Abstract
Background and Aims Water is an increasingly scarce resource that limits crop productivity in many parts of the world, and the frequency and severity of drought are predicted to increase as a result of climate change. Improving tolerance to drought stress is therefore important for maximizing future crop yields. The aim of this study was to compare the effects of drought on soybean (Glycine max) leaves and nodules in order to define phenotypic markers and changes in cellular redox state that characterize the stress response in different organs, and to characterize the relationships between leaf and nodule senescence during drought.
Methods Leaf and crown nodule metabolite pools were measured together with leaf and soil water contents, and leaf chlorophyll, total protein contents and chlorophyll a fluorescence quenching parameters in nodulated soybeans that were grown under either well-watered conditions or deprived of water for up to 21 d.
Key Results Ureides, ascorbate, protein, chlorophyll and the ratios of variable chlorophyll a fluorescence (Fv′) to maximal chlorophyll a fluorescence (Fm′) fell to levels below detection in the oldest leaves after 21 d of drought. While these drought-induced responses were not observed in the youngest leaf ranks, the Fv′/Fm′ ratios, pyridine nucleotide levels and the reduction state of the ascorbate pool were lower in all leaf ranks after 21 d of drought. In contrast to leaves, total nodule protein, pyridine nucleotides, ureides, ascorbate and glutathione contents increased as a result of the drought treatment. However, the nodule ascorbate pool was significantly less reduced as a result of drought. Higher levels of transcripts encoding two peroxiredoxins were detected in nodules exposed to drought stress but senescence-associated transcripts and other mRNAs encoding redox-related proteins were similar under both conditions.
Conclusions While the physiological impact of the drought was perceived throughout the shoot, stress-induced senescence occurred only in the oldest leaf ranks. At this stage, a number of drought-induced changes in nodule metabolites were observed but no metabolite or transcript markers of senescence could be detected. It is concluded that stress-induced senescence in the lowest leaf ranks precedes nodule senescence, suggesting that leaves of low photosynthetic capacity are sacrificed in favour of nodule nitrogen metabolism.
Keywords: Ascorbic acid, cysteine proteases, drought, Glycine max, nodules, peroxiredoxin, redox regulation, reactive oxygen species, ROS, soybean, senescence, ureides, water stress
INTRODUCTION
Water is an increasingly scarce resource that limits crop productivity in many parts of the world. Drought is a major limitation to crop growth and the productivity of current agriculture and is one of the most important environmental threats to food security worldwide (Cutforth et al., 2007). The frequency and severity of drought are predicted to increase as a result of climate change together with increases in the land areas experiencing drought (Jury and Vaux, 2007). While plant responses to drought are increasingly well understood (Claeys and Inzé, 2013), gaining a working knowledge of the genetic basis of drought tolerance remains more elusive. The acquisition of drought tolerance probably involves molecular, cellular, physiological and developmental adjustments (Yamaguchi-Shinozaki and Shinozaki, 2006; Manavalan et al., 2009). Drought-resistant cultivars are considered to produce higher yields than sensitive cultivars under conditions of water limitation (Fenta et al., 2012, 2014). Plants employ different strategies to avoid or tolerate drought. These are: (1) drought escape, e.g. reproduction before soil water becomes limiting in late-season drought; (2) dehydration tolerance, i.e. the ability of plant tissues to survive and recover from dehydration; and (3) dehydration avoidance, where plants use adaptive mechanisms to delay loss of turgor and the onset of dehydration. Plants can avoid the negative impacts of drought through reduced transpiration, osmotic adjustment or improved capture of water from the soil (Chaves and Oliveira, 2004; Blum, 2005). Rapid canopy development, and the timing and sensitivity of reproductive development in relation to seasonal droughts are considered to be important traits underpinning drought tolerance (Blum, 2005). In the first instance, drought is perceived by the root system from which signals are transmitted to the leaves, activating ‘water-saving’ strategies, including stomatal closure to control water loss by transpiration (Pastori and Foyer, 2002; Flexas et al., 2006; Cruz de Carvalho, 2008). Drought is also considered to increase the accumulation of reactive oxygen species (ROS; Iturbe-Ormaetxe et al., 1998; Tuteja, 2007) leading to oxidative signalling that interfaces with a network of hormonal responses (Noctor et al., 2014).
Soybean is the most important grain legume crop worldwide. It is used for human and animal consumption. As a legume, soybean is able to establish a symbiotic union with nitrogen-fixing soil bacteria of the genus Rhizobium that are housed with root nodules (Van Heerden et al., 2007). Symbiotic nitrogen fixation provides an important nitrogen source for agriculture, underpinning sustainable production. As in all legumes, exposure to drought leads to an inhibition of nodule nitrogen fixation and a breakdown of symbiosis that limits yields in many parts of the world (Ladrera et al., 2007). The yield of the soybean crop is decreased by drought, particularly when it occurs during flowering and early pod expansion (Pedersen et al., 2005), times when the nodules are already subject to a developmentally regulated senescence programme. Drought is therefore considered to be the greatest threat to soybean production and profitability worldwide (Neves-Borges et al., 2012). For example, in 2008–2009 drought-induced losses in total soybean production in Brazil, the second largest soybean producer, were estimated to be almost 11 million tonnes (Franchini et al., 2009). Hence, the delivery of high-yielding drought-tolerant varieties through marker-assisted selection programmes is essential to farmers. While functional genomics tools have allowed the dissection of the molecular mechanisms that define the drought stress response in soybean, there has been relatively little advance in shoot and root phenotyping to assist molecular genetics approaches (Fenta et al., 2012, 2014). Currently, traits such as rooting depth (root mass at depth), water use efficiency, nitrogen fixation and leaf wilting are important in the evaluation of soybean germplasm for drought tolerance.
In tropical legumes such as soybean, the primary products of symbiotic nitrogen fixation are ureides, such as allantoin and allantoate. Ureides, which are derived from urea, are less soluble than amides, which are the major products of symbiotic nitrogen fixation in temperate legumes. In some studies, ureides have been shown to accumulate in the nodules exposed to drought (Sinclair and Serraj, 1995; Serraj et al., 1999b). However, the drought-induced accumulation of ureides has not always been observed (Serraj et al., 1999a,b; King and Purcell, 2005; Todd et al., 2006; Ladrera et al., 2007). Moreover, no correlation was found between ureide accumulation and the stress-induced inhibition of nitrogen fixation (Ladrera et al., 2007).
The effects of drought on soybean nodules are well documented (Evans et al., 1999; Purcell et al., 2000; Streeter, 2003) as are the effects on leaves or leaves and roots (Liu et al., 2005; Stolf-Moreira et al., 2010). There is a strong association between root and nodule parameters and shoot biomass in drought-tolerant soybeans under both glasshouse and field conditions (Fenta et al., 2012, 2014). This finding led us to consider whether there was a relationship between leaf and nodule senescence under drought because this topic is poorly documented in the literature. The timing of the senescence genetic programmes in leaves and nodules appears to be considered as something like a ‘chicken and egg’ scenario in the overall plant stress response. This study was therefore undertaken to define the phenotypic markers of the drought response in soybean. Moreover, the relative progression of drought-induced changes in metabolism was measured in the different leaf ranks and in crown nodules to resolve the sequence of events with regard to the timing of senescence in different organs. The molecular and metabolic responses of the reduction/oxidation (redox) systems of leaves and nodules to drought were measured in relation to organ senescence.
MATERIALS AND METHODS
Plant material and growing conditions
Seeds of Glycine max ‘Williams 82’ were inoculated with 0·5 g per seed of a cell powder of Bradyrhizobium japonicum strain WB74-1 (Soygro bio-fertilized Ltd, Potchefstroom, South Africa) and placed one seed per pot (18 cm height and 22 cm diameter) filled to the rim with fine-grade (0·5–2 mm) vermiculite. Seedlings were watered twice per week with nitrogen-free Hoagland’s nutrient solution and demineralized water was added on the remaining days to maintain optimum conditions.
The plants were kept in a glasshouse with 16-h day length and 30/25 °C day/night temperature, with a light intensity of 350 µmol m–2 s–1.
Drought treatments
The drought experiment was performed on plants that had been grown for 5 weeks. At this stage the cotyledons had senesced and the plants had two unifoliolate lower leaves (L) and five trifoliolate (TF) leaves. A total of 30 plants were used in each experiment. Control plants received nutrients and water as in the first 5 weeks of growth. For the drought treatments, plants were deprived of both water and nutrients for 21 d.
