the ability of the virus to bind to its host cells, or (ii) MST-312 irreversibly inhibits the virions during binding such that they cannot complete their life cycles. Both of these explanations are consistent with MST-312 acting on the virus life cycle prior to 5 hpi.
The target for MST-312's antiviral activities is not yet defined. MST-312 is a chemical analogue of epigallocatechin gallate (EGCG), which is a natural compound found in the tea plant Camellia sinensis (13). That report described the synthesis of MST-312 and demonstrated that it has a 50% inhibitory concentration (IC50) of 0.67 μM for the inhibition of telomerase, compared to an IC50 of 1 μM for EGCG. MST-312 was also shown in that report to inhibit growth and reduce the telomere length of U937 cells with 60 to 90 days of treatment. Since that time, MST-312 has been utilized to reduce telomerase activities and inhibit the proliferation of medullary thyroid cancer (36), lung cancer (37), and ependymoma (30) cells. The data shown in Fig. 1 demonstrate that MST-312 can inhibit the activity of telomerase in the lysates of cells utilized for these experiments. Therefore, the effects of MST-312 on HSV replication and viral apoptosis could be linked to its effects on the telomerase enzyme. This compound appears to be relatively nontoxic to cultured cells at concentrations shown to dramatically reduce virus titers; therefore, it is unlikely to be acting solely through killing of infected cells prior to virion release. Moreover, the previously reported effects of MST-312 on cancer cell proliferation due to telomere shortening were observed with much longer exposure times (>72 h) than the treatment times required for antiviral activity (1 to 24 h). Perhaps, the virus utilizes telomerase activity in some portion of its life cycle. This is consistent with the variety of viral infections associated with altered telomerase activity levels (38), including HSV (3). Our results are consistent with a role for telomerase activation early in infection. Interestingly, HSV-1 has been shown to activate p38 mitogen-activated protein kinase (MAPK) in an ICP27-dependent manner (39, 40). This kinase can downregulate telomerase activity by inhibiting hTERT transcription (41). Therefore, it is possible that optimal HSV replication relies on the appropriate timing of telomerase activity. However, recent preliminary data generated in our laboratory suggest that inhibition of telomerase alone is insufficient to confer the dramatic antiviral activities observed in MST-312-treated infected cells (our unpublished data).
An additional or alternative mechanism whereby MST-312 may inhibit viral replication is through a mechanism similar to that of EGCG. EGCG has been reported to possess antiviral effects against HIV, HSV, hepatitis C virus, and adenovirus through multiple mechanisms (42). Isaacs et al. recently described studies in which HSV-1 virions that were exposed to EGCG for 1 h at 37°C displayed a 3-log10 reduction in plaque-forming ability (43). This reduction was accompanied by viral envelope damage. Additionally, incubation of purified gB and gD proteins with EGCG resulted in complexes with an aberrantly high apparent molecular weight. The possibility that MST-312 affects the envelope of virions would be consistent with our observation that MST-312 can suppress HSV-1 replication when it is added during the attachment phase of infection. However, one difference from MST-312 was that the addition of EGCG to HSV-infected cultures at 1 hpi did not inhibit virus replication. As shown in Fig. 10 and Table 1, MST-312 had a dramatic impact on HSV-1 replication when it was added at either 1 or 3 hpi. Therefore, it is possible that MST-312 possesses a novel antiviral activity that is distinct from those of both telomerase inhibition and EGCG. Side-by-side comparisons of the effects of EGCG and MST-312 on HSV infections would help to clarify the potential shared and distinct antiviral mechanisms of these compounds.
ACKNOWLEDGMENTS
We thank Aloysius Klingelhutz (University of Iowa) for expert advice and protocols for the TRAP assays.
These studies were supported by grants from the Iowa Osteopathic Education and Research Fund and the Iowa Science Foundation.
