Abstract
A complex feedback mechanism between parathyroid hormone (PTH), 1,25(OH)2D3 (1,25D), and fibroblast growth factor 23 (FGF-23) maintains mineral homeostasis, in part by regulating calcium and phosphate absorption/reabsorption. Previously, we showed that 1,25D regulates mineral homeostasis by repressing dentin matrix protein 1 (DMP1) via the vitamin D receptor pathway. Similar to 1,25D, PTH may modulate DMP1, but the underlying mechanism remains unknown. Immortalized murine cementoblasts (OCCM.30), similar to osteoblasts and known to express DMP1, were treated with PTH (1–34). Real-time quantitative polymerase chain reaction (PCR) and Western blot revealed that PTH decreased DMP1 gene transcription (85%) and protein expression (30%), respectively. PTH mediated the downregulation of DMP1 via the cAMP/protein kinase A (PKA) pathway. Immunohistochemistry confirmed the decreased localization of DMP1 in vivo in cellular cementum and alveolar bone of mice treated with a single dose (50 µg/kg) of PTH (1–34). RNA-seq was employed to further identify patterns of gene expression shared by PTH and 1,25D in regulating DMP1, as well as other factors involved in mineral homeostasis. PTH and 1,25D mutually upregulated 36 genes and mutually downregulated 27 genes by ≥2-fold expression (P ≤ 0.05). Many identified genes were linked with the regulation of bone/tooth homeostasis, cell growth and differentiation, calcium signaling, and DMP1 transcription. Validation of RNA-seq results via PCR array confirmed a similar gene expression pattern in response to PTH and 1,25D treatment. Collectively, these results suggest that PTH and 1,25D share complementary effects in maintaining mineral homeostasis by mutual regulation of genes/proteins associated with calcium and phosphate metabolism while also exerting distinct roles on factors modulating mineral metabolism. Furthermore, PTH may modulate phosphate homeostasis by downregulating DMP1 expression via the cAMP/PKA pathway. Targeting genes/proteins mutually governed by PTH and 1,25D may be a viable approach for designing new therapies for preserving mineralized tissue health.
Keywords: parathyroid hormone; 1,25D; dentin matrix protein 1; mineral homeostasis; RNA sequencing; hormonal regulation
Introduction
A complex feedback mechanism between parathyroid hormone (PTH), 1,25(OH)2D3 (1,25D), and fibroblast growth factor 23 (FGF23) maintains mineral homeostasis by regulating calcium and phosphate absorption/reabsorption (Krajisnik et al. 2007; Bergwitz and Jüppner 2010; Lavi-Moshayoff et al. 2010). PTH is the principal hormone that regulates serum calcium concentration by directly acting on the kidneys, bones, and small intestine (Mundy and Guise 1999). PTH targets the kidneys to increase reabsorption of calcium from distal tubules and increase production of 1,25D, which in turn targets the small intestine to enhance calcium and phosphate absorption. Furthermore, by indirectly mediating osteoclastic bone resorption, both PTH and 1,25D enhance calcium release from bones (McSheehy and Chambers 1987; Britto et al. 1994). In turn, the high serum calcium levels activate calcium-sensing receptors located on parathyroid cells to control PTH secretion, thereby creating a negative feedback mechanism to control PTH levels (Tfelt-Hansen and Brown 2005). Independent of the calcium-mediated negative feedback mechanism, an increase in 1,25D concentration represses PTH secretion to regulate PTH levels (Haussler et al. 2013). Thus, the intricate interaction between calcium, PTH, and 1,25D helps maintain calcium homeostasis.
FGF23 is the principal endocrine regulator of serum phosphate concentration and is primarily secreted by osteocytes and osteoblasts (Lanske and Razzaque 2014). FGF23 acts on the kidneys to increase phosphate excretion and decrease activity of the 25(OH)D-1α-hydroxylase, which converts 25(OH)D to 1,25D, thereby diminishing intestinal absorption of calcium and phosphate. Both systemic and local bone-derived factors regulate FGF23 promoter activity (Komaba and Fukagawa 2010). FGF23 levels are modulated by phosphate, 1,25D, PTH, and other mineral homeostasis factors, including phosphate regulating endopeptidase homolog, X-linked (PHEX), and the members of the small integrin-binding ligand interacting glycoproteins (SIBLING) family, including dentin matrix protein (DMP1) and matrix extracellular phosphoglycoprotein (MEPE) (Martin et al. 2011).
Previously, we showed that 1,25D regulates FGF23 expression by repressing DMP1 via the VDR pathway (Nociti et al. 2014). It has been hypothesized that similar to 1,25D, PTH may indirectly regulate FGF23 expression by modulating Dmp1 and/or Sost as an intermediary step (Bellido et al. 2005; Gooi et al. 2014). We aimed to define the molecular mechanisms involved in PTH-mediated regulation of Dmp1. Furthermore, using RNA sequencing (RNA-seq), we aimed to identify specific genes and signaling pathways mutually regulated by both PTH and 1,25D.
Materials and Methods
Cell Culture and Treatments
Immortalized murine cementoblasts (OCCM.30) were used in cell culture experiments (D’Errico et al. 2000; Nociti et al. 2014). Details of cell culture and PTH and 1,25D treatments are described in the Supplementary Materials and Methods in the Appendix.
Real-time Quantitative Polymerase Chain Reaction (RT-qPCR)
For gene expression analyses, total RNA was isolated using the Qiagen RNeasy Micro kit (Valencia, CA, USA). Complementary DNA (cDNA) synthesis and quantitative polymerase chain reaction were performed on the Roche Lightcycler 480 II System (Roche Applied Science, Indianapolis, IN, USA) using intron-spanning primers (Nociti et al. 2014). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene for the internal control.
