Abstract
Background and Aims The development of seedlings involves many morphological, physiological and biochemical processes, which are controlled by many factors. Some reactive oxygen and nitrogen species (ROS and RNS, respectively) are implicated as signal molecules in physiological and phytopathological processes. Pepper (Capsicum annuum) is a very important crop and the goal of this work was to provide a framework of the behaviour of the key elements in the metabolism of ROS and RNS in the main organs of pepper during its development.
Methods The main seedling organs (roots, hypocotyls and green cotyledons) of pepper seedlings were analysed 7, 10 and 14 d after germination. Activity and gene expression of the main enzymatic antioxidants (catalase, ascorbate–glutathione cycle enzymes), NADP-generating dehydrogenases and S-nitrosoglutathione reductase were determined. Cellular distribution of nitric oxide (·NO), superoxide radical (O2·–) and peroxynitrite (ONOO–) was investigated using confocal laser scanning microscopy.
Key Results The metabolism of ROS and RNS during pepper seedling development was highly regulated and showed significant plasticity, which was co-ordinated among the main seedling organs, resulting in correct development. Catalase showed higher activity in the aerial parts of the seedling (hypocotyls and green cotyledons) whereas roots of 7-d-old seedlings contained higher activity of the enzymatic components of the ascorbate glutathione cycle, NADP-isocitrate dehydrogenase and NADP-malic enzyme.
Conclusions There is differential regulation of the metabolism of ROS, nitric oxide and NADP dehydrogenases in the different plant organs during seedling development in pepper in the absence of stress. The metabolism of ROS and RNS seems to contribute significantly to plant development since their components are involved directly or indirectly in many metabolic pathways. Thus, specific molecules such as H2O2 and NO have implications for signalling, and their temporal and spatial regulation contributes to the success of seedling establishment.
Keywords: Antioxidant, Capsicum annuum, development, NADP dehydrogenases, nitric oxide, pepper, reactive oxygen species, RNS, ROS
INTRODUCTION
Plant growth and development constitute a highly regulated process in which many internal and external factors are involved, including availability of nutrients, hormones, pattern formation, cell polarity, cell cycle control and auxin transport. Bearing in mind that the final goal of a plant is to reproduce and be perpetuated in time, the early development steps are crucial in establishing the new seedling. In this sense, numerous aspects have been studied from different points of view, such as seed germination, lateral root initiation, cell differentiation, orientation, division, development, architecture of roots and leaves (Ten Tusscher and Scheres, 2011; De Smet et al., 2012; Niu et al., 2013; Axelrod and Bergmann, 2014; Kalve et al., 2014).
Reactive oxygen and nitrogen species (ROS and RNS, respectively) constitute two families of molecules that have been generally associated with the mechanism of responses against adverse stress conditions (Foyer and Noctor, 2005a; Gechev et al., 2006; Miller et al., 2008; Corpas et al., 2007, 2011; Airaki et al., 2012). However, this concept started to change when some key molecules such as hydrogen peroxide (H2O2) and nitric oxide (NO) came to be recognized as second messengers implicated in a variety of developmental processes, including germination, root growth, senescence, flower development and fruit ripening (Leshem and Pinchasov, 2000; Foyer and Noctor, 2005b; Bethke et al., 2006; Carol and Dolan, 2006; Corpas et al., 2006, 2013a; Pitzschke et al., 2006; Diaz-Vivancos et al., 2010; Zafra et al., 2010; Leterrier et al., 2011; Kocsy et al., 2013; Ishibashi et al., 2013; Chaki et al., 2015). Therefore, the relevance of ROS and RNS metabolism in the physiology of higher plants under optimal conditions has begun to be well documented. A good example is the progress made in the understanding of the mechanism of NO and interactions with other molecules (H2O2, auxin and gibberellins) in regulating primary and lateral root growth, which finally determines root architecture (Pagnussat et al., 2002; Correa-Aragunde et al., 2004; Fernández-Marcos et al. 2011; Duan et al., 2014). Moreover, the contributions of some of the enzymes involved in the regulation of ROS and RNS have been also studied in some specific physiological processes, such as germination, root formation, and hypocotyl development (Foreman et al., 2003; Mori and Schroeder, 2004; Carol and Dolan, 2006; Corpas et al., 2006; Bailly et al., 2008; Swanson and Gilroy, 2010; Šírová et al., 2011; Schippers et al., 2012).
In previous work we have studied the redox status and ROS and RNS levels in adult pepper plants at low temperature (Airaki et al., 2011, 2012) and pepper fruits during ripening (Mateos et al., 2009, 2013; Chaki et al., 2015). However, to our knowledge there is no report that has provided a wide framework for the behaviour of the key elements in the metabolism of ROS and RNS in the main organs during early plant development. Therefore, the main goal of this study was to provide a detailed analysis, using biochemical and cellular approaches, of the spatial and temporal regulation of ROS and RNS metabolism during the early development of pepper seedlings. Pepper is a plant with relevant agronomical interest since it is the second-most consumed vegetable worldwide according to its distribution and production (www.fao.org/publications; http://www.usaid.gov/sites/default/files/documents/1862/frutas_y_hortalizas.pdf). For this reason, key RNS and ROS parameters were selected, including the activity of enzymes involved in NO metabolism, such as S-nitrosoglutathione reductase (GSNOR), and different antioxidant enzymes (catalase and enzymes of the ascorbate–glutathione cycle). The cellular distributions of NO, the superoxide radical (O2·–) and peroxynitrite (ONOO–) were also studied.
MATERIALS AND METHODS
Plant materials and growth conditions
Pepper (Capsicum annuum L.) seeds, California type, were obtained from Syngenta Seeds S.A. (El Ejido, Almería, Spain). Seeds were surface-sterilized for 5 min in 50 % (v/v) commercial bleach, washed three times in sterile water and germinated in 100 × 100 mm plastic Petri dishes (six seeds per dish) containing Murashige and Skoog medium (Sigma) with a pH of 5.5 under sterile conditions. Then, they were sealed with Parafilm and placed in a vertical position for 5 d at 30 °C in the dark. The Petri dishes were then transferred to a growth chamber at 22/18 °C with a 16 h photoperiod. The seedlings were collected at different intervals between 7 and 14 d post-germination (Fig. 1).
Fig. 1.
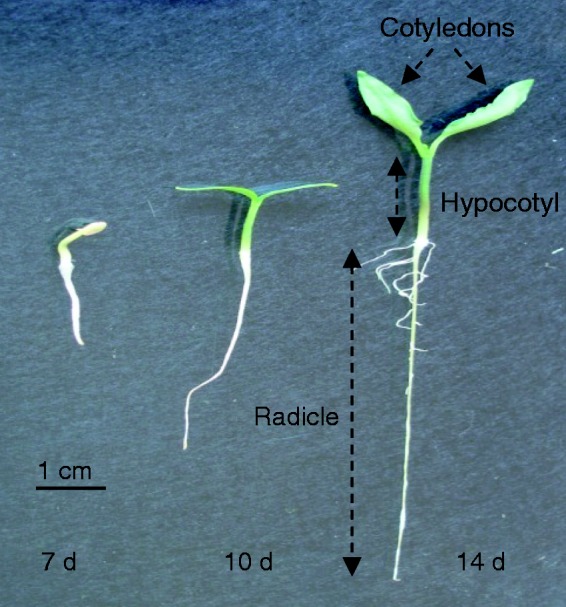
Appearance of a pepper seedling at different stages of development (7, 10 and 14 d old). Three main organs are distinguishable after 10 d: radicle (embryonic root), hypocotyl (embryonic shoot) and green cotyledons.
Crude extracts of plant organs
To measure enzyme activities, for each individual experiment around 20–25 pepper seedlings were collected, and the roots, hypocotyls and green cotyledons were separated, pooled and frozen in liquid N2 and ground in a mortar with a pestle. The powder was suspended in a homogenizing medium containing 50 mm Tris–HCl, pH 7.8, 0·1 mm EDTA, 5 mm dithiothreitol (DTT), 0·2 % (v/v) Triton X-100, 10 % (v/v) glycerol and 2 % (w/v) polyvinyl polypyrrolidone (PVPP). For the ascorbate peroxidase (APX) activity assay, 2 mm ascorbate was added to the homogenizing medium to preserve this activity (Miyake and Asada, 1996). Homogenates were centrifuged at 27 000 g for 25 min at 4 °C and supernatants were immediately used for the assays. For the GSNOR activity assay, the supernatants were passed through Sephadex G-25 gel filtration columns (NAP-10; GE Healthcare), which were equilibrated and eluted with the same buffer before the enzymatic assays (Barroso et al., 2006).
Enzymatic activity assays
Catalase (EC 1.11.1.6) activity was determined by measuring the disappearance of H2O2, as described by Aebi (1984). Ascorbate peroxidase (EC 1.11.1.11) was determined by monitoring the initial ascorbate oxidation by H2O2 at 290 nm (Hossain and Asada, 1984). To test the feasibility of the assay, 20 mm p-chloromercuriphenylsulphonic acid, a specific inhibitor of APX, was used (Mittler and Zilinskas, 1991). Monodehydroascorbate reductase (MDAR; 1.6.5.4) was assayed by measuring monodehydroascorbate-dependent NADH oxidation, and monodehydroascorbate was generated by the ascorbate/ascorbate oxidase system (Leterrier et al., 2005). The rate of monodehydroascorbate-independent NADH oxidation (without ascorbate and ascorbate oxidase) was subtracted from the monodehydroascorbate-dependent reaction. Glutathione reductase (GR; EC 1.6.4.2) was assayed by monitoring the NADPH oxidation coupled to the reduction of glutathione disulphide (GSSG) (Edwards et al., 1990). The reaction rate was corrected for the small, non-enzymatic oxidation of NADPH by GSSG. Activity of GSNOR was assayed spectrophotometrically at 25 °C by monitoring the oxidation of NADH at 340 nm (Barroso et al., 2006). Glucose-6-phosphate dehydrogenase (G6PDH; EC 1.1.1.49) activity was determined spectrophotometrically by recording the reduction of NADP at 340 nm. Assays were performed at 25 °C in a reaction medium (1 ml) containing 50 mm HEPES, pH 7.6, 2 mm MgCl2 and 0·8 mm NADP, and the reaction was initiated by the addition of 5 mm glucose-6-phosphate (G6P). For the determination of 6-phosphogluconate dehydrogenase (6PGDH; EC 1.1.1.44) activity, the reaction mixture was similar to that described for G6PDH, but the substrate was 5 mm 6-phosphogluconate (6PG) (Corpas et al., 1995, 1998). NADP isocitrate dehydrogenase (NADP-ICDH; EC 1.1.1.42) activity was measured by following NADP reduction according to Corpas et al (1999). The assay was performed at 25 °C in a reaction medium (1 ml) containing 50 mm HEPES, pH 7.6, 2 mm MgCl2 and 0·8 mm NADP, and the reaction was initiated by the addition of 10 mm 2 R,3 S-isocitrate. NADP-malic enzyme (NADP-ME; EC 1.1.1.40) activity was determined spectrophotometrically by recording the reduction of NADP at 340 nm using the same reaction mixture (1 ml), but in this case the reaction was initiated by the addition of 1 mm l-malate (Valderrama et al., 2006).
