Abstract
2′-5′-Oligoadenylate synthetase-like protein (OASL) is an interferon-inducible antiviral protein. Here we describe differential inhibitory activities of human OASL and the two mouse OASL homologs against respiratory syncytial virus (RSV) replication. Interestingly, nonstructural protein 1 (NS1) of RSV promoted proteasome-dependent degradation of specific OASL isoforms. We conclude that OASL acts as a cellular antiviral protein and that RSV NS1 suppresses this function to evade cellular innate immunity and allow virus growth.
TEXT
Cellular innate immunity against virus infection is primarily mediated by type I interferons (IFNs). In turn, the IFNs exert their pleiotropic effects through the induction of a variety of IFN-stimulated genes (ISGs) (1–4). Although the general antiviral roles of several ISGs have been demonstrated, the roles of individual ISGs and their effect on specific viruses have remained largely unidentified (5–9). On the other hand, the coevolution of the host and the virus has resulted in viral strategies to evade the host IFN response by targeting ISGs and other IFN pathway proteins (10). Oligoadenylate synthetases (OAS) are a family of ISGs characterized by their ability to synthesize 2′-5′-oligoadenylate (2-5A), which induces RNA degradation by activating RNase L (11, 12). Human oligoadenylate synthetase-like protein (OASL) is related to the OAS family by its N-terminal OAS-like domain but is devoid of 2-5A synthetase activity. Additionally, OASL contains two tandem ubiquitin-like (UBL) domains in the C terminus, which are absent in any of the other members of the OAS family (12–15). OASL is directly and rapidly induced by virus infection via interferon regulatory factor 3 (IRF3) as well as by IFN signaling (1, 12, 16, 17). Unlike in humans, two OASL isoforms have been identified in the mouse, Oasl1 and Oasl2. We have recently described the antiviral activities of human OASL and mouse Oasl2, which are mediated through the enhancement of RIG-I signaling (18). In the present study, we examined the antiviral activities of various human and mouse OASL proteins against respiratory syncytial virus (RSV), the founding member of the clinically significant Pneumovirus genus of the Paramyxoviridae family. We show that human OASL and mouse Oasl2 strongly inhibit RSV replication and that the RSV nonstructural protein 1 (NS1) specifically targets these two isoforms to promote viral replication.
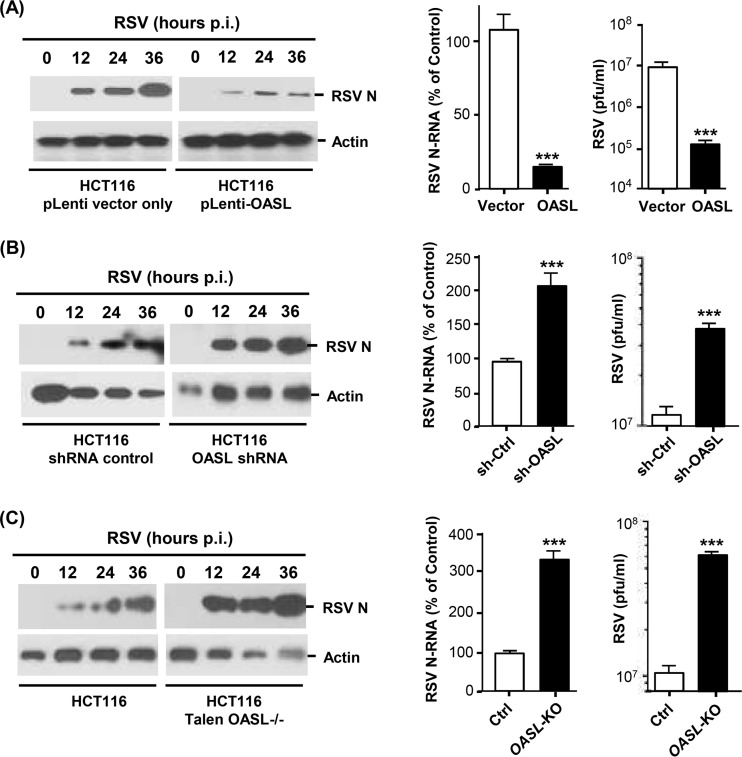
We first conducted a detailed analysis of the antiviral activity of human OASL against RSV. Recombinant OASL, stably expressed in HEK293 cells, strongly reduced RSV growth, measured by the reduction of progeny viral titer, which paralleled the reduction of intracellular viral RNA and proteins (Fig. 1), confirming our earlier findings (18) and establishing inhibition at the viral genome level. Similar antiviral activity of OASL was also observed using a different cell line, HCT116 (Fig. 2A). In complementary studies, enhanced RSV replication was seen when OASL expression was silenced by stable expression of the short hairpin RNA (shRNA) described previously (18) (Fig. 2B). To further corroborate these observations, we created OASL-deficient HCT116 cells by genome editing using the TALEN technology; RSV replication in these cells was also found to be highly elevated (Fig. 2C). Together, these results fully established the antiviral activity of OASL against RSV.
