Abstract
Background and Aims Vitamin E helps to control the cellular redox state by reacting with singlet oxygen and preventing the propagation of lipid peroxidation in thylakoid membranes. Both plant ageing and phosphorus deficiency can trigger accumulation of reactive oxygen species, leading to damage to the photosynthetic apparatus. This study investigates how phosphorus availability and vitamin E interact in the control of plant longevity in the short-lived annual Arabidopsis thaliana.
Methods The responses of tocopherol cyclase (VTE1)- and γ-tocopherol methyltransferase (VTE4)-null mutants to various levels of phosphorus availability was compared with that of wild-type plants. Longevity (time from germination to rosette death) and the time taken to pass through different developmental stages were determined, and measurements were taken of photosynthetic efficiency, pigment concentration, lipid peroxidation, vitamin E content and jasmonate content.
Key Results The vte1 mutant showed accelerated senescence under control conditions, excess phosphorus and mild phosphorus deficiency, suggesting a delaying, protective effect of α-tocopherol during plant senescence. However, under severe phosphorus deficiency the lack of α-tocopherol paradoxically increased longevity in the vte1 mutant, while senescence was accelerated in wild-type plants. Reduced photoprotection in vitamin E-deficient mutants led to increased levels of defence chemicals (as indicated by jasmonate levels) under severe phosphorus starvation in the vte4 mutant and under excess phosphorus and mild phosphorus starvation in the vte1 mutant, indicating a trade-off between the capacity for photoprotection and the activation of chemical defences (jasmonate accumulation).
Conclusions Vitamin E increases plant longevity under control conditions and mild phosphorus starvation, but accelerates senescence under severe phosphorus limitation. Complex interactions are revealed between phosphorus availability, vitamin E and the potential to synthesize jasmonates, suggesting a trade-off between photoprotection and the activation of chemical defences in the plants.
Keywords: Arabidopsis thaliana, jasmonates, longevity, P availability, photoprotection, plant defence trade-off, reactive oxygen species, ROS, senescence, vitamin E
INTRODUCTION
Phosphorus (P) is ubiquitous in life chemistry as it is involved in just about every function (White and Hammond, 2008; Frank, 2013). The chemical form of P taken up by plants, orthophosphate (H2PO4–), represents a very small fraction of available P in soils, and is generally immobile. However, as a macro-element, relatively significant quantities are required for biological function. Phosphorus deficiency produces many symptoms in plants: leaf tips look burnt and older leaves turn a dark green or reddish-purple colour. Poor availability of P in soils and consequent P deficiency are major constraints to crop production globally (Cordella et al., 2009).
During senescence, remobilization of photoassimilates and nutrients to other plant parts occurs, especially to more demanding parts, such as growing young leaves, reproductive structures (flowers and/or fruit under development) and storage tissues. After the initiation of leaf senescence, there is a transition to the so-called reorganization phase, during which cell components selectively re-differentiate, leading to nutrient remobilization. Once this is accomplished, programmed cell death occurs (Distelfeld et al., 2014; Juvany et al., 2014). During the reorganization phase, the extent of photo-oxidative stress in senescing leaves may be under tight control. Phosphatases, which are highly active during senescence, release phosphate from the nucleotide products of nuclease attack on RNA and DNA, and P can be redistributed from senescing tissues to other plant parts (Duff et al., 1994; Frank, 2013). Under P starvation, however, plants can accumulate sugars and starch in their leaves due to limitations in carbohydrate loading and/or unloading (Wingler et al., 1998; Hammond and White, 2008).
Lipid peroxidation can be suppressed by non-polar antioxidants such as vitamin E. The vitamin E group consists of eight compounds (four tocopherols and four tocotrienols, in each case termed α, β, γ and δ) that differ only in the saturation degree of the prenyl side chain and in the number of methyl groups in the chromanol ring. In photosynthetic tissues, the most important forms are α- and, to a lesser extent, γ-tocopherol. α-Tocopherol protects the photosynthetic membranes from the propagation of lipid peroxidation, helps to maintain membrane stability and, in a coordinated action with other antioxidants, enables the correct functioning of the photosynthetic machinery under stress conditions (Munné-Bosch, 2005; Munné-Bosch et al., 2013). γ-Tocopherol is the precursor of α-tocopherol and can accumulate in small quantities in young or senescing tissues only (Munné-Bosch and Alegre, 2002; Szymanska and Kruk, 2008). There is some controversy about whether or not tocopherols can have functions beyond their antioxidant role (Falk and Munné-Bosch, 2010). Some studies suggest that tocopherols can play a role in cell signalling, affecting processes outside chloroplasts (Cela et al., 2011). Studies of mutants with an altered tocopherol biosynthetic pathway suggested that altered antioxidant capacities led to an increase in anthocyanin accumulation in senescing leaves that may be linked to alterations in jasmonic acid (JA) levels (Munné-Bosch et al., 2007). Also, tocopherol-deficient mutants of Arabidopsis thaliana show alterations in photoassimilate transport at low temperatures as a result of alterations in callose deposition in the phloem (Maeda et al., 2006) and endoplasmic reticulum fatty acid metabolism (Maeda et al., 2008). Furthermore, it has recently been shown that vitamin E deficiency can accelerate senescence under optimal growth conditions, but paradoxically retard it under salt stress in tobacco plants (Abbasi et al., 2007; Asensi-Fabado et al., 2014).