Protein concentration was determined with the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA) using BSA as standard.
Partial cloning of GSNOR cDNA
Using plant glutathione-dependent formaldehyde dehydrogenase sequences from the data bank, degenerate oligonucleotides were designed in conserved domains of these sequences (Table 1). Total RNA was isolated from 10-d-old seedlings with Trizol Reagent (Gibco BRL, Paisley, UK) as described in the manufacturer’s manual, and RNA was quantified spectrophotometrically. Two micrograms of total RNA was used as a template for the reverse transcriptase reaction and added to a mixture containing 1·5 mm dNTPs, 1·6 µg polydT23 primer, 1 × reverse transcriptase buffer (25 mm Tris–HCl, pH 8.3, 5 mm MgCl2, 50 mm KCl and 2 mm DTT), 0·9 U RNasin ribonuclease inhibitor and 20 U avian myeloblastosis virus reverse transcriptase (Finnzymes, Espoo, Finland). The reaction was carried out at 42 °C for 40 min, followed by a 5-min step at 98 °C and cooling to 4 °C for 10 min. Then, PCR was carried out as follows: 1 µl of the cDNA was added to 250 µm dNTPs, 1·5 mm MgCl2, 1 × PCR buffer, 2·5 U of AmpliTaq Gold (Roche, Mannheim, Germany) and 0·5 µm of each primer (Table 1) in a final volume of 20 µl. Reactions were carried out in a Hybaid thermocycler (Ashford, UK). A first step of 2 min at 94 °C was followed by 30 cycles of 1 min at 94 °C, 1 min at 55 °C and 1 min at 65 °C, with a final extension of 10 min at 65 °C. Amplified PCR products were detected after electrophoresis in 1 % (w/v) agarose gels stained with ethidium bromide, and the visualized bands were cut and extracted from the gel (Qiaex II gel extraction kit; Qiagen, Madrid, Spain). The purified fragments were cloned into the pGEM-T Easy Vector (Promega, Madrid, Spain). A partial cDNA of glutathione-dependent formaldehyde dehydrogenase was obtained, confirmed by sequencing and deposited in the data bank with accession number EU652335.
Table 1.
Oligonucleotides used for the cloning and semiquantitative RT–PCR analysis of enzymes involved in the metabolism of ROS and RNS. Letters ‘F’ and ‘R’ correspond to forward and reverse oligonucleotides, respectively
| Name | Oligonucleotide sequence (5′–3′) | Product size (bp) | Accession number |
|---|---|---|---|
| cDNA cloning | |||
| F1-GSNOR | CTTGACAAAGTATGTGTCC | 588 | – |
| F2-GSNOR | AAGTCATTCGTTGCAAAGC | ||
| R-GSNOR | GTGAGTGTAGAACTTCTCC | 1121 | EU652335 |
| Semiquantitative RT–PCR | |||
| F-CAT | GATTTCTTCTCTTTCCTCC | ||
| R-CAT | CGATGTTCCTATTCAATACC | 418 | AF227952 |
| F-APX | TGTGCTCCTCTTATGCTCC | ||
| R-APX | CTCAAAACCAGAACGCTCC | 485 | X81376 |
| F-GR | TTTGGTTTATGGAGCTGCC | ||
| R-GR | CAGTGGGAGTTGCTTTCTG | 509 | AY547351 |
| F-MDAR | ATGGAGAGGGTGAAGTCCG | ||
| R-MDAR | GCCTTGACAGCCTGCTCAG | 279 | AY652702 |
| F-G6PDH | ATTTGTTGGTGCTGCGTT | ||
| R-G6PDH | CATTGATTGAAGGACCT | 255 | AY652703 |
| F-6PGDH | TGTAGTTATGCTCAGGGGATG | ||
| R-6PGDH | CTCTCATATGTGTGAGCCCC | 374 | AY532646 |
| F-ICDH | TTGTGCCAGAAGGTACAGAC′ | ||
| R-ICDH | CAGATTCCAGCCTCCTCGTA | 418 | AY572426 |
| F-ACT | ACTCTTAATCAATCCCTCC | ||
| R-ACT | GCACTGTATGACTGACACC | 573 | AY572427 |
Semiquantitative reverse transcription–PCR
Total RNA was extracted from whole seedlings with Trizol as described by the manufacturer (Gibco BRL, Life Technologies). Two micrograms of total RNA was used to produce cDNA by reverse transcription (RT)–PCR (Mateos et al., 2009). Semiquantitative RT–PCR amplification of actin cDNA from pepper was used as control. Catalase, GR, MDAR, APX, GSNOR, G6PDH, 6PGDH, NADP-ICDH, NADP-ME and actin cDNAs were amplified by the PCR as follows: 1 µl of each cDNA (30 ng) was added to 0·250 mm dNTPs, 2·5 mm MgCl2, 1 × PCR buffer, 0·5 U of HotMaster Taq™ DNA polymerase (Eppendorf, Madrid, Spain) and 0·5 µm of each primer (Table 1) in a final volume of 20 µl. Reactions were carried out in the Hybaid thermocycler. A first step of 10 min at 95 °C was followed by 28–33 cycles (depending on the gene) of 30 s at 95 °C, 30 s at 55 °C and 45 s at 65 °C. Then, PCR products were detected by electrophoresis in 1 % (w/v) agarose gels and staining with ethidium bromide. Quantification of the bands was performed using a Gel Doc system (Bio-Rad Laboratories) coupled with a high-sensitive charge-coupled device camera. Band intensity was expressed as relative absorbance units. The ratio between each specific gene and actin amplification was calculated to normalize for initial variations in sample concentration (Marone et al. 2001). Means and standard deviations were calculated after normalization to actin.
SDS–PAGE and immunoblot analysis
This was carried out in 10 % acrylamide slab gels. For western blot analysis, proteins were transferred to polyvinylidene difluoride (PVDF) membranes with a semi-dry Trans-Blot cell (Bio-Rad, Hercules, CA, USA). After transfer, membranes were used for cross-reactivity assays with a rabbit polyclonal antibody against 3-nitrotyrosine (Corpas et al., 2008) diluted 1 : 8000. For immunodetection, an affinity-purified goat anti-(rabbit IgG)–horseradish peroxidase conjugate (Bio-Rad) and an enhanced chemiluminescence kit (ECL Plus; Amersham, Piscataway, NJ, USA) were used. As positive control, commercial nitrated BSA (Sigma, St Louis, MO, USA) was used.
Detection of NO, O2·– and ONOO–by confocal laser scanning microscopy
Segments of pepper organs (roots, hypocotyls and green cotyledons) of ∼5 mm were taken from the elongation zone of roots and the middle area of hypocotyls and cotyledons. The segments were incubated in darkness with specific fluorescent probes. To detect NO, the organ segments were incubated at 25 °C for 1 h in darkness with 10 µm diaminofluorescein-FM diacetate (DAF-FM DA; Calbiochem) prepared in 10 mm Tris–HCl (pH 7.4) according to Corpas et al. (2006). For O2·–, segments were incubated at 37 °C for 30 min with 10 µm dihydroethidium (DHE; Fluka) as described by Valderrama et al. (2007). For ONOO–, the segments were incubated with 10 µm 3′-(p-aminophenyl) fluorescein (APF; Invitrogen) prepared in 10 mm Tris–HCl (pH 7.4) according to Chaki et al. (2009). The corresponding segments were then washed twice in the same buffer for 15 min each. After washing, sections were embedded in a mixture of 15 % acrylamide–bisacrylamide stock solution in 0·01 m PBS including 0·3 % tetramethylethylenediamine (TEMED) for 4 h and then polymerized in inclusion containers (1·5 × 0·9 × 0·5 mm; Sorvall Instruments) using 0·5 ml of a fresh 15 % acrylamide stock solution and 50 µl of 2 % persulphate ammonium. Then, 100-µm-thick sections, as indicated by the vibratome scale, were cut under 10 mm PBS. Sections were then soaked in glycerol : PBS (containing azide) (1 : 1; v : v) and mounted in the same medium for examination with a confocal laser scanning microscope (CLSM) system (Leica TCS SL), using standard filters and collection modalities for the different fluorescence probes (excitation at 495 nm, emission at 515 nm) (Corpas and Barroso, 2014).
Statistical analysis
Data are presented as the mean ± s.e.m. of at least three independent experiments. Pairwise analysis of variance (ANOVA) was used to detect differences among stage of development and organs. Values of P < 0·05 were considered statistically significant.
RESULTS
Pepper plants have a relative long period of growth. However, post-germination development is a key step in the establishment of the seedlings, a process involving various morphological, physiological, biochemical and molecular adaptations. Figure 1 shows a representative image of the appearance of pepper seedlings at the ages of development selected to carry out this work, i.e. 7, 10 and 14 d. These stages were selected because the major organs (roots, hypocotyls and green cotyledons) undergo significant changes in size, and hypocotyls and cotyledons become green, forming the first photosynthetic organs of the young plant. This step involves drastic biochemical changes. Table 2 summarizes length and biomass data for pepper seedlings between days 7 and 14 of development.
Table 2.
Growth parameters of roots, hypocotyls and green cotyledons of pepper seedlings at different stages (7–14 d) of development. Data are means ± s.e. for at least six independent seedlings
| Day | Root |
Hypocotyl length (cm) | Hypocotyl + cotyledon fresh weight (mg) | |
|---|---|---|---|---|
| Length (cm) | Fresh weight (mg) | |||
| 7 | 2·1 ± 0·1 | 12·0 ± 2·0 | 0·40 ± 0·05 | 11·8 ± 1·7 |
| 8 | 2·5 ± 0·1 | 14·7 ± 0·1 | 0·70 ± 0·05 | 15·3 ± 3·0 |
| 9 | 3·0 ± 0·3 | 16·3 ± 0·8 | 0·80 ± 0·03 | 19·5 ± 1·6 |
| 10 | 3·7 ± 0·2 | 18·6 ± 1·9 | 0·90 ± 0·04 | 29·5 ± 2·9 |
| 13 | 6·0 ± 0·1 | 29·3 ± 3·0 | 1·10 ± 0·10 | 50·9 ± 5·4 |
| 14 | 6·0 ± 0·1 | 32·0 ± 2·0 | 1·10 ± 0·10 | 62·7 ± 7·1 |
Activity of antioxidant enzymes and NADPH-generating dehydrogenases in the main organs during the development of pepper seedlings
The results of this are analysis shown in Fig. 2. Catalase activity increased during seedling development in hypocotyls and green cotyledons; however, in roots the activity did not show significant differences during the analysed period (Fig. 2A). Among all organs, the highest catalase activity was found in hypocotyls of 14-d-old seedlings. The APX activity was highest in roots of 7-d-old seedlings and was ∼25 % lower in roots of 10- and 14-d-old seedlings (Fig. 2B). Activity of MDAR had a different distribution; it was higher in roots than in other organs, the highest activity being found in the roots of 10-d-old seedlings (Fig. 2C). In hypocotyls and cotyledons MDAR activity increased with the age of the seedlings. Figure 2D shows that GR activity behaved differently among the three organs. Roots had the highest GR activity, but this activity decreased in 14-d-old seedlings. In hypocotyls, GR activity was 2-fold higher in 14-d-old seedlings compared with 7- and 10-d-old seedlings. Green cotyledons showed the lowest GR activity in comparison with roots and hypocotyls, and this activity was very similar in 10- and 14-d-old plants.