FIG 1.
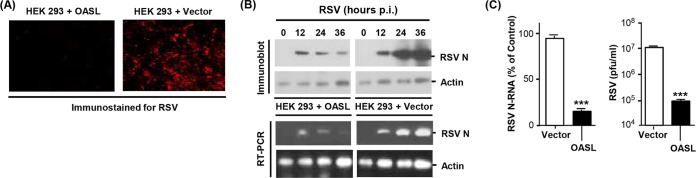
Inhibition of RSV replication in cells expressing human OASL. (A) HEK293 cells, stably transfected with V5-tagged OASL expression plasmid or the empty vector (pcDNA), as described before (18), were grown in monolayers on coverslips and infected with RSV Long at a multiplicity of infection of 3. At 18 h postinfection, cells were fixed and immunostained with mouse anti-RSV nucleoprotein (N) antibody (Abnova clone B023), followed by Alex Fluor 610-conjugated donkey anti-mouse IgG (Life Technologies). Images were captured in a Nikon AIRSI spectral confocal microscope system. (B) (Top) The same cell lines were infected as described above, and the total cell lysates were analyzed by immunoblotting using the same primary antibody described above and horseradish peroxidase (HRP)-conjugated secondary antibody, followed by ECL (enhanced chemiluminescence) detection. Actin is the loading control. (Bottom) Total RNA isolated from parallel cultures was subjected to quantitative reverse transcription-PCR (qRT-PCR), as described previously (38). The primers, synthesized by Integrated DNA Technologies (Coralville, IA), were as follows. RSV N gene, forward 5′-TGCAGGGCAAGTGATGTTAC-3′, and reverse, 5′-TTCCATTTCTGCTTGCACAC-3′; actin, forward, 5′-AGAAAATCTGGCACCACACC-3′, and reverse, 5′-GGGGTGTTGAAGGTCTCAAA-3′. A portion of the PCR sample was analyzed on 1.5% agarose gel, and the ethidium bromide-stained bands are shown. (C) (Left) Plot of the PCR results described in panel B. An average from three data sets with error bars is shown. (Right) The liberated virus in the medium at 24 h postinfection was assayed for PFU on HEp-2 monolayers as described previously (39). The asterisks indicate significance (P < 0.001).
FIG 2.
RSV replication in OASL-expressing and OASL-deficient cells. HCT116 cells, in which OASL was either expressed recombinantly or silenced by shRNA, have been described (18). The cells were infected with RSV, viral growth was assayed by quantitative immunoblot as described in the legend to Fig. 1B, and the data were plotted. (A) Inhibition of RSV growth in OASL-expressing cells. (B and C) Enhanced RSV growth in OASL-deficient cells compared to control cells. The asterisks indicate significance (P < 0.001).
