Abstract
Background Climate change predictions indicate a progressive increase in average temperatures and an increase in the frequency of heatwaves, which will have a negative impact on crop productivity. Over the last decade, a number of studies have addressed the question of how model plants or specific crops modify their metabolism when exposed to heat stress.
Scope This review provides an overview of the redox pathways that contribute to how plants cope with heat stress. The focus is on the role of reactive oxygen species (ROS), redox metabolites and enzymes in the signalling pathways leading to the activation of defence responses. Additional attention is paid to the regulating mechanisms that lead to an increase in specific ROS-scavenging systems during heat stress, which have been studied in different model systems. Finally, increasing thermo-tolerance in model and crop plants by exposing them to heat acclimation or to exogenous treatments is discussed.
Conclusions Although there is clear evidence that several strategies are specifically activated according to the intensity and the duration of heat stress, as well as the capacity of the different species or genotypes to overcome stress, an alteration in redox homeostasis seems to be a common event. Different mechanisms that act to enhance redox systems enable crops to overcome heat stress more effectively. Knowledge of thermo-tolerance within agronomic biodiversity is thus of key importance to enable researchers to identify new strategies for overcoming the impacts of climate change, and for decision-makers in planning for an uncertain future with new choices and options open to them.
Keywords: Antioxidants, biodiversity, climate change, heat stress, reactive oxygen species, ROS, redox homeostasis, thermo-tolerance
INTRODUCTION
In the near future climate changes will subject plants to more challenging environmental conditions. An increase of 0·2 °C in the average temperature has been predicted to occur over the next decades. Moreover, heatwaves, i.e. an increase of several degrees over the seasonal temperature for a sustained period of days, are projected to become more frequent (Meehl and Tebaldi, 2004; IPCC, 2014). Climate changes have already caused a fall in crop yield, and the expected further temperature alteration will also increase the negative effect on crop productivity by exacerbating other temperature-related stress conditions such as water shortages (Bita and Gerats, 2013).
Different phases of plant development and the timing of exposure to over-optimal temperatures are also critical for the response to heat stress (HS). Complete loss of grain production can be caused by an increase of a few degrees when plants are exposed to HS during flowering (Lobell et al., 2011). An increase in the average seasonal temperature of 1 °C induces a decrease in cereal productivity by 4–10 % (Wang et al., 2012), and similar behaviour has been observed in legumes (Young et al., 2004).
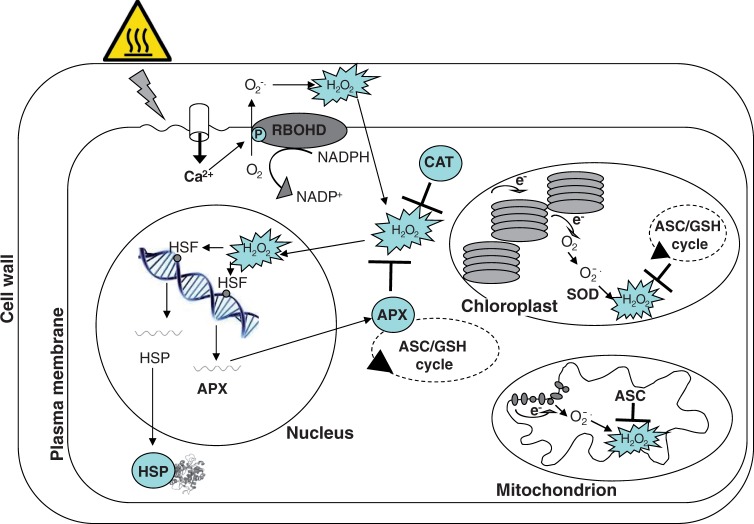
Plants possess several acclimation mechanisms that enable them to alleviate HS damage. Indeed, to cope with HS, resistant plants have evolved a series of strategies to sense temperature changes rapidly and adapt their metabolism, cellular structures as well as phenology accordingly. Different heat sensors have been suggested to be present in the plasma membrane, nucleus, cytosol and endoplasmic reticulum of plant cells (Sugio et al., 2009; Che et al., 2010; Mittler et al., 2012). However, a Ca2+ channel located in the plasma membrane has been indicated as the primary heat sensor in plants. Increments in temperature increase plasma membrane fluidity, activating the Ca2+ channel and Ca2+ influx to the cytosol (Mittler et al., 2012; Fig. 1). Studies with Ca2+ channel blockers suggest that Ca2+ accumulation in the cytosol is mainly responsible for the activation of downstream events leading to proteome, transcriptome and metabolome re-programming which results in thermo-tolerance acquisition (Larkindale and Knight, 2002; Mittler et al., 2012).
Fig. 1.
Redox systems in heat stress response. Schematic representation of the main redox events occurring in plant cells as a consequence of heat stress. Heat perception at the plasma membrane activates a Ca 2+ channel with a consequent increase in cytosolic levels of Ca 2+. Ca 2+ influx activates RBOHD by promoting its phosphorylation, leading to the increase of reactive oxygen species (ROS). Under heat stress, ROS production also occurs in chloroplasts and mitochondria. Hydrogen peroxide (H2O2) works as a second messenger and in the nucleus activates the expression of genes with heat shock elements in their promoters, such as heat shock proteins (HSPs) and cytosolic ascorbate peroxidase (APX). Other redox enzymes and metabolites, such as superoxide dismutase (SOD), catalase (CAT) and the ascorbate–glutathione (ASC–GSH) cycle, work in different cell compartments to maintain appropriate ROS levels.
The induced expression of heat shock proteins (HSPs) has been considered as a major re-programming event required for the acquisition of thermo-tolerance. HSPs recognize and bind unstable proteins, thus preventing their denaturation and misfolding pattern (Schöffl et al., 1998). Once plants have sensed HS, HSP expression is promptly activated by specific transcription factors (HSFs) binding the conserved sequence of the heat shock elements (HSEs) in the promoters of heat-responsive genes (Schöffl et al., 1998). The HSF–HSP network is a highly conserved molecular mechanism protecting organisms from the negative effects of HS on cellular structures and, consequently, functions, but it is not the only defence system operating in HS responses.