Three plants were sampled from each of the control and drought-treated plants at the times indicated on the figures and tables. Crown nodules were harvested and leaf discs were collected from each leaf rank on the plant. These were weighed and immediately frozen in liquid N2 until analysis. The roots and a soil core (10 cm length, 2·5 cm diameter) were taken from each pot in an area close to the plant. Each sample was weighed and then placed in an oven at 80 °C until a constant weight was reached, for measurement of dry weight values. The root and soil water contents were calculated from the fresh and dry weight measurements.
Protein content
The content of protein was determined following the method described by Bradford (1976).
Pigment content
Chlorophylls a and b and total carotenes were extracted in 95 % ethanol by homogenizing frozen leaf discs in a mortar that had been pre-cooled using liquid N2. The extracts were centrifuged at 12 000 g for 10 min at 4 °C. The absorbance of the supernatant solutions was then measured at 648·6 and 664·2 nm. The pigments were then quantified as described by Lichtenthaler (1987).
Chlorophyll a fluorescence
The quantum efficiency of photosystem II (PSII) reaction centres was determined by the ratios of variable chlorophyll a fluorescence (Fv′) to maximal chlorophyll a fluorescence (Fm′) in light-adapted leaves using a portable fluorometer (FluorPen FP100; Photon Systems Instruments, Brno, Czech Republic) using the equipment settings for quantum yield parameters.
Ascorbic acid, glutathione and pyridine nucleotides
Metabolites were measured in extracts from frozen plant material that had been homogenized in a pre-cooled mortar at liquid N2 temperatures. Frozen 1 m perchloric acid was then added and the mixtures were homogenized until they had thawed. After centrifugation at 14 000 g for 10 min (at 4 °C), the pellets were discarded and the supernatants were used for assay of ascorbate and glutathione contents. Total ascorbate and the ratio between reduced ascorbate and its oxidized form, dehydroascorbate, were determined in extracts of leaf discs and nodules as described by Foyer et al. (1983). Reduced glutathione (GSH) and homoglutathione (hGSH) were measured together with glutathione disulphide (GSSG) and homoglutathione disulphide (hGSSG) in extracts of leaf discs and nodules as described by Noctor and Foyer (1998). The spectrophometric technique used in these studies does not to discriminate between glutathione and homoglutathione. In these experiments, GSH was used to produce a standard curve and a correction factor of 2·6 was applied as described by Klapheck (1988) and has been used in similar studies (Pérez-Chaca et al., 2014).
The reduced and oxidized forms of pyridine nucleotides were extracted from leaf discs and nodules and assayed as described by Foyer et al. (2008). Oxidized pyridine nucleotides (NAD+ and NADP+) were extracted with acid as described for ascorbate and glutathione. Reduced pyridine nucleotides (NADH and NADPH) were extracted in 0·2 m NaOH and supernatant fractions were neutralized with 0·2 m HCl. Pyridine nucleotides were assayed using the phenazine methosulfate-catalysed reduction of dichlorophenolindophenol in the presence of ethanol and alcohol dehydrogenase (for NAD+ and NADH) or glucose 6-phosphate (G6P) and G6P dehydrogenase (for NADP+ and NADPH) as described by Queval and Noctor (2007).
Ureide contents
Ureide contents were determined in extracts of leaf discs and nodules according to the method of Young and Conway (1942). Leaf discs and nodules were ground in a mortar that had been precooled to liquid N2 temperatures and homogenised with frozen 0·2 m NaOH until the samples had thawed. Samples then were boiled for 20 min. After cooling, NaOH was added to the diluted extracts to ensure the conversion of allantoin to allantonic acid. The samples were then boiled to hydrolyse allantonic acid before phenylhydrazine and potassium ferricyanide solutions were added and the colour change was measured at 525 nm. Calibration curves were performed using allantoin as the standard.
RNA extraction, cDNA synthesis and qRT-PCR analysis
Real-time quantitative PCR (qRT-PCR) was performed as described previously (Pellny et al., 2009). Frozen nodule material was ground with a mortar and pestle in liquid nitrogen and about 100 mg fresh material was used for mRNA extraction using an RNEasy Plant Minikit (Qiagen, France) according to the manufacturer’s instructions. RNA reverse transcription and quantitative PCR were performed on an Eppendorf Realplex2 real-time PCR system by one-step RT-PCR using Quantifast SYBR Green RTPCR Kit (Qiagen) following the manufacturer’s instructions. The data was analysed using the 2−ΔΔCT method (Livak and Schmittgen, 2001). Relative expression was normalized against the constitutively expressed β-actin or soybean elongation factor. Melting curves were determined for each sequence to confirm the identity of amplification products. Accession numbers and sequences used for forward and reverse primers used are shown in Table 1.
Table 1.
Primer sequences for amplification of different vacuolar, papain-like cysteine proteases and redox-associated proteins; primers for γECS, hGS, GR MDAAR and DHAR were designed based on NCBI reference sequences
| Phytozome ID | Forward primer (5′ to 3′) | Reverse primer (3′ to 5′) |
|---|---|---|
| Glyma18g52780 (Actin 1) | TGTTCCCTGGTATTGCTGAC | AAGGTGCTAAGAGATGCCAAG |
| Glyma02g44460 (ElF 1-β) | GTGGTACGATGCTGTCTCTC | CCACTGAATCTTACCCCTTGAG |
| γECS (XM_006586262.1) | CTCAAACAGGGGAAGCAGAG | CACTTTGGCTGGAAACCAAT |
| hGS (NM_001250121.1) | AATTCTGGTCGTGGTTCAGG | TGAAATTGCTTGTCCATCCA |
| MDAR (XM_006599044.1) | GTGGTGGGAATTGGAATACG | TGCTTTGACTGGAAATGCTG |
| DHAR (NM_001250000.1) | GAGATTGCTTTGGGGCATTA | TTCCACTTTAGGACGCCAAC |
| Glyma06g41610 (Tx1) | CAGTGGATAAAATTGTGGGTGC | GGCAACTGTTCAATGTGTTCG |
| Glyma07g09240 (Prx1) | TGAAAGGAAAGGGTGTGGAC | GAGGGCATTGGTGTATTTGG |
| Glyma09g32540 (Prx2) | ACGTTCCTGGCTTCATTGAG | TTCTCTGGGAATGTTTTGGC |
| Glyma12g13920(Grx1) | TTCCTTATAGGTCATGGCAACC | GTGTGGGCTAATTGTGAAGTG |
| Glyma16g07870 (Grx2) | ACCTATTGCCCTTTCTGC | TCCGTCCACTCAACTAATGC |
| Glyma17g14680 (VPE1) | CTACGGAAACTACAGGCATC | GTTCTCCGTCGTCACATTAT |
| Glyma05g04230 (VPE2) | CACCATCCCTTGTAAATTGT | GGGGTTTCAGTGCATAATAA |
| Glyma14g10620 (VPE3) | GGTCGTGGATGTTGCTGAGG | ATCTGCTTGATGCCTGTAGTTTCC |
| Glyma04g04400 (CP1) | GATCTTTAATGGCCACGATCCTCAT | CAGCACCTTGAAAGGGGTAATCCT |
| Glyma17g05670 (CP2) | GCTTGTCACTGCTCATTTTCGC | TTTTCCGGTGTAGGGATATGC |
| Glyma10g35100 (CP3) | GAGGCCATGCCCTCATGT | TCACCTCTCTCCCCAGTGTAGG |
| Glyma14g40670 (CP4) | ATATGGAGCGTGTGACTCGG | GTAATATCCATTCTCTCCCCAGCTC |
| Glyma04g03090 (CP5) | AAGCTGTGGTGCATGTTGGG | AGTGGCGCTTGTCTTTGCAG |
Data analysis
The data presented are the means ± s.e. of three independent plants per treatment and per time point. For comparisons and statistical analysis, a t-test was performed for each level and time point between control and drought plants, and significance levels are indicated with asterisks (*P < 0·05, **P < 0·01, ***P < 0·001).