Fig. 2.
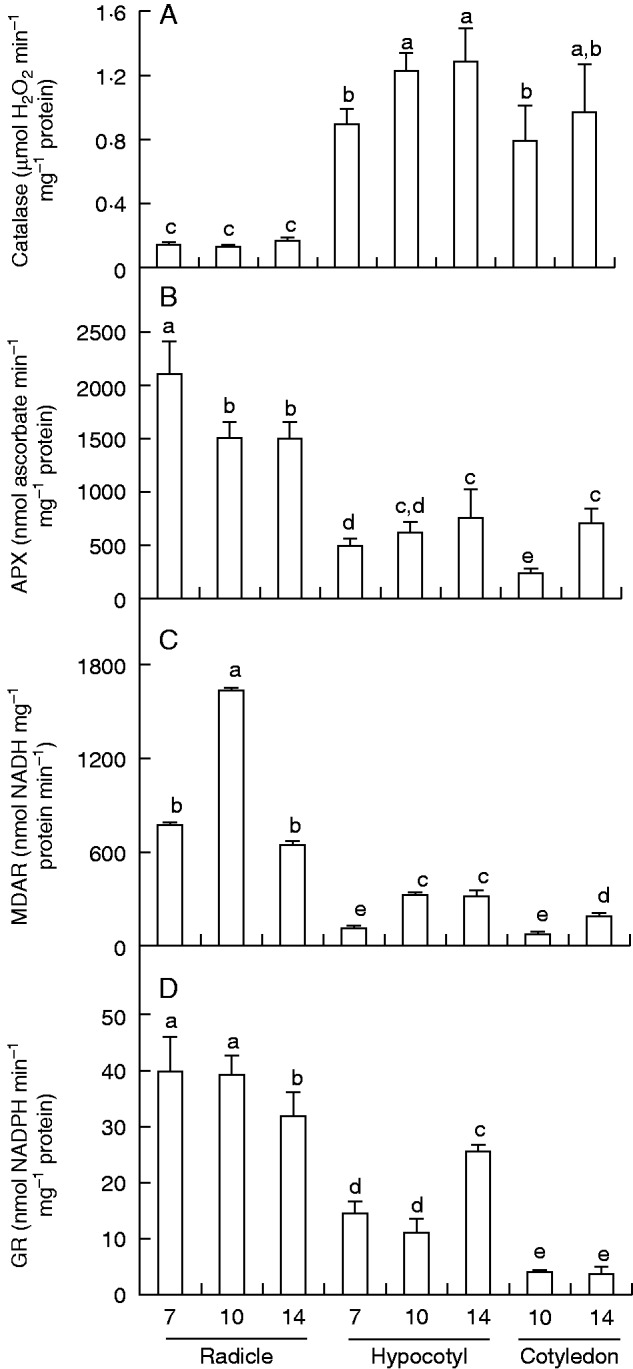
Activity of catalase and ascorbate–glutathione cycle enzymes in radicles, hypocotyls and green cotyledons of pepper seedlings at different stages of development (7, 10 and 14 d old). (A) Catalase. (B) Ascorbate peroxidase (APX). (C) Monodehydroascorbate reductase (MDAR). (D) Glutathione reductase (GR). Data are mean ± s.e.m. of at least three experiments. Different letters indicate significant differences between stages and organs (P ≤ 0·05).
The activity of the main NADP dehydrogenases in the different pepper organs during plant development is represented in Fig. 3. Activity of G6PDH was highest in hypocotyls of 10-d-old seedlings (Fig. 3A). In roots, G6PDH activity did not show significant differences between growth stages. In hypocotyls, activity was up to 1.5-fold higher in 10- and 14-d-old seedlings compared with 7-d-old seedlings. Green cotyledons had the lowest activity, but activity was 2·2-fold higher in cotyledons of 14-d-old seedlings compared with those of 10-d-old seedlings. The activity of 6PGDH is shown in Fig. 3B. The highest activity was observed in roots but there were no significant changes during the period analysed. In hypocotyls and green cotyledons, activity was 2·2-fold higher in 14-d-old seedlings than at younger ages. Activity of NADP-ICDH (Fig. 3C) showed different behaviour compared with both dehydrogenases of the pentose phosphate pathway (G6PDH and 6PGDH). Thus, in roots and hypocotyls NADP-ICDH activity significantly decreased from 7 to 14 d (4-fold and 2·4-fold, respectively). Among all organs and stages, the highest NADP-ICDH activity was found in roots of 7-d-old seedlings and the lowest activity in green cotyledons. The behaviour of NADP-ME differed among organs (Fig. 3D). In roots, activity decreased with age, being reduced by half in 14-d-old compared with 7-d-old seedlings. However, in hypocotyls activity increased 4-fold from 7 to 14 d. No difference in activity was observed in green cotyledons.
Fig. 3.
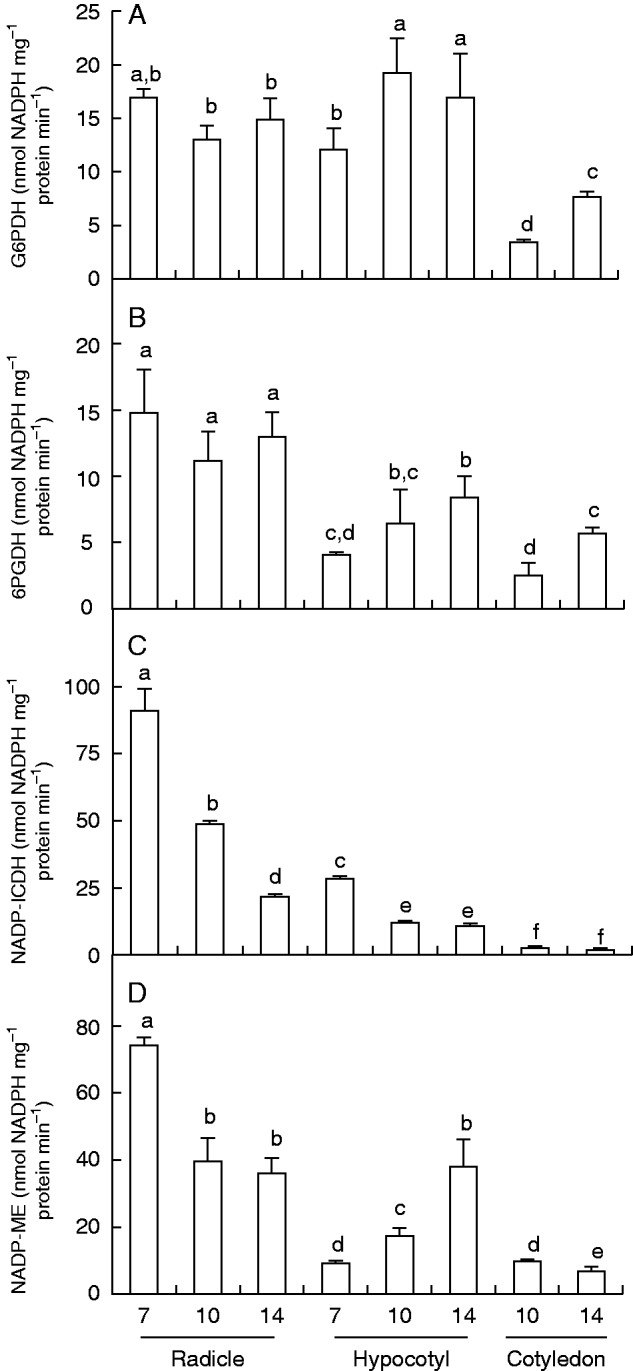
Activity of NADP dehydrogenases in radicles, hypocotyls and green cotyledons of pepper seedlings at different stages of development (7, 10 and 14 d old). (A) Glucose-6-phosphate dehydrogenase (G6PDH). (B) 6-Phosphogluconate dehydrogenase (6PGDH). (C) NADP isocitrate dehydrogenase (NADP-ICDH). (D) NADP-malic enzyme (NADP-ME). Data are mean ± s.e.m. of at least three experiments. Different letters indicate significant differences between stages and organs (P ≤ 0·05).
Transcript analysis of catalase, three enzymes of the antioxidative ascorbate-glutathione cycle (APX, MDAR and GR) and the family of NADP dehydrogenases, was performed by semiquantitative RT–PCR using whole seedlings of different ages (Fig. 4). No significant changes in antioxidant transcripts were observed, with the exception of GR, which was lower in 14-d-old seedlings. Expression of NADP dehydrogenase mRNAs was lower at 14 d than at younger ages, and in the case of NADP-ICDH there was maximum expression in 10-d-old seedlings.
Fig. 4.
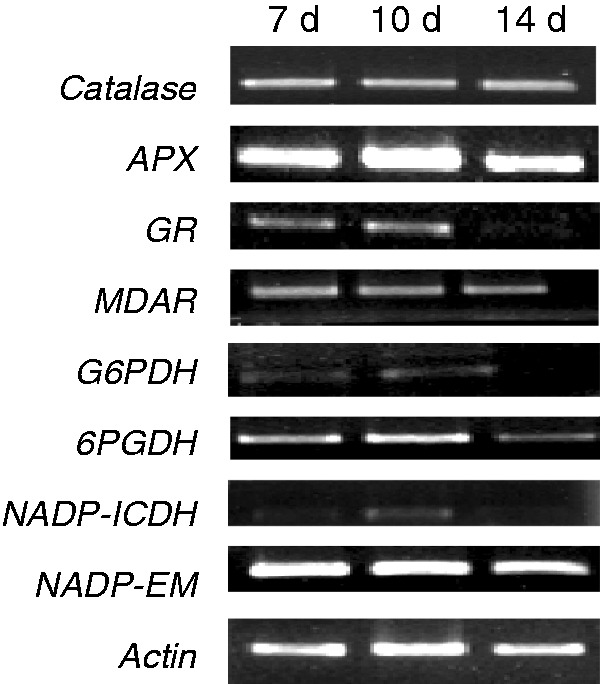
mRNA expression of catalase, ascorbate peroxidase (APX), glutathione reductase (GR), monodehydroascorbate reductase (MDAR) and NADP dehydrogenases in whole pepper seedlings at different stages of development (7, 10 and 14 d old). Semiquantitative RT–PCR was performed on total RNA isolated from whole seedlings. Representative agarose electrophoresis gels of the amplification products visualized by ethidium bromide staining under UV light. Actin was used as internal control.
Metabolism of RNS in the main organs during pepper seedlings development
As part of the analysis of RNS metabolism in pepper seedlings, we studied GSNOR activity and its mRNA expression (Fig. 5A, B), and the pattern of protein tyrosine nitration (NO2-Tyr) in the different organs (Fig. 6).