Oxidative stress emerging as a consequence of HS also seems to play a role in triggering defence mechanisms. Indeed, reactive oxygen species (ROS) and ROS-scavenging systems are part of signalling pathways leading to the activation of defence responses against HS.
This review discusses the recent findings underlying the role of plant redox systems in HS responses and their relevance in thermo-tolerance acquisition.
REACTIVE OXYGEN SPECIES DURING HEAT STRESS
One of the major consequences of HS is the impairment of redox homeostasis. ROS levels are altered and this activates the defence responses in resistant plants or induces oxidative structural or functional damage in sensitive plants. ROS levels and types, as well as endogenous ROS-scavenging systems or the presence of other reactive species in the sites of ROS production/accumulation, are key factors for organism/cell fate (de Pinto et al., 2006).
Several HS-dependent ROS production sites have been described. It has been suggested that the respiratory burst oxidase homologue D (RBOHD), a ROS-generating NADPH oxidase located in the plasma membrane, could have a key role in the oxidative burst occurring during HS (Suzuki et al., 2011; Fig. 1). In arabidopsis and tobacco cell cultures, the increase in H2O2 production, occurring during the early phase of HS, can be blocked by the application of an inhibitor of the enzyme NADPH oxidase (Volkov et al., 2006; Konigshofer et al., 2008). It has also been shown that RBOHD has a central role in HS survival and HS signal transduction (Larkindale et al., 2005; Miller et al., 2009). The activity of this protein is regulated by phosphorylation via protein kinases such as cyclin-dependent protein kinases (CDPKs) and by the direct binding of calcium. Therefore, the cytosolic Ca2+ influx, which occurs as a consequence of heat perception, could activate RBOHD and result in the accumulation of ROS (Miller et al., 2009; Fig. 1).
Production of ROS during HS can also occur in mitochondria (Fig. 1). An impaired mitochondrial metabolism seems to be responsible for oxidative bursts occurring in tobacco cells undergoing heat-induced programmed cell death (PCD) (Vacca et al., 2004; Valenti et al., 2007). During HS, calcium uptake by mitochondria and ROS generation occur (Rhoads et al., 2006; Rikhvanov et al., 2007). It has been suggested that the source of ROS resides mainly in the inner membrane of mitochondria where hyperpolarization occurs. Agents able to prevent an increase in electrochemical potential of the inner mitochondrial membrane have also been shown to inhibit thermo-tolerance (Rikhvanov et al., 2007).
In green tissues, chloroplasts probably act as the main ROS-producing site, since photosynthesis is very sensitive to temperature (Crafts-Brandner and Salvucci, 2002; Foyer and Noctor, 2003; Yamamoto et al., 2008; Zinta et al., 2014; Fig. 1). Apart from the direct effect on photochemical reactions in thylakoid membranes, the increase in temperature induces stomatal closure, particularly when water is scarce. The consequent alterations in CO2 and O2 concentrations within mesophyll cells affect the Calvin cycle, thus increasing the impairment in photosynthetic electron flux and leading to a further increase in ROS production. The aim of the water–water cycle is to cope with the ROS overproduction of chloroplasts. However, when HS is persistent or of a particular intensity, such a cycle cannot maintain ROS under control, and oxidative damage occurs (Asada 1999).
Peroxisomes are another site of ROS production, in particular under conditions that increase photorespiration, such as stomatal closure (Vanderauwera et al., 2011).
In HS conditions, ROS play an important role as molecular signals by activating downstream pathways leading to protective effects. There are several redox-sensitive transcriptional factors whose activities rely on redox changes (Tron et al., 2002; Heine et al., 2004; Shaikhali et al., 2008, 2012). The heat shock factor HSFA4a, which rapidly responds to H2O2, can function as a direct ROS sensor and rely on a transcription factor function acting upstream of ZAT12 and APX1, two genes with an HSE in their promoters (Miller and Mittler, 2006; Fig. 1).
In relation to the capacity of ROS to act as signals that activate metabolic responses, a temporal–spatial concept of the ROS wave has been proposed that describes ROS signalling as a dynamic process occurring within cells between different organelles, as well as between cells over long distances (Mittler et al., 2011). Starting in the tissues subjected to HS or other stresses and propagating to distal parts of plants, ROS waves induce systemic acquired acclimation, enabling tissues that have not been exposed to HS to increase thermo-tolerance in arabidopsis (Suzuki et al., 2013a).
Under certain conditions, an increase in HS-dependent ROS induces an increase in the cellular systems aimed at restoring redox balance (Fig. 1). This strategy seems to be commonly correlated to thermo-tolerance, as discussed in the next section.
INVOLVEMENT OF REDOX SYSTEMS IN HEAT STRESS RESPONSES
Changes in redox regulation allow resistant plants to survive or to maintain their productivity under HS. These thermo-tolerance properties are based on changes in the levels of redox metabolites, and on the expression/activities of enzymes able to scavenge ROS or to maintain their substrates in the reduced state, even under oxidative conditions. A set of reactions involved in thermo-tolerance is the ascorbate–glutathione (ASC–GSH) cycle. ASC and GSH are the major soluble redox metabolites in plant cells. They act as ROS scavengers directly or as substrates of redox enzymes (Foyer et al., 2009). In the cycle, ASC is used as an electron donor by ascorbate peroxidase (APX), a typical plant enzyme that controls H2O2 levels. Oxidation of ASC generates monodehydroascorbate (MDHA), a radical molecule unable to react with oxygen, which spontaneously undergoes dismutation, thus producing ASC and dehydroascorbic acid (DHA). MDHA and DHA are reduced to ASC by two specific reductases using pyridine nucleotides and GSH as electron donors, respectively. In the same cycle, the oxidized form of glutathione, GSSG, is reduced to GSH by GSSG reductase (GR), another enzyme using a pyridine nucleotide as an electron donor. Interestingly, the ASC–GSH cycle is enhanced under HS in almost all organelles producing ROS (Locato et al., 2009; Fig. 1). Other peroxidases (PODs), catalases (CATs) and superoxide dismutases (SODs) are present, with several isoenzymes acting in different cellular compartments and further regulating ROS levels (De Gara et al., 2010).