SDS-PAGE and Western Blot Analysis
OCCM.30 cells were treated with PTH (1–34) under serum-free conditions for 48 h (Nociti et al. 2014). Rabbit anti-DMP1 C-terminus antibodies (Huang et al. 2008) and IRDye secondary antibodies (LI-COR, Lincoln, NE, USA) were used to detect DMP1 in Western blot. An imaging system (ODYSSEY CLx; LI-COR) with Image Studio Software (LI-COR) was used to quantify protein levels.
Immunofluorescence
OCCM.30 cells were treated with PTH (1–34) for 48 h, then fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) for 15 min and permeabilized with 0.25% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) for 10 min. The cells were blocked in 1% bovine serum albumin (BSA) with 0.3 M glycine (Sigma-Aldrich) and incubated with rabbit anti-DMP1 C-terminus antibodies for 1 h at room temperature. ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to quantify staining.
Immunohistochemistry
Animal studies were approved by the University of Michigan Committee on the Use and Care of Animals (UCUCA). Mandibles were collected from mice treated with a once-daily subcutaneous injection of 50 µg/kg PTH (1–34) (n = 5) or vehicle (n = 3) at age 16 wk for 6 wk (Novince et al. 2012). Decalcified samples were embedded in paraffin and 5-µm serial sections were prepared for immunohistochemistry using primary antibody against the DMP1 C-terminus. Antibody detection was performed using DAB Peroxidase (HRP) Substrate Kit (Vector Laboratories, Burlingame, CA, USA). Sections were counterstained with hematoxylin.
RNA-Sequencing (RNA-seq)
Total RNA was extracted as described above, and RNA integrity was determined with the Bioanalyzer 2100 using the RNA 6000 Nano kit (Agilent Technologies, Palo Alto, CA, USA). RNA-seq methods are described in detail in the Supplementary Materials and Methods in the Appendix.
Statistical Analysis
Intergroup differences were evaluated by 1-way analysis of variance (ANOVA) followed by the post hoc Tukey test or by a Student’s t test (Prism; GraphPad Software, La Jolla, CA, USA).
Results
PTH Downregulates DMP1 Expression in Cementoblasts
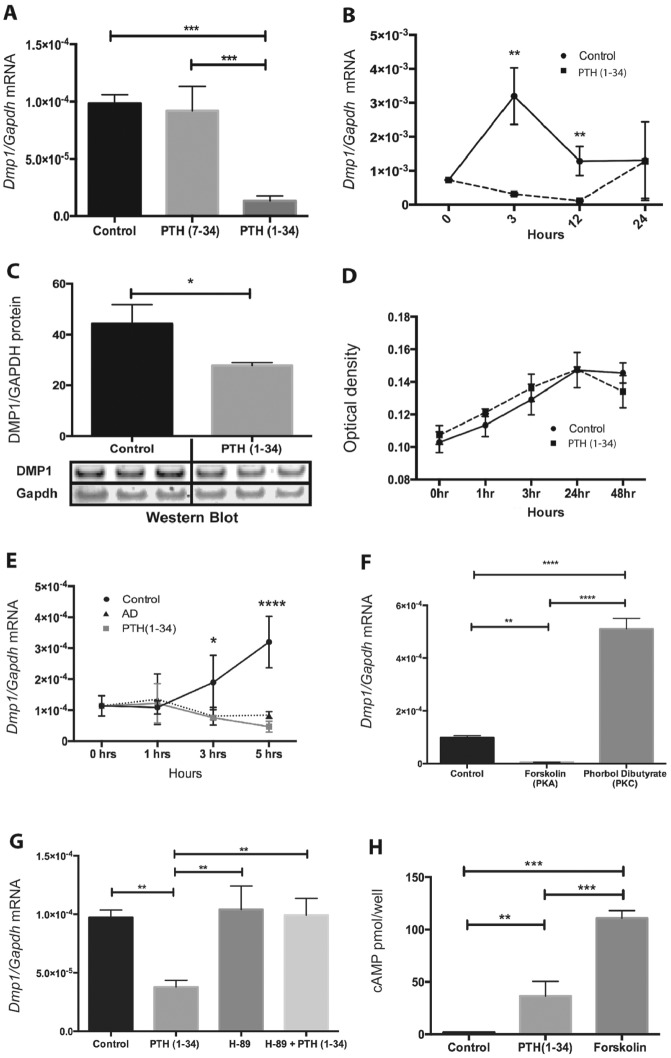
PTH (1–34) at 10–7 M significantly downregulated (86%) Dmp1 messenger RNA (mRNA) expression at 3 h in OCCM.30 cells (Fig. 1A). The PTH/PTHrP receptor antagonist, PTH (7–34), had no effect on Dmp1 mRNA. The inhibitory effect of PTH on Dmp1 expression was time dependent, with the most consistent potent effect noted at 3 h following treatment (Fig. 1B). Western blot analysis confirmed a 33% decrease of DMP1 protein in cells treated with PTH (1–34) for 48 h (Fig. 1C). Cell numbers over time (48 h) were not affected by PTH treatment (Fig. 1D). Furthermore, PTH (1–34) at 10–7 M downregulated Dmp1 in osteocyte-like MLO-A5 cells (data not shown) similar to 1,25D (Nociti et al. 2014), confirming the ability of both 1,25 and PTH to downregulate Dmp1 in “cyte”-like cells.
Figure 1.