RESULTS
Water content in soybean tissues during drought treatment
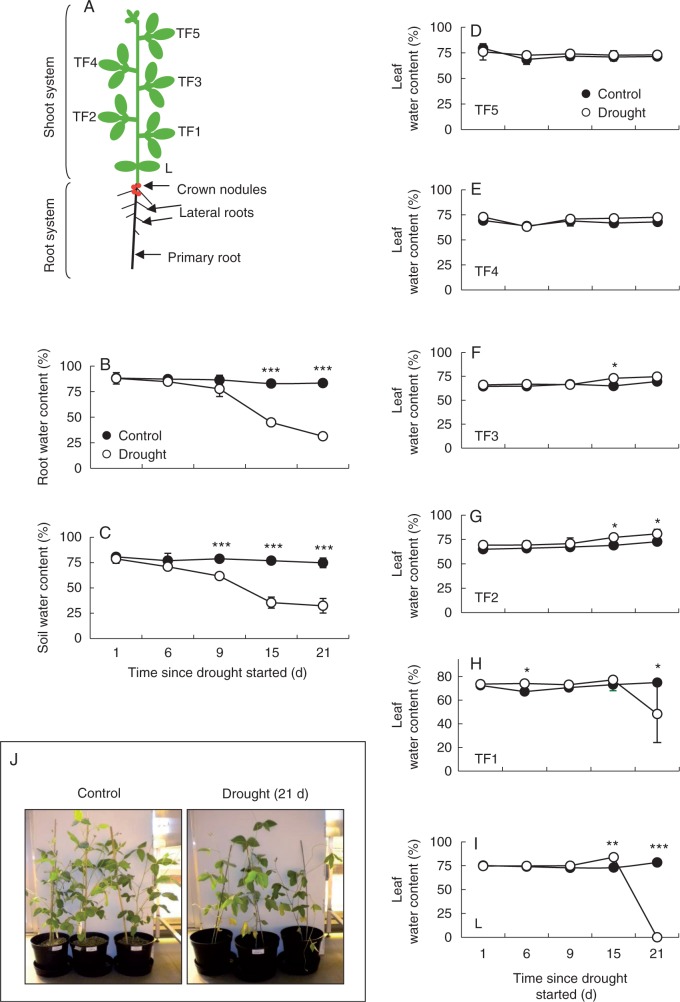
Nodulated soybean plants were grown to the fifth trifoliate leaf stage, as illustrated in Fig. 1A. At this stage, the plants had lost their cotyledons and had two unifoliolate leaflets (L) and five trifoliolate leaves (TF). Batches of plants were then subjected to drought by depriving the plants of water for 21 d. In comparison with well-watered controls, the water contents of roots (Fig. 1B) and the vermiculite planting medium (Fig. 1B, C) decreased from day 9 onwards in plants experiencing drought (Fig. 1B, C). In contrast, leaf water contents were similar to the well-watered controls in all but the lowest leaf ranks of the plants subjected to drought. The lowest leaf rank, L, contained little water after 21 d of drought (Fig. 1I). Similarly, TF1 had significant lower water contents than control plants after 21 d of drought (Fig. 1D). Visual inspection showed that the L leaves were dry and yellow, and the TF1 leaves were flaccid and yellow (Fig. 1J).
Fig. 1.
The effects of drought on leaf and soil water contents. (A) Schematic representation of a soybean plant indicating the different leaf ranks at the time of the experiment (L, unifoliolate leaves; TF, trifoliolate leaves). (B–J) root water content (B), soil water content (C), the water content of leaves at different ranks on the stem (D–I) and the phenotype of the plants after 21 d of treatment (J). Data are mean ± s.e. of three different samples. Statistical differences for each time point between the control and drought are denoted by asterisks (*P < 0·05, **P < 0·01, ***P < 0·001).
Photosynthesis and leaf chlorophyll contents
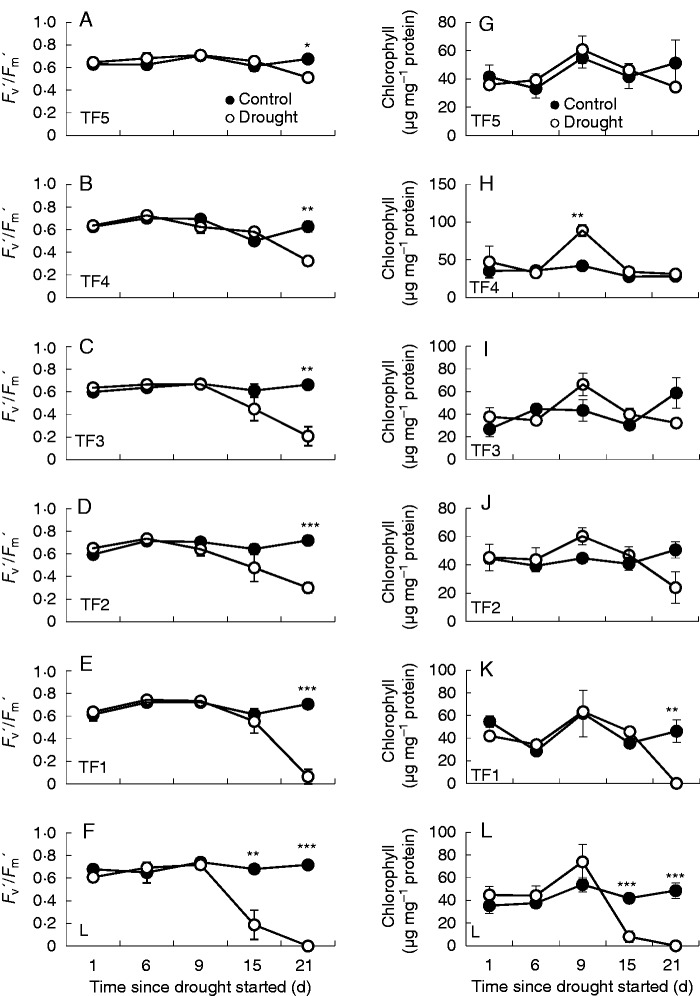
The ratio of light-adapted variable chlorophyll a fluorescence (Fv′) to maximal chlorophyll a fluorescence (Fm′) was used as a measure of the photosynthetic capacity. Decreases in Fv′/ Fm′ indicate that fewer open reaction centres are available in PSII to undertake photochemistry. Despite the observation that the water contents of all but the lowest leaf ranks were similar in the plants experiencing drought to well-watered controls, the physiological impact of the imposed stress was observed by the decreased Fv′/Fm′ ratios in all leaf ranks after 21 d of drought treatment (Fig. 2A–F). However, in contrast to other leaves, the Fv′/Fm′ ratios of the TF1 leaf ranks were no longer measurable at day 21, indicating complete inhibition of photosynthesis (Fig. 2E). Leaf rank L showed significant decreases in Fv′/Fm′ from day 15 of the drought treatment. The Fv′/Fm′ ratios were below the levels of detection at day 21. This observation is consistent with the loss of chlorophyll from leaf rank L (Fig. 2G–L). In contrast, the levels of chlorophyll expressed on a protein basis were similar in other leaf ranks (TF3, TF4 and TF5) under both well-watered and drought conditions (Fig. 2A–D).
Fig. 2.
The effects of drought on photosystem II efficiency (Fv′/Fm′) in light-adapted leaves (A–F) and chlorophyll levels (G–L) in the different leaf ranks. Data are means ± s.e. of three different samples. Statistical differences for each time point between the control and drought are denoted by asterisks (*P < 0·05, **P < 0·01, ***P < 0·001).
