Abstract
Dental enamel formation is an intricate process tightly regulated by ameloblast cells. The correct spatiotemporal patterning of enamel matrix protein (EMP) expression is fundamental to orchestrate the formation of enamel crystals, which depend on a robust supply of Ca2+. In the extracellular milieu, Ca2+-EMP interactions occur at different levels. Despite its recognized role in enamel development, the molecular machinery involved in Ca2+ homeostasis in ameloblasts remains poorly understood. A common mechanism for Ca2+ influx is store-operated Ca2+ entry (SOCE). We evaluated the possibility that Ca2+ influx in enamel cells might be mediated by SOCE and the Ca2+ release-activated Ca2+ (CRAC) channel, the prototypical SOCE channel. Using ameloblast-like LS8 cells, we demonstrate that these cells express Ca2+-handling molecules and mediate Ca2+ influx through SOCE. As a rise in the cytosolic Ca2+ concentration is a versatile signal that can modulate gene expression, we assessed whether SOCE in enamel cells had any effect on the expression of EMPs. Our results demonstrate that stimulating LS8 cells or murine primary enamel organ cells with thapsigargin to activate SOCE leads to increased expression of Amelx, Ambn, Enam, Mmp20. This effect is reversed when cells are treated with a CRAC channel inhibitor. These data indicate that Ca2+ influx in LS8 cells and enamel organ cells is mediated by CRAC channels and that Ca2+ signals enhance the expression of EMPs. Ca2+ plays an important role not only in mineralizing dental enamel but also in regulating the expression of EMPs.
Keywords: dental enamel, calcification, physiology, Fura-2, calcium channels, calcium signaling
Introduction
Enamel formation is a complex process requiring the tight spatiotemporal expression of a number of enamel matrix proteins (EMPs), including amelogenin (Amelx), ameloblastin (Ambn), and enamelin (Enam). These EMPs are synthesized and secreted by ameloblast cells and are presented to the extracellular domain where they mediate the development of hydroxyapatite-like crystals. Mutations in the genes encoding these EMPs result in amelogenesis imperfecta in humans.
Ca2+ is an important constituent element in mineralized enamel and participates in the formation of enamel crystals at several levels. Extracellular Ca2+ is critical for the precipitation of ions from fluid; hence, Ca2+ is required for enamel crystal growth (Simmer and Fincham 1995). The physiologic involvement of Ca2+ in enamel formation is also evident by the presence of enamel defects in vitamin D–deficient animals, which disrupts normal plasma Ca2+ concentration resulting in hypocalcemia (Descroix et al. 2010). The contributions of extracellular Ca2+ to build enamel crystals and to participate as critical regulators of associated processes is becoming better defined, yet the mechanisms enabling Ca2+ influx prior to its extrusion into the extracellular space remain poorly understood. Store-operated Ca2+ entry (SOCE) is one of the main Ca2+ influx mechanisms in many cell types. SOCE activation requires depletion of Ca2+ stores from the endoplasmic reticulum (ER) via inositol 1,4,5-trisphosphate receptors and/or ryanodine receptors (RyRs). Depletion of Ca2+ from the ER activates store-operated plasma membrane Ca2+ channels that mediate Ca2+ influx into the cytoplasm resulting in an increase of the cytosolic Ca2+ concentration ([Ca2+]c) (Feske 2009) and a refilling of ER Ca2+ stores via the sarco/ER Ca2+-ATPase (SERCA). The best-studied mechanism of SOCE is mediated by the CRAC (Ca2+ release-activated Ca2+) channel (Feske 2009). The CRAC channel has been linked to enamel development, as patients with mutations in genes encoding the CRAC channel proteins ORAI1 and STIM1 present with severely hypocalcified enamel (McCarl et al. 2009; Picard et al. 2009; Wang et al. 2014). Intracellular Ca2+ is a second messenger involved in many cellular processes, such as gene regulation, cell division, and cell death (Putney 2001). Despite the well-established role of Ca2+ as a universal second messenger, the functions associated with changes in [Ca2+]c in enamel cells are poorly understood.
A previous study showed that human ameloblast lineage cells cultured in varying [Ca2+]c resulted in enhanced cell differentiation with upregulation of Amelx expression, thus suggesting a link between Ca2+ and enamel gene expression in vitro (Chen et al. 2009). Several immortalized cell lines are available to study enamel development. One of these lines is the mouse-derived ameloblast-like LS8 cell line, which has been widely used in studies of Amelx and Ambn function as well as circadian activity and endocytosis (Zhou and Snead 2000; Dhamija and Krebsbach 2001; Lacruz, Hacia, et al. 2012; Lacruz et al. 2013). When compared with other available ameloblast-like cell lines, LS8 cells express mRNA for enamel genes associated with the secretory stage of amelogenesis (Sarkar et al. 2014), making them an appropriate cell model system to study possible links between [Ca2+]c changes and enamel genes. In the present study, we explored whether ameloblast-like LS8 cells express Ca2+-handling molecules, especially those encoding the CRAC channel. We also investigated the potential effects of [Ca2+]c changes mediated by SOCE on the expression of enamel genes in LS8 cells and in primary enamel organ (EO) cells.