Parathyroid hormone (PTH) downregulates Dmp1 via cAMP/protein kinase A (PKA) signaling in cementoblasts. (A) PTH (1–34) significantly downregulated Dmp1 messenger RNA (mRNA) expression by 86% in OCCM.30, while the antagonist PTH (7–34) expression had no effect. (B) PTH (1–34) (10–7 M) significantly down-regulates Dmp1 mRNA expression at 3 h and 12 h, with potent effect noted at 3 h. (C) Western blot demonstrates significant reduction in dentin matrix protein 1 (DMP1) by 33% in the total cell lysate of OCCM.30 harvested 48 h after PTH (1–34) (10–7M) treatment. (D) Cell enumeration assays showed that OCCM.30 cell numbers over time were not affected by PTH (1–34) (10–7 M) treatment. (E) Pretreatment of OCCM.30 with transcription inhibitor actinomycin D (AD) (5 µg/mL) revealed that PTH (1–34) (10–7 M) did not affect Dmp1 mRNA stability, suggesting a direct effect at the transcriptional level. (F) Forskolin (10 µM), a PKA activator, had similar effects as PTH (1–34), decreasing Dmp1 expression by 94%, while the protein kinase C (PKC) activator phorbol dibutyrate increased Dmp1 expression by ~5-fold. (G) PKA inhibitor H-89 (10 µM) completely inhibited PTH (1–34)–mediated downregulation of Dmp1 in OCCM.30. (H) cAMP levels were elevated in PTH (1–34) and forskolin-treated cells. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 as calculated by 1-way analysis of variance.
cAMP/PKA Signaling Is Required for PTH-Mediated Downregulation of Dmp1 in Cementoblasts
PTH treatment of OCCM.30 cells pretreated with actinomycin D did not accelerate the degradation of Dmp1 mRNA (Fig. 1E), indicating that PTH effects on Dmp1 were at the transcription level. Treatment of OCCM.30 cells with the protein kinase A (PKA) agonist, forskolin, had effects similar to PTH, while the protein kinase C (PKC) agonist, phorbol dibutyrate, enhanced Dmp1 mRNA levels (Fig. 1F), suggesting an effect of PKC on basal Dmp1 levels. In addition, the PKA antagonist H-89 blocked the PTH-mediated downregulation of Dmp1 (Fig. 1G). The increase in cAMP levels observed in both PTH and forskolin treatments (Fig. 1H) confirmed that the cAMP/PKA pathway was the primary mechanism involved in PTH-mediated downregulation of Dmp1.
PTH Reduces DMP1 Protein Localization in Cementoblasts and Dentoalveolar Tissues
Previously, our group and others have published that intermittent PTH administration in rats increases bone formation in the mandible (Hunziker et al. 2000; Kuroshima et al. 2013). The mice used in this study displayed anabolic actions of PTH based on histomorphometric measures as well as serum biochemical data (Novince et al. 2012). Immunofluorescence staining showed that the DMP1 C-terminal peptide was localized in both the nucleus and cytoplasm of vehicle-treated OCCM .30 cells (Fig. 2A, B). Treatment of cells with PTH (1–34) for 48 h resulted in a significant decrease (P < 0.05) in DMP1 C-terminal peptide localization in the nucleus (18%) and cytoplasm (38%) (Fig. 2C, D). Decreased nuclear expression of DMP1 in cementoblasts treated with PTH indicates a potential regulatory function of DMP1 in the nucleus of cells from mineralized tissue that needs to be further investigated. Furthermore, immunohistochemistry revealed decreased localization of DMP1 C-terminal peptide in matrices of alveolar bone, odontoblasts, and cellular cementum of PTH (1–34)–treated mice (Fig. 2E–H).
Figure 2.
Parathyroid hormone (PTH) (1–34) reduces dentin matrix protein 1 (DMP1) levels in mineralized tissue in situ and in cementoblasts in vitro. (A–D) OCCM.30 were treated with 10–7 M PTH or vehicle for 48 h. Compared with the nuclear and cytoplasmic localization of DMP1 C-terminal peptide (green) in controls (A), PTH (1–34) treatment for 48 h decreased DMP1 C-terminal peptide in both the nucleus and cytoplasm (B). The nucleus area was circled by blue lines marked by DAPI staining (not shown). DMP1 C-terminal peptides were downregulated by 18% in the nucleus (C) and by 38% in the cytoplasm (D) of OCCM.30, confirming that PTH (1–34) treatment significantly decreased DMP1 protein expression in both subcellular compartments. (E–H) Immunohistochemistry (IHC) staining of mandible sections from control (n = 3) or PTH (1–34)–treated mice (n = 5) revealed that PTH (1–34) decreased DMP1 localization in matrices of both cellular cementum and alveolar bone (indicated by yellow arrows). Bars indicate 100 µm in panels A and B. DEN, dentin; CC, cellular cementum; PDL, periodontal ligament; AB, alveolar bone. ***P < 0.001, ****P < 0.0001 as calculated by the Student’s t test.
RNA-seq Analysis of Patterns of Gene Expression Shared by PTH and 1,25D
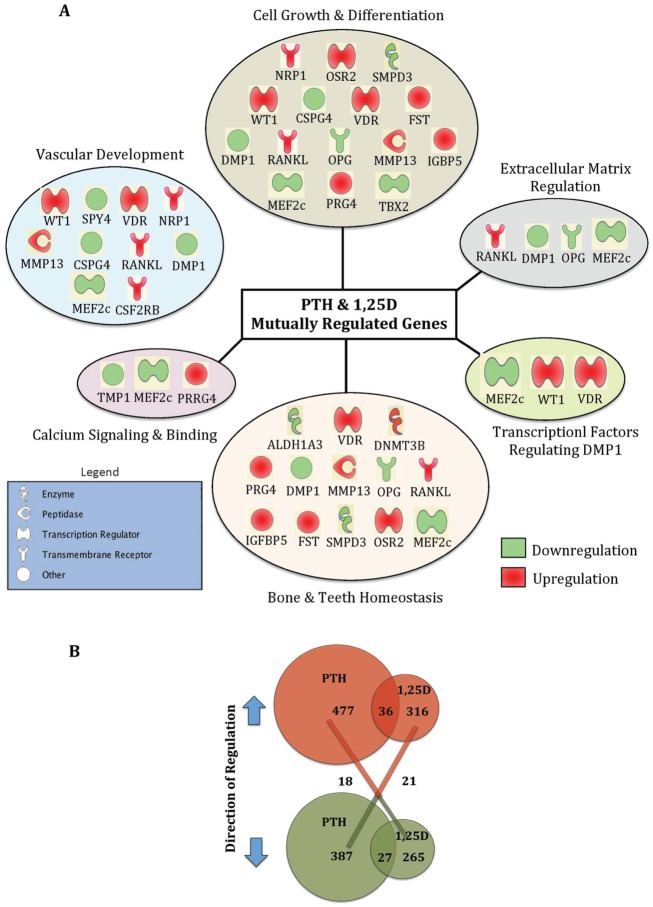
Using RNA-seq analysis, we identified patterns of gene expression shared by PTH and 1,25D in regulating calcium and phosphate homeostasis, including those associated with cementogenesis. A total of 24,700 genes were differentially expressed in the PTH treatment group, including 477 genes significantly upregulated and 316 genes significantly downregulated ≥2-fold (P < 0.05) (Figure 3). A total of 24,273 genes were differentially expressed in the 1,25D treatment group, including 387 genes significantly upregulated and 265 genes significantly downregulated ≥2-fold (P < 0.05).