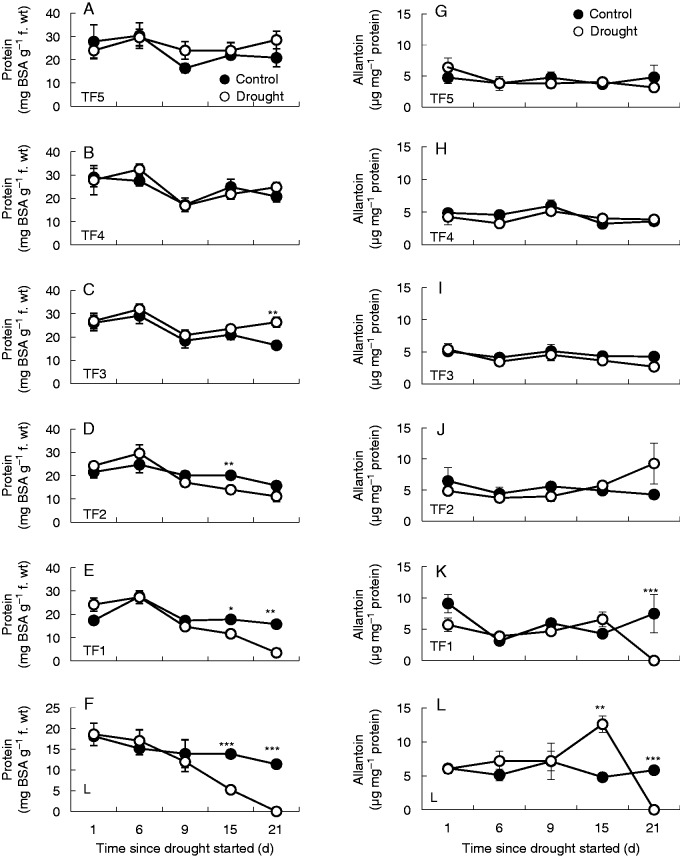
Leaf protein and ureide contents
Leaf protein contents were significantly decreased from day 15 in the TF1 and L leaf ranks (Fig. 3E, F). In contrast, protein levels were similar in the youngest leaf ranks (TF2–TF5) of plants experiencing drought stress and well-watered controls (Fig. 3A–D). A similar trend was observed with regard to the ureide contents of the youngest leaf ranks (Fig. 3H–K). However, the TF1 and L leaves accumulated ureides under drought stress relative to well-watered controls (Fig. 3M)
Fig. 3.
The effects of drought on the protein (A–G) and ureide (H–N) contents of leaves at different leaf ranks. Data are the mean ± s.e. of three different samples. Statistical differences for each time point between the control and drought are denoted by asterisks (*P < 0·05, **P < 0·01, ***P < 0·001).
Leaf ascorbate, glutathione and pyridine nucleotide contents
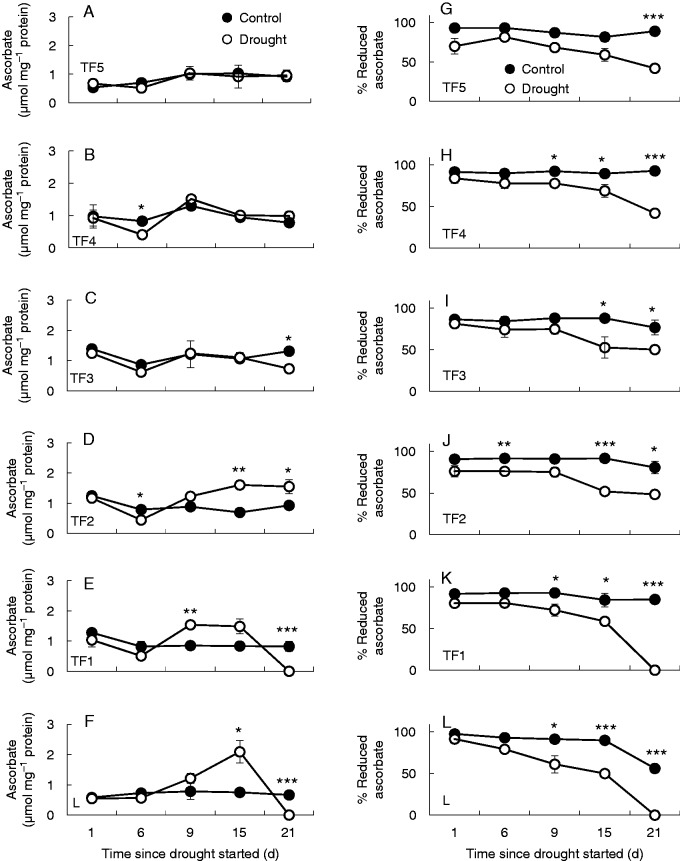
The levels of total ascorbate (Fig. 4E) were similar in the youngest leaf ranks of well-watered controls and drought-stressed plants (TF3–TF5; Fig. 4A–C). In contrast, the lower leaf ranks tended to accumulate ascorbate in response to drought (Fig 4D–F). However, the reduction state of the ascorbate pool expressed as the percentage of pool that was present as the reduced form was decreased in all the leaf ranks after 21 d of drought (Fig. 4G–L).
Fig. 4.
The effects of drought on the total (reduced plus oxidized) ascorbate levels (A–F) and on the percentage present in the reduced form (G–M) of the leaves at different leaf ranks. Data are the means ± s.e. of three different samples. Statistical differences for each time point between the control and drought are denoted by asterisks (*P < 0·05, **P < 0·01, ***P < 0·001).
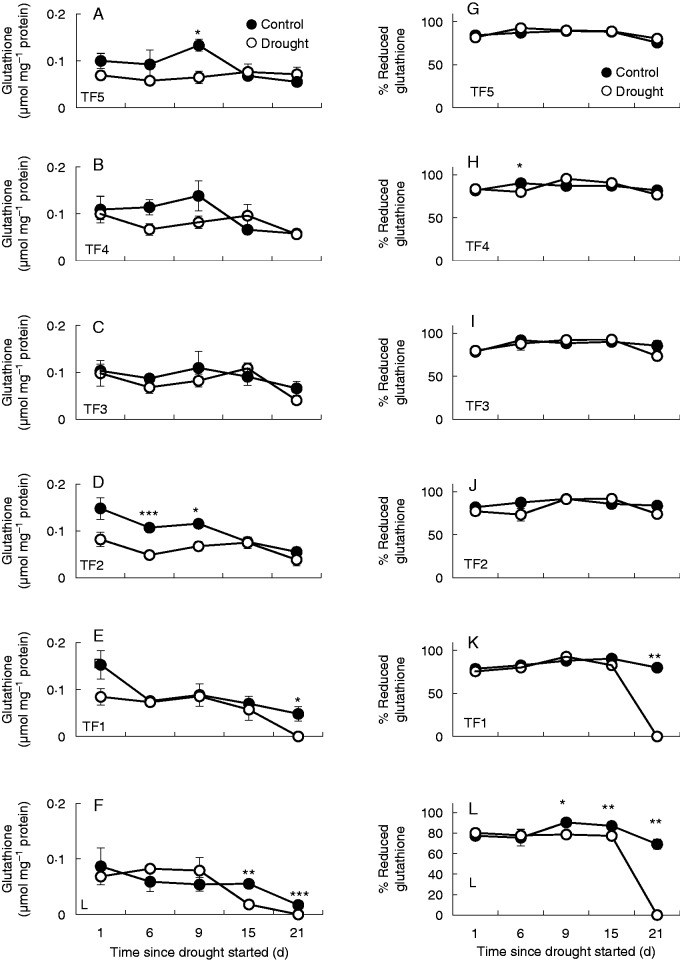
The levels of total glutathione (GSH plus GSSG) and homoglutathione (hGSH plus hGSSG) tended to decrease with leaf development, a feature that was observed in all but the youngest leaf ranks (Fig. 5). Drought had little effect on the abundance of glutathione (GSH plus GSSG) and homoglutathione (hGSH plus hGSSG) in all but the lowest leaf ranks, which had significantly lower levels of these antioxidants after 21 s of the drought stress treatment relative to well-watered controls (Fig. 5F). Moreover, the reduction state of the glutathione pool expressed as the percentage of the pool that was present as GSH plus hGSH was lower in the TF1 leaf ranks relative to well-watered controls after 21 d of drought (Fig. 5K).
Fig. 5.
The effects of drought on the total glutathione and homoglutathione (GSH and hGSH plus GSSG and hGSSG) pool (A–F) and percentage of reduced form (G–M) in the leaves at different leaf ranks. Data are the means ± s.e. of three different samples. Statistical differences for each time point between the control and drought are denoted by asterisks (*P < 0·05, **P < 0·01, ***P < 0·001).
Levels of NAD and NADH were significantly decreased in the youngest leaf ranks (TF2–TF5) of plants exposed to drought stress compared with well-watered controls (Table 2). In contrast, levels of NADP and NADPH were similar in the youngest leaf ranks of drought-treated plants and well-watered controls (Table 2).
Table 2.