Materials and Methods
Animal Tissue Dissection
Mixed samples of male and female Sprague-Dawley rats or C57B1 mice were used at ~4 wk of age. EO cells were collected from the region equivalent to the secretory stage EO as described (Smith et al. 2011).
Cell Preparation and Western Blot
Ameloblast-like LS8 cells were cultured in standard culture conditions as described (Chen et al. 1992). For some experiments, LS8 cells were pretreated with thapsigargin (1 µM; Sigma-Aldrich, St. Louis, MO, USA) and 2-aminoethyldiphenyl borate (2-APB; 50 μM; Sigma-Aldrich) using conditions previously described for other cell types (Takemura et al. 1989; Murakami et al. 2012). Thapsigargin is widely used as an inhibitor of SERCA pumps resulting in relatively fast (minutes) Ca2+ depletion of the ER and induction of SOCE (Takemura et al. 1989). Given the quick physiologic response generated by thapsigargin and that prolonged inhibition of SERCA results in ER stress and possibly cell death (Hu et al. 2004), we exposed EO cells to short incubation times that are sufficient to induce gene expression changes (30 min, 1 h, 1.5 h). Cell culture and Western blot protocols are found in the Appendix and as previously described using rat EO cells from the secretory stage (Lacruz et al. 2013). ImageJ software was used to assess changes in optical density.
Real-time Polymerase Chain Reaction
Total RNA extraction and reverse transcription from LS8 and EO cells were as described (Lacruz, Smith, et al. 2012). All experiments were performed in triplicates. The Hprt1 gene was used to standardize baseline level of RNA sample. Relative quantification of gene expression used the ΔCT and ΔΔCt method (Pfaffl 2001). Primer sequences used can be found in the Appendix Table.
Immunofluorescence Staining
LS8 or EO cells were grown for 24 to 48 h on a coverglass and were fixed with 4% paraformaldehyde before incubation with 0.2% Triton-X (in phosphate-buffered saline) for 20 min at room temperature. After blocking for 30 min with 2% bovine serum albumin in phosphate-buffered saline, sections were incubated overnight at 4 oC with appropriate antibodies (Appendix). After washing in phosphate-buffered saline, samples were incubated with secondary antibodies for 30 min, washed, and mounted using Prolong Gold Mounting Media containing Dapi (Invitrogen, USA).
Intracellular Ca2+ Measurements
To determine cytosolic [Ca2+]c, LS8 cells were loaded with Fura-2/AM (5 µM, Life Technology Invitrogen, Grand Island, NY, USA) in DMEM medium containing 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% glutamine for 30 min at room temperature, as this is a standard protocol used in many other cell types (Feske et al. 2001). After loading, cells were washed and dispensed in 96-well black glass-bottom plates (Molecular Devices). Average fluorescence was measured by FlexStation III (Molecular Devices, Sunnyvale, CA, USA), a 96-well fluorescence spectrometer, at 340- and 380-nm excitation wavelengths and a 510-nm emission wavelength as previously described (Gupta et al. 2009) at 25 °C. Data acquisition was performed using SoftMax Pro (Molecular Devices). For each experiment, the slopes (velocity) and peaks (magnitude) of changes in the 340/380-nm Fura-2 ratio were determined after treatment of cells with 1.25µM thapsigargin (Sigma-Aldrich) in Ca2+-free Ringer solution and addition of 2mM extracellular Ca2+ to measure ER store depletion and SOCE, respectively. For some experiments, LS8 cells were treated with pharmacologic inhibitors of CRAC channels (Parekh 2010). Cells were preincubated with 3μM Synta-66 (provided by S. Feske) for 6 h, 50μM 2-APB (Sigma-Aldrich) for 30 min, and 300nM 3,5-bis (trifluoromethyl) pyrazole (BTP-2; Sigma-Aldrich) for 15 min as described (Bird et al. 2008; Putney 2010) before initiating the experiment. For the solutions used, see the Appendix.
Statistics
Data are provided as mean ± SEM; n represents the number of independent experiments. Differences were tested for significance using Student’s unpaired 1-tailed t test or analysis of variance. P < 0.05 was considered statistically significant.