Given the centrality of lipid peroxidation in plant defence against both biotic and abiotic stresses, we hypothesized that lack of vitamin E might influence interactions with P availability in the control of longevity and the capacity to synthesize jasmonates (JAs). Here, we examined the effects of alterations in vitamin E composition on the longevity, extent of photoprotection and JA profile to unravel possible trade-offs between vitamin E and defence signal production in plants.
MATERIALS AND METHODS
Plant material and sampling
Seeds of Arabidopsis thaliana Columbia ecotype (Col-0) and vte1 and vte4 mutants, which were provided by Kathleen Brückner (University of Kiel, Germany), were used in this study. The vte1 and vte4 mutants have T-DNA insertions in the VTE1 and VTE4 genes, which encode tocopherol cyclase and γ-tocopherol methyltransferase, respectively. The vte1 mutant lacks both α-tocopherol and γ-tocopherol, and the vte4 mutant lacks α-tocopherol but accumulates γ-tocopherol in the leaves (Porfirova et al., 2002; Bergmüller et al., 2003). Seeds were sown in 0·1-L pots containing a mixture of peat : perlite : vermiculite (1 : 1 : 1, v/v/v) and exposed to 4°C for 1 week. Then, plants were transferred to a constant environment chamber (16-h photoperiod, 90–110 µmol quanta m–2 s–1, air temperature 21–23 °C). When plants had developed their first two leaves (stage 1·02, with two rosette leaves >1 mm in length; Boyes et al., 2001), they were exposed to four nutrient solution treatments. The control (C) treatment contained 5 mm Ca(NO3)2, 5 mm KNO3, 2 mm MgSO4, 1 mm KH2PO4 and 5 g/L iron chelate (EDTA FeNa). The treatment enriched in phosphorus (+P) contained the same but with 2 m KH2PO4, while the –P1 and –P2 treatments contained the same but with 0·5 and 0·1 mm KH2PO4 (50 % and 90 % less P, respectively), compared with controls. The –P1 and –P2 treatments were supplied with 0·5 and 0·1 mm KCl, respectively, to compensate for K deficiency. Plants were watered with freshly prepared nutrient solution every 2–3 d. All nutrient solutions were adjusted to a pH of 6·5–7·0.
To estimate longevity (time from germination to rosette death) and the time elapsed between different developmental stages (time to germination, from sowing to germination; time to flowering, from germination to first flower at anthesis; and senescence period, time to rosette death minus time to flowering), we used 35 individuals per treatment and genotype. The rosette was considered dead when all leaves had completely senesced. For biomass estimation, chlorophyll fluorescence measurements and biochemical analyses on whole-rosette leaves, the entire rosettes of five individuals were used. These biochemical analyses were performed at the fruiting stage, when the first mature silique was visible. For pigment, vitamin E, lipid peroxidation and JA analyses, leaves were collected, immediately frozen in liquid nitrogen and stored at –80 °C until analysis.
Biomass estimation
Rosette and reproductive biomasses were estimated by weighing all leaves from the entire rosette and the whole reproductive part, respectively.
The Fv/Fm ratio
The maximum efficiency of photosystem II photochemistry [Fv/Fm ratio, an indicator of the photoinhibition of photosystem II (PSII)] was determined using a pulse-modulated fluorimeter (Mini-PAM; Walz, Effeltrich, Germany) after 1 h of dark adaptation, as described (Genty et al., 1989).
Pigment analyses
Samples were extracted in methanol and assayed spectrophotometrically to estimate chlorophyll and carotenoid contents. Specific absorption coefficients reported by Lichtenthaler and Wellburn (1983) were used.
Lipid peroxidation assay
Lipid peroxidation was determined by estimating lipid hydroperoxide content following the FOX assay described by Bou et al. (2010). Leaves were ground with a mortar and pestle with liquid nitrogen, homogenized in 50 mmol L−1 potassium phosphate buffer (pH 6·0) and centrifuged at 20 000 g for 25 min at 4 °C. To 50 µL of supernatant an equal volume of 10 mm triphenylphosphine (TPP) in methanol was added and incubated for 30 min in darkness at room temperature. Then, the sample was incubated in 190 µL of freshly prepared FOX-2 reagent (Sigma-Aldrich, Steinheim, Germany) with 10 µL of sample (and the respective negative controls, as well as standards ranging from 0 to 20 µm H2O2 used for calibration) and the absorbance was measured at 560 nm.
Vitamin E analyses
The extraction and analyses of tocopherols were performed as described by Amaral et al., (2005). Briefly, leaf samples were ground in liquid nitrogen and extracted with methanol until the extract was colourless. After 45 min in a Branson 2510 ultrasonic cleaner (Bransonic, Danbury, CT, USA), the samples were centrifuged for 15 min at 4 °C and transferred to HPLC vials. The HPLC equipment consisted of a system with a Waters 600 controller pump, a Waters 714 Plus autosampler and an FP-1520 fluorescence detector (Jasco, Tokyo, Japan). For the separation of both tocopherols, an Inertsil 100A (5 mm, 30 × 250 mm; GL Sciences Inc.) normal-phase column operating at room temperature was used. The mobile phase was a mixture of n-hexane and 1,4-dioxane (95·5 : 4·5, v/v). The system worked a flow rate of 0·7 mL min–1, injecting 10 mL per sample. Detection was carried out at 295 nm for excitation and at 330 nm for emission. Quantification was based on the fluorescence signal response compared with authentic standards of α- and γ-tocopherol (Sigma-Aldrich).