Relevance of biodiversity
The HS tolerance of plants seems to be strictly linked to their redox homeostatic capacity, and species-specific differences in antioxidant regulation could play an adaptive role to cope with elevated temperatures. A comparative study performed in plants species with contrasting life forms, such us woody perennial Populus trichocarpa, annual legume Glycine max or annual forb Arabidopsis thaliana, suggests a relationship between heat shock-induced transcriptional networks, ROS-scavenging systems and photosynthesis efficiency (Weston et al., 2011). Weston’s study shows that soybean has a higher temperature optimum than arabidopsis and poplar. Transcriptomic analyses demonstrate that ROS scavenging and HSP network transcripts are clustered into the same module for arabidopsis and poplar, and in different modules for soybean, thus suggesting a divergence in ROS regulation among these species. The species-specific variation in the antioxidant regulation in response to heat is confirmed by the enzyme activities of SOD, GR and POD. In arabidopsis and poplar, enzyme activities increase gradually with an increase in temperature, whereas in soybean a rapid but only transient increase in POD and GR activities occurs (Weston et al., 2011).
Maize genotypes are more able than rice genotypes to maintain their growth under HS conditions due to their greater capacity to manage oxidative damage. Indeed, the expression of CAT, APX and GR, as well as ASC and GSH contents, are higher in maize plants compared with rice plants during HS. The different antioxidant capacities of maize and rice could reflect the relative sensitivity of C4 and C3 plants to HS (Kumar et al., 2012a).
Several studies performed with sensitive and thermo-tolerant genotypes within the same species underline a clear correlation between thermo-tolerance and the ability to increase one or more ROS-scavenging enzymes. In four different wheat genotypes, heat tolerance, estimated by the capacity to maintain photosynthetic efficiency in seedlings exposed to HS, is correlated with the capability of APX and CAT to dissipate H2O2 co-operatively (Dash and Mohanty, 2002). Similarly, studies conducted by exposing different wheat genotypes to HS show that ROS-scavenging enzymes have a positive correlation with chlorophyll content and a negative correlation with membrane damage and heat susceptibility index (Almeselmani et al., 2006; Hameed et al., 2012). Analogous results have been reported in cotton, where genotypic differences in thermo-tolerance have been linked to antioxidant protection of the photosynthetic machinery (Snider et al., 2010).
The association between antioxidant enzymes and heat tolerance has also been shown in Kentucky bluegrass using two different genotypes. During HS, the heat-tolerant genotype ‘Midnight’ maintained significantly higher activities of SOD, CAT, POD, APX and GR than the heat-sensitive genotype ‘Brilliant’ (Du et al., 2013).
Relevance of heat stress characteristics
Changes in the activities of antioxidant enzymes under HS also strongly depend on stress duration and intensity.
Generally, a short period of HS enhances ROS-scavenging systems, which enable plants to survive by containing oxidative damage. However, when the HS is prolonged, the proteins and metabolites involved in redox control are overwhelmed, with an irreversible compromise of plant growth and survival. A study on tobacco BY-2 cell culture, a well-known plant model system, shows that short-term HS at 35 °C causes a transient increase in ASC content and in enzyme activity in terms of H2O2 removal and in terms of recycling the oxidized forms of ASC and GSH.
The rapid enrichment of antioxidant systems enables tobacco BY-2 cells to avoid oxidizing conditions and to maintain a normal growth rate. On the other hand, under prolonged HS at the same temperature, the redox systems involved in defence mechanisms, in particular enzymatic ones, are initially induced but subsequently suppressed. The decline of these enzymes causes H2O2 to increase within cells and leads to the onset of oxidative stress, and consequently cell death (Sgobba et al., 2015). In apple leaves, the ASC–GSH cycle is upregulated in response to high temperatures, but, after reaching the maximum, it declines with an increase in the duration of treatment (Ma et al., 2008).
In wheat, a transcriptomic study has shown that most of the genes involved in the ROS-scavenging pathway, including alternative oxidases, APX3, glutathione peroxidases and thioredoxin, have higher expression levels during short-term heat shock than during long-term heat treatments. The data suggest that plants scavenge ROS generated by heat shock, while the oxidative stress induced by long-term HS impairs a plant’s capacity to increase these salvage mechanisms (Qin et al., 2008). A study conducted on alfalfa showed that the APX transcript increases after 24 and 48 h of exposure at 48 °C, but drastically decreases after 72 h of HS. Interestingly, no phenotypic effects were observed after 24 h of heat treatment, whereas leaves were scorched after 72 h of exposure to HS (Li et al., 2013). An increase in genes coding for redox enzymes, in particular APX and DHA reductase, has also been reported in grapevine leaves exposed to 45 °C for a short period (Liu et al., 2012). In lily plants, the high capacity to overcome short periods of exposure to high temperatures seems to be due to the increase in antioxidant molecules and enzymes, which prevent oxidative damage (Yin et al., 2008).
Long-term HS is probably critical for thermo-tolerance since it negatively affects the scavenger capacity of ROS of the exposed tissues. The decrease in SOD and CAT in the leaves of two creeping bentgrass genotypes after long-term HS is responsible for the damage to cell membranes and leaf senescence (Liu and Huang, 2000). The decline in SOD and CAT activities with increasing root-zone temperatures in creeping bentgrass negatively affects turf quality (Wang et al., 2003). The reduction in SOD activity after long-term HS is responsible for the heat sensitivity of the ‘Brillant’ Kentucky bluegrass cultivar (He and Huang, 2010), and causes membrane damage and decreases the photosynthetic rate in sorghum leaves (Djanaguiraman et al., 2010), as well as oxidative damage and growth reduction in cucumber plants (Zhang et al., 2012).