Results
ER Ca2+ Homeostasis in LS8 and EO Cells
A hallmark of SOCE is its activation following release of Ca2+ from ER stores. This process is receptor mediated, and the 2 main release channels in the ER are IP3Rs and RyRs (Stathopulos et al. 2012). In LS8 cells, transcripts of all 3 IP3R isoforms are expressed, with IP3R3 being the most abundant homologue (Fig. 1A). IP3R3 protein expression was confirmed by immunofluorescence and showed an intracellular distribution pattern consistent with the ER location of IP3R3 (Fig. 1D). Of all the 3 RyR homologues, only RyR2 mRNA was detectable in significant quantities in LS8 cells (Fig. 1B), although we could not detect the protein by immunofluorescence (data not shown), possibly due to low affinity of the antibody used. Following ER Ca2+ depletion and subsequent increases in cytosolic [Ca2+]c, SERCAs pump Ca2+ back into the ER and refill the stores (Lytton et al. 1992). SERCA2 mRNA was the predominant SERCA isoform, whereas SERCA1 and SERCA3 were not detectable (Fig. 1C). We confirmed the expression of SERCA2 protein by immunofluorescence (Fig. 1E). SERCA2 in LS8 cells showed an intracellular distribution pattern consistent with its location in the ER and colocalized with calnexin (CNX), an ER-localized chaperone protein. However, whereas SERCA showed a wide distribution throughout the ER, CNX distribution was more restricted to a limited perinuclear region of the ER, which might correspond to the rough ER given its role as a chaperone. Heterogeneous distribution of ER proteins is not uncommon given the differences in the compartmentalization and function of this organelle and the extent of sarco/ER in various cells (Meldolesi and Pozzan 1998). The cellular localization of STIM1, IP3R3, and SERCA2 was also investigated in primary EO cells. As in LS8 cells, these proteins are expressed in EO cells showing a cytoplasmic localization (Appendix Fig. 1).
Figure 1.

Expression level of mRNAs for IP3R, RyR, and sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and cellular localization of IP3R3 and SERCA in LS8 cells. Relative quantification of mRNA levels for inositol receptors (IP3R 1 to 3) (A), ryanodine receptors (RyR 1 to 3) (B), and SERCA 1 to 3 (C) in LS8 cells. Each experiment was repeated a minimum of 3 times. Hprt1 was used as the reference gene. (D) Confocal microscopy showing cellular localization of IP3R3 (green) in LS8 cells. (E) Confocal microscopy showing SERCA2 and calnexin (CNX) in LS8 cells. SERCA2 expression is more widely distributed than CNX in the endoplasmic reticulum of these cells. DAPI is shown as blue nuclear staining. Images in panels D and E are representative of a minimum of 3 independent experiments.
Molecular Components of CRAC Channels in LS8 Cells
To investigate the expression of CRAC channel components in LS8 cells, we surveyed their mRNA repertoire for the expression of Stim1, Stim2, Orai1, Orai2, and Orai3 genes. Results show that mRNA transcripts for all Orai and Stim genes can be identified (Fig. 2A, B). For Orai, we found that the predominant homologue was Orai2, but Orai1 also showed high abundance, whereas Stim1 was more abundant than Stim2. Immunofluorescence analysis shows that STIM1 protein is present and has an intracellular localization pattern consistent with its localization in the ER (Fig. 2C). Compared with CNX, which is concentrated in the perinuclear ER, STIM1 is expressed throughout the entire cell, which might be a feature of LS8 cells where CNX distribution is limited.
Figure 2.

mRNA expression levels of Orai and Stim genes and subcellular localization of STIM1 in LS8 cells. Relative mRNA quantification of Orai (1 to 3; A) and Stim (1 and 2; B) in LS8 cells. Each experiment was repeated a minimum of 3 times. Hprt1 was used as the reference gene. (C) Confocal microscopy showing localization of STIM1 (red) and the endoplasmic reticulum marker calnexin (CNX; green) in LS8 cells. DAPI is shown as blue nuclear staining. Panels A and B are representative of a minimum of 3 independent experiments.