Fig. 5.
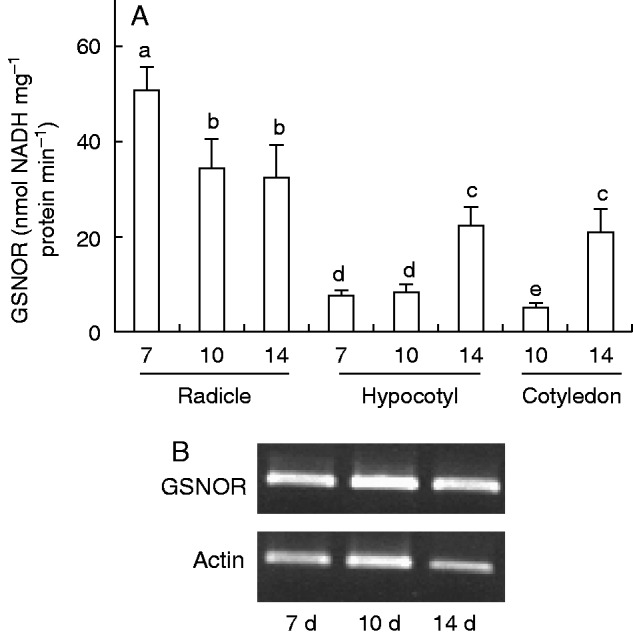
Activity of S-nitrosoglutathione reductase (GSNOR) and GSNOR gene expression in radicles, hypocotyls and green cotyledons of pepper seedlings at different stages of development (7, 10 and 14 d old). (A) Spectrophotometric assay of GSNOR activity. Data are mean ± s.e.m. of at least three experiments. Different letters indicate significant differences between stages and organs (P ≤ 0·05). (B) mRNA expression of GSNOR in whole seedlings. Semiquantitative RT-PCR was performed on total RNA isolated from whole seedlings. Representative agarose electrophoresis gels of the amplification products visualized by ethidium bromide staining under UV light. Actin was used as internal control.
Fig. 6.
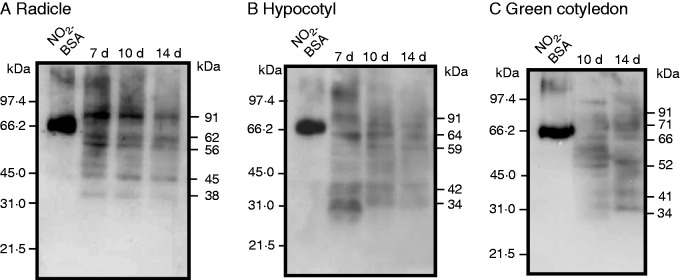
Tyrosine nitration pattern in radicles, hypocotyls and green cotyledons of pepper seedlings at different stages of development (7, 10 and 14 d old). (A) Radicle samples (5 μg per lane). (B) Hypocotyl samples (10 μg per lane). (C) Cotyledon samples (20 μg per lane). Commercial nitrated BSA (NO2-BSA; 2 µg of protein) was used as a positive control. Numbers to the right of the immunoblots indicate relative molecular masses of the detected immunoreactive bands, and relative molecular masses of the protein markers are shown to the left. Western blots were probed with a rabbit anti-nitrotyrosine polyclonal antibody at 1 : 8000 dilution.
The activity of GSNOR was highest in roots of 7-d-old seedlings, and decreased with age, being ∼6-fold lower in roots of 14-d-old plants (Fig. 5A). However, its behaviour was different in hypocotyls and green cotyledons, as activity was 1·8-fold and 3·4-fold higher, respectively, in 14-d-old seedlings compared with 7- and 10-d-old seedlings. To analyse the mRNA expression of GSNOR by semiquantitative PCR, it was necessary to prepare a partial cDNA of this pepper gene. Thus, a cDNA of 1000 bp (accession number EU652335) that coded for a polypeptide of 333 amino acids was identified. This protein showed 96 % identity with alcohol dehydrogenase 3 of Solanum tuberosum (P14675), 91 % identity with Lactuca sativa (BAA07911) and 89 % identity with Ricinus communis (XP_002534157). Semiquantitative RT–PCR of mRNA expression of GSNOR is shown in Fig. 5B. The mRNA did not show any significant change at any of the stages of development analysed.
The tyrosine nitration pattern in the three organs of pepper seedlings analysed during natural development is presented in Fig. 6. The profile of immunoreactive bands differed among the three organs. In roots, the number of immunoreactive bands apparently did not change with the age of seedlings; however, the intensity of the immunoreactive bands of 91, 62, 56 and 38 kDa seemed to diminish (Fig. 6A). A similar result was observed in hypocotyls, with the immunoreactive bands of 91, 64, 42 and 34 kDa diminishing and only the 59-kDa band increased (Fig. 6B). In cotyledons the tyrosine nitration profile was different in comparison with the other organs (Fig. 6C).
Cellular detection of NO, O2·– and ONOO– by CLSM
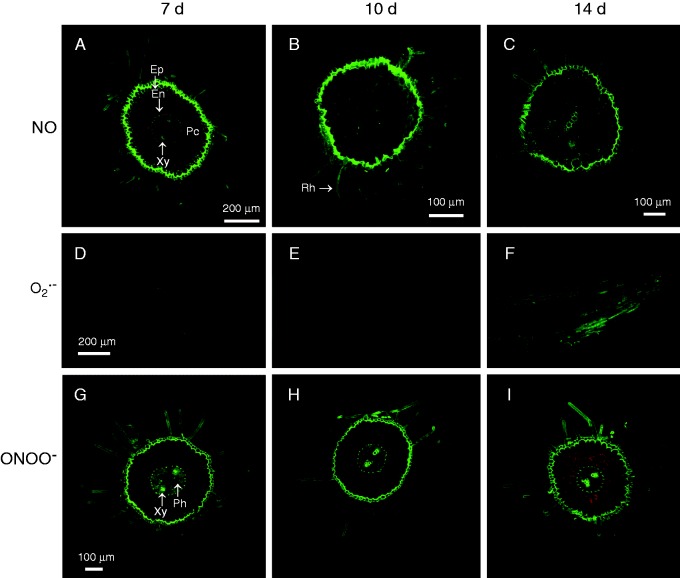
Figure 7 illustrates the cellular localization of NO (panels A–C), O2·– (panels D–F) and ONOO– (panels G–I) in roots of 7-, 10- and 14-d-old pepper seedlings. Nitric oxide, observed as intense green fluorescence, was localized mainly in epidermal cells and root hairs, and was less prominent in vascular tissue of roots from 7-, 10- and 14-d-old seedlings (Fig. 7A–C). However in roots of 10-d-old seedlings, slight green fluorescence was also generally distributed in parenchyma cells of the cortex (Fig. 7B). The orange colour corresponds to autofluorescence, which was clearly observed in the parenchyma cells of the cortex (Fig. 7A, B). The superoxide radical, observed as green fluorescence in longitudinal root sections, showed a general distribution in the epidermal cells of roots, with a slight intensification in 14-d-old seedlings (Fig. 7F) in comparison with 7- and 10-d-old seedlings (Fig. 7D and E, respectively). Peroxynitrite, observed as intense green fluorescence, was localized in almost all cell types in the root cross-sections, including root hairs, epidermal cells, parenchyma cells of the cortex, endodermis, xylem and phloem of 7-d-old seedlings (Fig. 7G). Similar localization of ONOO– was observed in roots of 10- and 14-d-old seedlings, but with a lower intensity of green fluorescence in the parenchyma cells of the cortex (Fig. 7H, I). The orange colour corresponds to the autofluorescence distributed in almost all parenchyma cells of the cortex (Fig. 7I).
Fig. 7.
Representative images of radicles of pepper seedlings illustrating confocal laser scanning microscopy (CLSM) detection and visualization of endogenous nitric oxide (NO), superoxide radical (O2·–) and peroxynitrite (ONOO–) at different stages of development (7, 10 and 14 d old). (A–C) Detection of endogenous NO using diaminofluorescein-FM diacetate as a fluorescent probe in cross-sections of pepper radicles. (D–F) Detection of endogenous O2·– using dihydroethidium as a fluorescent probe in root tips. (G–I) Detection and visualization of ONOO– using 3′-(p-aminophenyl) fluorescein as a fluorescent probe in transverse sections of pepper radicles. Strong and bright green fluorescence corresponds to each specific fluorescent probe in the corresponding panels. Each picture was prepared from 30 sections, which were analysed by CLSM. The orange–yellow colour corresponds to autofluorescence. En, endodermis; Ep, epidermis; Pc, parenchyma cells of the cortex; Ph, phloem; Rh, root hair; Xy, xylem.
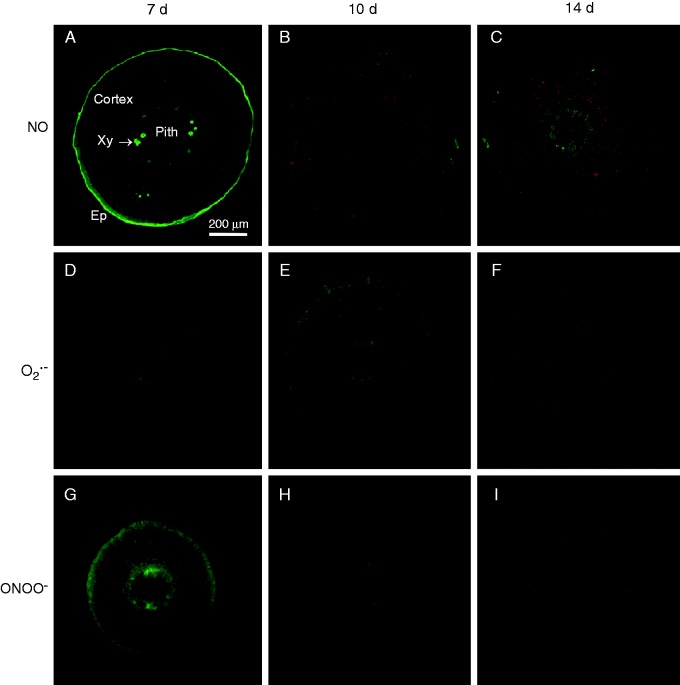
Figure 8 shows the cellular localization of NO (panels A–C), O2·– (panels D–F) and ONOO– (panels G–I) in transverse sections of hypocotyls of 7-, 10- and 14-d-old pepper seedlings. Nitric oxide, observed as intense green fluorescence, was localized mainly in epidermal cells and xylem, and was less prominent in cortex cells of hypocotyl sections of 7-d-old seedlings (Fig. 8A). In the case of 10- and 14-d-old seedlings, green fluorescence was distributed in all cell types, although it was less intense in epidermal cells (Fig. 8B and C, respectively). The orange colour corresponds to autofluorescence. Superoxide, observed as green fluorescence, was distributed in almost all cell types of the cortex in the hypocotyls of 7-d-old seedlings (Fig. 8D). In hypocotyls of 10-d-old seedlings, fluorescence was less intense (Fig. 8E) and in hypocotyls of 14-d-old seedlings it was again distributed in all cell types (Fig. 8F).