JA profiling
The extraction and analyses of 12-oxo-phytodienoic acid (OPDA), which is the JA precursor, JA and JA-isoleucine (JA-Ile) were performed by ultra-high performance liquid chromatography coupled to mass spectrometry in tandem (UHPLC–MS/MS) as described by Müller and Munné-Bosch (2011), with some modifications. Leaf samples (50 mg) were ground using liquid nitrogen and 30 µL of internal standard solution (d5-OPDA, d5-JA and d5-JA-Ile, 1 ppm) and 1·5 mL of methanol were added, and the samples were incubated for 30 min in a Branson 2510 ultrasonic cleaner (Bransonic) and centrifuged for 15 min at 4 °C. Re-extraction was performed with methanol, repeating the same procedure. Both supernatants were mixed, filtered through a 0·22 µm PTFE filter (Waters Milford, MA, USA) and injected into the UHPLC–MS/MS system. The UHPLC system consisted of an Acquity Waters binary pump equipped with an autosampler and a UV detector. For analysis of the extracts, a HALO C18 column (2·7 mm, 2·1 × 75 mm) was used with a binary solvent system comprising acetonitrile (A) and deionized water (B), both containing 0·005 % (v/v) glacial acetic acid. Separation was performed using a gradient of increasing acetonitrile content, at a constant flow rate of 0·4 mL min–1. The following gradient was used: 0 min, 1 % A; 0–4·5 min, increasing to 45 % A; 4·5–5 min, increasing to 99 % A; 5–6 min, 99 % A; 6–6·2 min, decreasing to 1 % A; 6·2–7 min, 1 % A. The MS/MS analyses were performed on an API 3000 triple quadrupole mass spectrometer (PE Sciex, Framingham, MA, USA). Results were corrected taking into account the specific recovery rates using internal deuterated standards.
Statistical analyses
Differences between treatments were evaluated by one- or two-way analysis of variance (ANOVA) and were considered significant at a probability level of P ≤ 0·05.
RESULTS
Longevity of A. thaliana vte1 and vte4 mutants under contrasting P availabilities
The vte4 and vte1 mutants were morphologically indistinguishable, despite the vte1 mutant lacking both α-tocopherol and γ-tocopherol, and vte4 lacking α-tocopherol and accumulating γ-tocopherol instead. The vte1 mutant grown under control conditions, excess P and mild P deficiency showed reduced longevity, due to both accelerated flowering and senescence (Fig. 1). However, longevity decreased by 25 % (and senescence was accelerated by 40 %) in wild-type plants under severe P deficiency (–P2) compared with both vte1 and vte4 mutants. The vte4 mutant, which accumulates γ- instead of α-tocopherol, showed a phenotype similar to the wild type under control conditions, excess P and mild P deficiency, thus indicating a similar role for both α- and γ-tocopherol in the control of longevity under these conditions. However, under severe P deficiency (–P2), senescence was accelerated in the wild type, and both vte mutants did respond similarly, thus indicating that α-tocopherol, but not γ-tocopherol, promotes senescence under severe P limitation (Fig. 1). It is also worth mentioning that flowering occurred on average 25, 27 and 30 d after germination in the wild type, vte4 and vte1 mutants, respectively, under control conditions, indicating delayed flowering in the vte mutants relative to the wild type (Fig. 1). Furthermore, rosette and reproductive biomasses of wild-type plants and both vte mutants were reduced by 70–80 % in response to severe P deficiency (Fig. 2). Vegetative (rosette) and reproductive biomass was higher in the vte1 mutant compared with the other two genotypes under mild P limitation and excess P (–P1 and +P, respectively; Fig. 2).
Fig. 1.
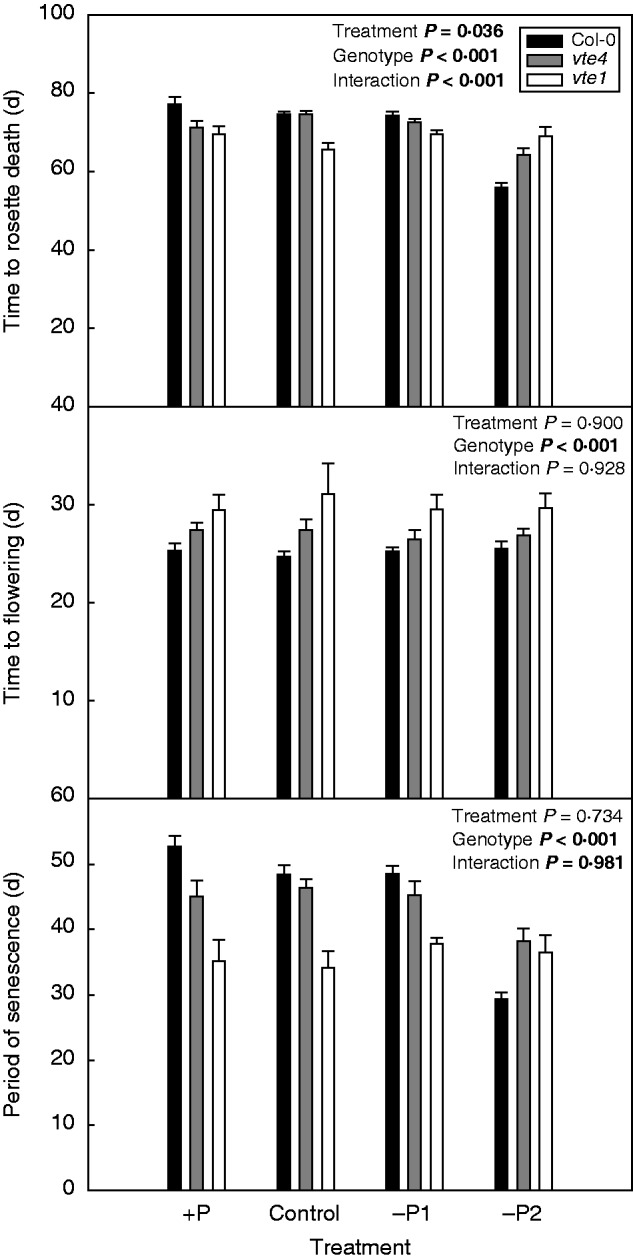
Longevity (time from germination to rosette death), time to flowering (first flower) and senescence period (time to rosette death minus time to flowering) of wild type (Col-0) and vte4 and vte1 mutants (see key) of A. thaliana grown under various P availabilities (+P, C, –P1 and –P2 represent various P concentrations in the nutrient solution; see Materials and methods for details). Data represent the mean ± s.e. of n = 35 individuals. Significant differences between treatments, genotypes and their interaction (treatment × genotype) were analysed by two-way ANOVA (P ≤ 0·05).