Figure 3.
Parathyroid hormone (PTH) and 1,25(OH)2D3 (1,25D) regulate extensive target genes in cementoblasts. (A) Selected genes mutually regulated by both PTH and 1,25D were manually grouped by category and delineated based on their degree of regulation (red, upregulated; green, downregulated). (B) Venn diagram of PTH and 1,25D upregulated (red), downregulated (green), and inversely regulated genes.
Genes mutually regulated by PTH and 1,25D
PTH and 1,25D mutually upregulated 36 genes and downregulated 27 genes ≥2-fold (P < 0.05). Functional categories with subsets of representative genes altered by both PTH and 1,25D are outlined in Figure 3A with a complete gene list in the Table. In addition, PTH and 1,25D inversely regulated 39 genes; that is, 18 genes upregulated by PTH were downregulated by 1,25D, and 21 genes downregulated by PTH were upregulated by 1,25D (Fig. 3B). Ingenuity pathway analysis of genes significantly upregulated and downregulated by both PTH and 1,25D were categorized as hits into functional categories associated with bone and teeth homeostasis, cell growth and differentiation, extracellular matrix regulation, calcium signaling and binding, and transcription factors that potentially modulate Dmp1 expression (Fig. 3A). We also identified gene categories involving growth of connective tissue and vascular development, indicating that PTH and 1,25D may be involved in novel functional aspects of skeletal biology or in paracrine regulation of other cell types. In addition, some of the key markers for mineralized tissue such as bone sialoprotein (Bsp), osterix (Osx), and bone γ-carboxyglutamate protein (Bglap) were also identified by RNA-seq.
Table.
Genes Mutually Regulated by Parathyroid Hormone (PTH) and 1,25(OH)2D3 (1,25D).
| Gene Symbol | Gene Name | Type | PTH Fold Changea | 1,25D Fold Changea |
|---|---|---|---|---|
| Adam11 | ADAM metallopeptidase domain 11 | Peptidase | +2.59 | +3.00 |
| Csf2rb | Colony stimulating factor 2 receptor, beta, low-affinity (granulocyte-macrophage) | Transmembrane receptor | +5.50 | +7.16 |
| Csf2rb2 | Colony stimulating factor 2 receptor, beta, low-affinity (granulocyte-macrophage) | Transmembrane receptor | +3.43 | +14.43 |
| Dnmt3b | DNA (cytosine-5-)–methyltransferase 3 beta | Enzyme | +2.03 | +12.58 |
| E330013P04Rik | RIKEN cDNA E330013P04 gene | Other | +2.67 | +2.59 |
| Fgl2 | Fibrinogen-like 2 | Peptidase | +2.34 | +11.96 |
| Fmo1 | Flavin containing monooxygenase 1 | Enzyme | +5.20 | +12.89 |
| Fst | Follistatin | Other | +2.58 | +11.98 |
| Gfra1 | GDNF family receptor alpha 1 | Transmembrane receptor | +7.90 | +4.32 |
| Glyat | Glycine-N-acyltransferase | Enzyme | +5.66 | +38.00 |
| Gm10445 | Predicted gene 10445 | Other | +15.78 | +46.18 |
| Gzmb | Granzyme B | Peptidase | +33.72 | +6.83 |
| Gzmc | Granzyme C | Peptidase | +10.93 | +2.31 |
| Igfbp5 | Insulin-like growth factor binding protein 5 | Other | +6.72 | +22.30 |
| Il2rb | Interleukin 2 receptor, beta | Transmembrane receptor | +5.23 | +2.73 |
| Mfap3l | Microfibrillar-associated protein 3-like | Other | +4.51 | +4.68 |
| Mmp13 | Matrix metallopeptidase 13 (collagenase 3) | Peptidase | +4.83 | +3.16 |
| Nrp1 | Neuropilin 1 | Transmembrane receptor | +3.33 | +2.08 |
| Osr2 | Odd-skipped related 2 (Drosophila) | Transcription regulator | +2.71 | +4.69 |
| Parm1 | Prostate androgen-regulated mucin-like protein 1 | Other | +5.89 | +2.87 |
| Pls1 | Plastin 1 | Other | +2.01 | +3.76 |
| Prg4 | Proteoglycan 4 | Other | +8.82 | +196.62 |
| Prrg4 | Proline rich Gla (G-carboxyglutamic acid) 4 | Other | +17.12 | +125.72 |
| Rad52 | RAD52 homolog (Saccharomyces cerevisiae) | Other | +2.79 | +2.23 |
| Slc25a19 | Solute carrier family 25 (mitochondrial thiamine pyrophosphate carrier), member 19 | Transporter | +4.96 | +6.02 |
| Slc37a2 | Solute carrier family 37 (glycerol-3-phosphate transporter), member 2 | Transporter | +4.01 | +3.31 |
| Slco1a6 | Solute carrier organic anion transporter family, member 1a6 | Transporter | +4.64 | +7.27 |
| Slpi | Secretory leukocyte peptidase inhibitor | Other | +3.15 | +81.53 |
| Spata31d1b | Family with sequence similarity 75, member D1 | Other | +7.58 | +45.21 |
| Tnfsf11/RANKL | Tumor necrosis factor (ligand) superfamily, member 11 | Cytokine | +8.39 | +4.93 |
| Tsnaxip1 | Translin-associated factor X interacting protein 1 | Other | +2.00 | +2.58 |