Detection of Functional CRAC Channels in LS8 Cells
Having identified CRAC channel components and SERCA pumps in LS8 cells, we tested whether CRAC channels are functionally active in these cells by measuring SOCE directly. Fura-2 ratios in LS8 cells are shown in Figure 3. In zero Ca2+ conditions, this ratio is comparable with other cell types, such as HEK293 cells (Luo et al. 2001). The addition of the SERCA inhibitor thapsigargin to LS8 cells in the absence of extracellular Ca2+ resulted in an increase of [Ca2+]c, reflecting the release of Ca2+ from ER stores (Fig. 3A, B, gray tracings). This Ca2+ is either extruded or bound by cytosolic proteins, hence decreasing the Fura-2 fluorescence ratio as observed in Figure 3A. Following the return of [Ca2+]c to near basal conditions, the readdition of extracellular Ca2+ at that point resulted in a rapid and sustained increase of [Ca2+]c, which was due to SOCE. The peak of [Ca2+]c in LS8 cells after readdition of extracellular Ca2+ associated with SOCE was significantly greater (P < 0.01) than the Ca2+ peak after thapsigargin-mediated store release, suggesting that Ca2+ influx via SOCE is the predominant Ca2+ signal in LS8 cells. To confirm that Ca2+ influx was CRAC channel mediated, we assessed whether LS8 cells treated with pharmacologic inhibitors of CRAC channel function (Synta-66, BTP-2, 2-APB) had any effect on [Ca2+]c. None of these inhibitors affected the slope or peak of thapsigargin-induced Ca2+ release from the ER relative to untreated controls (Fig. 3B). However, all of these compounds inhibited Ca2+ entry almost completely after readdition of extracellular Ca2+ when compared with untreated control cells (gray tracings). These data demonstrate that CRAC channels are key mediators of Ca2+ influx in LS8 cells.
Figure 3.

Ca2+ entry is inhibited by CRAC (Ca2+ release-activated Ca2+) channel blockers in ameloblast-like LS8 cells. (A) Ca2+ measurements in LS8 cells. Representative Ca2+ traces showing the Fura-2 fluorescence ratios (340/380 nm) of Fura-2/AM-loaded control LS8 cells (white tracings) and cells pretreated with the CRAC channel inhibitors Synta-66 (3 µM, black), BTP-2 (300 nM, blue), and 2-APB (50µM, red). Where indicated, 1.25µM thapsigargin (TG) was added to the nominally Ca2+-free extracellular bath solution to measure the release of Ca2+ from endoplasmic reticulum stores followed by the addition of 2mM extracellular Ca2+ to induce store-operated Ca2+ entry (SOCE). Arithmetic means ± SEM of the peak (left) and slope (right) of the change in Fura-2 ratio following addition of TG (Ca2+ release; B) and readdition of 2mM Ca2+ (SOCE; C) of extracellular Ca2+ in LS8 cells. Untreated control (n = 6 independent experiments, white bars), Synta-66 pretreated (n = 6, black bars), BTP-2 pretreated (n = 6, blue bars), 2-APB pretreated (n = 6, red bars). ***P < 0.001. Analysis of variance.
Expression of Enamel Genes Is SOCE Dependent
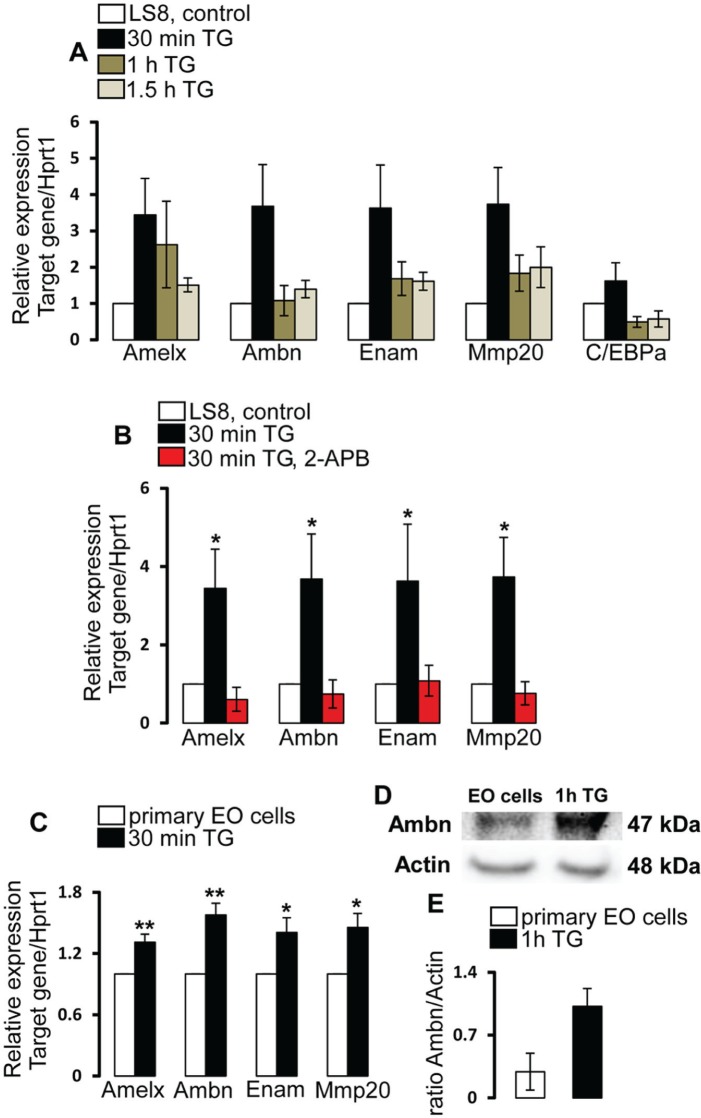
To investigate whether SOCE regulates the expression of enamel genes, we treated LS8 cells with thapsigargin to activate SOCE and analyzed expression of key genes by real-time polymerase chain reaction at different time points. LS8 cells are known to express Amelx, Ambn, Enam, and Mmp20 (Sarkar et al. 2014). LS8 cells were stimulated with thapsigargin for 30 min, 1 h, and 1.5 h, and potential cell stress was visually assessed at the longest time point (1.5 h). Thapsigargin did not appear to affect cell morphology (Appendix Fig. 2). We next turned