Fig. 2.
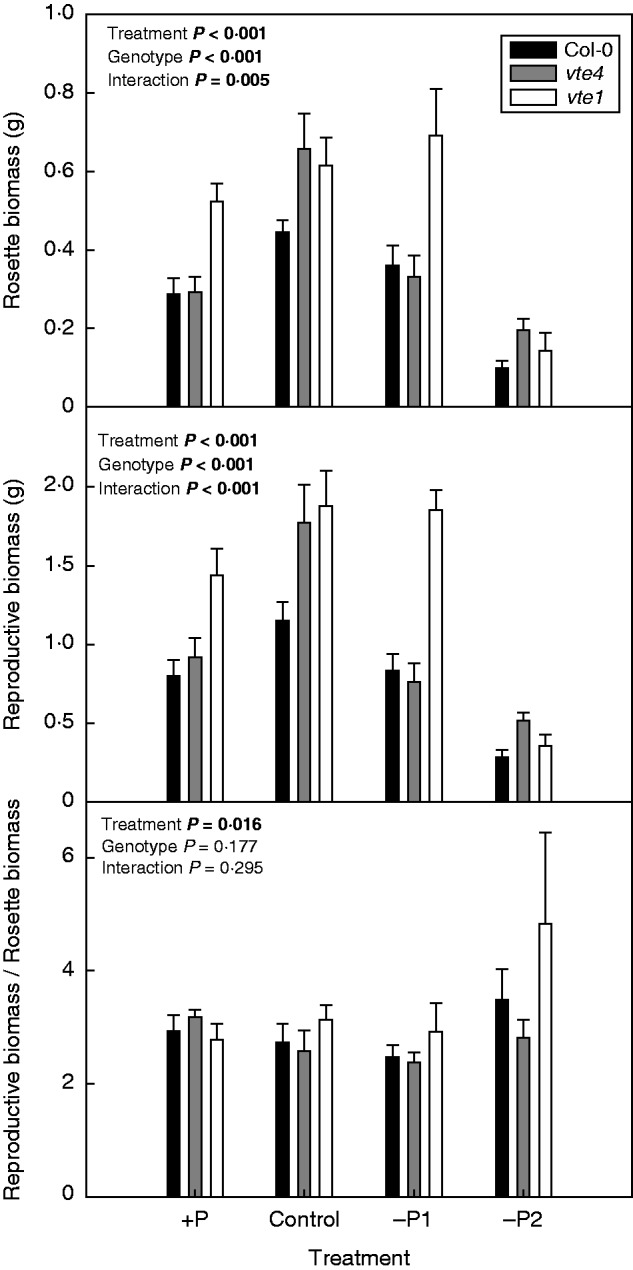
Rosette biomass, reproductive biomass and ratio of reproductive to rosette biomass in wild type (Col-0) and vte4 and vte1 mutants (see key) of A. thaliana grown under various P availabilities (+P, C, –P1 and –P2 represent various P concentrations in the nutrient solution; see Materials and methods for details). Data represent the mean ± s.e. of n = 35 individuals. Significant differences between treatments, genotypes and their interaction (treatment × genotype) were analysed by two-way ANOVA (P ≤ 0·05).
Vitamin E increased PSII inactivation in the wild type under severe P limitation
α-Tocopherol accumulated in leaves of the wild type both under mild and severe P starvation, while γ-tocopherol accumulated in the vte4 mutant under severe P limitation only (Fig. 3). As expected, the vte1 mutant accumulated neither α- nor γ-tocopherol in leaves (Fig. 3). Various photo-oxidative stress markers, including pigment (both chlorophyll and carotenoid) levels, the efficiency of PSII photochemistry and the extent of lipid peroxidation (measured as lipid hydroperoxide accumulation) revealed some differences in photoprotection strategies between the three genotypes (Fig. 4). Chlorophyll and lipid hydroperoxide contents of the vte mutants were similar to those of wild-type plants in all tested conditions, except for reduced chlorophyll and lipid hydroperoxide levels in the vte4 mutant under severe P limitation (Fig. 4). The maximum efficiency of PSII photochemistry (Fv/Fm ratio), which is an indicator of inhibition of the photosynthetic apparatus, decreased more in wild-type plants (by 59 %) than in the vte mutants (20–25 %) under severe P deficiency. Increased longevity of the vte1 and vte4 mutants under severe P deficiency was therefore associated with improved PSII efficiency. In other words, accelerated senescence under severe P deficiency in the wild type was associated with photoinhibition of PSII. Interestingly, wild-type plants accumulated not only more α-tocopherol but also more carotenoids in senescing tissues under severe P limitation (Fig. 4). Therefore, increased PSII inactivation was paradoxically observed despite higher tocopherol and carotenoid accumulation in wild-type plants under severe P starvation.