Fig. 8.
Representative images of transverse sections of hypocotyl of pepper seedlings illustrating confocal laser scanning microscopy (CLSM) detection and visualization of endogenous nitric oxide (NO), superoxide radical (O2·–) and peroxynitrite (ONOO–) at different stages of development (7, 10 and 14 d old). (A–C) Detection of endogenous NO using diaminofluorescein-FM diacetate as a fluorescent probe. (D–F) Detection of endogenous O2·– using dihydroethidium as a fluorescent probe. (G–I) Detection of ONOO– using 3′-(p-aminophenyl) fluorescein as a fluorescent probe in transverse sections. Strong and bright green fluorescence correspond to each specific fluorescent probe in the corresponding panels. Each picture was prepared from 30 sections, which were analysed by CLSM. The orange–yellow colour corresponds to autofluorescence. En, endodermis; Ep, epidermis; Xy, xylem.
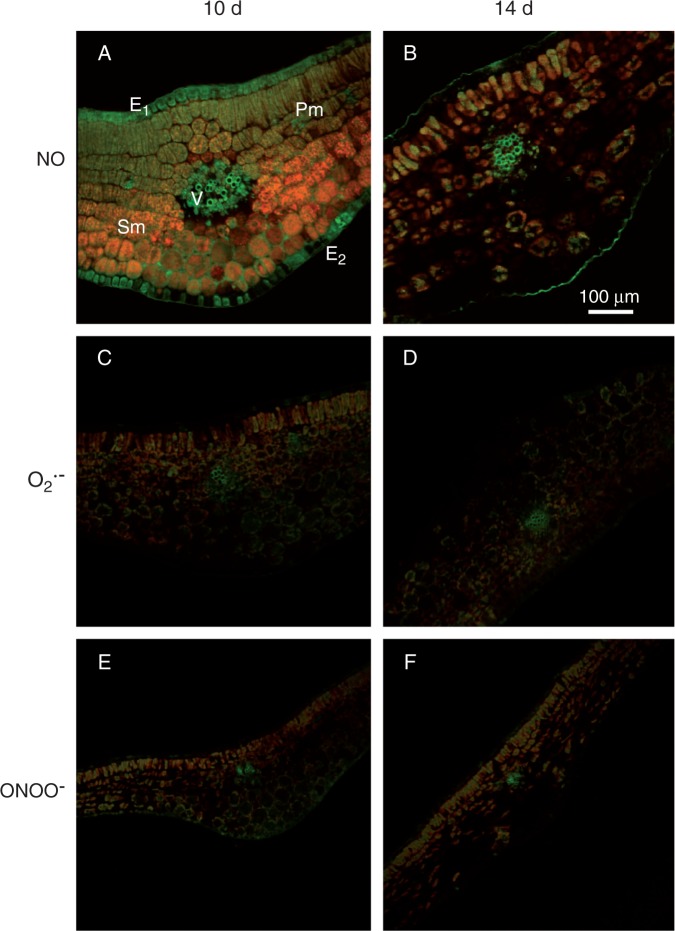
Figure 9 shows the cellular localization of NO (panels A and B), O2·– (panels C and D) and ONOO– (panels E and F) in transverse sections of green cotyledons of 10- and 14-d-old pepper seedlings. Nitric oxide, observed as intense green fluorescence, was localized in vascular tissue, epidermal and palisade mesophyll cells of green cotyledons of 10-d-old seedlings (Fig. 9A). In 14-d-old seedlings, green fluorescence intensity had diminished in all cell types (Fig. 9B). The orange colour corresponds to autofluorescence. Superoxide, observed as green fluorescence, showed a discrete distribution in spongy mesophyll cells and in vascular tissue in the cotyledons of 10- and 14-d-old seedlings (Fig. 9C, D). In the case of ONOO–, green fluorescence was also present in spongy mesophyll cells and in vascular tissue in the cotyledons of 10-d-old seedlings (Fig. 9E) but was reduced in the green cotyledons of 14-d-old seedlings (Fig. 9F).
Fig. 9.
Representative images of transverse sections of green cotyledons of pepper seedlings illustrating confocal laser scanning microscopy (CLSM) detection of endogenous nitric oxide (NO), superoxide radical (O2·–) and peroxynitrite (ONOO–) at different stages of development (10 and 14 d old). (A, B) Detection of endogenous NO using diaminofluorescein-FM diacetate as a fluorescent probe. (C, D) Detection of endogenous O2·– using dihydroethidium as a fluorescent probe in root tips. (E, F) Detection and visualization of ONOO– using 3′-(p-aminophenyl) fluorescein as a fluorescent probe. Strong and bright green fluorescence corresponds to each specific fluorescent probe in the corresponding panels. Each picture was prepared from 30 sections analysed by CLSM. The orange–yellow colour corresponds to autofluorescence. E1, adaxial epidermis; E2, abaxial epidermis; Pm, palisade mesophyll; Sm, spongy mesophyll; V, main vein.
DISCUSSION
The pepper plant has enormous agronomical relevance since its fruits are the second most consumed vegetable worldwide and are also characterized for their high levels of vitamin C, provitamin A and calcium (Howard et al., 1994). Thus, according to a Dietary Reference Intakes report, a medium-sized green bell pepper contains 177 % of the recommended dietary allowance of vitamin C (http://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/), which is necessary to maintain good cardiac and vascular health (Vanderslice and Higgs, 1991; Kirsh et al., 2006). In previous studies we analysed some aspects of the metabolism of ROS and RNS in 30-d-old pepper plants exposed to low temperature during different periods of time that provoked nitro-oxidative stress (Airaki et al., 2012) and in fruits during the ripening process (Mateos et al., 2009, 2013). Considering that ROS and RNS are also involved in other physiological processes, including plant growth and development, in this work the key enzymatic elements involved in the metabolism of ROS and RNS during the early stages of pepper seedlings (7–14 d old) were analysed in the main organs (roots, hypocotyls and green cotyledons) of this plant. It must be pointed out that, due to the small size of the different organs during the developmental stage, of seedlings, it was decided to use individual organs for the analysis of biochemical parameters, whereas whole seedlings were used for the gene expression analyses. To our knowledge there are no reports that provide a wide overview of these components and its modulation in the establishment of higher plant seedlings, something which is critical for the future success of the plant and its fruit quality.
Catalase has the highest activity in the aerial part of pepper seedlings, whereas the enzymatic components of the ascorbate glutathione cycle show higher activity in roots
Reactive oxygen species are produced during normal cellular metabolism, being involved in many physiological aspects. For example in seed physiology, ROS are involved in the disruption of the seed dormancy (Bailly, 2004; Bailly et al., 2004; Oracz et al., 2007, 2009; Müller et al., 2009) since some ROS serve as messenger molecules in seedling growth and developmental processes (Rodríguez et al., 2002; Foreman et al., 2003; Carol and Dolan, 2006; Swanson and Gilroy, 2010).
The main function of catalase is the removal of H2O2, either generated inside peroxisomes due to their own metabolism or accumulated in this organelle through cell metabolism. Our results on catalase activity in the early days of development of pepper seedlings showed that there were significant differences among the analysed organs, roots having the lowest activity. Thus, catalase is probably the main antioxidant enzyme involved in the control of H2O2 in the aerial part of pepper seedlings during development. Similar behaviour of catalase activity has been reported in other plant species, such as maize (Zea mays) seedlings (Redinbaugh et al., 1990). In other species, the inhibition of catalase has been reported to contribute to a higher level of H2O2, which was necessary to break the seed dormancy in lettuce (Lactuca sativa) (Hendricks and Taylorson, 1975). In other cases, such as oil seedlings during the transition from dark to light conditions, catalase activity and/or isozyme number are differentially modulated. This modulation has been described in cotton (Gossypium hirsutum) (Ni et al., 1990; Ni and Trelease, 1991) and pumpkin (Yamaguchi et al., 1995). However, APX, which is part of the ascorbate–glutathione cycle, showed the highest activity in roots in the present study, suggesting that these two systems are complementary in regulating the level of H2O2 during seedling development. The activities of the other enzymes of the ascorbate–glutathione cycle, MDAR and GR, followed the same pattern as APX activity, being higher in the roots than in the other organs. Taking these results together, the highest antioxidant activity of these enzymes, which are differentially modulated in roots, hypocotyls and cotyledons, might allow speculation about the possible involvement of ROS in the processes of growth and development in pepper seedlings. The involvement of antioxidant enzymes has also been reported in other plants. During germination and the early stages of root development in Ipomoea triloba, the activities of catalase, superoxide dismutase and guaiacol peroxidase were probably the crucial enzymes involved in the neutralization of ROS, since they had higher levels of activity compared with other enzymes, such as APX and GR (Pergo and Ishii-Iwamoto, 2011). In germinating pea (Pisum sativum) seeds, increases in catalase and superoxide dismutase activities have also been reported, probably due to the increased oxygen uptake which is accompanied by a set of events (mobilization of fatty acids, cell elongation and division in the embryo, radical protrusion, etc.) including the release of ROS (Wojtyla et al., 2006).
Roots have the highest capacity to regenerate NADPH via NADP dehydrogenases
NADPH is an essential cofactor in cell growth, proliferation and detoxification in eukaryotic cells. It is remarkable that, among the three pepper organs analysed, the activity of NADP-ICDH and NADP-ME was highest in roots and especially in 7-d-old seedlings, suggesting a higher demand of NADPH for growth in this period. NADP-ICDH catalyses the oxidative decarboxylation of isocitrate to 2-oxoglutarate, with the concomitant production of the reduced coenzyme NADPH and it has therefore been associated with the metabolism of amino acids and ammonia (Fieuw et al., 1995; Gálvez and Gadal, 1995; Palomo et al., 1998). In this context, the high NADP-ICDH activity found in roots of 7-d-old seedlings must be related to the relevance of this enzyme in the metabolism of nitrogen, which is critical for plant growth. These results are in a good agreement with previous data in 3-week-old pea plants, in which NADP-ICDH activity was 3-fold higher in roots than in green leaves (Chen et al., 1988), and in 6-week old Arabidopsis plants, in which NADP-ICDH activity in roots was 3.2-fold higher than in leaves (Leterrier et al., 2012).
On the other hand, during the imbibition and germination process in soybean seeds there was an increase in the NADPH/NADP ratio, which was mainly due to NADP-ICDH activity and to a lesser extent to G6PDH (Duke et al., 1977). In pine, NADP-ICDH is also implicated in the primary development of seedlings (Palomo et al., 1998; Pascual et al., 2008). NADP-ME catalyses the reaction of malate with NADP to yield α-oxaloacetate and NADPH. Thus, this activity is involved in different processes, including pyruvate metabolism, carbon fixation and lignin biosynthesis (Schaaf et al., 1995). Therefore, the high NADP-ME activity in roots could also be correlated with the demand for both NADPH and α-oxalacetate by biosynthetic pathways such as gluconeogenesis and the amino acid and fatty acid synthesis necessary for root growth. Activities of G6PDH and 6PGDH were also higher in roots and hypocotyl seedlings in the early days of development.