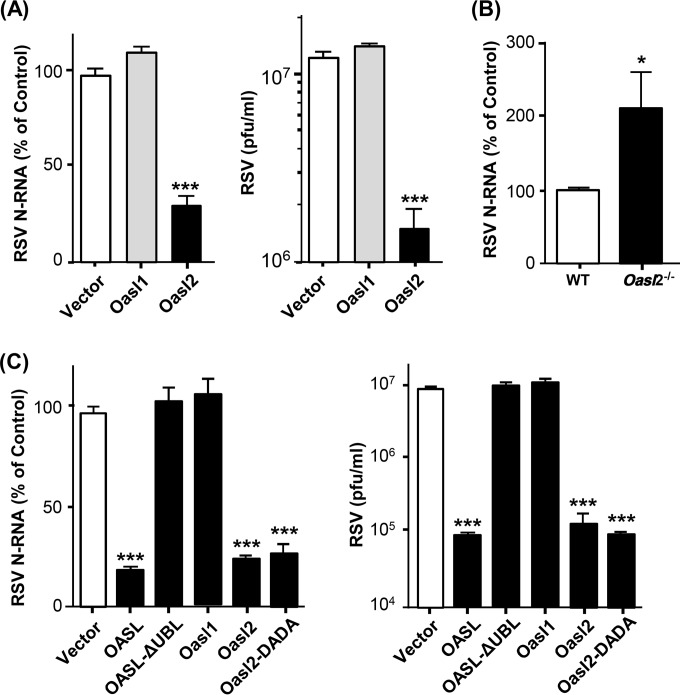
As human OASL lacks the enzymatic activity of OAS, it is incapable of generating the second messenger 2-5A and thereby does not activate RNase L. It is thus likely that OASL exerts its anti-RSV effect through a novel mechanism that is 2-5A independent. The existence of two mouse orthologs—the catalytically inactive Oasl1 and catalytically active Oasl2 (18, 19)—provided us with an opportunity to test this. However, whereas Oasl1 expression failed to inhibit RSV activity, Oasl2 did (Fig. 3A); moreover, primary bone marrow-derived macrophages (BMDMs), isolated from Oasl2−/− mice, supported much more robust RSV growth than cells from isogenic wild-type mice (Fig. 3B). To abolish the catalytic activity of Oasl2, we then mutated two of the three conserved Asp residues in its core catalytic triad (19) to create the mutant designated “DADA.” As shown (Fig. 3C), these mutations did not reduce the anti-RSV activity of Oasl2. Thus, although mouse Oasl2 is enzymatically active, its antiviral activity is clearly not due to the generation of 2-5A.
FIG 3.
Interrogation of the antiviral role of two mouse Oasl isoforms. HEK293 cells were transfected with the indicated expression plasmids (and empty pcDNA vector controls) and then infected with RSV as in Fig. 1B. (A) Replication of RSV was measured by qRT-PCR assay of N gene mRNA, as in Fig. 1B, and by plaque assay as in Fig. 1C. The asterisks indicate significance (P < 0.0001). (B) Bone marrow-derived macrophages from Oasl2−/− and wild-type mice were tested for RSV growth as in panel A. *, P < 0.05. (C) Human OASL, mouse Oasl1 and Oasl2, and the indicated mutant versions of these proteins were expressed by transfection in mouse embryonic fibroblasts, and RSV growth was assayed as in panel A. ***, P < 0.001.
We have recently shown that the unique UBL domain of OASL, absent in classic OAS enzymes, is important for antiviral activity against vesicular stomatitis virus (VSV) and Sendai virus (SeV) (18). We found this is also true for RSV, since loss of the UBL domain of OASL also led to the total loss of anti-RSV activity (Fig. 3C). Overall, we conclude that the antiviral activity of OASL proteins is independent of 2-5A but requires the UBL domain.