| Ttc39b | Tetratricopeptide repeat domain 39B | Other | +2.07 | +2.00 |
| Usp2 | Ubiquitin specific peptidase 2 | Peptidase | +3.50 | +2.26 |
| Vdr | Vitamin D (1,25-dihydroxyvitamin D3) receptor | Nuclear receptor | +8.23 | +6.54 |
| Wdr72 | WD repeat domain 72 | Other | +46.48 | +32.63 |
| Wt1 | Wilms tumor 1 | Transcription regulator | +21.83 | +38.61 |
| 4833403I15Rik | Laeverin | Peptidase | −2.85 | −2.34 |
| Adamts15 | ADAM metallopeptidase with thrombospondin type 1 motif, 15 | Peptidase | −6.75 | −33.15 |
| Aldh1a3 | Aldehyde dehydrogenase 1 family, member A3 | Enzyme | −2.23 | −2.03 |
| Astn1 | Astrotactin 1 | Other | −2.81 | −2.76 |
| Cacna1i | Calcium channel, voltage-dependent, T type, alpha 1I subunit | Ion channel | −2.44 | −2.48 |
| Ces1g | Carboxylesterase 1G | Enzyme | −2.06 | −2.52 |
| Cspg4 | Chondroitin sulfate proteoglycan 4 | Other | −2.21 | −2.58 |
| Dmp1 | Dentin matrix acidic phosphoprotein 1 | Other | −5.42 | −2.52 |
| Fam198b | Family with sequence similarity 198, member B | Other | −2.57 | −3.39 |
| Gcnt2 | Glucosaminyl (N-acetyl) transferase 2, I-branching enzyme (I blood group) | Enzyme | −8.11 | −2.35 |
| Grhl3 | Grainyhead-like 3 (Drosophila) | Other | −2.18 | −10.21 |
| Kcnf1 | Potassium voltage-gated channel, subfamily F, member 1 | Ion channel | −2.94 | −2.44 |
| Macc1 | Metastasis associated in colon cancer 1 | Other | −3.08 | −2.70 |
| Mef2c | Myocyte enhancer factor 2C | Transcription regulator | −2.21 | −2.49 |
| Odf3l1 | Outer dense fiber of sperm tails 3-like 1 | Other | −2.07 | −26.91 |
| Olfr874 | Olfactory receptor, family 8, subfamily B, member 12 | G protein–coupled receptor | −3.12 | −47.69 |
| Ptger4 | Prostaglandin E receptor 4 (subtype EP4) | G protein–coupled receptor | −2.23 | −2.59 |
| Rab11fip4 | RAB11 family interacting protein 4 (class II) | Other | −6.48 | −2.40 |
| Scml4 | Sex comb on midleg-like 4 (Drosophila) | Other | −3.02 | −3.05 |
| Smpd3 | Sphingomyelin phosphodiesterase 3, neutral membrane (neutral sphingomyelinase II) | Enzyme | −3.46 | −6.11 |
| Spns2 | Spinster homolog 2 (Drosophila) | Other | −2.18 | −2.79 |
| Spry4 | Sprouty homolog 4 (Drosophila) | Other | −2.16 | −2.41 |
| Tbx2 | T-box 2 | Transcription regulator | −3.16 | −3.30 |
| Tnfrsf11b/OPG | Tumor necrosis factor receptor superfamily, member 11b | Transmembrane receptor | −2.28 | −2.42 |
| Tpm1 | Tropomyosin 1 (alpha) | Other | −2.03 | −2.39 |
| Usp43 | Ubiquitin specific peptidase 43 | Peptidase | −2.40 | −2.54 |
| Xaf1 | XIAP associated factor 1 | Other | −2.61 | −2.28 |
+ represents upregulation and − represents downregulation (P < 0.05).
Both PTH and 1,25D regulated several genes encoding components of signaling pathways, including Wnt/β-catenin, BMP, NF-κB, VDR/RXR activation, and calcium signaling. In addition, PTH activated the PKA signaling pathway, considered to have anabolic effects on bone (Siddappa et al. 2008). These gene lists and extended results can be found in Supplementary Tables 2 and 4, available online. Genes encoding specific transcription factors regulated by PTH and 1,25D are listed in Supplementary Tables 3 and 5, among which WT1, VDR, and Mef2C were mutually regulated by both PTH and 1,25D. Based on data analysis using Genomatix (Ann Arbor, MI, USA), WT1 (binding sequence: tagtggg CGGGagacatgg), VDR (binding seq uence: cagcGAAGttctcg aagaggg at ct), and MEF2C (binding sequence: ttgctttCTAAatatatatcttt), all have potential binding sites on the Dmp1 promoter.
We also noted that both PTH and 1,25D upregulated proteoglycan 4 (Prg4) by 9-fold and 197-fold, respectively. These results are consistent with our previous study that shows PTH enhances endochondral bone formation and the attainment of peak trabecular bone mass by upregulating Prg4 mRNA expression in bone and liver (Novince et al. 2012). RNA-seq results from the present study suggest that 1,25D may also regulate Prg4, contributing to skeletal/cementum homeostasis.
We also explored endogenous factors important for phosphate regulation. PTH downregulated expression of Dmp1 (5-fold), Sost (4-fold), and Mepe (4-fold) in OCCM.30, but only Dmp1 downregulation was statistically significant (P < 0.05) (Table). Confirming our previous study using real-time quantitative polymerase chain reaction (RT-qPCR) (Nociti et al. 2014), 1,25D treatment significantly downregulated Dmp1 (3-fold) and Phex (2-fold) and significantly upregulated Fgf23 mRNA (48-fold).