The highest activity of the NADP dehydrogenases observed in roots of 7-d-old seedlings again suggests a higher demand for NADPH by biosynthetic pathways, such as sugar biosynthesis, fatty acid and carotenoid biosynthesis. Moreover, the presence of both dehydrogenases (G6PDH and 6PGDH) of the pentose phosphate pathway also supports the efficient recycling of NADPH for its subsequent use in the ascorbate–glutathione cycle, which would help to reduce a potential overproduction of H2O2 in the cell (Mateos et al., 2013). Furthermore, it is also well recognized that NADPH, together with glutathione and ascorbate, modulates the redox state of the cell and transmits the necessary information that regulates signalling pathways (Noctor, 2006; Gallie, 2013). Therefore, the generated NADPH may be used by the plasma membrane NADPH oxidase of plant cells to produce ROS, which are involved in cell expansion through the activation of Ca2+ channels (Foreman et al., 2003).
We observed a discrepancy between the expression of genes encoding antioxidant enzymes and NADP dehydrogenases and their corresponding activities in the different seedling growth stages analysed (7, 10 and 14 d) since gene expression was not affected, with the exception of GR, G6PDH and ICDH, which showed slight modulation. This could suggest that the modulation of these enzymes during this period is due to post-translational regulations. This behaviour has previously been observed in pepper plants exposed to low temperature (Airaki et al., 2012) and in pepper fruits during the ripening process (Mateos et al., 2009, 2013). However, it will be possible to interpret these observations more precisely when the different isoenzymes of these enzymatic systems have been identified and localized.
The NO content of pepper seedlings is modulated differentially during development depending on the organ
Nitric oxide plays an important role in a wide range of physiological responses during growth and development of plants (Lamattina et al., 2003; Shapiro, 2005; Corpas et al., 2006; Wilson et al., 2008). This gas can regulate plant growth depending on its concentration, such that low concentrations stimulate growth but high concentrations may inhibit it (Anderson and Mansfield, 1979; Hufton et al., 1996; Leshem and Haramaty, 1996; Gouvea et al., 1997; Jin et al., 2009). The NO molecule seems to be synthesized mainly in tissues that are in the growth phase, such as the embryonic axis and cotyledons, whereas lower levels are produced in mature and senescent organs (Beligni and Lamattina, 2001). In roots the application of exogenous NO induces root elongation (Kopyra and Gwóźdź, 2003) and causes the formation of roots spontaneously (Pagnussat et al., 2002). Our experimental model showed the presence of NO in the root epidermis of 7- and 10-d-old seedlings and this may indicate the involvement of NO in a signalling cascade during development and the formation of new roots. Kopyra and Gwóźdź (2003) reported that NO donors counteract the inhibitory effects of heavy metals and salinity on the root growth, and this suggests that the NO detected in roots may have a protective role in abiotic stress conditions. Furthermore, production of NO in the root could be involved in the interaction of the plant with beneficial soil microorganisms, including mycorrhiza and rhizobacteria (Stöhr and Ullrich, 2002; Horchani et al., 2011) and as a mechanism of defence against pathogens (Murakami et al., 2011).
The analysis of tissue NO showed that each organ had a different content depending on the age of the seedling. The hypocotyl of 7-d-old seedling had a clearly higher NO content, which could participate in elongation, meristem development and communications between organs through the vascular system. Similar behaviour was reported during the early development of the main organs of pea seedlings at 5, 7, 9, 11, 13 and 15 d of growth, and stems of 11-d-old seedlings showed the highest NO production (Corpas et al., 2006). On the other hand, the cellular localization of NO showed that this free radical is mainly located in epidermal cells and vascular tissue, which makes sense considering that NO production has been reported to be involved in the formation of xylem (Gabaldón et al., 2005). In roots, NO clearly accumulated in the root hairs, which also correlates well with the involvement of NO in determining lateral root development (Correa-Aragunde et al., 2004), nitrate perception and root elongation (Manoli et al., 2014). The cellular localization of peroxynitrite and the superoxide radical follows a similar pattern, especially in roots, where they may be involved in defence mechanisms or lignification, considering that the superoxide radical, some peroxidases and NO participate in the lignification of vascular tissue (Puntarulo et al., 1991; Ogawa et al., 1997; Gabaldón et al., 2005; Gómez-Ros et al., 2012). In addition, it has been reported recently that one of the primary events in initially differentiating tracheary elements during root xylem development is a burst of NO in thin-walled cells, followed by synthesis of H2O2 (Bagniewska-Zadworna et al., 2014).
Currently, it is well established that S-nitrosoglutathione (GSNO) is the preferred physiological substrate of the glutathione-dependent enzyme formaldehyde dehydrogenase (Leterrier et al., 2011; Corpas et al., 2013a), which catalyses the NADH-dependent reduction of GSNO to GSSG and NH3. For this reason, this enzyme has been renamed as S-nitrosoglutathione reductase (GSNOR). The presence of GSNOR activity in plants has been conclusively demonstrated in many species (Sakamoto et al., 2002; Feechan et al., 2005; Barroso et al., 2006; Espunya et al., 2006; Leterrier et al., 2011); this GSNOR activity is necessary for normal plant development under optimal growth conditions (Lee et al., 2008; Kwon et al., 2012).
In Arabidopsis, analysis of the activity and expression of GSNOR by immunolocalization and histochemical methods showed that this protein was differentially expressed, being higher in roots and leaves from the earliest stages of development (Espunya et al., 2006). Our results are in good agreement with these data because roots of 7-d-old seedlings showed the highest activity, and activity diminished with seedling age. Conversely, GSNOR activity increased with seedling age in hypocotyls and cotyledons. Furthermore, over-expression and deletion of GSNOR in transgenic Arabidopsis plants produced a short root phenotype that correlated with a low level of intracellular glutathione and altered spatial distribution in the roots. This suggested that GSNOR and, consequently GSNO, could be involved in the regulation of the redox state of the root (Espunya et al., 2006). In this context, to our knowledge this is the first time that the GSNOR gene has been reported in pepper plants. Moreover, the analysis of this gene in other plant species, including Arabidopsis, tomato and tobacco, has shown that it is present in a single copy (Díaz et al., 2003; Lee et al. 2008; Xu et al., 2013; Kubienová et al., 2013).
Nitration of tyrosine is a covalent modification of proteins by the addition of a nitro group (-NO2) forming 3-nitrotyrosine (Gow et al., 2004; Radi, 2004). In plants, this modification of proteins has been used as a reliable marker of nitro-oxidative stress situations (Corpas et al., 2007, 2013b; Valderrama et al., 2007; Airaki et al., 2012). However, the presence of this post-translation modification mediated by NO-derived molecules in plants under normal physiological conditions suggests that nitration could have an additional function. In pepper seedlings grown under optimal conditions, the pattern of protein nitration in roots, hypocotyls and green cotyledons during the different stages of development was quite different. Several reports have identified some protein targets of this post-translation modification mediated by NO-derived molecules such as peroxynitrite. Thus, nitrated proteins have been identified in 9-d-old sunflower hypocotyls (Chaki et al., 2009), 2-week old Arabidopsis seedlings (Lozano-Juste et al., 2011), bitter orange (Citrus aurantium) roots (Tanou et al., 2009) and 71-d-old pea roots (Begara-Morales et al., 2013). Interestingly, some antioxidant enzymes were found among the identified nitrated proteins, such as catalase, APX, MDAR, GR and NADP-ICDH. In some cases, it has been demonstrated that nitration provokes inactivation of the enzyme activity (Corpas et al., 2015), which reflects a clear interplay between ROS and RNS metabolism. Moreover, in rice seedlings grown under normal conditions it has been reported that the tyrosine nitration of α-tubulin inhibits cell division and, consequently, cell growth (Jovanović et al., 2010), which provides evidence for the interconnection between post-translational modifications mediated by NO during plant development.
In summary, the present results show that during the development of pepper seedlings there is differential regulation of the metabolism of ROS, NO and NADP dehydrogenases, depending on the plant organ. Under these circumstances, it can be concluded that the metabolism of ROS and RNS seems to contribute significantly to plant development since their components are involved directly or indirectly in many metabolic pathways (β-oxidation, carbon and nitrogen metabolism, etc.). Thus, specific molecules such as H2O2 and NO have signalling implications (regulating gene expression and mediating post-translational modifications, such as nitration and S-nitrosylation, which affect protein function) (Begara et al., 2013, 2014) and their temporal and spatial regulation must contribute to the success of seedling establishment. Additionally, considering the NO profile during seedling development, it seems that this gaseous radical must be involved in the signalling cascade that allows root formation and elongation of pepper plants, as has been proposed for tomato (Correa-Aragunde et al., 2004) and sunflower plants (Corti-Monzón et al., 2014).
ACKNOWLEDGEMENTS
M.A. acknowledges a PhD fellowship from the Junta de Andalucía, Spain. This work was supported by ERDF-cofinanced grants from the Ministry of Science and Innovation (BIO2009-12003-C02-01 and BIO2009-12003-C02-02) and Junta de Andalucía (project P06-CVI-1820), Spain. The provision of pepper seeds by Sierra Bacarizo (Syngenta Seeds Ltd.) is acknowledged. Technical and personal support provided by CICT of Universidad de Jaén (UJA, MINECO, Junta de Andalucía, FEDER) is gratefully acknowledged. The valuable technical assistance of Carmelo Ruiz and M. Jesús Campos is also appreciated.