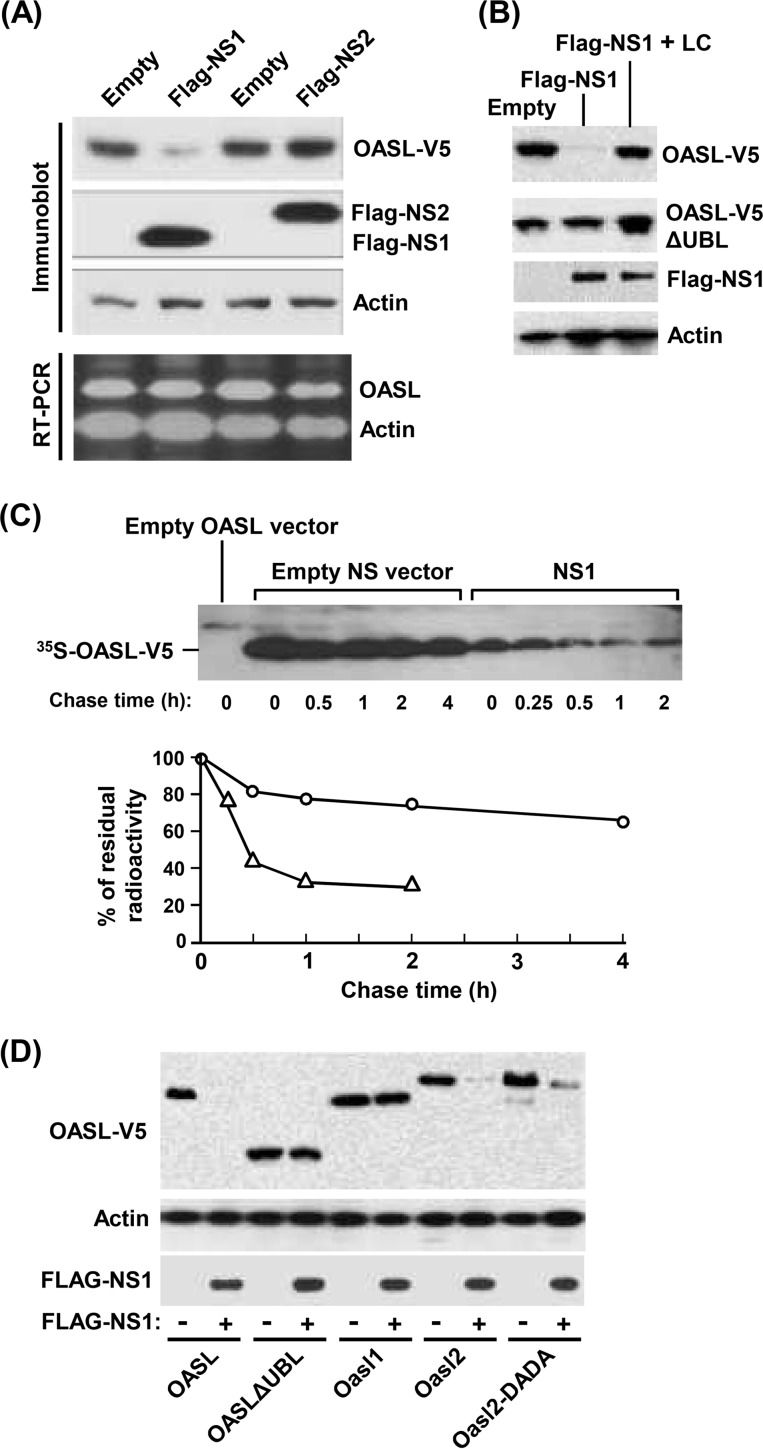
The nonstructural proteins of RSV are known to suppress the host cell's IFN response (20–27), thus promoting robust virus growth and pathogenesis. Although the exact molecular mechanism of the suppression is still being actively pursued, we and others have shown that NS proteins target multiple members of the IFN induction and response pathways, such as RIG-I, IRF3, IRF7, and STAT2 (28–37). We also showed that NS1 and/or NS2 promotes the degradation of these substrates, in part by recruiting a proteasomal activity (37). It was, therefore, logical to investigate whether the NS proteins may also target OASL for degradation, in an attempt to counteract the antiviral function of OASL. Indeed, when coexpressed, recombinant NS1 strongly reduced OASL protein levels (Fig. 4A), whereas NS2 was ineffective. This activity of NS1 could be inhibited by lactacystin, indicating proteasomal degradation as the mechanism (Fig. 4B). Furthermore, a pulse-chase experiment showed that the in vivo half-life of OASL is reduced from >4 h to about 30 min in the presence of NS1, confirming a posttranslational effect (Fig. 4C). Finally, a mutated OASL from which the UBL domain was deleted (ΔUBL OASL) failed to be targeted by NS1 (Fig. 4D), suggesting that this ubiquitin-like domain plays a cardinal, potentially novel, role in the proteasomal targeting of OASL. Like human OASL, mouse Oasl2 was targeted by NS1, but interestingly, mouse Oasl1 was not (Fig. 4D). The enzymatically defective DADA mutant of Oasl2 was also NS1 sensitive (Fig. 4D). Based on these results, it appears that the viral NS1 protein has evolved to specifically target the RSV-inhibitory OASL and Oasl2 proteins and that such targeting is dependent on the presence of the ubiquitin-like domain in these proteins. However, our study for the first time points out unique properties in the UBL domains of Oasl1 and Oasl2, which render one resistant to NS1 while making the other sensitive. Finer mapping of the specific residues in the UBL domains responsible for this differential sensitivity will shed light on the molecular mechanism of the NS1-mediated targeting and help create clinically useful inhibitors of NS1.