The intensity of HS is another pivotal parameter for thermo-tolerance. The increase in temperature stimulates the activity and the expression of antioxidant enzymes until a threshold temperature is reached. If the temperature is higher than the threshold, which changes considerably among species and cultivars, it causes a decrease in redox metabolites and proteins and a consequent decrease in growth (Locato et al., 2008; Chakraborty and Pradhan, 2011; Kumar et al., 2012a).
MECHANISMS REGULATING ROS-SCAVENGING SYSTEMS DURING HEAT STRESS
In model systems, such as arabidopsis plants or cultured cells, the regulatory mechanisms that lead to an increase in specific ROS-scavenging systems during HS have been studied in depth. In this section the mechanisms regulating ASC biosynthesis, APX and thiol-containing proteins are analysed.
Ascorbate biosynthesis
The ASC level influences plant responses to heat stress. The arabidopsis mutants vtc1 and vtc2, with a low ASC content (Conklin et al., 2000), have a lower level of basal thermo-tolerance than the wild type. The low levels of ASC increase the heat-dependent oxidative damage found in the vitc1 and vitc2 mutants, also in HS conditions where there is a small decrease in survival (Larkindale et al., 2005).
The role of AtDjB1, a member of the arabidopsis J-protein family, in the acquisition of thermo-tolerance seems to be related to the control of the ASC concentration (Zhou et al., 2012). In the AtDjB1 knockout plants atj1-1, the ASC content is lower than in the wild type, which leads to a high accumulation of oxidative products after HS. Interestingly, the exogenous addition of ASC confers thermo-tolerance to atj1-1 plants. AtDjB1 is localized in mitochondria and, interacting with the mitochondrial HSP70 mtHSC70-1, acts as an ATPase. As a consequence, the accumulation of ATP, which occurs in atj1-1 plants, inhibits the electron transport chain (Zhou et al., 2012).
Interestingly, the last step of ASC synthesis depends on the electron transport chain (Bartoli et al., 2000) since, l-galactone-γ-lactone dehydrogenase (GLDH), the last enzyme of ASC biosynthesis, is an integral part of plant mitochondrial complex I (Millar et al., 2003). Therefore, AtDjB1 could act by modulating ASC concentrations through an indirect effect on GLDH activity. The accumulation of H2O2, occurring as a consequence of an ASC decrease and the impairment of the electron transport chain, could be responsible for decreased thermo-tolerance in atj1-1 plants (Zhou et al., 2012).
The importance of ASC in the HS response has also been shown in tobacco BY-2 cells. During the HS-induced PCD, a decrease in ASC content occurs (Vacca et al., 2004; Locato et al., 2008). The decrease in ASC observed in tobacco BY-2 cells undergoing HS-induced PCD could be due, at least in part, to an impairment of GLDH (Valenti et al., 2007). Moreover, the exposure of tobacco BY-2 cells to a long moderate HS determines an inhibition of cell growth and a decrease in cell viability. The exogenous addition of GL, the last precursor of ASC, increases the ASC content and enhances basal thermo-tolerance (Sgobba et al., 2015).
Ascorbate peroxidase
Ascorbate peroxidase plays a key role in heat shock response. Arabidopsis thaliana possess eight APX isoenzymes with different localizations: APX1, APX2 and APX6 are soluble cytosolic isoenzymes; APX3, APX4 and APX5 are microsomial isoenzymes, and sAPX and tAPX are localized in the chloroplast (Panchuk et al., 2005).
Treatments inducing heat shock responses (1–2 h at 37 °C) increase the mRNA levels of all APX genes; however, the effects change qualitatively and quantitatively for different genes (Panchuk et al., 2002; Koussevitzky et al., 2008).
The APX1 and APX2 promoters contain an HSE, binding the HSF, characteristic for heat shock genes (Storozhenko et al., 1998; Panchuk et al., 2002; Fig. 1). APX3 and APX5 possess a similar sequence to HSE but are unable to bind HSF (Panchuk et al., 2002). However, microsomal APXs are involved in the response to HS (Shi et al., 2001).
In arabidopsis, APX1 is regulated by HS in an HSF-dependent mode, and APX1 may link the antioxidant pathway to HS-induced protection of general cellular functions (Storozhenko et al., 1998). HS is also able to trigger APX2 gene expression at the mRNA level, and this correlates with the appearance of a new thermo-stable APX isozyme, APXS. The contribution of HSF to the induction of APX2 is supported by the constitutive levels of APX2 mRNA and APXS activity in HSF3 transgenic plants under non-stress conditions. APX2/APXS is probably required to compensate for the HS-dependent decline of APX1 activity in the cytosol (Panchuk et al., 2002). AtHSFA2 is also a key regulator of APX2 (Nishizawa et al. 2006). A tomato cytosolic APX, homologous to arabidopsis APX2, is also considerably upregulated in pollen during HS (Frank et al., 2009).
In arabidopsis subjected to HS, a deficiency in APX2 may induce signals with a greater role than signals activated by a deficiency in APX1 (Suzuki et al., 2013b). Consistently, in arabidopsis, HS affects root growth more severely in the double mutants apx1/apx2 and in the mutant apx2 than in wild-type or apx1 plants. It has also been proposed that the signals related to APX2 deficiency play a key role in the regulation of plant productivity during HS (Suzuki et al., 2013b).