LITERATURE CITED
- Aebi H. 1984. Catalase in vitro. Methods in Enzymology 105: 121–126. [DOI] [PubMed] [Google Scholar]
- Airaki M, Leterrier M, Mateos RM, et al. 2012. Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant, Cell & Environment 35: 281–295. [DOI] [PubMed] [Google Scholar]
- Airaki M, Sánchez-Moreno L, Leterrier M, Barroso JB, Palma JM, Corpas FJ. 2011. Detection and quantification of S-nitrosoglutathione (GSNO) in pepper (Capsicum annuum L.) plant organs by LC-ES/MS. Plant & Cell Physiology 52: 2006–2015. [DOI] [PubMed] [Google Scholar]
- Anderson S, Mansfield TA. 1979. The effects of nitric oxide pollution on the growth of tomato. Environmental Pollution 20: 113–121. [Google Scholar]
- Axelrod JD, Bergmann DC. 2014. Coordinating cell polarity: heading in the right direction? Development 141: 3298–3302. [DOI] [PubMed] [Google Scholar]
- Bagniewska-Zadworna A, Arasimowicz-Jelonek M, Smoliński DJ, Stelmasik A. 2014. New insights into pioneer root xylem development: evidence obtained from Populus trichocarpa plants grown under field conditions. Annals of Botany 113: 1235–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C. 2004. Active oxygen species and antioxidants in seed biology. Seed Science Research 14: 93–107. [Google Scholar]
- Bailly C, Leymarie J, Lehner A, Rousseau S, Côme D, Corbineau F. 2004. Catalase activity and expression in developing sunflower seeds as related to drying. Journal of Experimental Botany 55: 475–483. [DOI] [PubMed] [Google Scholar]
- Bailly C, El-Maarouf-Bouteau H, Corbineau F. 2008. From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. Comptes Rendus Biologies 331: 806–814. [DOI] [PubMed] [Google Scholar]
- Barroso JB, Corpas FJ, Carreras A, et al. 2006. Localization of S-nitrosoglutathione and expression of S-nitrosoglutathione reductase in pea plants under cadmium stress. Journal of Experimental Botany 57: 1785–1793. [DOI] [PubMed] [Google Scholar]
- Begara-Morales JC, Chaki M, Sánchez-Calvo B, et al. 2013. Protein tyrosine nitration in pea roots during development and senescence. Journal of Experimental Botany 64: 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begara-Morales JC, Sánchez-Calvo B, Chaki M, et al. 2014. Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. Journal of Experimental Botany 65: 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. 2001. Nitric oxide in plants: the history is just beginning. Plant, Cell & Environment 24: 267–278. [Google Scholar]
- Bethke PC, Libourel IG, Jones RL. 2006. Nitric oxide reduces seed dormancy in Arabidopsis. Journal of Experimental Botany 57: 517–526. [DOI] [PubMed] [Google Scholar]
- Carol RJ, Dolan L. 2006. The role of reactive oxygen species in cell growth: lessons from root hairs. Journal of Experimental Botany 57: 1829–1834. [DOI] [PubMed] [Google Scholar]
- Chaki M, Fernández-Ocaña AM, Valderrama R, et al. 2009. Involvement of reactive nitrogen and oxygen species (RNS and ROS) in sunflower-mildew interaction. Plant & Cell Physiology 50: 265–279. [DOI] [PubMed] [Google Scholar]
- Chaki M, Álvarez de Morales P, Ruiz C, et al. 2015. Ripening of pepper (Capsicum annuum L.) fruits is characterized by an enhancement of protein tyrosine nitration. Annals of Botany 16: 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Le Maréchal P, Vidal J, Jacquot JP, Gadal P. 1988. Purification and comparative properties of the cytosolic isocitrate dehydrogenases (NADP) from pea (Pisum sativum) roots and green leaves. European Journal of Biochemistry 175: 565–572. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, Barroso JB. 2014. Peroxynitrite (ONOO–) is endogenously produced in Arabidopsis peroxisomes and is overproduced under cadmium stress. Annals of Botany 113: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ, García-Salguero L, Peragón J, Lupiáñez JA. 1995. Kinetic properties of hexose-monophosphate dehydrogenases. I. Isolation and partial purification of glucose-6-phosphate dehydrogenase from rat liver and kidney cortex. Life Sciences 56: 179–189. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, Barroso JB, Sandalio LM, et al. 1998. A dehydrogenase-mediated recycling system of NADPH in plant peroxisomes. Biochemical Journal 330: 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ, Barroso JB, Sandalio LM, Palma JM, Lupiáñez JA, del Río LA. 1999. Peroxisomal NADP-dependent isocitrate dehydrogenase. Characterization and activity regulation during natural senescence. Plant Physiology 121: 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ, Barroso JB, Carreras A, et al. 2006. Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta 224: 246–254. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, del Río LA, Barroso JB. 2007. Need of biomarkers of nitrosative stress in plants. Trends in Plant Science 12: 436–438. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, Chaki M, Fernández-Ocaña A, et al. 2008. Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant & Cell Physiology 49: 1711–1722. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, Leterrier M, Valderrama R, et al. 2011. Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Science 181: 604–611. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, Alché JD, Barroso JB. 2013a. Current overview of S-nitrosoglutathione (GSNO) in higher plants. Frontiers in Plant Science 4: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ, Palma JM, del Río LA, Barroso JB. 2013b. Protein tyrosine nitration in higher plants grown under natural and stress conditions. Frontiers in Plant Science 4: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ, Begara-Morales JC, Sánchez-Calvo B, Chaki M, Barroso JB. 2015. Nitration and S-nitrosylation: Two post-translational modifications (PTMs) mediated by reactive nitrogen species (RNS) and their role in signalling processes of plant cells. In: Gupta KJ, Igamberdiev AU, eds. Reactive oxygen and nitrogen species signaling and communication in plants, signaling and communication in plants. Vol. 23. Switzerland: Springer International Publishing, 267–281. doi: 10.1007/978-3-319-10079-1_13. [Google Scholar]
- Correa-Aragunde N, Graziano M, Lamattina L. 2004. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218: 900–905. [DOI] [PubMed] [Google Scholar]
- Corti-Monzón G, Pinedo M, Di Rienzo J, et al. 2014. Nitric oxide is required for determining root architecture and lignin composition in sunflower. Supporting evidence from microarray analyses. Nitric Oxide 39: 20–28. [DOI] [PubMed] [Google Scholar]
- De Smet I, White PJ, Bengough AG, et al. 2012. Analyzing lateral root development: how to move forward. Plant Cell 24: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz M, Achkor H, Titarenko E, Martínez MC. 2003. The gene encoding glutathione-dependent formaldehyde dehydrogenase/GSNO reductase is responsive to wounding, jasmonic acid and salicylic acid. FEBS Letters 543: 136–139. [DOI] [PubMed] [Google Scholar]
- Diaz-Vivancos P, Wolff T, Markovic J, Pallardó FV, Foyer CH. 2010. A nuclear glutathione cycle within the cell cycle. Biochemical Journal 431: 169–178. [DOI] [PubMed] [Google Scholar]
- Duan X, Li X, Ding F, et al. 2014. Interaction of nitric oxide and reactive oxygen species and associated regulation of root growth in wheat seedlings under zinc stress. Ecotoxicology and Environmental Safety 113C: 95–102. [DOI] [PubMed] [Google Scholar]
- Duke SH, Schrader LE, Miller MG. 1977. Low temperature effects on soybean (Glycine max Merr. cv. Wells) mitochondrial respiration and several dehydrogenases during imbibition and germination. Plant Physiology 60: 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EA, Rawsthorne S, Mullineaux PM. 1990. Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 180: 278–284. [DOI] [PubMed] [Google Scholar]
- Espunya MC, Diaz M, Moreno-Romero J, Martinez MC. 2006. Modification of intracellular levels of glutathione-dependent formaldehyde dehydrogenase alters glutathione homeostasis and root development. Plant, Cell & Environment 29: 1002–1011. [DOI] [PubMed] [Google Scholar]
- Feechan A, Kwon E, Yun BW, Wang Y, Pallas JA, Loake GJ. 2005. A central role for S-nitrosothiols in plant disease resistance. Proceedings of the National Academy of Sciences of the USA 102: 8054–8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Marcos M, Sanz L, Lewis DR, Muday GK, Lorenzo O. 2011. Nitric oxide causes root apical meristem defects and growth inhibition while reducing PINFORMED 1 (PIN1)-dependent acropetal auxin transport. Proceedings of the National Academy of Sciences of the USA 108: 18506–18511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieuw S, Müller-Röber B, Gálvez S, Willmitzer L. 1995. Cloning and expression analysis of the cytosolic NADP-dependent isocitrate dehydrogenase from potato. Implications for nitrogen metabolism. Plant Physiology 107: 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. 2005a. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17: 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. 2005b. Oxidant and antioxidant signaling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell & Environment 28: 1056–1071. [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, et al. 2003. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446. [DOI] [PubMed] [Google Scholar]
- Gabaldón C, Gómez Ros LV, Pedreño MA, Ros Barceló A. 2005. Nitric oxide production by the differentiating xylem of Zinnia elegans. New Phytology 165: 121–130. [DOI] [PubMed] [Google Scholar]
- Gallie DR. 2013. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. Journal of Experimental Botany 64: 433–443. [DOI] [PubMed] [Google Scholar]
- Gálvez S, Gadal P. 1995. On the function of the NADP-dependent isocitrate dehydrogenase isoenzymes in living organisms. Plant Science 105: 1–14. [Google Scholar]
- Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C. 2006. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28: 1091–1101. [DOI] [PubMed] [Google Scholar]
- Gómez-Ros LV, Gabaldón C, López Núñez-Flores MJ, et al. 2012. The promoter region of the Zinnia elegans basic peroxidase isoenzyme gene contains cis-elements responsive to nitric oxide and hydrogen peroxide. Planta 236: 327–342. [DOI] [PubMed] [Google Scholar]
- Gouvea CM, Souza CP, Magalhaes CAN, Martin IS. 1997. NO-releasing substances that induce growth elongation in maize root segments. Plant Growth Regulation 21: 183–187. [Google Scholar]
- Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. 2004. Biological significance of nitric oxide-mediated protein modifications. American Journal of Physiology. Lung Cellular and Molecular Physiology 287: L262–L268. [DOI] [PubMed] [Google Scholar]
- Hendricks SB, Taylorson RB. 1975. Breaking of seed dormancy by catalase inhibition . Proceedings of the National Academy of Sciences of the USA 72: 306–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horchani F, Prévot M, Boscari A, et al. 2011. Both plant and bacterial nitrate reductases contribute to nitric oxide production in Medicago truncatula nitrogen-fixing nodules. Plant Physiology 155: 1023–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Asada K. 1984. Inactivation of ascorbate peroxidase in spinach chloroplasts on dark addition of hydrogen peroxide: its protection by ascorbate. Plant & Cell Physiology 25: 1285–1295. [Google Scholar]
- Howard LR, Smith RT, Wagner AB, Villalon B, Burns EE. 1994. Provitamin A and ascorbic acid content of fresh pepper cultivars (Capsicum annuum) and processed jalapenos. Journal of Food Science 59: 362–365. [Google Scholar]
- Hufton CA, Besford RT, Wellburn AR. 1996. Effects of NO (+NO2) pollution on growth, nitrate reductase activities and associated protein contents in glasshouse lettuce grown hydroponically in winter CO2 enrichment. New Phytologist 133: 495–501. [Google Scholar]
- Ishibashi Y, Koda Y, Zheng SH, Yuasa T, Iwaya-Inoue M. 2013. Regulation of soybean seed germination through ethylene production in response to reactive oxygen species. Annals of Botany 111: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, Du ST, Zhang YS, Tang C, Lin XY. 2009. Atmospheric nitric oxide stimulates plant growth and improves the quality of spinach (Spinacia oleracea). Annals of Applied Biology 155: 113–120. [Google Scholar]