our attention to possible changes in mRNA levels induced by thapsigargin. Results in Figure 4 show a significant (P < 0.05) increase in the expression of all enamel genes analyzed after 30 min of stimulation with thapsigargin (3- to 4-fold increase; Fig. 4A). At the later time points (1 h, 1.5 h), the increase in gene expression was less pronounced compared with 30-min stimulation, and the fold change was smaller. As the increase in gene expression peaked 30 min after thapsigargin stimulation, we used this time point to assess whether inhibiting Ca2+ entry with the CRAC channel blocker 2-APB in the presence of thapsigargin had any effect on the expression of enamel genes. In treated cells, we found no increases in mRNA levels for any of the enamel genes, indicating that expression is dependent on CRAC channel activation (Fig. 4B).
Figure 4.
Store-operated Ca2+ entry–dependent expression of enamel matrix proteins in ameloblast-like LS8 and mouse enamel organ (EO) cells. (A) Relative mRNA expression of Amelx, Ambn, Enam, Mmp20, and C/EBPa in LS8 cells treated with thapsigargin (TG) for 30 min (black bars), 1 h (green bars), and 1.5 h (gray bars) or left untreated (white bars). Each experiment was repeated a minimum of 3 times. Hprt1 was used as a reference gene. Arithmetic means ± SEM of LS8 cells. (B) Relative mRNA expression of Amelx, Ambn, Enam, and Mmp20 in LS8 cells left untreated (control, white bars), treated with TG for 30 min (black bars), and treated with TG and preincubated with 2-APB (red bars). Each experiment was repeated a minimum of 3 times. Hprt1 was used as a reference gene. Arithmetic means ± SEM of LS8 cells untreated (control, white bars) and TG pretreated (back bars). *P < 0.05. Analysis of variance. (C) Relative mRNA expression of Amelx, Ambn, Enam, and Mmp20 in TG-treated (30 min) and untreated (control) mouse primary EO cells. Each experiment was repeated a minimum of 3 times. Hprt1 was used as a reference gene. Arithmetic means ± SEM of mice EOs left untreated (control, white bars) and treated with TG for 30 min (black bars). *P < 0.05. **P < 0.01. Student’s t test. (D) Western blot analysis of Ambn protein expression and actin as loading control in untreated and TG-treated rat EO cells. (E) Quantification of Ambn protein expression normalized to actin in primary EO cells using ImageJ software to measure the optical density of bands in Western blot experiments. Ameloblastin andtibody against C-terminus and actin antibody used were AMBN (1:1,000, 48 kDa; Santa Cruz, Dallas, TX, USA) and ACTB (1:2,000, 47 kDa; Santa Cruz). Error bars represent ±SEM. Quantification of a minimum of 3 independent experiments.
We also tested whether mRNA expression of CCAAT/enhancer-binding protein alpha (C/EBPa), a transcription factor family that enhances Amelx transcription (Xu et al. 2006; Xu et al. 2007), was upregulated in LS8 cells after thapsigargin stimulation and found a nonsignificant upregulation at 30 min. No mRNA increases were detected at 1 or 1.5 h (Fig. 4A). To test whether SOCE also regulates enamel gene expression in primary EO cells, we stimulated these cells isolated from mouse EOs with thapsigargin for 30 min. We found a significant increase (P < 0.05) in the expression of Amelx, Ambn, Enam, and Mmp20 mRNA compared with nonstimulated EO cells, consistent with similar findings in the LS8 cells. Induction of SOCE with thapsigargin furthermore increased protein levels of Ambn in rat EO cells when analyzed by Western blot analysis (Fig. 4C).