FIG 4.
Reduction of OASL expression by RSV NS1. (A) (Top) Recombinant V5-tagged human OASL (18) and pCAGGS plasmids expressing Flag-tagged NS1 or NS2 (34) were cotransfected into HEK293 cells, and the levels of the proteins were determined by immunoblotting at 24 h posttransfection. Actin serves as the loading control. (Bottom) qRT-PCR of OASL and actin mRNA from the cells indicated above was conducted, and the products were analyzed as in Fig. 1B. Primers for human OASL were as follows: forward, 5′-AAAGAGAGGCCCATCATCC-3′; reverse, 5′-ATCTGGGTAACCCCTCTGC-3′. (B) The experiment was done as in panel A using either full-length OASL or OASL with UBL deleted cotransfected with NS1 plasmid. Where indicated, lactacystin (LC [10 μM]) was added to the medium. Actin is the loading control. (C) Pulse-chase experiment to determine the effect of NS1 on the half-life of OASL protein. Cells were transfected with NS1 expression plasmid (or empty pCAGGS vector) as in panel A and 18 h later pulse-labeled with [35S]-Met-Cys (Trans-35S-label; MP Biomedicals) in Met-Cys-free Dulbecco's modified Eagle's medium (DMEM) (Life Technologies) for 1 h, followed by chase with complete DMEM supplemented with Met and Cys (10 mg/ml each). At the indicated time points, the cells were processed for standard immunoprecipitation with V5 antibody and protein A-Sepharose, followed by SDS-PAGE and autoradiography. (Top) Autoradiograph. (Bottom) Plot of the band intensities in the autoradiograph (○, no NS1; △, with NS1). (D) The experiment was done as in panel B using the indicated V5-tagged human and mouse Oasl homologs and their mutants cotransfected with NS1. Actin was the loading control, as used before.
ACKNOWLEDGMENTS
We thank Rune Hartmann for the mouse Oasl1 and Oasl2 expression constructs.
This work was supported in part by grants AI082673 (S.N.S.) and AI109569 (S.B.) from the NIAID/NIH, by University of Pittsburgh Cancer Institute Startup funds (S.N.S.), and by grant S10 OD010381 (S.B.) from the NIH.