- Jovanović AM, Durst S, Nick P. 2010. Plant cell division is specifically affected by nitrotyrosine. Journal of Experimental Botany 61: 901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalve S, De Vos D, Beemster GT. 2014. Leaf development: a cellular perspective. Frontiers in Plant Science 5: 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsh VA, Hayes RB, Mayne ST, et al. 2006. Supplemental and dietary vitamin E, beta-carotene, and vitamin C intakes and prostate cancer risk. Journal of the National Cancer Institute 98: 245–254. [DOI] [PubMed] [Google Scholar]
- Kocsy G, Tari I, Vanková R, et al. 2013. Redox control of plant growth and development. Plant Science 211: 77–91. [DOI] [PubMed] [Google Scholar]
- Kopyra M, Gwóźdź EA. 2003. Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiology and Biochemistry 41: 1011–1017. [Google Scholar]
- Kubienová L, Kopečný D, Tylichová M, et al. 2013. Structural and functional characterization of a plant S-nitrosoglutathione reductase from Solanum lycopersicum. Biochimie 95: 889–902. [DOI] [PubMed] [Google Scholar]
- Kwon E, Feechan A, Yun BW, et al. 2012. AtGSNOR1 function is required for multiple developmental programs in Arabidopsis. Planta 236: 887–900. [DOI] [PubMed] [Google Scholar]
- Lamattina L, García-Mata C, Graziano M, Pagnussat G. 2003. Nitric oxide: the versatility of an extensive signal molecule. Annual Review of Plant Biology 54:109–136. [DOI] [PubMed] [Google Scholar]
- Lee U, Wie C, Fernández BO, Feelisch M, Vierling E. 2008. Modulation of nitrosative stress by S-nitrosoglutathione reductase is critical for thermotolerance and plant growth in Arabidopsis. Plant Cell 20: 786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem YY, Haramaty E. 1996. The characterization and contrasting effects of the nitric oxide free radical in vegetative stress and senescence of Pisum sativum Linn. foliage. Journal of Plant Physiology 148: 258–263. [Google Scholar]
- Leshem YY, Pinchasov Y. 2000. Non-invasive photoacoustic spectroscopic determination of relative endogenous nitric oxide and ethylene content stoichiometry during the ripening of strawberries Fragaria anannasa (Dutch.) and avocados Persea americana (Mill.). Journal of Experimental Botany 51: 1471–1473. [PubMed] [Google Scholar]
- Leterrier M, Corpas FJ, Barroso JB, Sandalio LM, del Río LA. 2005. Peroxisomal monodehydroascorbate reductase. Genomic clone characterization and functional analysis under environmental stress conditions. Plant Physiology 138: 2111–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier M, Barroso JB, Palma JM, Corpas FJ. 2012. Cytosolic NADP-isocitrate dehydrogenase in Arabidopsis leaves and roots Biologia Plantarum 56: 705–710. [Google Scholar]
- Leterrier M, Chaki M, Airaki M, et al. 2011. Function of S-nitrosoglutathione reductase (GSNOR) in plant development and under biotic/abiotic stress. Plant Signaling & Behavior 6: 789–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Juste J, Colom-Moreno R, León J. 2011. In vivo protein tyrosine nitration in Arabidopsis thaliana . Journal of Experimental Botany 62: 3501–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli A, Begheldo M, Genre A, Lanfranco L, Trevisan S, Quaggiotti S. 2014. NO homeostasis is a key regulator of early nitrate perception and root elongation in maize. Journal of Experimental Botany 65: 185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marone M, Mozzetti S, De Ritis D, Pierelli L, Scambia G. 2001. Semiquantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biological Procedures Online 3: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos RM, Bonilla-Valverde D, del Río LA, Palma JM, Corpas FJ. 2009. NADP-dehydrogenases from pepper fruits: effect of maturation. Physiologia Plantarum 135: 130–139. [DOI] [PubMed] [Google Scholar]
- Mateos RM, Jiménez A, Román P, et al. 2013. Antioxidant systems from pepper (Capsicum annuum L.): involvement in the response to temperature changes in ripe fruits. International Journal of Molecular Sciences 14: 9556–9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Shulaev V, Mittler R. 2008. Reactive oxygen signaling and abiotic stress. Physiologia Plantarum 133: 481–489. [DOI] [PubMed] [Google Scholar]
- Mittler R, Zilinskas BA. 1991. Purification and characterization of pea cytosolic ascorbate peroxidase. Plant Physiology 97: 962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake C, Asada K. 1996. Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate: hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant & Cell Physiology 36: 661–668. [Google Scholar]
- Mori IC, Schroeder JI. 2004. Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiology 135: 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Leubner-Metzger G. 2009. In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiology 150: 1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami E, Nagata M, Shimoda Y, et al. 2011. Nitric oxide production induced in roots of Lotus japonicus by lipopolysaccharide from Mesorhizobium loti. Plant & Cell Physiology 52: 610–617. [DOI] [PubMed] [Google Scholar]
- Ni W, Trelease RN. 1991. Post-transcriptional regulation of catalase isozyme expression in cotton seeds. Plant Cell 3: 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Trelease RN, Eising R. 1990. Two temporally synthesized charge subunits interact to form the five isoforms of cottonseed (Gossypium hirsutum) catalase. Biochemical Journal 269: 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu YF, Chai RS, Jin GL, Wang H, Tang CX, Zhang YS. 2013. Responses of root architecture development to low phosphorus availability: a review. Annals of Botany 112: 391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G. 2006. Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant, Cell & Environment 29:409–425. [DOI] [PubMed] [Google Scholar]
- Van Norman JM, Breakfield NW, Benfey PN. 2011. Intercellular communication during plant development. Plant Cell 23: 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Kanematsu S, Asada K. 1997. Generation of superoxide anion and localization of CuZn-superoxide dismutase in the vascular tissue of spinach hypocotyls: their association with lignification. Plant & Cell Physiology 38: 1118–1126. [DOI] [PubMed] [Google Scholar]
- Oracz K, El-Maarouf Bouteau H, Farrant JM, et al. 2007. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant Journal 50: 452–465. [DOI] [PubMed] [Google Scholar]
- Oracz K, El-Maarouf-Bouteau H, Kranner I, Bogatek R, Corbineau F, Bailly C. 2009. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiology 150: 494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L. 2002. Nitric oxide is required for root organogenesis. Plant Physiology 129: 954–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomo J, Gallardo F, Suarez MF, Cánovas FM. 1998. Purification and characterization of NADP-linked isocitrate dehydrogenase from scots pine. Evidence for different physiological roles of the enzyme in primary development. Plant Physiology 118: 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual MB, Molina-Rueda JJ, Cánovas FM, Gallardo F. 2008. Spatial distribution of cytosolic NADP-isocitrate dehydrogenase in pine embryos and seedlings. Tree Physiology 28: 1773–1782. [DOI] [PubMed] [Google Scholar]
- Pergo EM, Ishii-Iwamoto EL. 2011. Changes in energy metabolism and antioxidant defense systems during seed germination of the weed species Ipomoea triloba L. and the responses to allelochemicals. Journal of Chemical Ecology 37: 500–513. [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Forzani C, Hirt H. 2006. Reactive oxygen species signaling in plants. Antioxidants & Redox Signaling 8: 1757–1764. [DOI] [PubMed] [Google Scholar]
- Puntarulo S, Galleano M, Sanchez RA, Boveris A. 1991. Superoxide anion and hydrogen peroxide metabolism in soybean embryonic axes during germination. Biochimica et Biophysica Acta 1074: 277–283. [DOI] [PubMed] [Google Scholar]
- Radi R. 2004. Nitric oxide, oxidants, and protein tyrosine nitration. Proceedings of the National Academy of Sciences of the USA 101: 4003–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh MG, Sabre M, Scandalios JG. 1990. The distribution of catalase activity, isozyme protein, and transcript in the tissues of the developing maize seedling. Plant Physiology 92: 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez AA, Grunberg KA, Taleisnik EL. 2002. Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiology 129: 1627–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A, Ueda M, Morikawa H. 2002. Arabidopsis glutathione-dependent formaldehyde dehydrogenase is an S-nitrosoglutathione reductase. FEBS Letters 515: 20–24. [DOI] [PubMed] [Google Scholar]
- Shapiro AD. 2005. Nitric oxide signaling in plants. Vitamins & Hormones 72: 339–398. [DOI] [PubMed] [Google Scholar]
- Schaaf J, Walter MH, Hess D. 1995. Primary metabolism in plant defense (regulation of a bean malic enzyme gene promoter in transgenic tobacco by developmental and environmental cues). Plant Physiology 108: 949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers JH, Nguyen HM, Lu D, Schmidt R, Mueller-Roeber B. 2012. ROS homeostasis during development: an evolutionary conserved strategy. Cellular and Molecular Life Sciences 69: 3245–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šírová J, Sedlářová M, Piterková J, Luhová L, Petřivalský M. 2011. The role of nitric oxide in the germination of plant seeds and pollen. Plant Science 181: 560–572. [DOI] [PubMed] [Google Scholar]
- Stöhr C, Ullrich WR. 2002. Generation and possible roles of NO in plant roots and their apoplastic space. Journal of Experimental Botany 53: 2293–2303. [DOI] [PubMed] [Google Scholar]
- Swanson S, Gilroy S. 2010. ROS in plant development. Physiologia Plantarum 138: 384–392. [DOI] [PubMed] [Google Scholar]
- Tanou G, Job C, Rajjou L, et al. 2009. Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. Plant Journal 60: 795–804. [DOI] [PubMed] [Google Scholar]
- ten Tusscher KHWJ, Scheres B. 2011. Joining forces: feedback and integration in plant development. Current Opinion in Genetics and Development 21: 799–805. [DOI] [PubMed] [Google Scholar]
- Valderrama R, Corpas FJ, Carreras A, et al. 2006. The dehydrogenase-mediated recycling of NADPH is a key antioxidant system against salt-induced oxidative stress in olive plants. Plant, Cell & Environment 29: 1449–1459. [DOI] [PubMed] [Google Scholar]
- Valderrama R, Corpas FJ, Carreras A, et al. 2007. Nitrosative stress in plants. FEBS Letters 581: 453–461. [DOI] [PubMed] [Google Scholar]
- Vanderslice JT, Higgs DJ. 1991. Vitamin C content of foods: sample variability. American Journal of Clinical Nutrition 54: 1323S–1327S. [DOI] [PubMed] [Google Scholar]
- Wilson ID, Neill SJ, Hancock JT. 2008. Nitric oxide synthesis and signaling in plants. Plant, Cell & Environment 31: 622–631. [DOI] [PubMed] [Google Scholar]
- Wojtyla Ł, Garnczarska M, Zalewski T, Bednarski W, Ratajczak L, Jurga S. 2006. A comparative study of water distribution, free radical production and activation of antioxidative metabolism in germinating pea seeds. Journal of Plant Physiology 163: 1207–1220. [DOI] [PubMed] [Google Scholar]
- Xu S, Guerra D, Lee U, Vierling E. 2013. S-nitrosoglutathione reductases are low-copy number, cysteine-rich proteins in plants that control multiple developmental and defense responses in Arabidopsis. Frontiers in Plant Science 4: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Mori H, Nishimura M. 1995. A novel isoenzyme of ascorbate peroxidase localized on glyoxysomal and leaf peroxisomal membranes in pumpkin. Plant & Cell Physiology 36: 1157–1162. [DOI] [PubMed] [Google Scholar]
- Zafra A, Rodríguez-García M I, Alché J D. 2010. Cellular localization of ROS and NO in olive reproductive tissues during flower development. BMC Plant Biology 10:36 doi: 10.1186/1471-2229-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]