Discussion
Increases in intracellular [Ca2+]c are important signaling mechanisms that are known to modulate gene expression in many cell types (Berridge 1993). Here we investigated Ca2+ dynamics and changes in gene expression in ameloblast-like LS8 cells, an immortalized nonexcitable cell line, and in primary EO cells. We first investigated if LS8 cells express proteins required for Ca2+ homeostasis.
The ER is a reservoir for Ca2+ that can be released into the cytosol as needed through receptor-mediated mechanisms. We show that the ER release channels IP3Rs and RyRs are expressed in these cells at the mRNA and protein levels, with IP3R3 and RyR2 being the predominant homologues. As RyRs are most commonly identified in excitable cells (Bennett et al. 1996), it is likely that ER Ca2+ release is mediated by IP3Rs in LS8 cells. These results are supported by immunofluorescence localization of IP3R but not RyR. Moreover, we found that SERCA proteins are also expressed in LS8 cells, with SERCA2 being the predominant homologue (Fig. 1C), as also reported in EO cells (Franklin et al. 2001). We confirmed the expression of STIM1, SERCA2, and IP3R3 in EO cells (Appendix Fig. 1). These data demonstrate that LS8 cells and primary EO cells are equipped with proteins required for proper Ca2+ homeostasis, enabling the release of Ca2+ from intracellular stores and the refilling of these stores.
The present study also reveals that LS8 cells express CRAC channel components STIM and ORAI1. Depletion of ER Ca2+ stores leads the Ca2+ sensor in the ER STIM1 to undergo conformational changes aggregating at specific regions of the ER known as puncta. This process recruits the plasma membrane protein ORAI1, the pore subunit of the channel, resulting in Ca2+ influx (Shaw and Feske 2012). The biophysical properties of the CRAC channel indicate that it is highly selective for Ca2+ (Parekh and Putney 2005). We found that the predominant Orai genes expressed in LS8 cells are Orai2 and Orai3. Mutations in the human STIM1 gene are associated with enamel defects (Picard et al. 2009; Wang et al. 2014), and we found that Stim1 mRNA expression in LS8 cells is greater than that of Stim2. To assess if Ca2+ influx in LS8 cells has characteristics associated with SOCE, we stimulated LS8 cells with thapsigargin, as is frequently used to induce SOCE (Putney 2009). We identified [Ca2+]c increase followed by a more prominent rise upon readdition of extracellular Ca2+. Release of ER Ca2+ followed by Ca2+ entry is a hallmark of SOCE, and LS8 cells behave similar to other cell types, including fibroblasts or parotid acinar cells (Takemura et al. 1989; Feske et al. 2006). In LS8 cells pretreated with several pharmacologic CRAC channel inhibitors, Ca2+ influx was nearly abolished (Fig. 3A–C), demonstrating that SOCE in LS8 cells is mediated by CRAC channels. These data are also in keeping with the identification of severely hypocalcified enamel in patients with mutations in the CRAC channel genes STIM1 and ORAI1 (McCarl et al. 2009; Picard et al. 2009; Wang et al. 2014). As SOCE is disabled in these patients, other potential alternative Ca2+ influx channels in ameloblasts are unable to compensate for this loss of function, disrupting the flow of the Ca2+ transport system; accordingly, ameloblasts cannot provide the growing enamel crystals with the required levels of Ca2+, and this tissue becomes hypocalcified. This effect is not obvious in the skeleton of patients with mutations in CRAC channel genes, although Orai1-deficient mice show decreased bone deposition, and CRAC channels might also be involved in bone growth (Robinson et al. 2012).
No previous associations between SOCE activity and EMPs have been reported. To assess the potential roles of SOCE in the regulation of EMP expression, we stimulated LS8 and EO cells with thapsigargin to activate SOCE. We identified significant increases in mRNA levels of the enamel genes Amelx, Ambn, Enam, and Mmp20 after thapsigargin treatment. In contrast, upregulation of gene expression was prevented when LS8 cells were treated with thapsigargin in the presence of 2-APB, which inhibits CRAC channel function (Fig. 4B), indicating that CRAC channels are involved in regulating enamel gene expression. Upregulation in Ambn mRNA level was supported by Western blot analysis (Fig. 4D). Although the transcription factor C/EBPa showed a moderate increase in expression, this change was not significant. Thus, the mechanisms by which an increase in [Ca2+]c via CRAC channels regulates expression of EMPs, by either C/EBPa or other pathways, have yet to be identified.