REFERENCES
- 1.Whitley RJ, Roizman B. 2009. Herpes simplex viruses, p 409–436. In Richman DD, Whitley RJ, Hayden FG (ed), Clinical virology, 3rd ed ASM Press, Washington, DC. [Google Scholar]
- 2.Schillinger JA, Xu F, Sternberg MR, Armstrong GL, Lee FK, Nahmias AJ, McQuillan GM, Louis ME, Markowitz LE. 2004. National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976-1994. Sex Transm Dis 31:753–760. doi: 10.1097/01.olq.0000145852.43262.c3. [DOI] [PubMed] [Google Scholar]
- 3.Yang CT, Song J, Bu X, Cong YS, Bacchetti S, Rennie P, Jia WW. 2003. Herpes simplex virus type-1 infection upregulates cellular promoters and telomerase activity in both tumor and nontumor human cells. Gene Ther 10:1494–1502. doi: 10.1038/sj.gt.3302005. [DOI] [PubMed] [Google Scholar]
- 4.Bellon M, Nicot C. 2008. Regulation of telomerase and telomeres: human tumor viruses take control. J Natl Cancer Inst 100:98–108. doi: 10.1093/jnci/djm269. [DOI] [PubMed] [Google Scholar]
- 5.Fragnet L, Blasco MA, Klapper W, Rasschaert D. 2003. The RNA subunit of telomerase is encoded by Marek's disease virus. J Virol 77:5985–5996. doi: 10.1128/JVI.77.10.5985-5996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harnack U, Lehmann C, Matthes E, Pecher G. 2001. Up-regulation of telomerase activity in herpesvirus saimiri immortalized human T-lymphocytes. Anticancer Res 21:3969–3972. [PubMed] [Google Scholar]
- 7.Knight JS, Cotter MA II, Robertson ES. 2001. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus transactivates the telomerase reverse transcriptase promoter. J Biol Chem 276:22971–22978. doi: 10.1074/jbc.M101890200. [DOI] [PubMed] [Google Scholar]
- 8.Pagnini U, De Martino L, Montagnaro S, Diodato A, Longo M, Pacelli F, Pisanelli G, Iovane G. 2006. Bovine herpesvirus type 1 (BHV-1) up-regulates telomerase activity in MDBK cells. Vet Microbiol 113:231–236. doi: 10.1016/j.vetmic.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Straat K, Liu C, Rahbar A, Zhu Q, Liu L, Wolmer-Solberg N, Lou F, Liu Z, Shen J, Jia J, Kyo S, Bjorkholm M, Sjoberg J, Soderberg-Naucler C, Xu D. 2009. Activation of telomerase by human cytomegalovirus. J Natl Cancer Inst 101:488–497. doi: 10.1093/jnci/djp031. [DOI] [PubMed] [Google Scholar]
- 10.Terrin L, Dal Col J, Rampazzo E, Zancai P, Pedrotti M, Ammirabile G, Bergamin S, Rizzo S, Dolcetti R, De Rossi A. 2008. Latent membrane protein 1 of Epstein-Barr virus activates the hTERT promoter and enhances telomerase activity in B lymphocytes. J Virol 82:10175–10187. doi: 10.1128/JVI.00321-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma SC, Borah S, Robertson ES. 2004. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus up-regulates transcription of human telomerase reverse transcriptase promoter through interaction with transcription factor Sp1. J Virol 78:10348–10359. doi: 10.1128/JVI.78.19.10348-10359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen ML, Kraft RM, Aubert M, Goodwin E, DiMaio D, Blaho JA. 2007. p53 and hTERT determine sensitivity to viral apoptosis. J Virol 81:12985–12995. doi: 10.1128/JVI.01485-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seimiya H, Oh-hara T, Suzuki T, Naasani I, Shimazaki T, Tsuchiya K, Tsuruo T. 2002. Telomere shortening and growth inhibition of human cancer cells by novel synthetic telomerase inhibitors MST-312, MST-295, and MST-1991. Mol Cancer Ther 1:657–665. [PubMed] [Google Scholar]
- 14.Rhim JS, Schell K, Creasy B, Case W. 1969. Biological characteristics and viral susceptibility of an African green monkey kidney cell line (Vero). Proc Soc Exp Biol Med 132:670–678. doi: 10.3181/00379727-132-34285. [DOI] [PubMed] [Google Scholar]
- 15.Nelson-Rees WA, Zhdanov VM, Hawthorne PK, Flandermeyer RR. 1974. HeLa-like marker chromosomes and type-A variant glucose-6-phosphate dehydrogenase isoenzyme in human cell cultures producing Mason-Pfizer monkey virus-like particles. J Natl Cancer Inst 53:751–757. [DOI] [PubMed] [Google Scholar]
- 16.Chen TR. 1988. Re-evaluation of HeLa, HeLa S3, and HEp-2 karyotypes. Cytogenet Cell Genet 48:19–24. doi: 10.1159/000132579. [DOI] [PubMed] [Google Scholar]
- 17.Yedowitz JC, Blaho JA. 2005. Herpes simplex virus 2 modulates apoptosis and stimulates NF-kappaB nuclear translocation during infection in human epithelial HEp-2 cells. Virology 342:297–310. doi: 10.1016/j.virol.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 18.Soliman TM, Sandri-Goldin RM, Silverstein SJ. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J Virol 71:9188–9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wege H, Chui MS, Le HT, Tran JM, Zern MA. 2003. SYBR green real-time telomeric repeat amplification protocol for the rapid quantification of telomerase activity. Nucleic Acids Res 31:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim NW, Wu F. 1997. Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic Acids Res 25:2595–2597. doi: 10.1093/nar/25.13.