Validation of RNA-seq by PCR Array
We validated RNA-seq results using the PCR array for a panel of 39 genes. PCR results confirmed transcriptional changes similar to RNA-seq, including genes coregulated in the same direction (e.g., Dmp1, Dspp, Vdr, Tnfsf11, and Spp1) and those inversely regulated by PTH and 1,25D (e.g., Runx2, Fgfr2, and Hdac7). Transcription factors such as Mef2c and Wt1 implicated in regulation of Dmp1 expression were also validated by PCR. A subset of representative genes validated by PCR array is shown in Figure 4, with a complete gene list provided in Supplementary Table 6. Notably, most of the genes in Supplementary Table 6 were consistent with RNA-seq results, with 85% matching in the PTH-treated group and 90% matching in the 1,25D group. However, PCR array results for Fgf23, Phex, and Sost were inconsistent with RNA-seq results.
Figure 4.
Custom polymerase chain reaction (PCR) array validates the RNA-seq data and identifies complex responses to parathyroid hormone (PTH) (1–34) or 1,25(OH)2D3 (1,25D). Cementoblasts treated with vehicle (control), PTH (1–34) (10–7 M) (A), or 1,25D (10 nM) (B) were evaluated for expression of the indicated genes. Samples were analyzed in triplicate with standard deviations. *P < 0.05, **P < 0.01, ****P < 0.0001, as calculated by the Student’s t test.
Discussion
Mineral homeostasis is maintained by a complex feedback mechanism driven by PTH, 1,25D, and FGF23, but many molecular links between these factors remain unidentified and poorly understood (Krajisnik et al. 2007; Lavi-Moshayoff et al. 2010). We demonstrate for the first time that PTH, like 1,25D, downregulates Dmp1 expression in murine cementoblasts via PKA/cAMP signaling and decreases DMP1 protein in mouse dentoalveolar tissues. Furthermore, through RNA-seq analysis, we identified transcriptomes mutually regulated by PTH and 1,25D that implicate gene networks involved in bone and tooth homeostasis, calcium signaling and binding, and regulation of extracellular matrix. Based on these data, we hypothesize that PTH and 1,25D both repress Dmp1 as an intermediary step for modulating downstream targets, including FGF23, and also regulate multiple aspects of skeletal and dental development and homeostasis. However, downregulation of Dmp1 may not be a universal phenomenon in mineralized tissues, and there may be cell-specific differences and additional local modifiers that affect cell response.
Dmp1 Is a Target Gene Regulated by PTH
DMP1 is an extracellular matrix protein expressed in osteocytes, osteoblasts, odontoblasts, and cementoblasts (D’Souza et al. 1997; MacDougall et al. 1998; Feng et al. 2002). DMP1 plays an important role in mineral homeostasis via its direct effect on mineralization and indirect effect on systemic phosphate homeostasis that are not well understood (He and George 2004; Ling et al. 2005). Dmp1–/– mice exhibit hypomineralized bones and teeth as a consequence of hypophosphatemia related to elevated FGF23 levels (Ye et al. 2004; Feng et al. 2006; Zhang et al. 2011).
Because of the profound and pivotal function of DMP1 in regulating FGF23 expression and systemic phosphate metabolism, we aimed to better understand how 1,25D and PTH regulate DMP1 expression. Previously, we reported that 1,25D represses Dmp1 expression via the VDR pathway and that class I histone deacetylase (HDAC) (1/2/3) is required (Nociti et al. 2014). Here we show that PTH downregulates Dmp1 expression in cementoblasts via a PKA/cAMP-dependent mechanism and decreases DMP1 protein in alveolar bone and cellular cementum of mice. In agreement with our findings, Bellido et al. (2005) reported that PTH downregulated DMP1 and osteocalcin in osteocytes, both of which are targets of transcription factor RUNX2. Runx2 mRNA was not significantly altered in the present study, although both PTH and 1,25D significantly regulated expression of transcription factors Mef2c, Wt1, and Vdr, which we identified as potential regulators of Dmp1 transcription. Leupin et al. (2007) demonstrated that PTH downregulates Mef2c activation by PKA-mediated control of HDAC5 translocation to the nucleus (Wein et al. 2014), where it interacts with MEF2c, while our past studies suggested that the 1,25D-responsive Dmp1 promoter sequence is located between 2.5 and 4 kb upstream of the transcription start site (Nociti et al. 2014). A search for predicted transcription factor binding sites using Genomatix suggested that WT1 might have a potential binding site in the Dmp1 promoter region, but this novel in silico finding requires further validation. Other transcription factors known to regulate Dmp1, including OSX, KLF4, and RUNX2 (Miyazaki et al. 2008; Lin et al. 2013; Liu et al. 2013), were not significantly regulated in our RNA-seq studies.
DMP1 May Serve as an Intermediary Link for 1,25D and PTH Regulation of FGF23
Interactions between PTH, 1,25D, and FGF23 direct calcium and phosphate mineral homeostasis, but several aspects of these interactions remain poorly understood. Like DMP1, PHEX is an endopeptidase-processing extracellular matrix protein expressed in bones and teeth (Rowe 2012). Loss-of-function mutations in either DMP1 or PHEX can result in increased circulating levels of FGF23, hypophosphatemia, an inappropriately normal level of 1,25D, and inherited rachitic conditions such as autosomal recessive hypophosphatemic rickets (ARHR) and X-linked hypophosphatemic rickets (XLH) (Rowe 2012). These data suggest that PHEX and DMP1 may act upstream of FGF23, but the underlying mechanisms remain unclear.
Previously, PTH and PTHrP (1–34) have been shown to downregulate PHEX through the activation of the PKA transduction pathway, resulting in increased Fgf23 expression (Alos and Ecarot 2005). PTH downregulation of DMP1 may represent an additional link between PTH and FGF23, which parallels the ability for 1,25D to downregulate PHEX and DMP1 (Rowe 2012) and provides 2 mechanisms for feed-forward regulation of FGF23.
Genes That Are Mutually Regulated by PTH and 1,25D in Cementoblasts
Beyond the finding that 1,25D and PTH both decrease DMP1, existing data support complementary roles for PTH and 1,25D in modulation of mineral homeostasis. Thus, we also aimed to identify shared patterns of gene expression of PTH and 1,25D using RNA-seq.