Ameloblast-like LS8 cells are thus a suitable model to study Ca2+ dynamics related to enamel formation, although it should be pointed out that enamel formation is a 2-step process involving the secretory and maturation stages. As some of the proteins under study here have been previously observed to increase expression during maturation (i.e., STIM1, SERCA2; Franklin et al. 2001; Lacruz, Smith, et al. 2012), LS8 cells provide a window into Ca2+ modeling that might be better suited to the secretory stage. Ca2+ uptake must be balanced by appropriate Ca2+ extrusion pathways. In ameloblasts, we and others (Okumura et al. 2010) have detected the expression of Ca2+ exchangers (NCX, NCKX) in enamel cells, which likely involves these proteins in Ca2+ efflux.
The data presented here highlight the role of CRAC channels as key modulators of Ca2+ influx in LS8 cells and directly link the resulting increases in [Ca2+]c with the expression of Amelx, Ambn, Enam, and Mmp20 genes. Taken together, our results provide important new information on how Ca2+ signals control the formation of dental enamel and validate this methodology to analyze physiologic properties of enamel-forming cells.
Author Contributions
M.K. Nurbaeva, M. Eckstein, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; M.L. Snead, contributed to conception, design, and data interpretation, drafted and critically revised the manuscript; S. Feske, R.S. Lacruz, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was funded by National Institutes of Health (NIH) / National Institutes of Dental and Craniofacial Research K99/R00 award (DE022799) to R.S.L., NIH grant AI097302 to S.F., and NIH grant DE13045 to M.L.S.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bennett DL, Cheek TR, Berridge MJ, De Smedt H, Parys JB, Missiaen L, Bootman MD. 1996. Expression and function of ryanodine receptors in nonexcitable cells. J Biol Chem. 271(11):6356–6362. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 1993. Inositol trisphosphate and calcium signalling. Nature. 361(6410):315–325. [DOI] [PubMed] [Google Scholar]
- Bird GS, DeHaven WI, Smyth JT, Putney JW., Jr. 2008. Methods for studying store-operated calcium entry. Methods. 46(3):204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang Y, Mendoza J, Denbesten P. 2009. Calcium-mediated differentiation of ameloblast lineage cells in vitro. J Exp Zool B Mol Dev Evol. 312(5):458–464. [DOI] [PubMed] [Google Scholar]
- Chen LS, Couwenhoven RI, Hsu D, Luo W, Snead ML. 1992. Maintenance of amelogenin gene expression by transformed epithelial cells of mouse enamel organ. Arch Oral Biol. 37(10):771–778. [DOI] [PubMed] [Google Scholar]
- Descroix V, Kato S, Lezot F, Berdal A. 2010. Physiopathology of dental rickets in vitamin D receptor-ablated mice. J Dent Res. 89(12):1427–1432. [DOI] [PubMed] [Google Scholar]
- Dhamija S, Krebsbach PH. 2001. Role of Cbfa1 in ameloblastin gene transcription. J Biol Chem. 276(37):35159–35164. [DOI] [PubMed] [Google Scholar]
- Feske S. 2009. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 231(1):189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. 2001. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2(4):316–324. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. 2006. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 441(7090):179–185. [DOI] [PubMed] [Google Scholar]
- Franklin IK, Winz RA, Hubbard MJ. 2001. Endoplasmic reticulum Ca2+-ATPase pump is up-regulated in calcium-transporting dental enamel cells: a non-housekeeping role for SERCA2b. Biochem J. 358(Pt 1):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Singh RK, Nanda K, Chatterjee M, Tiwari A, Sundaram S, Gupta D, Chugh A, Dastidar S, Ray A. 2009. Ratiometric Ca+2 measurement in human recombinant muscarinic receptor subtypes using the Flexstation scanning fluorometer. J Recept Signal Transduct Res. 29(2):100–106. [DOI] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Exton JH. 2004. Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem. 279(47):49420–49429. [DOI] [PubMed] [Google Scholar]
- Lacruz RS, Brookes SJ, Wen X, Jimenez JM, Vikman S, Hu P, White SN, Lyngstadaas SP, Okamoto CT, Smith CE, et al. 2013. Adaptor protein complex 2–mediated, clathrin-dependent endocytosis, and related gene activities, are a prominent feature during maturation stage amelogenesis. J Bone Miner Res. 28(3):672–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Hacia JG, Bromage TG, Boyde A, Lei Y, Xu Y, Miller JD, Paine ML, Snead ML. 2012. The circadian clock modulates enamel development. J Biol Rhythms. 27(3):237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Smith CE, Bringas P, Jr, Chen YB, Smith SM, Snead ML, Kurtz I, Hacia JG, Hubbard MJ, Paine ML. 2012. Identification of novel candidate genes involved in mineralization of dental enamel by genome-wide transcript profiling. J Cell Physiol. 227(5):2264–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Broad LM, Bird GS, Putney JW., Jr. 2001. Signaling pathways underlying muscarinic receptor-induced [Ca2+]i oscillations in HEK293 cells. J Biol Chem. 276(8):5613–5621. [DOI] [PubMed] [Google Scholar]
- Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH. 1992. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem. 267(20):14483–14489. [PubMed] [Google Scholar]
- McCarl CA, Picard C, Khalil S, Kawasaki T, Rother J, Papolos A, Kutok J, Hivroz C, Ledeist F, Plogmann K, et al. 2009. ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J Allergy Clin Immunol. 124(6):1311–1318.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J, Pozzan T. 1998. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem Sci. 23(1):10–14. [DOI] [PubMed] [Google Scholar]
- Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T. 2012. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 109(28):11282–11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura R, Shibukawa Y, Muramatsu T, Hashimoto S, Nakagawa K, Tazaki M, Shimono M. 2010. Sodium-calcium exchangers in rat ameloblasts. J Pharmacol Sci. 112(2):223–230. [DOI] [PubMed] [Google Scholar]
- Parekh AB. 2010. Store-operated CRAC channels: function in health and disease. Nat Rev Drug Discov. 9(5):399–410. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr. 2005. Store-operated calcium channels. Physiol Rev. 85(2):757–810. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C, McCarl CA, Papolos A, Khalil S, Luthy K, Hivroz C, LeDeist F, Rieux-Laucat F, Rechavi G, Rao A, et al. 2009. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med. 360(19):1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW. 2009. Capacitative calcium entry: from concept to molecules. Immunol Rev. 231(1):10–22. [DOI] [PubMed] [Google Scholar]
- Putney JW. 2010. Pharmacology of store-operated calcium channels. Mol Interv. 10(4):209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr. 2001. Cell biology: channelling calcium. Nature. 410(6829):648–649. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Mancarella S, Songsawad D, Tourkova IL, Barnett JB, Gill DL, Soboloff J, Blair HC. 2012. Gene disruption of the calcium channel Orai1 results in inhibition of osteoclast and osteoblast differentiation and impairs skeletal development. Lab Invest. 92(7):1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J, Simanian EJ, Tuggy SY, Bartlett JD, Snead ML, Sugiyama T, Paine ML. 2014. Comparison of two mouse ameloblast-like cell lines for enamel-specific gene expression. Front Physiol. 5:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Feske S. 2012. Regulation of lymphocyte function by ORAI and STIM proteins in infection and autoimmunity. J Physiol. 590(Pt 17):4157–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmer JP, Fincham AG. 1995. Molecular mechanisms of dental enamel formation. Crit Rev Oral Biol Med. 6(2):84–108. [DOI] [PubMed] [Google Scholar]
- Smith CE, Hu Y, Richardson AS, Bartlett JD, Hu JC, Simmer JP. 2011. Relationships between protein and mineral during enamel development in normal and genetically altered mice. Eur J Oral Sci. 119 Suppl 1:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopulos PB, Seo MD, Enomoto M, Amador FJ, Ishiyama N, Ikura M. 2012. Themes and variations in ER/SR calcium release channels: structure and function. Physiology. 27(6):331–342. [DOI] [PubMed] [Google Scholar]
- Takemura H, Hughes AR, Thastrup O, Putney JW., Jr. 1989. Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells: evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 264(21):12266–12271. [PubMed] [Google Scholar]
- Wang S, Choi M, Richardson AS, Reid BM, Seymen F, Yildirim M, Tuna E, Gencay K, Simmer JP, Hu JC. 2014. STIM1 and SLC24A4 are critical for enamel maturation. J Dent Res. 93(7):94S-100S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhou YL, Gonzalez FJ, Snead ML. 2007. CCAAT/enhancer-binding protein delta (C/EBPdelta) maintains amelogenin expression in the absence of C/EBPalpha in vivo. J Biol Chem. 282(41):29882–29889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhou YL, Luo W, Zhu QS, Levy D, MacDougald OA, Snead ML. 2006. NF-Y and CCAAT/enhancer-binding protein alpha synergistically activate the mouse amelogenin gene. J Biol Chem. 281(23):16090–16098. [DOI] [PubMed] [Google Scholar]
- Zhou YL, Snead ML. 2000. Identification of CCAAT/enhancer-binding protein alpha as a transactivator of the mouse amelogenin gene. J Biol Chem. 275(16):12273–12280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.