REFERENCES
- 1.Sarkar SN, Sen GC. 2004. Novel functions of proteins encoded by viral stress-inducible genes. Pharmacol Ther 103:245–259. doi: 10.1016/j.pharmthera.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Sen GC, Sarkar SN. 2007. The interferon-stimulated genes: targets of direct signaling by interferons, double-stranded RNA, and viruses. Curr Top Microbiol Immunol 316:233–250. [DOI] [PubMed] [Google Scholar]
- 3.Sadler AJ, Williams BR. 2008. Interferon-inducible antiviral effectors. Nat Rev Immunol 8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan N, Chen ZJ. 2012. Intrinsic antiviral immunity. Nat Immunol 13:214–222. doi: 10.1038/ni.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. 2011. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoggins JW. 2014. Interferon-stimulated genes: roles in viral pathogenesis. Curr Opin Virol 6:40–46. doi: 10.1016/j.coviro.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider WM, Chevillotte MD, Rice CM. 2014. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol 32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond MS, Farzan M. 2013. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat Rev Immunol 13:46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fensterl V, Sen GC. 2015. Interferon-induced Ifit proteins: their role in viral pathogenesis. J Virol 89:2462–2468. doi: 10.1128/JVI.02744-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowie AG, Unterholzner L. 2008. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol 8:911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristiansen H, Gad HH, Eskildsen-Larsen S, Despres P, Hartmann R. 2011. The oligoadenylate synthetase family: an ancient protein family with multiple antiviral activities. J Interferon Cytokine Res 31:41–47. doi: 10.1089/jir.2010.0107. [DOI] [PubMed] [Google Scholar]
- 12.Zhu J, Ghosh A, Sarkar SN. 2015. OASL—a new player in controlling antiviral innate immunity. Curr Opin Virol 12:15–19. doi: 10.1016/j.coviro.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebouillat D, Marié I, Hovanessian AG. 1998. Molecular cloning and characterization of two related and interferon-induced 56-kDa and 30-kDa proteins highly similar to 2′-5′ oligoadenylate synthetase. Eur J Biochem 257:319–330. doi: 10.1046/j.1432-1327.1998.2570319.x. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann R, Olsen HS, Widder S, Jorgensen R, Justesen J. 1998. p59OASL, a 2′-5′ oligoadenylate synthetase like protein: a novel human gene related to the 2′-5′ oligoadenylate synthetase family. Nucleic Acids Res 26:4121–4128. doi: 10.1093/nar/26.18.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eskildsen S, Justesen J, Schierup MH, Hartmann R. 2003. Characterization of the 2′-5′-oligoadenylate synthetase ubiquitin-like family. Nucleic Acids Res 31:3166–3173. doi: 10.1093/nar/gkg427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sen GC, Peters GA. 2007. Viral stress-inducible genes. Adv Virus Res 70:233–263. doi: 10.1016/S0065-3527(07)70006-4. [DOI] [PubMed] [Google Scholar]
- 17.Melchjorsen J, Kristiansen H, Christiansen R, Rintahaka J, Matikainen S, Paludan SR, Hartmann R. 2009. Differential regulation of the OASL and OAS1 genes in response to viral infections. J Interferon Cytokine Res 29:199–207. doi: 10.1089/jir.2008.0050. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Zhang Y, Ghosh A, Cuevas RA, Forero A, Dhar J, Ibsen MS, Schmid-Burgk JL, Schmidt T, Ganapathiraju MK, Fujita T, Hartmann R, Barik S, Hornung V, Coyne CB, Sarkar SN. 2014. Antiviral activity of human OASL protein is mediated by enhancing signaling of the RIG-I RNA sensor. Immunity 40:936–948. doi: 10.1016/j.immuni.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eskildsen S, Hartmann R, Kjeldgaard NO, Justesen J. 2002. Gene structure of the murine 2′-5′-oligoadenylate synthetase family. Cell Mol Life Sci 59:1212–1222. doi: 10.1007/s00018-002-8499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng MN, Collins PL. 1999. Altered growth characteristics of recombinant respiratory syncytial viruses which do not produce NS2 protein. J Virol 73:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin H, Zhou H, Cheng X, Tang R, Munoz M, Nguyen N. 2000. Recombinant respiratory syncytial viruses with deletions in the NS1, NS2, SH, and M2-2 genes are attenuated in vitro and in vivo. Virology 273:210–218. doi: 10.1006/viro.2000.0393. [DOI] [PubMed] [Google Scholar]