On a transcriptome-wide scale, RNA-seq results confirm that both PTH and 1,25D regulate genes known to direct mineral homeostasis, including transcripts associated with skeletal system development, extracellular matrix mineralization, cell-cell adhesion, ion transportation, and vascular development. A subset of genes overlapped significantly between PTH and 1,25D transcriptomes. We identified these as genes cooperatively regulated by PTH and 1,25D and further analyzed this subset for gene networks involved in regulating calcium and phosphate homeostasis. Both PTH and 1,25D coregulated genes involved in signaling pathways such as Wnt/β-catenin, BMP, NF-κB, VDR/RXR activation, and calcium signaling. In support of these findings, other studies on osteocytes have noted that both PTH and 1,25D opposed differentiation-mediated changes in gene expression during osteoblast-to-osteocyte transition but reinforced mature osteocyte markers (St. John et al. 2014; St. John et al. 2015). Furthermore, our study revealed that PTH regulated its target genes via the PKA-mediated signaling pathway and also through alternative activation pathways, similar to the findings noted by St. John et al. (2015).
Proteoglycan 4 (Prg4), which plays an important role in joint lubrication and synovial homeostasis, is a principal regulator of skeletal homeostasis and PTH anabolism. Initially, PRG4 was identified as a novel PTH responsive gene in bone marrow stromal cells and was investigated as a potential regulator of immune cells and the action of PTH on hematopoietic progenitor cells. Previously, we showed that Prg4 supports endochondral bone formation and trabecular bone mass in the developing skeleton and indirectly supports skeletal homeostasis and PTH anabolic actions by protecting joint function in the mature skeleton (Novince et al. 2012). A single subcutaneous injection of PTH (1–34) (1 µg/g) in C57BL6 wild-type mice significantly increased Prg4 mRNA in calvaria and long bone as well as liver, indicating Prg4 is a PTH-responsive gene not only in bone but also in liver and that both marrow- and liver-derived Prg4 are candidate regulators of skeletal remodeling and the anabolic actions of PTH. In the present study, we noted that both PTH and 1,25D upregulated Prg4 by 9-fold and 197-fold, respectively. These results suggest that 1,25D upregulation of Prg4 may contribute to skeletal/cementum homeostasis, but the underlying mechanism remains to be determined.
Treatments with 1,25D or PTH induce elevated FGF23 expression in cells in vitro, including osteocytes, osteoblasts, and cementoblasts (Lavi-Moshayoff et al. 2010; Nociti et al. 2014). Our RNA-seq analysis revealed that 1,25D upregulated Fgf23 transcripts significantly at 24 h. The limited effect of PTH on Fgf23 and Sost at 3 h could be attributed in part to 1) the variable influence PTH exerts on cells based on treatment time and duration (e.g., 3 h may be ideal for detecting Dmp1 changes but not other genes), 2) low basal levels of certain genes preventing detection and/or statistical assessment, and 3) the experimental design (e.g., in our previous study examining effects of 1,25D on DMP1 expression, cells were seeded at 1.0 × 104/cm2 for 5 d prior to addition of 1,25D) (Nociti et al. 2014). In contrast, in the current study, cells were seeded at a higher density (2.0 × 105/cm2) for 2 d, prior to adding PTH. Both methods generated consistent results in terms of PTH effect on DMP1 expression, although minor differences were observed in expression of other genes such as Sost and Fgf23. Future studies should explore the possibility of a time-dependent transient regulation of genes by PTH and 1,25D as well as their effects on mineralization in vitro and in vivo. A further in-depth investigation to understand factors, hormones, and pathways controlling calcium and phosphate homeostasis will have a significant impact on the diagnosis and treatment of disorders of bone and mineral metabolism.
Author Contributions
L. Wang, A.B. Tran, F.H. Nociti Jr, C.C. Krieger, K.R. Kantovitz, M.J. Somerman, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; V. Thumbigere-Math, B.L. Foster, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript; C.M. Novince, A.J. Koh, L.K. McCauley, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank Dr. Chunlin Qin (Baylor College of Dentistry) for providing the DMP1 antibody, Gustavo Gutierrez-Cruz and Steve Brooks from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) for RNA-seq analysis, and Mudita Patel (NIAMS) for technical assistance.