On the other hand, cytosolic APX1 seems to play a key role in the acclimation of plants to a combination of drought and HS. During drought, ROS are thought to be produced in the chloroplast and peroxisomes (Asada et al., 2006; Van Breusegem and Dat, 2006), whereas, during HS, ROS are mainly produced in chloroplasts and mitochondria (Suzuki and Mittler, 2006). In plants subjected to a combination of drought and HS, ROS can also be produced in the apoplast. In the drought and HS combination, APX1 could affect the diffusion of ROS from the various compartments to the nuclei. Consequently, during the depletion of APX1 caused by multiple stresses, more ROS could reach the nuclei and negatively affect cellular survival (Koussevitzky et al., 2008) or modify the pattern of gene expression (Miller and Mittler, 2006).
Cytosolic APX6, an APX isoenzyme replacing APX1 in developing and germinating seeds, may play a role in the heat tolerance of arabidopsis seeds (Chen et al., 2014). Indeed seeds lacking APX6 accumulate high levels of ROS, exhibit increased oxidative damage, and show reduced germination under control conditions. These effects increase under HS. In addition, ripening APX6-deficient seeds exposed to HS display reduced germination vigour (Chen et al., 2014)
The involvement of APX in HS responses has also been extensively studied in tobacco BY-2 cells. A short-term exposure (10 min) of tobacco BY-2 cells at 35 or 55 °C causes an increase or decrease, respectively, in total APX activity (Locato et al., 2008). The increase in APX activity induced by exposure at 35 °C is only due to an increase in cytosolic isoenzymes. On the other hand, the short exposure at 55 °C determines the decline of cytosolic, plastidial and mitochondrial isoenzymes (Locato et al., 2009). The changes in cytosolic APX activity are also accompanied by changes in gene expression (Locato et al., 2008, 2009). However, the regulation of the cytosolic APX activity under HS inducing PCD (10 min at 55 °C) occurs more precociously than the regulation of its gene expression, and seems to be due to nitrosylation, ubiquitination and consequent proteasome-dependent degradation of the protein (Vacca et al., 2004; de Pinto et al., 2013).
In tobacco BY-2 cells, APX also plays a role in response to a moderate HS of different durations. Cytosolic APX activity and expression increase in the first 3 d of exposure at 35 °C. However, when the HS is prolonged, there is a decline in cytosolic APX, which is responsible for the consequent increase in oxidative damage leading to cell death (Sgobba et al., 2015).
Thiol-containing proteins
Several thiol-containing proteins are involved in redox homeostasis and ROS scavenging, and their levels and activities are regulated by HS-dependent mechanisms.
Glutaredoxins (GRXs) are small ubiquitous proteins of the thioredoxin (TRX) family which mediate a reversible reduction in the disulphide bonds of their substrate proteins in the presence of GSH via a dithiol or monothiol mechanism (Rouhier et al., 2006). GRXs could play a key role in oxidative stress responses since they are able to regulate the cellular redox state. Various studies have reported that GRXs are involved in the HS response. For instance, the expression of PvGRX5, a GRX of the fern Pteris vittata, in arabidopsis increases the thermo-tolerance (Sundaram and Rathinasabapathi, 2010). AtGRXS17, a monothiol GRX of arabidopsis, is involved in the control of temperature-dependent post-embryonic growth. AtGRXS17 expression is induced by elevated temperatures, and an alteration in AtGRXS17 expression leads to hypersensitivity to high temperatures. In atgrxs17 mutants, HS induces higher levels of ROS and membrane damage than in the wild type.
The sensitivity of atgrxs17 mutants to high temperatures is dependent on both the duration and degree of the temperature treatment. At 22 °C, atgrxs17 mutants display minor growth defects, at 25 °C more severe phenotypes, and at 28 °C the growth of atgrxs17 mutants is severely repressed. These different effects are linked to the different levels of accumulation of ROS in the growing tissues (Cheng et al., 2011). Experiments conducted in tobacco and tomato expressing a green fluorescent protein (GFP)–AtGRXS17 fused protein show that this GRX is localized in the cytoplasm and the nuclear envelope under optimal temperature, and migrates into the nucleus during HS. In addition, during HS, AtGRXS17-expressing tomato plants show an upregulation of HSF and HSPs, suggesting that the increase in the nuclear pool of AtGRXS17 increases the expression of the HS-related transcription factor (Wu et al., 2012).
The expression of AtGRXS17 in tomato plants also reduces oxidative damage, and the increased thermo-tolerance is linked with increased CAT activity and reduced H2O2 accumulation. AtGRXS17 may even protect CAT activity directly, by physically interacting with this protein, or indirectly, by increasing HSP transcription (Wu et al., 2012).
Peroxiredoxins (PRXs) are a family of thiol-based peroxidases that can be divided into three classes: 1Cys-PRX, typical 2Cys-PRX and atypical 2Cys-PRX (Rhee et al., 2005).
Peroxiredoxins are able to enhance plant tolerance to HS (Kim et al., 2010). In A. thaliana, induction of the PER1 gene, which encodes 1Cys-PRX, is mediated by the interaction of FCA, a plant-specific RNA-binding protein of the autonomous flowering pathway, with ABA-INSENSITIVE 5 (Lee et al., 2015). FCA could be involved in environmental adaptation responses through RNA processing and chromatin modification (Kumar et al., 2011b). FCA could play a role in the stimulation of thermo-tolerance by enhancing antioxidant activity. The increase in antioxidant capacity during HS in arabidopsis plants overexpressing FCA could be explained, at least in part, by the transcriptional regulation of the PER1 gene by FCA. Indeed, the PER1 gene is induced by heat and FCA overexpression, whereas in fca mutants, the observed decrease in total antioxidant activity may be due to the failure of the heat induction of the PER1 gene (Lee et al., 2015).