- 22.Schlender J, Bossert B, Buchholz U, Conzelmann KK. 2000. Bovine respiratory syncytial virus nonstructural proteins NS1 and NS2 cooperatively antagonize alpha/beta interferon-induced antiviral response. J Virol 74:8234–8242. doi: 10.1128/JVI.74.18.8234-8242.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng MN, Whitehead SS, Bermingham A, St Claire M, Elkins WR, Murphy BR, Collins PL. 2000. Recombinant respiratory syncytial virus that does not express the NS1 or M2-2 protein is highly attenuated and immunogenic in chimpanzees. J Virol 74:9317–9321. doi: 10.1128/JVI.74.19.9317-9321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin H, Zhou H, Cheng X, Tang R, Munoz M, Nguyen N. 2003. Evaluation of recombinant respiratory syncytial virus gene deletion mutants in African green monkeys for their potential as live attenuated vaccine candidates. Vaccine 21:3647–3652. doi: 10.1016/S0264-410X(03)00426-2. [DOI] [PubMed] [Google Scholar]
- 25.Valarcher JF, Furze J, Wyld S, Cook R, Conzelmann KK, Taylor G. 2003. Role of alpha/beta interferons in the attenuation and immunogenicity of recombinant bovine respiratory syncytial viruses lacking NS proteins. J Virol 77:8426–8439. doi: 10.1128/JVI.77.15.8426-8439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spann KM, Tran KC, Chi B, Rabin RL, Collins PL. 2004. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J Virol 78:4363–4369. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramaswamy M, Shi L, Varga SM, Barik S, Behlke MA, Look DC. 2006. Respiratory syncytial virus nonstructural protein 2 specifically inhibits type I interferon signal transduction. Virology 344:328–339. doi: 10.1016/j.virol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Barik S. 2013. Respiratory syncytial virus mechanisms to interfere with type 1 interferons. Curr Top Microbiol Immunol 372:173–191. doi: 10.1007/978-3-642-38919-1_9. [DOI] [PubMed] [Google Scholar]
- 29.Bossert B, Marozin S, Conzelmann KK. 2003. Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3. J Virol 77:8661–8668. doi: 10.1128/JVI.77.16.8661-8668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo MS, Brazas RM, Holtzman MJ. 2005. Respiratory syncytial virus nonstructural proteins NS1 and NS2 mediate inhibition of Stat2 expression and alpha/beta interferon responsiveness. J Virol 79:9315–9319. doi: 10.1128/JVI.79.14.9315-9319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spann KM, Tran KC, Collins PL. 2005. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-κB, and proinflammatory cytokines. J Virol 79:5353–5362. doi: 10.1128/JVI.79.9.5353-5362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elliott J, Lynch OT, Suessmuth Y, Qian P, Boyd CR, Burrows JF, Buick R, Stevenson NJ, Touzelet O, Gadina M, Power UF, Johnston JA. 2007. Respiratory syncytial virus NS1 protein degrades STAT2 by using the elongin-cullin E3 ligase. J Virol 81:3428–3436. doi: 10.1128/JVI.02303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ling Z, Tran KC, Teng MN. 2009. Human respiratory syncytial virus nonstructural protein NS2 antagonizes the activation of beta interferon transcription by interacting with RIG-I. J Virol 83:3734–3742. doi: 10.1128/JVI.02434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swedan S, Musiyenko A, Barik S. 2009. Respiratory syncytial virus nonstructural proteins decrease levels of multiple members of the cellular interferon pathways. J Virol 83:9682–9693. doi: 10.1128/JVI.00715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren J, Liu T, Pang L, Li K, Garofalo RP, Casola A, Bao X. 2011. A novel mechanism for the inhibition of interferon regulatory factor-3-dependent gene expression by human respiratory syncytial virus NS1 protein. J Gen Virol 92:2153–2159. doi: 10.1099/vir.0.032987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swedan S, Andrews J, Majumdar T, Musiyenko A, Barik S. 2011. Multiple functional domains and complexes of the two nonstructural proteins of human respiratory syncytial virus contribute to interferon suppression and cellular location. J Virol 85:10090–10100. doi: 10.1128/JVI.00413-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goswami R, Majumdar T, Dhar J, Chattopadhyay S, Bandyopadhyay SK, Verbovetskaya V, Sen GC, Barik S. 2013. Viral degradasome hijacks mitochondria to suppress innate immunity. Cell Res 23:1025–1042. doi: 10.1038/cr.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazumder B, Poddar D, Basu A, Kour R, Verbovetskaya V, Barik S. 2014. Extraribosomal L13a is a specific innate immune factor for antiviral defense. J Virol 88:9100–9110. doi: 10.1128/JVI.01129-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bitko V, Barik S. 2001. Phenotypic silencing of cytoplasmic genes using sequence-specific double-stranded short interfering RNA and its application in the reverse genetics of wild type negative-strand RNA viruses. BMC Microbiol 1:34. doi: 10.1186/1471-2180-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]