Levels of pyridine nucleotides and percentage of the reduced forms in the leaves and nodules of well-watered plants and on plants deprived of water for 1 and 21 d (plant leaf level codes are as in Fig. 1)
| (a) | ||||||
|---|---|---|---|---|---|---|
| Plant leaf level | NAD + NADH (nmol mg–1 protein) |
% NADH |
||||
| Well-watered | Drought (day 1) | Drought (day 21) | Well-watered | Drought (day 1) | Drought (day 21) | |
| TF5 | 1·87 ± 0·33 | 1·80 ± 0·13 | 1·05 ± 0·14 | 20·8 ± 0·9 | 24·1 ± 5·5 | 26·3 ± 7·8 |
| TF4 | 1·71 ± 0·43 | 2·46 ± 0·48 | 1·25 ± 0·09 | 24·0 ± 2·6 | 19·2 ± 2·9 | 21·9 ± 1·4 |
| TF3 | 1·42 ± 0·25 | 1·99 ± 0·14 | 0·97 ± 0·02 | 20·0 ± 1·0 | 23·8 ± 2·5 | 23·4 ± 4·6 |
| TF2 | 1·42 ± 0·30 | 0·98 ± 0·23 | 1·98 ± 0·27 | 23·4 ± 1·8 | 31·5 ± 5·6 | 27·7 ± 1·9 |
| TF1 | 1·77 ± 0·17 | 1.89 ± 0·16 | 0·00 ± 0·00*** | 20·0 ± 1·2 | 19·3 ± 3·7 | 0·0 ± 0·0*** |
| L | 1·76 ± 0·27 | 2·15 ± 0·19 | 0·00 ± 0·00*** | 24·5 ± 1·6 | 20·2 ± 2·2 | 0·0 ± 0·0*** |
| Nodule | 6·85 ± 0·41 | 7·01 ± 0·66 | 5·34 ± 0·70 | 23·8 ± 0·6 | 25·3 ± 3·0 | 21·8 ± 0·2* |
| (b) |
||||||
|---|---|---|---|---|---|---|
| Plant leaf level | NADP + NADPH (nmol mg–1 protein) |
% NADPH |
||||
| Well-watered | Drought (day 1) | Drought (day 21) | Well-watered | Drought (day 1) | Drought (day 21) | |
| TF5 | 1·69 ± 0·22 | 1·84 ± 0·29 | 1·09 ± 0·07 | 44·6 ± 5·8 | 40·8 ± 12·5 | 58·3 ± 6·6 |
| TF4 | 1·33 ± 0·07 | 1·23 ± 0·04 | 1·15 ± 0·16 | 44·3 ± 5·2 | 21·5 ± 3·2 | 50·8 ± 8·7 |
| TF3 | 1·18 ± 0·17 | 1·48 ± 0·22 | 0·99 ± 0·16 | 44·4 ± 6·1 | 28·2 ± 1·9 | 50·6 ± 8·1 |
| TF2 | 1·48 ± 0·12 | 1·40 ± 0·17 | 1·64 ± 0·37 | 42·5 ± 4·5 | 29·6 ± 4·3 | 56·3 ± 4·8 |
| TF1 | 1·57 ± 0·16 | 1·35 ± 0·14 | 0·00 ± 0·00*** | 35·1 ± 4·6 | 30·2 ± 4·3 | 0·0 ± 0·0*** |
| L | 1·26 ± 0·17 | 1·55 ± 0·16 | 0·00 ± 0·00*** | 37·7 ± 3·3 | 27·4 ± 5·9 | 0·0 ± 0·0*** |
| Nodule | 1·04 ± 0·05 | 0·99 ± 0·07 | 1·58 ± 0·14** | 30·5 ± 3·4 | 33·6 ± 6·0 | 32·8 ± 4·6 |
Data are the mean ± s.e. Significant differences of each drought time point compared with the well-watered plants are indicated by asterisks (*P < 0·05, **P < 0·01, ***P < 0·001).
The effects of drought on nodule parameters
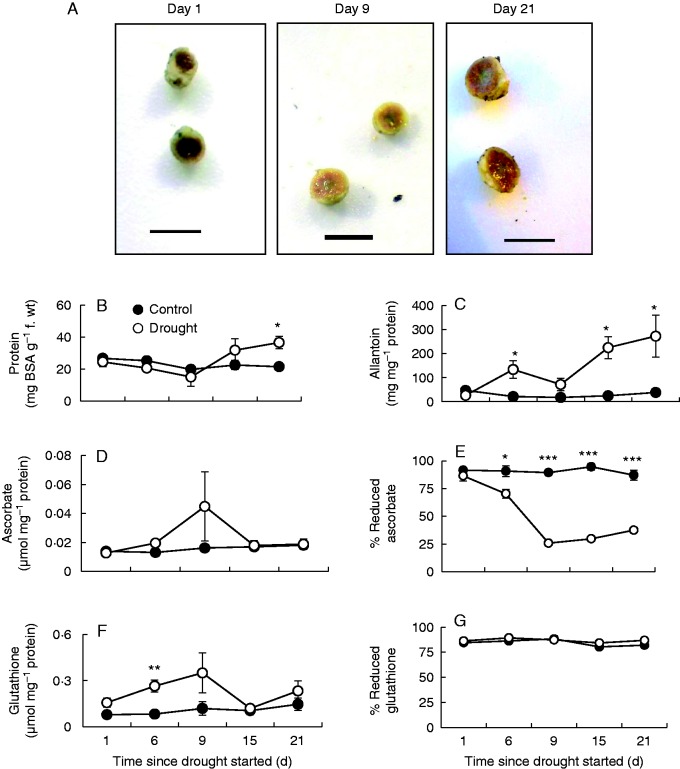
Crown nodules harvested throughout the 21 d of the drought stress treatment were not greatly visually different from the crown nodules of the well-watered controls (Fig. 6A), except that they were slightly less pink in the central zone, where developmental senescence is known to be initiated (Puppo et al., 2005). In contrast to leaves, nodule protein (Fig. 6B) ureide (Fig. 6C) contents increased as a result of drought treatment. While the reduction state of the nodule ascorbate pool was significantly lower than in well-watered controls even on the first few days of the stress treatment (Fig. 6E), the reduction state of the nodule glutathione pool (Fig. 6G) was similar in the nodules of well-watered controls and plants experiencing drought. While pyridine nucleotides were not detectable in the TF1 leaf ranks after 21 d of drought, nodule pyridine nucleotide levels were either similar to well-watered controls or even increased (Table 2).
Fig. 6.
The effects of drought on nodule phenotype (A) 1, 9 and 21 d after the onset of drought treatment, (B) protein content, (C) ureide content, measured as allantoin, (D) total ascorbate, (E) the percentage of reduced ascorbate, (F) total glutathione plus homoglutathione content, and (G) percentage of reduced glutathione plus homoglutathione. In B–G data are the means ± s.e. of three independent nodule samples. Statistical differences for each time point between the control and drought are denoted by asterisks (*P < 0·05, **P < 0·01, ***P < 0·001). Scale bar = 0·5 cm.
To explore the effects of drought further, we selected a number of nodule-expressed sequences encoding different proteins associated with redox regulation and senescence [cysteine proteases (CPs) and vacuolar processing enzymes (VPEs)]. The abundance of transcripts encoding a thioredoxin (TRX1, Glyma06g41610) and two glutaredoxins (Grx1, Glyma12g13920; and Grx2, Glyma16g07870) were similar in the nodules from well-watered and drought-stressed plants. The abundance of transcripts encoding two peroxiredoxins (Prx1, Glyma07g09240; and Prx2, Glyma09g32540), however, was higher in the plants exposed to drought for 15 d than the well-watered controls (Fig. 7A). Of the selected transcripts encoding CPs (Fig. 7B) and VPEs (Fig. 7C) only CP4 (Glyma14g40670) and VPE3 (Glyma14g10620) were significantly reduced as a result of drought treatment (Fig. 7B, C). Although values were not significant, CP3 transcripts tended to increase, possibly indicating the onset of nodule senescence (Fig. 7B). Of the transcripts encoding nodule antioxidant enzymes that were selected for further analysis, only transcripts encoding dehydroascorbate reductase (DHAR) were significantly changed as a result of drought treatment (Fig. 8). DHAR transcripts were decreased in the nodules of plants exposed to drought for 9 and 21 d compared with those of 1 d of drought (Fig. 8).
Fig. 7.