Fig. 3.
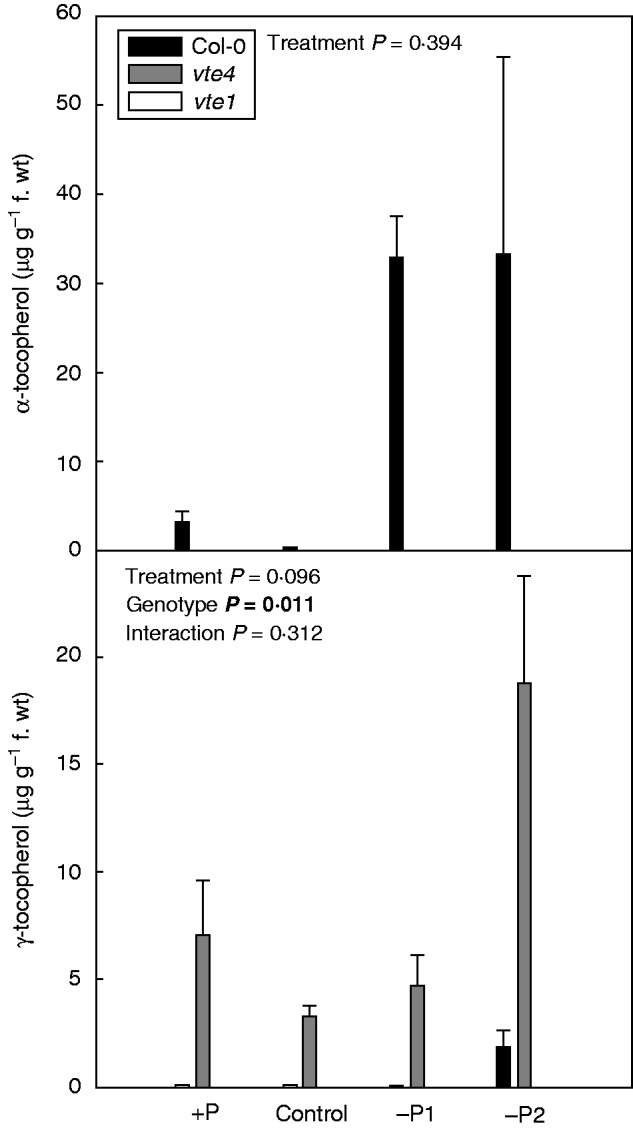
Levels of α- and γ-tocopherol in wild type (Col-0) and vte4 and vte1 mutants (see key) of A. thaliana grown under various P availabilities (+P, C, –P1 and –P2 represent various P concentrations in the nutrient solution; see Materials and methods for details). Data represent the mean ± s.e. of n = 5 individuals. Significant differences between treatments, genotypes and their interaction (treatment × genotype) were analysed by two-way ANOVA (P ≤ 0·05). Note that both tocopherols were absent in the vte1 mutant, while the vte4 mutant accumulated γ- instead of α-tocopherol.
Fig. 4.
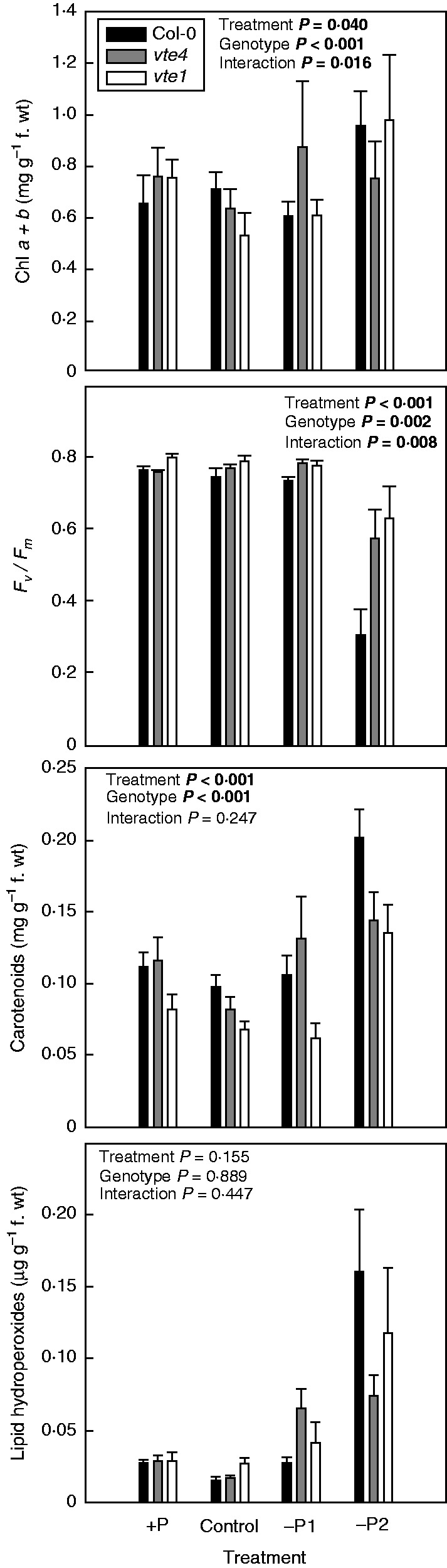
Chlorophyll (Chl) a + b levels, maximum PSII efficiency of PSII photochemistry (Fv/Fm ratio), total carotenoid levels and extent of lipid peroxidation (estimated as hydroperoxide levels) in wild type (Col-0) and vte4 and vte1 mutants (see key) of A. thaliana grown under various P availabilities (+P, C, –P1 and –P2 represent various P concentrations in the nutrient solution; see Materials and methods for details). Data represent the mean ± s.e. of n = 5 individuals. Significant differences between treatments, genotypes and their interaction (treatment × genotype) were analysed by two-way variance ANOVA (P ≤ 0·05).