A C-type NADPH-dependent thioredoxin reductase (NTRC) has been proposed as an efficient electron donor to 2Cys-PRX (Moon et al., 2006; Pérez-Ruiz et al., 2006). NTRC is induced in response to environmental stress (Serrato et al., 2004). Overexpression of NTRC in arabidopsis determines enhanced thermo-resistance against heat shock. In contrast, in NTRC-deficient arabidopsis (ntrc1), growth inhibition and plant death occur during HS. NTRC can act as a disulphide reductase, with both foldase and holdase activities. These multiple functions are heat shock regulated. HS induces a switch from a low molecular weight to a high molecular weight complex of NTRC. This switch, which is reversible, increases the redox-dependent holdase chaperone function for NTRC, and provides enhanced thermo-tolerance (Chae et al., 2013).
IMPROVEMENT IN PLANT THERMO-TOLERANCE
The involvement of antioxidant systems in the defence response activated by plants against HS has also been proved by studies demonstrating that many treatments able to increase the redox capacity of plants reduce heat-dependent damage by increasing thermo-tolerance.
Heat acclimation
Heat acclimation is obtained by priming, i.e. the exposure to a temperature lower than the threshold temperature or to a short-term HS, and generally enhances antioxidant systems and improves thermo-tolerance, thus making plants more resistant to a subsequent higher HS.
In Orchardgrass, heat acclimation, achieved by a short treatment at 38/30 °C (day/night) and a subsequent recovery at 25/20 °C, increases thermo-tolerance to a subsequent prolonged treatment at 38/30 °C. Acclimated plants show a higher activity and expression of several ROS-scavenging genes than non-acclimated plants. The improvements in membrane stabilization and in the efficiency of water use and photosynthesis have also been attributed to the enhancement of these antioxidant enzymes (Zhao et al., 2014). Similarly, heat-acclimated turfgrass species with higher ASC and GSH contents are able to overcome a subsequent HS of almost 15–20 °C higher than optimal temperatures by maintaining higher membrane thermo-stability, lower lipid peroxidation and lower chloroplast damage than non-acclimated plants (Xu et al., 2006).
The reproductive phase is particularly sensitive to HS. Pre-anthesis heat acclimation alleviates the photosynthetic and oxidative damage caused by post-anthesis HS in wheat flag leaves. Again, the beneficial effects can in part be attributed to changes in the activity and expression of antioxidant enzymes. The upregulation of mitochondrial Mn-SOD and chloroplastic Cu/Zn-SOD is of particular relevance, which suggests an important role for the organelles in the thermo-tolerance of wheat (X Wang et al., 2011).
The enhancement of antioxidant enzymes in specific organelles in conferring thermo-tolerance after heat acclimation is also important in wheat seedlings. Heat acclimation improves the redox homeostasis of seedlings exposed to a later high temperature stress, by increasing the activities of SOD in chloroplasts and of GR and POD in mitochondria. As a consequence, chloroplasts and mitochondria of acclimated plants show a lower superoxide radical production rate and lipid peroxidation than non-acclimated plants. The improvement in antioxidant capacities of chloroplasts may contribute to the higher photochemical efficiency of the acclimated seedlings (Wang et al., 2014).
Exogenous treatments
Several studies report the capacity of different plant growth regulators to reduce the oxidative damage occurring when plants are exposed to high temperatures. Treatments with abscisic acid (ABA), 1-aminocyclopropane 1-carboxylic acid (ACC) and salicylic acid (SA) reduce HS-dependent ROS accumulation and lipid peroxidation in Agrostis stolonifera (Larkindale and Huang, 2004). The use of the arabidopsis insensitive mutants abi1 and etr1, as well as the transgenic line expressing the bacterial gene nahG, coding for salicylate hydroxylase, further demonstrates a positive correlation between the impairment in ABA-, ethylene- or SA-dependent signalling pathways and HS susceptibility (Larkindale and Knight, 2002).
Interestingly, all these plant growth regulators are well known for their role in the activation of plant defence responses against different kinds of stress, including ABA in drought, ACC in flooding, and SA in plant–pathogen interactions (Grichko and Glick, 2001; Riera et al., 2005; An and Mou, 2011). The defence mechanism activated by these regulators seems to involve the enhancement of ROS-scavenging systems, such as an increase in the activities of the redox enzymes APX, POD, CAT and SOD, which improves the thermo-tolerance (Larkindale and Knight, 2002; Larkindale and Huang, 2004; Wang and Li, 2006; Ding et al., 2010; Kumar et al., 2012b). However, there are some contradictions in the literature, possibly depending on the varying sensitivities of different species to these molecules. In soybean leaves, treatments with 1-methyl-cyclopropene, an inhibitor of ethylene biosynthesis, reduced senescence symptoms caused by HS, by decreasing ROS and increasing CAT and SOD activities. This suggests that an inverse correlation exists between ethylene and HS resistance, at least in this species (Djanaguiraman et al., 2011).
Different signalling molecules have also been tested for their capacity to increase thermo-tolerance in plants. Calcium pre-treatment reduced H2O2 and superoxide anion levels by increasing the activities of APX and SOD, respectively, in tomato plants exposed to HS (Ding et al., 2012). The involvement of calcium in the activation of defence responses against HS has also been demonstrated by the use of Ca2+ chelators and Ca2+ channel blockers that are able to inhibit the Ca2+ protective effect against HS (Larkindale and Knight, 2002; Ding et al., 2012).
Pharmacological approaches have been used to show that nitric oxide (NO) acts as a Ca2+ upstream signal in HS responses (Wang et al., 2014). Treatments with sodium nitroprusside, an NO donor, demonstrate that NO is able to reduce lipid peroxidation in Chrysanthemum morifolium plants exposed to HS, by the upregulation of the antioxidant enzymes SOD, CAT, POX and APX (Yang et al., 2011). NO is also involved in the signalling pathway leading to HS-dependent HSP expression, acting upstream of H2O2, a key molecule of the HS response (Wang et al., 2014). As previously mentioned, ROS, and in particular H2O2, are involved in the activation of HSP expression. In arabidopsis, ROS scavengers inhibit HSP expression, and HSP expression is also strongly reduced in rboh mutants. In both cases, the impairment of H2O2 production decreases plant thermo-tolerance (Larkindale et al., 2005; Volvok et al., 2006). In line with this, pre-treatments with H2O2 protect thylakoidal membranes of Cucumis sativum from lipid peroxidation occurring during HS (Gao et al., 2010). This protective mechanism seems to depend on the acclimation induced by H2O2, which increases the activities of chloroplastic SOD and ASC–GSH cycle enzymes, thus preparing these organelles to cope with the following HS (Gao et al., 2010).