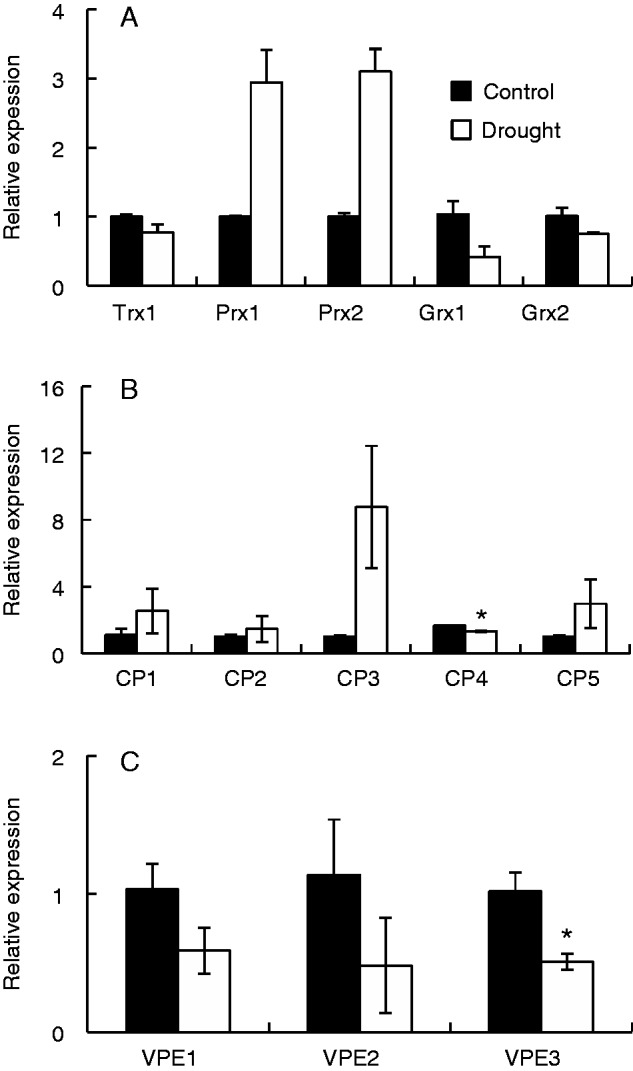
The effects of drought on the abundance of selected transcript in nodules. (A) Thioredoxin (TRX1, Glyma06g41610), peroxiredoxins (Prx1, Glyma07g09240; Prx2, Glyma09g32540) and glutaredoxins (Grx1, Glyma12g13920; Grx2, Glyma16g07870). (B) Cysteine proteases (CP1–CP5, Glyma04g04400, Glyma17g05670, Glyma10g35100, Glyma14g40670 and Glyma04g03090, respectively). (C) Vacuolar processing enzymes (VPE1–VPE3, Glyma17g14680, Glyma05g04230 and Glyma14g10620, respectively). Data are the means ± s.e. of three different samples of nodules harvested from either well-watered (Control) or drought-treated plants at day 15 of the experiment Statistical differences for each time point between the control and drought are denoted by an asterisk (*P < 0·05).
Fig. 8.
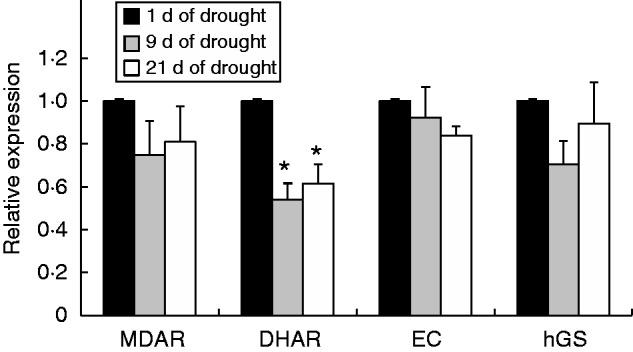
The effects of drought on the abundance of selected transcripts encoding antioxidant enzymes. Samples of nodules were harvested from either well-watered or drought-treated plants on days 1, 9 and 21 of the experiment. Data are the means ± s.e. of three different samples. Statistical differences for each time point between the control and drought are denoted by an asterisk (*P < 0·05).
DISCUSSION
The negative impact of drought on symbiotic N2 fixation in soybean root nodules is well documented (Sinclair and Serraj, 1995; King and Purcell, 2005; Marino et al., 2007). Several mechanisms could account for the stress-induced inhibition of nitrogenase activity, including (1) carbohydrate depletion, (2) changes in the oxygen diffusion barrier and (3) feedback regulation by ureide accumulation (Serraj et al., 1999a; Van Heerden et al., 2008). Drought-induced decreases in nitrogenase activity may result from changes in carbon (Gordon et al., 1999) and/or nitrogen metabolism (Ladrera et al., 2007). While we have not measured nitrogenase activity in the present study, the data presented in Fig. 6C support the evidence from earlier studies (Vadez et al., 2000; Ladrera et al., 2007) showing that ureides accumulate in the nodules of plants experiencing drought stress. Therefore, if nitrogenase activity was inhibited as a result of the imposition of drought, the extent of inhibition experienced under these conditions was not sufficient to prevent ureide accumulation in the nodules. Legume species that produce ureides are known to be more sensitive to drought than those that produce and transport amides because of lower ureide solubility. It is possible that ureide accumulation in the nodules causes a negative feedback inhibition of symbiotic N2 fixation (Sinclair and Serraj, 1995). However, the drought-induced increases in nodule ureides measured in the present study were accompanied by higher levels of nodule protein, pyridine nucleotides, ascorbate and glutathione (Fig. 6). The accumulation of these low-molecular-weight antioxidants suggests that the nodule response to the imposition of drought is to shore-up the defences against perturbations in cellular redox state.
It is now well established that glutathione and homoglutathione play important roles in the nodulation process and in drought tolerance (Matamoros et al., 1999; Frendo et al., 2005). While we were not able to measure the homoglutathione pool in these studies, the redox state of the glutathione pool was similar in the nodules of well-watered plants and those that were experiencing drought stress. The drought response measured in the present study showed that the nodule appears to be rich in metabolites, antioxidants and protein. Drought-induced structural changes in the oxygen diffusion barrier have been reported to alter nodule permeability to O2 lowering in-symbiosome O2 concentrations ([Oi]) leading to inhibition of nitrogenase activity indirectly because of lower nodule respiratory activity (Serraj and Sinclair, 1996). Conversely, stress-induced changes to nodule O2 homeostasis can also lead to an increase in [Oi] and the fractional oxygenation of leghaemoglobin (Kuzma et al., 1995). Low symbiosome [Oi] would favour decreased ROS production and high levels of reduction of the nodule ascorbate and glutathione/homogluthaione pools. The data presented here show that the nodule ascorbate pool was significantly less reduced in drought-stressed plants than in well-watered controls. This might suggest that nodule ROS production was increased rather than decreased and hence nodule [Oi] was increased following the imposition of drought stress.
After 21 d of drought, the stress had an impact on photosynthesis (as measured by the Fv′/Fm′ ratio) and cellular redox state (as indicated by the enhanced oxidation of the ascorbate pool) of leaves at all ranks on the stem, the negative impacts of drought being most severe in the TF1 and L leaf ranks. The imposition of drought stress leads to a genome-wide preprograming of gene expression regulation in leaves (Molina et al., 2008). However, the effects of drought on the nodule transcriptome are much less well characterized (Afonso-Grunz et al., 2014). A similar sequence of events has been suggested to occur in leaf and nodule senescence programmes in which catabolic nutrients are recycled, followed by organ degeneration (Van de Velde et al., 2006). The most abundantly expressed genes in the senescent zones of the Medicago truncatula nodule were CPs that were highly homologous to SAG12, a well-characterized marker of leaf senescence (Van de Velde et al., 2006). CPs are synthesized in legume nodules during senescence (Asp et al., 2004). To determine whether the drought stress treatment used in these studies triggered nodule senescence, we selected five CP sequences from the soybean database (www.soybase.org), which we have designated CP1–CP5 (Glyma04g04400, Glyma17g05670, Glyma10g35100, Glyma14g40670 and Glyma04g03090, respectively) and three sequences encoding VPEs which we have designated VPE1–VPE3 (Glyma17g14680, Glyma05g04230 and Glyma14g10620, respectively). We selected these proteases because levels of the transcripts encoding these enzymes were changed during nodule senescence as measured by RNaseq analysis (data not shown; S. van Wyk, University of Pretoria, unpubl. res.). The abundance of transcripts encoding CPs Glyma04g04400, Glyma17g05670 and Glyma10g35100 was decreased during the process of developmental nodule senescence, while Glyma14g40670 and Glyma04g03090 and the mRNAs encoding the VPEs measured here was increased as determined in our RNaseq analysis (S. van Wyk, unpubl, res,). Similarly, the genes related to redox processes that were measured in this study were selected because their transcripts were changed in abundance by more than 50-fold as a result of developmental nodule senescence in our RNaseq analysis (S. van Wyk, University of Pretoria, unpubl. res.).