Trade-offs between vitamin E and JA accumulation in senescing leaves
We investigated JA levels in leaves to unravel the effects of P availability and tocopherol accumulation on the potential to synthesize defence chemicals. The levels of OPDA, JA and JA-Ile increased under severe P deficiency in all genotypes (Fig. 5). The extent of this accumulation differed, however, between genotypes depending on treatment. Under severe P limitation the vte4 mutant showed a very sharp increase in OPDA levels, attaining 2-fold higher levels than the vte1 mutant and the wild type. Note that lipid hydroperoxide levels were lower in the vte4 mutant compared with vte1 and the wild type under these conditions (Fig. 4), thus suggesting increased conversion of lipid hydroperoxides to OPDA in the vte4 mutant under severe P deficiency. Interestingly, this increased OPDA production did not result in concomitant increases in JA or JA-Ile levels under severe P limitation (Fig. 5). Furthermore, JA levels were higher in the vte1 mutant compared with the other two genotypes under mild P limitation and excess P (–P1 and +P, respectively; Fig. 5). Higher JA levels were observed in parallel with increased vegetative and reproductive growth in the vte1 mutant compared with the other two genotypes under these conditions (Fig. 2).
Fig. 5.
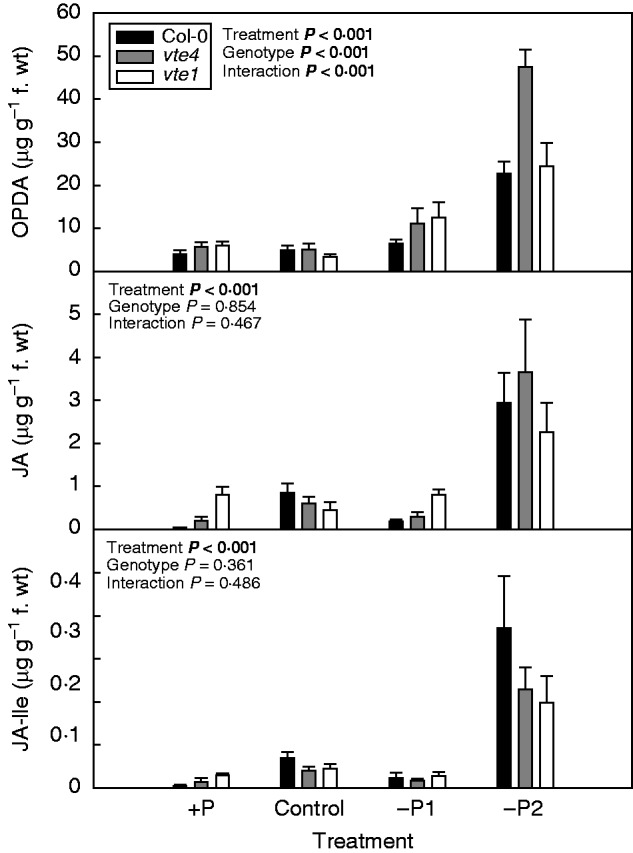
Jasmonate profiling, including levels of OPDA (JA precursor), JA, and JA-Ile in the rosette leaves of wild type (Col-0) and vte4 and vte1 mutants (see key) of A. thaliana grown under various P availabilities (+P, C, –P1 and –P2 represent various P concentrations in the nutrient solution; see Materials and methods for details). Data represent the mean ± s.e. of n = 5 individuals. Significant differences between treatments, genotypes and their interaction (treatment × genotype) were analysed by two-way ANOVA (P ≤ 0·05).
DISCUSSION
Longevity of A. thaliana vte1 and vte4 mutants under contrasting P availabilities
α-Tocopherol deficiency in the vte1 mutant reduced longevity under control conditions, excess P and mild P deficiency, but it paradoxically increased longevity under severe P deficiency. Interestingly, accelerated senescence was also observed in transgenic tobacco plants with α-tocopherol deficiency (Abbasi et al., 2007), and the same plants showed delayed senescence under salt stress (Asensi-Fabado et al., 2014). It therefore appears that growth conditions strongly modulate the effects of vitamin E in the control of longevity. Although little is known about the specific roles of α- and γ-tocopherol in the control of longevity and senescence, our results suggest similar roles for both α- and γ-tocopherol in the control of longevity under some conditions (control, excess P and mild P deficiency) and different roles in others (severe P starvation). This suggests that the effects of tocopherols in the modulation of longevity under control conditions, excess P and mild P deficiency may have an important antioxidant component, since the antioxidant capacity of both tocopherol forms is similar in biological membranes (Kamal-Eldin and Appelqvist, 1996), but α-tocopherol may exert an additional specific role, which could not be mimicked by γ-tocopherol, under severe P limitation. Since Asensi-Fabado et al. (2014) have shown that sugar signalling (and not oxidative stress) is a key component of the observed phenotype in salt-stressed tobacco plants, it is tempting to speculate that altered cell signalling may be responsible for the increased longevity observed in the present study in the vte1 mutant under severe P starvation, an aspect that warrants further investigation.