Treatment with ASC may also reduce H2O2 overaccumulation during HS, leading to a reinforcement of the antioxidant systems in mungbean plants (Kumar et al., 2011a). ASC protects plants by stimulating the increase of proline in the tissue (Kumar et al., 2011a). Proline is an osmoprotectant that protects enzymes from inactivation by HS. Treatments with osmoprotectants such as proline, glycine betaine and trehalose have also been shown to increase thermo-tolerance in different plants, by promoting membrane stability and reducing oxidative stress in the HS-exposed tissues (Rasheed et al., 2011; Kaushal et al., 2011; Kumar et al., 2012b).
However, the ability of proline to protect from HS may vary according to the developmental phases in which the stress is imposed. For instance, in arabidopsis seedlings ectopically expressing the d(1)-pyrroline-5-carboxylate synthetase 1 gene under the control of a heat shock promoter, proline accumulation under HS decreases the thermo-tolerance. The inhibition of thermo-tolerance in these conditions seems to be due to an increased ROS production via the inhibition of ABA and ethylene biosynthesis (Lv et al., 2011).
Polyamines are also metabolites involved in plant responses during environmental stresses. They seem to act as ROS scavengers and promoters of antioxidant defences (Gill and Tuteja, 2010). Spermidine treatment increases the viability of tobacco cells under severe oxidative stress conditions and during lethal HS (Marsoni et al., 2010; Vannini et al., 2012). Putresceine application to Triticum aestivum plants exposed to HS reduces lipid peroxidation by increasing the redox shield in grains, roots and shoots (Asthir et al., 2012).
p-Hydroxybenzoic acid has also been reported to induce thermo-tolerance in cucumber plants by increasing redox-dependent defences (Zhang et al., 2012).
PERSPECTIVES AND CONCLUSIONS
An increase in average temperatures and further heatwaves and other extreme events will have a strong impact on food production in the future. A better understanding of the eco-physiological and molecular mechanisms that enable plants to overcome these climate changes is thus fundamental in order to minimize the negative impact on plant yield. The use of model systems, such as cultured cells or arabidopsis plants, and crops has led to the identification of redox systems among the metabolic pathways activated in response to HS (Fig. 1) and more generally to climate change.
However, it is clear that in each species and, within species, different genotypes react to HS by activating the specific redox defences in different ways, thus showing a diverse ability to react to temperature increases. We thus need to improve our knowledge of the capacity of specific crops to activate redox defences in order to overcome climate change, by performing experimental studies in which the putative changes are simulated as realistically as possible. Further studies are also required in order to identify exogenous treatments that are able to alleviate the oxidative damage due to HS in specific species or genotypes.
The complexity of these studies has increased due to the fact that in a naturally changing environment more parameters have to be taken into consideration. For example, heatwaves can be accompanied by either humid or dry conditions, thus with substantially different impacts on plant physiology. Climatic variability also strongly depends on regional differences, and in different regions different plants are more suitable for cultivation.
A better evaluation of agronomic biodiversity is also key in enabling researchers to identify new strategies to overcome climate changes and in assisting decision-makers to plan for an uncertain future with new choices and options.
LITERATURE CITED
- Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP. 2006. Protective role of antioxidant enzymes under high temperature stress. Plant Science 171: 382–388. [DOI] [PubMed] [Google Scholar]
- An CF, Mou ZL. 2011. Salicylic acid and its function in plant immunity. Journal of Integrative Plant Biology 53: 412–428. [DOI] [PubMed] [Google Scholar]
- Asada K. 1999. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology 50: 601–639. [DOI] [PubMed] [Google Scholar]
- Asada K. 2006. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology 141: 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthir B, Koundal A, Bains NS. 2012. Putrescine modulates antioxidant defense response in wheat under high temperature stress. Biologia Plantarum 56: 757–761. [Google Scholar]
- Bartoli CG, Pastori GM, Foyer CH. 2000. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiology 123: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bita CE, Gerats T. 2013. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Frontiers in Plant Science 4: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae HB, Moon JC, Shin MR, et al. 2013. Thioredoxin reductase type C (NTRC) orchestrates enhanced thermotolerance to Arabidopsis by its redox-dependent holdase chaperone function. Molecular Plant 6: 323–36. [DOI] [PubMed] [Google Scholar]
- Chakraborty U, Pradhan D. 2011. High temperature-induced oxidative stress in Lens culinaris, role of antioxidants and amelioration of stress by chemical pre-treatments. Journal of Plant Interactions 6: 43–52. [Google Scholar]
- Che P, Bussell JD, Zhou WX, Estavillo GM, Pogson BJ, Smith SM. 2010. Signaling from the endoplasmic reticulum activates brassinosteroid signaling and promotes acclimation to stress in Arabidopsis. Science Signaling 3: ra69. [DOI] [PubMed] [Google Scholar]