VPEs are involved in protein re-mobilization during leaf senescence and seed-set (Muntz and Shutov, 2002). None of the selected transcripts encoding CPs or VPEs (Fig. 7B, C) was increased in abundance in the nodules as a result of drought treatment. This suggests that there had been no substantial triggering of the nodule senescence programme at this stage of the drought stress treatment. We also selected sequences from the soybean database that encode proteins involved in redox-related processes in the nodules (Figs 7 and 8) to determine whether the drought-induced enhanced oxidation of the nodule ascorbate pool was accompanied by changes in the levels of redox-related transcripts. While the abundance of very few redox-related transcripts was changed in response to drought, as has been noted previously in leaves (Noctor et al., 2014), the abundance of transcripts encoding two peroxiredoxins, which we have called Prx1 (Glyma07g09240) and Prx2 (Glyma09g32540), was significantly higher in the plants exposed to drought than in the well-watered controls (Fig. 7A). Prx1 has homology to PrxIIC, expression of which is induced by oxidants such as hydrogen peroxide and lipid peroxides and also by the addition of ascorbate (Horling et al., 2003). Prx2 (also called TPX1 in the database) encodes a thioredoxin-dependent peroxidase of unknown function. These findings may support the view that oxidative stress in nodules is caused by enhanced ROS formation rather than a decrease in the antioxidant defences (Evans et al., 1999).
Leaf senescence is a highly regulated catabolic process in which leaf constituents are remobilized and transported to other plant organs. The progression of leaf senescence in different ranks on the stem is influenced by the availability of light and hence photosynthetic carbon metabolism that underpins the fitness of plants, particularly when they are grown within dense canopies (Brunel-Muguet et al., 2013). Senescence begins with the lowest leaves on the shoot. The light gradient or partial shading of lower leaves is considered to regulate the order of the senescence process to allow remobilization of resources toward leaves that are exposed to higher light and hence have higher photosynthetic capacities (Boonman et al., 2006). The partial shading of leaves in dense canopies is thought to lower transpiration rates and hence cytokinin delivery to lower leaves, resulting in lower photosynthetic activity that in some way targets the leaves for early senescence (Boonman et al., 2006). The programme of drought-induced senescence observed in this study clearly targets the lowest leaves on the shoot, which had lost all their protein, chlorophyll and photosynthetic capacity after 21 d of water deprivation. The data presented here clearly demonstrate that the senescence programme is complete in the lower leaves before any molecular or metabolic markers (such as loss of protein) are observed in the crown nodules. This suggests that senescence of the lower leaves precedes that of the nodules and younger leaves. This is important in understanding the stress response because it appears that symbiotic N2 fixation and ureide production are maintained in a similar manner to the younger more photosynthetically competent leaves by re-allocation of carbon resources from the lower leaves.
The data presented here show that soybean plants were not deprived of nitrogen as a result of drought because the products of symbiotic nitrogen fixation were abundant in the leaves even after 21 d of exposure to this stress. While we cannot rule out a deficiency in other nutrients such as phosphate under the conditions used in this study, we consider that the experimental design reflects the natural situation that occurs when plants are deprived of water. Changes in sub-sets of transcript profiles can be useful indicators of deficiencies in different nutrients. We have performed an RNaseq analysis in similar studies on drought in soybean and we have not found any transcript changes that would indicate major deficiencies in essential nutrients. We have not included phenotypic parameters for soybean growth in response to drought in this study because we have previously extensively characterized the effects of drought on a range of shoot and root parameters in different soybean cultivars grown under glasshouse or field conditions (Fenta et al., 2012, 2014). Such studies have demonstrated the importance of maintaining shoot growth, photosynthesis and symbiotic nitrogen fixation in drought tolerance in soybean (Fenta et al., 2012, 2014).
CONCLUSIONS
While plant responses to drought are complex and need to be analysed at system levels using genomics and physiological approaches, it is also important to understand the order and sequence of events that underpin survival strategies. The concept that the nodules are the first target of drought stress is well established in the literature but the data presented here demonstrate that this is not the case. Rather, the soybean plants balance the provision of nitrogen and carbon resources and the lower leaves are sacrificed before the nodules. Preserving the nodules rather than leaves of low photosynthetic capacity clearly has a physiological advantage in stress situations. The next step is therefore to characterize the genes that expressed in the nodule to preserve symbiotic N2 fixation at the early stages of drought. Here we have identified transcripts encoding two thioredoxin-dependent peroxidases as being important in the drought-induced response programme. While little is known about the functions of thioredoxin-dependent peroxidases, many are thought to have signalling as well as defensive functions. These proteins are therefore potential new targets for further study in relation to signalling and defence functions at the crucial early stages of the nodule response to drought. This new knowledge adds to the body of information required for the improvement of soybeans that is required to sustain the wide range of soya applications in the food and animal feed industries.
ACKNOWLEDGEMENTS
This work was funded by FP7-PIRSES-GA-2008-230830 (LEGIM) and PIIF-GA-2011-299347 (Soylife; K.K.). B.M.G. thanks Subprograma Estancias de Movilidad posdoctoral en centros extranjeros (2009), Ministerio de Educación (Spain). J.W.C. thanks BBSRC for a CASE studentship (BB/K501839/1). D.S. thanks BBSRC for a CASE studentship (BB/J011363/1). M.Q. thanks the Schlumberger Foundation Faculty for the Future Award for her fellowship.
LITERATURE CITED
- Afonso-Grunz F, Molina C, Hoffmeier K, et al. 2014. Genome-based analysis of the transcriptome from mature chickpea root nodules. Frontiers in Plant Science 5: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asp T, Bowra S, Borg S, Holm PB. 2004. Cloning and characterisation of three groups of cysteine protease genes expressed in the senescing zone of white clover (Trifolium repens) nodules. Plant Science 167: 825–837. [Google Scholar]
- Blum A. 2005. Drought resistance, water-use efficiency, and yield potential – are they compatible, dissonant, or mutally exclusive? Australian Journal of Agriculture Research 56: 1159–1168. [Google Scholar]
- Boonman A, Anten NPR, Dueck TA, et al. 2006. Functional significance of shade-induced leaf senescence in dense canopies: an experimental test using transgenic tobacco. The American Naturalist 168: 597–606. [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Brunel-Muguet S, Beauclair P, Bataille MP, et al. 2013. Light restriction delays leaf senescence in winter oilseed rape (Brassica napus L.) Journal of Plant Growth Regulation 32: 506–518. [Google Scholar]
- Chaves MM, Oliveira MM. 2004. Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. Journal of Experimental Botany 55: 2365–2383. [DOI] [PubMed] [Google Scholar]
- Claeys H, Inzé D. 2013. The agony of choice: how plants balance growth and survival under water-limiting conditions. Plant Physiology 162: 1768–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz de Carvalho MH. 2008. Drought stress and reactive oxygen species. Plant Signalling & Behavior 3: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutforth HW, McGinn SM, McPhee KE, Miller PR. 2007. Adaptation of pulse crops to the changing climate of the northern Great Plains. Agronomy Journal 99: 1684–1699. [Google Scholar]
- Evans PJ, Gallesi D, Mathieu C, et al. 1999. Oxidative stress occurs during soybean nodule senescence. Planta 208: 73–79. [Google Scholar]
- Fenta BA, Driscoll SP, Kunert KJ, Foyer CH. 2012. Characterization of drought tolerance traits in nodulated soybeans: the importance of maintaining photosynthesis and shoot biomass under drought-induced limitations on nitrogen metabolism. Journal of Agronomy and Crop Physiology 198: 92–103. [Google Scholar]
- Fenta BA, Beebe SE, Kunert KK, et al. 2014. Field phenotyping of soybean roots for drought stress tolerance. Agronomy 4: 418–435. [Google Scholar]