Vitamin E paradoxically increased PSII inactivation in the wild type under severe P limitation
Tocopherols are unique in terminating lipid peroxidation chain reactions (Munné-Bosch and Alegre, 2002; Havaux et al., 2005; Schneider, 2005; Traber and Stevens, 2011) and control singlet oxygen levels in thylakoid membranes in cooperation with carotenoids, thus preventing irreversible photo-inactivation of PSII (Munné-Bosch and Alegre, 2002; Havaux et al., 2005). Previous studies using vitamin E-deficient A. thaliana mutants have shown that tocopherols can modulate extraplastidic polyunsaturated fatty acid (PUFA) metabolism, this effect strongly modulating low-temperature adaptation (Maeda et al., 2008). In addition, it has been suggested that vitamin E may be involved in cellular signalling by modulating the expression levels of some genes related to ethylene biosynthesis, perception and signalling (Cela et al., 2011). Increased PSII inactivation, as indicated by drastic reductions in the Fv/Fm ratio, was observed in the wild type under severe P deficiency, thus indicating that vitamin E, and α-tocopherol in particular, accelerates senescence under severe P limitation. Since this effect appears to be specific for α-tocopherol and is not observed in the vte4 mutant, it is likely that PSII inactivation results from accelerated autophagy during senescence irrespective of the antioxidant capacity of α-tocopherol in chloroplasts to protect the photosynthetic apparatus. It should be noted that wild-type plants contained not only more α-tocopherol but also more carotenoids, thus suggesting that the antioxidant capacity to control singlet oxygen accumulation is not only not compromised, but enhanced. It therefore appears that the senescence-promoting effect of α-tocopherol under severe P starvation is not associated with oxidative stress, but with non-antioxidant effects, as happens in salt-stressed tobacco plants (Asensi-Fabado et al., 2014). The specific role of α-tocopherol in the control of autophagy processes, in particular those linked to sugar signalling, warrants further investigation.
Linking vitamin E and JAs in plants
Evidence obtained thus far in mutants defective in thermal energy dissipation and antioxidant protection suggest a link between increased reactive oxygen species levels, increased oxylipin levels and increased defences against certain pests or pathogens, so that a defect in photoprotective capacity can lead to altered responses to biotic stress (for a reviews see Demmig-Adams et al., 2013, 2014). In our study, increased JA accumulation was observed in the vte1 and vte4 mutants, particularly in the vte4 mutant, in which there was a 2-fold increase in OPDA level under severe P limitation, and in the vte1 mutant, which showed sharp increases in both JA and JA-Ile levels under excess P, and additionally in JA levels under mild P starvation. The increased OPDA level in the vte4 mutant under severe P limitation did not result in increased JA and/or JA-Ile levels, but was associated with reduced lipid hydroperoxide levels, thus suggesting increased channelling of oxylipins towards OPDA under these conditions. OPDA serves as a precursor of JA, but it also activates biotic defence independently of JA (Danon et al., 2005; Taki et al., 2005). However, it is unknown why OPDA, and not other JAs, specifically accumulated in this mutant. By contrast, JA and/or JA-Ile accumulated in response to excess P or mild P deficiency in the vte1 mutant. This was particularly true for JA, the vte1 mutant showing 5- and 10-fold higher levels than the wild type under excess P and mild P starvation, respectively. A similar response was also observed in another vte1 A. thaliana mutant line exposed to a combination of high light and low temperatures (Munné-Bosch et al., 2007). It therefore appears that reduced photoprotection in vitamin E-deficient mutants favours the capacity to synthesize chemical defences, such as JAs, under abiotic stress conditions, thus suggesting a trade-off between different defence pathways in plants (photoprotection versus potential chemical defence to necrotrophs through JAs).
It is concluded that tocopherols play an important role in controlling longevity, the effects depending on P availability. While both α- and γ-tocopherol prevent senescence under control conditions, excess P or mild P starvation, α-tocopherol, but not γ-tocopherol, accelerates senescence under severe P deficiency. Furthermore, vitamin E deficiency increases JAs levels, suggesting a trade-off between photoprotection and the activation of biotic defences.
ACKNOWLEDGEMENTS
We are very grateful to the Serveis Científico-tècnics (University of Barcelona) for technical assistance and Kathleen Brückner (University of Kiel, Germany) for providing seeds of vte1 and vte4 mutants. We are also indebted to Melanie Morales, Maren Müller and Laura Siles (University of Barcelona) for their support in hormone and vitamin E analyses. Support for the research was received through grant BFU2012-32057 from the Ministry of Science and Innovation of the Spanish Government, and the ICREA Academia prize to S.M.B., funded by the Catalan Government. B.S. holds a fellowship from the Ministry of Science and Innovation of the Spanish Government.