Footnotes
This research was supported in part by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (MJS) and the National Institute of Diabetes and Digestive and Kidney Diseases R01DK53904 (LKM).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Alos N, Ecarot B. 2005. Downregulation of osteoblast Phex expression by PTH. Bone. 37(4):589–598. [DOI] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL. 2005. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 146(11):4577–4583. [DOI] [PubMed] [Google Scholar]
- Bergwitz C, Jüppner H. 2010. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 61:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto JM, Fenton AJ, Holloway WR, Nicholson GC. 1994. Osteoblasts mediate thyroid hormone stimulation of osteoclastic bone resorption. Endocrinology. 134(1):169–176. [DOI] [PubMed] [Google Scholar]
- D’Errico JA, Berry JE, Ouyang H, Strayhorn CL, Windle JJ, Somerman MJ. 2000. Employing a transgenic animal model to obtain cementoblasts in vitro. J Periodontol. 71(1):63–72. [DOI] [PubMed] [Google Scholar]
- D’Souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB, MacDougall M. 1997. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res. 12(12):2040–2049. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Zhang J, Dallas SL, Lu Y, Chen S, Tan X, Owen M, Harris SE, Macdougall M. 2002. Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J Bone Miner Res. 17(10):1822–1831. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, et al. 2006. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 38(11):1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooi JH, Chia LY, Walsh NC, Karsdal MA, Quinn JM, Martin TJ, Sims NA. 2014. Decline in calcitonin receptor expression in osteocytes with age.J Endocrinol. 221(2):181–191. [DOI] [PubMed] [Google Scholar]
- Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, Jurutka PW. 2013. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 92(2):77–98. [DOI] [PubMed] [Google Scholar]
- He G, George A. 2004. Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J Biol Chem. 279(12):11649–11656. [DOI] [PubMed] [Google Scholar]
- Huang B, Maciejewska I, Sun Y, Peng T, Qin D, Lu Y, Bonewald L, Butler WT, Feng J, Qin C. 2008. Identification of full-length dentin matrix protein 1 in dentin and bone. Calcif Tissue Int. 82(5):401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker J, Wronski TJ, Miller SC. 2000. Mandibular bone formation rates in aged ovariectomized rats treated with anti-resorptive agents alone and in combination with intermittent parathyroid hormone. J Dent Res. 79(6):1431–1438. [DOI] [PubMed] [Google Scholar]
- Komaba H, Fukagawa M. 2010. FGF23-parathyroid interaction: implications in chronic kidney disease. Kidney Int. 77(4):292–298. [DOI] [PubMed] [Google Scholar]
- Krajisnik T, Björklund P, Marsell R, Ljunggren Ö, Åkerström G, Jonsson KB, Westin G, Larsson TE. 2007. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 195(1):125–131. [DOI] [PubMed] [Google Scholar]
- Kuroshima S, Kovacic BL, Kozloff KM, McCauley LK, Yamashita J. 2013. Intra-oral PTH administration promotes tooth extraction socket healing. J Dent Res. 92(6):553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanske B, Razzaque MS. 2014. Molecular interactions of FGF23 and PTH in phosphate regulation. Kidney Int. 86(6):1072–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. 2010. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 299(4):F882–F889. [DOI] [PubMed] [Google Scholar]
- Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, Keller H. 2007. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res. 22(12):1957–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Liu H, Sun Q, Yuan G, Zhang L, Chen Z. 2013. KLF4 promoted odontoblastic differentiation of mouse dental papilla cells via regulation of DMP1. J Cell Physiol. 228(10):2076–2085. [DOI] [PubMed] [Google Scholar]
- Ling Y, Rios HF, Myers ER, Lu Y, Feng JQ, Boskey AL. 2005. DMP1 depletion decreases bone mineralization in vivo: an FTIR imaging analysis. J Bone Miner Res. 20(12):2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Lin H, Zhang L, Sun Q, Yuan G, Zhang L, Chen S, Chen Z. 2013. miR-145 and miR-143 regulate odontoblast differentiation through targeting Klf4 and Osx genes in a feedback loop. J Biol Chem. 288(13):9261–9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall M, Gu TT, Luan X, Simmons D, Chen J. 1998. Identification of a novel isoform of mouse dentin matrix protein 1: spatial expression in mineralized tissues. J Bone Miner Res. 13(3):422–431. [DOI] [PubMed] [Google Scholar]
- Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, Quarles LD. 2011. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 25(8):2551–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSheehy PM, Chambers TJ. 1987. 1,25-Dihydroxyvitamin D3 stimulates rat osteoblastic cells to release a soluble factor that increases osteoclastic bone resorption. J Clin Invest. 80(2):425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Kanatani N, Rokutanda S, Yoshida C, Toyosawa S, Nakamura R, Takada S, Komori T. 2008. Inhibition of the terminal differentiation of odontoblasts and their transdifferentiation into osteoblasts in Runx2 transgenic mice. Arch Histol Cytol. 71(2):131–146. [DOI] [PubMed] [Google Scholar]
- Mundy GR, Guise TA. 1999. Hormonal control of calcium homeostasis. Clin Chem. 45(8 Pt 2):1347–1352. [PubMed] [Google Scholar]
- Nociti FH, Foster BL, Tran AB, Dunn D, Presland RB, Wang L, Bhattacharyya N, Collins MT, Somerman MJ. 2014. Vitamin D represses dentin matrix protein 1 in cementoblasts and osteocytes. J Dent Res. 93(2):148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novince CM, Michalski MN, Koh AJ, Sinder BP, Entezami P, Eber MR, Pettway GJ, Rosol TJ, Wronski TJ, Kozloff KM, et al. 2012. Proteoglycan 4: a dynamic regulator of skeletogenesis and parathyroid hormone skeletal anabolism. J Bone Miner Res. 27(1):11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe PS. 2012. Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit Rev Eukaryot Gene Expr. 22(1):61–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddappa R, Martens A, Doorn J, Leusink A, Olivo C, Licht R, van Rijn L, Gaspar C, Fodde R, Janssen F, et al. 2008. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc Natl Acad Sci USA. 105(20):7281–7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John HC, Bishop KA, Meyer MB, Benkusky NA, Leng N, Kendziorski C, Bonewald LF, Pike JW. 2014. The osteoblast to osteocyte transition: epigenetic changes and response to the vitamin D3 hormone. Mol Endocrinol. 28(7):1150–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John HC, Meyer MB, Benkusky NA, Carlson AH, Prideaux M, Bonewald LF, Pike JW. 2015. The parathyroid hormone–regulated transcriptome in osteocytes: parallel actions with 1,25-dihydroxyvitamin D3 to oppose gene expression changes during differentiation and to promote mature cell function. Bone. 72:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tfelt-Hansen J, Brown EM. 2005. The calcium-sensing receptor in normal physiology and pathophysiology: a review. Crit Rev Clin Lab Sci. 42(1):35–70. [DOI] [PubMed] [Google Scholar]
- Wein MN, Spatz J, Nishimori S, Doench J, Root D, Babij P, Nagano K, Baron R, Brooks D, Bouxsein M, et al. 2014. HDAC5 controls MEF2C-driven sclerostin expression in osteocytes. J Bone Miner Res. 30(3):400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. 2004. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 279(18):19141–19148. [DOI] [PubMed] [Google Scholar]
- Zhang R, Lu Y, Ye L, Yuan B, Yu S, Qin C, Xie Y, Gao T, Drezner MK, Bonewald LF, et al. 2011. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J Bone Miner Res. 26(5):1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.