- Flexas J, Bota J, Galmes J, Medrano H, Ribas-Carbo M. 2006. Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiologia Plantarum 127: 343–352. [Google Scholar]
- Foyer CH, Rowell J, Walker D. 1983. The effect of sucrose on the rate of de novo sucrose biosynthesis in leaf protoplasts from spinach, wheat and barley . Archives of Biochemistry and Biophysics 220: 232–238. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Pellny TK, Locato V, De Gara L. 2008. Analysis of redox relationships in the plant cell cycle: determinations of ascorbate, glutathione and poly (ADPribose) polymerase (PARP) in plant cell cultures. In: Hancock J, ed. Redox mediated signal transduction: methods in molecular biology series. New York: The Humana Press Inc, 199–215. [DOI] [PubMed] [Google Scholar]
- Franchini JC, Debias H, Sacoman A, Nepomuceno AL, Farias JRB. 2009. Manejo do Solo para Redução das Perdas de Produtividade pela Seca. Londrina: Embrapa Soja. [Google Scholar]
- Frendo P, Harrison J, Norman C, et al. 2005. Glutathione and homoglutathione play a critical role in the nodulation process of Medicago truncatula. Molecular Plant Microbe Interactions 18: 254–259. [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Minchin FR, James CL, Komina O. 1999. Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiology 120: 867–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horling F, Lamkemeyer P, Konig J, et al. 2003. Divergent light-, ascorbate-, and oxidative stress-dependent regulation of expression of the peroxiredoxin gene family in Arabidopsis. Plant Physiology 131: 317.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe I, Escudero PR, Arrese-Igor C, Becana M. 1998. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiology 116: 173–181. [Google Scholar]
- Jury WA, Vaux HJ. 2007. The emerging global water crisis: managing scarcity and conflict between water users. Advances in Agronomy 95: 1–76. [Google Scholar]
- King CA, Purcell LC. 2005. Inhibition of N2 fixation in soybean is associated with elevated ureides and amino acids. Plant Physiology 137: 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapheck S. 1988. Homoglutathione: isolation, quantification and occurrence in legumes. Physiologia Plantarum 74: 727–732. [Google Scholar]
- Kuzma MM, Topunov AF, Layzell DB. 1995. Effects of temperature on infected cell O2 concentration and adenylate levels in attached soybean nodules. Plant Physiology 107: 1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK. 1987. Chlorophylls and carenoids: pigments of photosynthetic biomembranes. Methods in Enzymology 148: 350–382. [Google Scholar]
- Ladrera R, Marino D, Larrainzar E, Gonzalez EM, Arrese-Igor C. 2007. Reduced carbon availability to bacteroids and elevated ureides in nodules, but not in shoots, are involved in the nitrogen fixation response to early drought in soybean. Plant Physiology 145: 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Andersen MN, Jacobsen SE, Jensen CR. 2005. Stomatal control and water use efficiency of soybean (Glycine max L. Merr.) during progressive soil drying. Environmental and Experimental Botany 54: 33–40. [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Matamoros MA, Moran JF, Iturbe-Ormaetxe I, Rubio MC, Becana M. 1999. Glutathione and homoglutathione synthesis in legume root nodules. Plant Physiology 121: 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavalan LP, Guttikonda SK, Tran LSP, Nguyen HT. 2009. Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiology 50: 1260–1276. [DOI] [PubMed] [Google Scholar]
- Marino D, Frendo P, Ladrera R, et al. 2007. Nitrogen fixation control under drought stress. Localized or systemic? Plant Physiology 143: 1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina C, Rotter B, Horres R, et al. 2008. SuperSAGE: the drought stress-responsive transcriptome of chickpea roots. BMC Genomics 9: e553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntz K, Shutov AD. 2002. Legumains and their function in plants. Trends in Plant Science 7: 340–344. [DOI] [PubMed] [Google Scholar]
- Neves-Borges AC, Guimarães-Dias F, Cruz F, et al. 2012. Expression pattern of drought stress marker genes in soybean roots under two water deficit systems. Genetics and Molecular Biology 35: 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. 1998. Simultaneous measurement of foliar glutathione, gamma-glutamylcysteine, and amino acids by high-performance liquid chromatography: comparison with two other assay methods for glutathione. Analytical Biochemistry 264: 98–110. [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Foyer CH. 2014. The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiology 164: 1636–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori GM, Foyer CH. 2002. Common components, networks, and pathways of cross-tolerance to stress. The central role of ‘Redox’ and abscisic acid-mediated controls. Plant Physiology 129: 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen P, Kumudini S, Board J, Conley S. 2005. Soybean growth and development. In: Dorrance AE, Draper MA, Hershman DE, eds. Using foliar fungicides to manage soybean rust. Columbus: Ohio State University, 41–47. [Google Scholar]
- Pellny TK, Locato V, Diaz Vivancos P, et al. 2009. Pyridine nucleotide cycling and control of intracellular redox state in relation to poly(ADP-ribose) polymerase activity and nuclear localization of glutathione during exponential growth of Arabidopsis cells in culture. Molecular Plant 2: 442–456. [DOI] [PubMed] [Google Scholar]
- Pérez-Chaca MV, Rodríguez-Serrano M, Molina AS, et al. 2014. Cadmium induces two waves of reactive oxygen species in Glycine max (L.) roots. Plant, Cell and Environment 37: 1672–1687. [DOI] [PubMed] [Google Scholar]
- Puppo A, Groten K, Bastian F, et al. 2005. Legume nodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytologist 165: 683–701. [DOI] [PubMed] [Google Scholar]
- Purcell LC, King CA, Ball RA. 2000. Soybean cultivar differences in ureides and the relationship to drought tolerant nitrogen fixation and manganese nutrition. Crop Science 40: 1062–1070. [Google Scholar]
- Queval G, Noctor G. 2007. A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: application to redox profiling during Arabidopsis rosette development. Analytical Biochemistry 363: 58–69. [DOI] [PubMed] [Google Scholar]
- Serraj R, Sinclair TR. 1996. Inhibition of nitrogenase activity and nodule oxygen permeability by water deficit. Journal of Experimental Botany 47: 1067–1073. [Google Scholar]
- Serraj R, Vadez V, Deninson RF, Sinclair TR. 1999a. Involvement of ureides in nitrogen fixation inhibition in soybean. Plant Physiology 119: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serraj R, Sinclair TR, Purcell LC. 1999b. Symbiotic N2 fixation response to drought. Journal of Experimental Botany 50: 143–155. [Google Scholar]
- Sinclair TR, Serraj R. 1995. Legume nitrogen fixation and drought. Nature 378: 344. [Google Scholar]
- Stolf-Moreira R, Medri ME, Neumaier N, et al. 2010. Soybean physiology and gene expression during drought. Genetics and Molecular Research 9: 1946–1956. [DOI] [PubMed] [Google Scholar]
- Streeter JG. 2003. Effects of drought on nitrogen fixation in soybean root nodules. Plant, Cell and Environment 26: 1199–1204. [Google Scholar]
- Vadez V, Sinclair TR, Serraj R. 2000. Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiologia Plantarum 110: 215–223. [Google Scholar]
- Van Heerden PDR, De Beer M, Mellet DJ, Maphike HS, Foit W. 2007. Growth media effects on shoot physiology, nodule numbers and symbiotic nitrogen fixation in soybean. South African Journal of Botany 73: 600–605. [Google Scholar]
- Van Heerden PDR, Kiddle G, Pellny TK, et al. 2008. Roles for the regulation of respiration and the oxygen diffusion barrier in soybean in the protection of symbiotic nitrogen fixation from chilling-induced inhibition and shoots from premature senescence. Plant Physiology 148: 316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd CD, Tipton PA, Blevins DG, Piedras P, Pineda M, Polacco JC. 2006. Update on ureide degradation in legumes. Journal of Experimental Botany 57: 5–12. [DOI] [PubMed] [Google Scholar]
- Tuteja N. 2007. Abscisic acid and abiotic stress signalling. Plant Signalling & Behavior 2: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. 2006. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annual Review Plant Biology 57: 781–803. [DOI] [PubMed] [Google Scholar]
- Van de Velde W, Perez Guerra JC, De Keyser A, et al. 2006. Aging in legume symbiosis. A molecular view on nodule senescence in Medicago truncatula. Plant Physiology 141: 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GE, Conway CF. 1942. On the estimation of allantoin by the rimini-schryver reaction. Journal of Biological Chemistry 142: 839–853. [Google Scholar]