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen ML, Kraft RM, Blaho JA. 2005. African green monkey kidney Vero cells require de novo protein synthesis for efficient herpes simplex virus 1-dependent apoptosis. Virology 336:274–290. doi: 10.1016/j.virol.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Sanfilippo CM, Blaho JA. 2006. ICP0 gene expression is a herpes simplex virus type 1 apoptotic trigger. J Virol 80:6810–6821. doi: 10.1128/JVI.00334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowles RN, Yedowitz JC, Blaho JA. 2008. Reconsideration of viral protein immunoblotting for differentiation of human herpes simplex viruses. Diagn Microbiol Infect Dis 62:167–176. doi: 10.1016/j.diagmicrobio.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaho JA, Morton ER, Yedowitz JC. 2005. Herpes simplex virus: propagation, quantification, and storage. Curr Protoc Microbiol Chapter 14:Unit 14E.1. doi: 10.1002/9780471729259.mc14e01s00. [DOI] [PubMed] [Google Scholar]
- 25.Cotter CR, Nguyen ML, Yount JS, Lopez CB, Blaho JA, Moran TM. 2010. The virion host shut-off (vhs) protein blocks a TLR-independent pathway of herpes simplex virus type 1 recognition in human and mouse dendritic cells. PLoS One 5:e8684. doi: 10.1371/journal.pone.0008684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLean CA. 1998. HSV entry and spread. Methods Mol Med 10:9–18. doi: 10.1385/0-89603-347-3:9. [DOI] [PubMed] [Google Scholar]
- 27.Cliffe AR, Knipe DM. 2008. Herpes simplex virus ICP0 promotes both histone removal and acetylation on viral DNA during lytic infection. J Virol 82:12030–12038. doi: 10.1128/JVI.01575-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.L'Allemain G. 1999. Multiple actions of EGCG, the main component of green tea. Bull Cancer 86:721–724. [PubMed] [Google Scholar]
- 29.Naasani I, Seimiya H, Tsuruo T. 1998. Telomerase inhibition, telomere shortening, and senescence of cancer cells by tea catechins. Biochem Biophys Res Commun 249:391–396. doi: 10.1006/bbrc.1998.9075. [DOI] [PubMed] [Google Scholar]
- 30.Wong VC, Morrison A, Tabori U, Hawkins CE. 2010. Telomerase inhibition as a novel therapy for pediatric ependymoma. Brain Pathol 20:780–786. doi: 10.1111/j.1750-3639.2010.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou M, Xu D, Bjorkholm M, Gruber A. 2001. Real-time quantitative telomeric repeat amplification protocol assay for the detection of telomerase activity. Clin Chem 47:519–524. [PubMed] [Google Scholar]
- 32.Kerr JF, Wyllie AH, Currie AR. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honess RW, Roizman B. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol 14:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honess RW, Roizman B. 1975. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A 72:1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roizman B, Knipe DM, Whitley RJ. 2013. Herpes simplex viruses, p 1823–1897. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed, vol 2 Lippincott Williams &Wilkins, Philadelphia, PA. [Google Scholar]
- 36.Stadler G, Wieser M, Streubel B, Stift A, Friedl J, Gnant M, Niederle B, Beham A, Katinger H, Pfragner R, Grillari J, Voglauer R. 2008. Low telomerase activity: possible role in the progression of human medullary thyroid carcinoma. Eur J Cancer 44:866–875. doi: 10.1016/j.ejca.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Serrano D, Bleau AM, Fernandez-Garcia I, Fernandez-Marcelo T, Iniesta P, Ortiz-de-Solorzano C, Calvo A. 2011. Inhibition of telomerase activity preferentially targets aldehyde dehydrogenase-positive cancer stem-like cells in lung cancer. Mol Cancer 10:96. doi: 10.1186/1476-4598-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen ML, Blaho JA. 2011. Telomerase activity during herpesvirus infection. Future Virol 6:901–904. doi: 10.2217/fvl.11.65. [DOI] [Google Scholar]
- 39.Gillis PA, Okagaki LH, Rice SA. 2009. Herpes simplex virus type 1 ICP27 induces p38 mitogen-activated protein kinase signaling and apoptosis in HeLa cells. J Virol 83:1767–1777. doi: 10.1128/JVI.01944-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hargett D, McLean T, Bachenheimer SL. 2005. Herpes simplex virus ICP27 activation of stress kinases JNK and p38. J Virol 79:8348–8360. doi: 10.1128/JVI.79.13.8348-8360.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harada G, Neng Q, Fujiki T, Katakura Y. 2014. Molecular mechanisms for the p38-induced cellular senescence in normal human fibroblast. J Biochem 156:283–290. doi: 10.1093/jb/mvu040. [DOI] [PubMed] [Google Scholar]
- 42.Steinmann J, Buer J, Pietschmann T, Steinmann E. 2013. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol 168:1059–1073. doi: 10.1111/bph.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isaacs CE, Wen GY, Xu W, Jia JH, Rohan L, Corbo C, Di Maggio V, Jenkins EC Jr, Hillier S. 2008. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob Agents Chemother 52:962–970. doi: 10.1128/AAC.00825-07. [DOI] [PMC free article] [PubMed] [Google Scholar]