LITERATURE CITED
- Abbasi AR, Hajirezaei M, Hofius D, Sonnewald U, Voll LM. 2007. Specific roles of α- and γ-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiology 143: 1720–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral JS, Casal S, Torres D, Seabra RM, Oliveria BPP. 2005. Simultaneous determination of tocopherols and tocotrienols in hazelnuts by a normal phase liquid chromatographic method. Analytical Science 21: 1545–1548. [DOI] [PubMed] [Google Scholar]
- Asensi-Fabado MA, Ammon A, Sonnewald U, Munné-Bosch S, Voll LM. 2014. Tocopherol deficiency reduces sucrose export from salt-stressed potato leaves independently of oxidative stress and symplastic obstruction by callose. Journal of Experimental Botany 66: 957–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmüller E, Porfirova S, Dörmann P. 2003. Characterization of an Arabidopsis mutant deficient in γ-tocopherol methyltransferase. Plant Molecular Biology 52: 1181–1190. [DOI] [PubMed] [Google Scholar]
- Bou R, Chen B, Guardiola F, Codony R. 2010. Determination of lipid and protein hydroperoxides using the fluorescent probe. Food Chemistry 123: 892–900. [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, et al. 2001. Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cela J, Chang C, Munné-Bosch S. 2011. Accumulation of γ- rather than α-tocopherol alters ethylene signaling gene expression in the vte4 mutant of Arabidopsis thaliana. Plant and Cell Physiology 52: 1389–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordella D, Drangerta J-O, White S, et al. 2009. The story of phosphorus: Global security and food for thought. Global Environmental Change 19: 292–305. [Google Scholar]
- Danon A, Miersch O, Felix G, Camp RG, Apel K. 2005. Concurrent activation of cell death-regulating signalling pathways by singlet oxygen in Arabidopsis thaliana. Plant Journal 41: 68–80. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Cohu CM, Amiard V, et al. 2013. Emerging trade-offs – impact of photoprotectants (PsbS, xanthophylls, and vitamin E) on oxylipins and biotic defense. New Phytologist 197: 720–729. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Stewart JJ, Adams WW., III 2014. Chloroplast photoprotection and the trade-off between abiotic and biotic defense. In: Demmig-Adams B, Garab G, Adams W, III, Govindjee eds. Non-photochemical quenching and energy dissipation in plants, algae and cyanobacteria. Advances in Photosynthesis and Respiration, Vol. 40 Dordrecht: Springer. [Google Scholar]
- Distelfeld A, Avni R, Fischer AM. 2014. Senescence, nutrient remobilization, and yield in wheat and barley. Journal of Experimental Botany 65: 3783–3798. [DOI] [PubMed] [Google Scholar]
- Duff SMG, Sarath G, Plaxton WC. 1994. The role of acid phosphatases in plant phosphorus metabolism. Physiologia Plantarum 90: 791–800. [Google Scholar]
- Falk J, Munné-Bosch S. 2010. Tocochromanol functions in plants: antioxidation and beyond. Journal of Experimental Botany 61: 1549–1566. [DOI] [PubMed] [Google Scholar]
- Frank A. 2013. Chemistry of plant phosphorus compounds. Waltham, MA: Elsevier. [Google Scholar]
- Genty B, Briantais JL, Baker NR. 1989. The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92. [Google Scholar]
- Hammond JP, White PJ. 2008. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. Journal of Experimental Botany 59: 93–109. [DOI] [PubMed] [Google Scholar]
- Havaux M, Eymery F, Porfirova S, Rey P, Dörmann P. 2005. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 17: 3451–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvany M, Müller M, Munné-Bosch S. 2014. Photo-oxidative stress in emerging and senescing leaves: a mirror image? Journal of Experimental Botany 64: 3087–3098. [DOI] [PubMed] [Google Scholar]
- Kamal-Eldin A, Appelqvist L-Å. 1996. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31: 671–701. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Wellburn AR. 1983. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions 11: 591–592. [Google Scholar]
- Maeda H, Song W, Sage TL, DellaPenna D. 2006. Tocopherols play a crucial role in low-temperature adaptation and phloem loading in Arabidopsis. Plant Cell 18: 2710–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Sage TL, Isaac G, Welti R, DellaPennaa D. 2008. Tocopherols modulate extraplastidic polyunsaturated fatty acid metabolism in Arabidopsis at low temperature. Plant Cell 20: 452–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Munné-Bosch S. 2011. Rapid and sensitive hormonal profiling of complex plant samples by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Plant Methods 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S. 2005. The role of α-tocopherol in plant stress tolerance. Journal of Plant Physiology 162: 743–748. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L. 2002. The function of tocopherols and tocotrienols in plants. Critical Reviews in Plant Sciences 57: 21–31. [Google Scholar]
- Munné-Bosch S, Weiler EW, Alegre L, Muller M, Duchting P, Falk J. 2007. α-Tocopherol may influence cellular signaling by modulating jasmonic acid levels in plants. Planta 225: 681–691. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Queval G, Foyer CH. 2013. The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiology 161: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porfirova S, Bergmüller E, Tropf S, Lemke R, Dörmann P. 2002. Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proceedings of the National Academy of Sciences of the USA 99: 12495–12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. 2005. Chemistry and biology of vitamin E. Molecular Nutrition & Food Research 49: 7–30. [DOI] [PubMed] [Google Scholar]
- Szymanska R, Kruk J. 2008. γ-Tocopherol dominates in young leaves of runner bean (Phaseolus coccineus) under a variety of growing conditions: the possible functions of γ-tocopherol. Phytochemistry 79: 2142–2148. [DOI] [PubMed] [Google Scholar]
- Taki N, Sasaki-Sekimoto Y, Obayashi T, et al. 2005. 12-Oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiology 139: 1268–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber MG, Stevens JF. 2011. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radical Biology and Medicine 51: 1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Hammond JP. 2008. The ecophysiology of plant-phosphorus interactions. Dordrecht: Springer. [Google Scholar]
- Wingler A, Von Schaewen A, Leegood RC, Lea PJ, Paul Quick W. 1998. Regulation of leaf senescence by cytokinin, sugars, and light: effects on NADH-dependent hydroxypyruvate reductase. Plant Physiology 116: 329–335. [Google Scholar]