- Chen C, Letni I, Hacham Y, et al. 2014. ASCORBATE PEROXIDASE6 protects Arabidopsis desiccating and germinating seeds from stress and mediates cross talk between reactive oxygen species, abscisic acid, and auxin. Plant Physiology 166: 370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Liu JZ, Liu X, et al. 2011. Arabidopsis monothiol glutaredoxin, AtGRXS17, is critical for temperature-dependent postembryonic growth and development via modulating auxin response. Journal of Biological Chemistry 286: 20398–20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL. 2000. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 154: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME. 2002. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiology 129: 1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash S, Mohanty N. 2002. Response of seedlings to heat-stress in cultivars of wheat: growth temperature-dependent differential modulation of photosystem 1 and 2 activity, and foliar antioxidant defense capacity. Journal of Plant Physiology 159: 49–59. [Google Scholar]
- De Gara L, Locato V, Dipierro S, de Pinto MC. 2010. Redox homeostasis in plants. The challenge of living with endogenous oxygen production. Respiratory Physiology and Neurobiology 173: S13–S19. [DOI] [PubMed] [Google Scholar]
- Ding HD, Zhang HJ, Zhu XH, Liu H, Liang JS, Lu B, 2012. Involvement of calcium and calmodulin signaling in adaptation to heat stress-induced oxidative stress in Solanum lycopersicum L. leaves. African Journal of Biotechnology 11: 3259–3269. [Google Scholar]
- Ding W, Song L, Wang X, Bi Y. 2010. Effect of abscisic acid on heat stress tolerance in the calli from two ecotypes of Phragmites communis. Biologia Plantarum 54: 607–613. [Google Scholar]
- Djanaguiraman M, Prasad PVV, Seppanen M. 2010. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiology and Biochemistry 48: 999–1007. [DOI] [PubMed] [Google Scholar]
- Djanaguiraman M, Prasad PVV, Al-Khatib K. 2011. Ethylene perception inhibitor 1-MCP decreases oxidative damage of leaves through enhanced antioxidant defense mechanisms in soybean plants grown under high temperature stress. Environmental and Experimental Botany 71: 215–223. [Google Scholar]
- Du HM, Zhou P, Huang BR. 2013. Antioxidant enzymatic activities and gene expression associated with heat tolerance in a cool-season perennial grass species. Environmental and Experimental Botany 87: 159–166. [Google Scholar]
- Foyer CH, Noctor G. 2003. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiologia Plantarum 119: 355–364. [Google Scholar]
- Foyer CH, Bloom AJ, Queval G, Noctor G. 2009. Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annual Review of Plant Biology 60: 455–484. [DOI] [PubMed] [Google Scholar]
- Frank G, Pressman E, Ophir R, et al. 2009. Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. Journal of Experimental Botany 60: 3891–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Guo YK, Lin SH, Fang YY, Bai JG. 2010. Hydrogen peroxide pretreatment alters the activity of antioxidant enzymes and protects chloroplast ultrastructure in heat-stressed cucumber leaves. Scientia Horticulturae 126: 20–26. [Google Scholar]
- Gill SS, Tuteja N. 2010. Polyamines and abiotic stress tolerance in plants. Plant Signaling and Behavior 5: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichko VP, Glick BR. 2001. Ethylene and flooding stress in plants. Plant Physiology and Biochemistry 39: 1–9. [Google Scholar]
- Hameed A, Goher M, Iqbal N. 2012. Heat stress-induced cell death, changes in antioxidants, lipid peroxidation, and protease activity in wheat leaves. Journal of Plant Growth Regulation 31: 283–291. [Google Scholar]
- He YL, Huang BR. 2010. Differential responses to heat stress in activities and isozymes of four antioxidant enzymes for two cultivars of Kentucky bluegrass contrasting in heat tolerance. Journal of the American Society for Horticultural Science 135: 116–124. [Google Scholar]
- Heine GF, Hernandez JM, Grotewold E. 2004. Two cysteines in plant R2R3 MYB domains participate in REDOX-dependent DNA binding. Journal of Biological Chemistry 279: 37878–37885. [DOI] [PubMed] [Google Scholar]
- IPCC., 2014. Climate change 2014: impacts, adaptation and vulnerability. Working group II contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- Kaushal N, Gupta K, Bhandhari K, Kumar S, Thakur P, Nayyar H. 2011. Proline induces heat tolerance in chickpea (Cicer arietinum L.) plants by protecting vital enzymes of carbon and antioxidative metabolism. Physiology and Molecular Biology of Plants 17: 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Alam I, Lee KW, et al. 2010. Enhanced tolerance of transgenic tall fescue plants overexpressing 2-Cys peroxiredoxin against methyl viologen and heat stresses. Biotechnology Letters 32: 571–576. [DOI] [PubMed] [Google Scholar]
- Konigshofer H, Tromballa HW, Loppert HG. 2008. Early events in signalling high-temperature stress in tobacco BY2 cells involve alterations in membrane fluidity and enhanced hydrogen peroxide production. Plant, Cell and Environment 31: 1771–1780. [DOI] [PubMed] [Google Scholar]
- Koussevitzky S, Suzuki N, Huntington S, et al. 2008. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. Journal of Biological Chemistry 283: 34197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kaur R, Kaur N, et al. 2011a. Heat-stress induced inhibition in growth and chlorosis in mungbean (Phaseolus aureus Roxb.) is partly mitigated by ascorbic acid application and is related to reduction in oxidative stress. Acta Physiologiae Plantarum 33: 2091–2101. [Google Scholar]
- Kumar S, Jiang SL, Jami SK, Hill RD. 2011b. Cloning and characterization of barley caryopsis FCA. Physiologia Plantarum 143: 93–106. [DOI] [PubMed] [Google Scholar]
- Kumar S, Gupta D, Nayyar H. 2012a. Comparative response of maize and rice genotypes to heat stress: status of oxidative stress and antioxidants. Acta Physiologiae Plantarum 34: 75–86. [Google Scholar]
- Kumar S, Kaushal N, Nayyar H, Gaur P. 2012b. Abscisic acid induces heat tolerance in chickpea (Cicer arietinum L.) seedlings by facilitated accumulation of osmoprotectants. Acta Physiologiae Plantarum 34: 1651–1658. [Google Scholar]
- Larkindale J, Huang B. 2004. Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. Journal of Plant Physiology 161: 405–413. [DOI] [PubMed] [Google Scholar]
- Larkindale J, Knight MR. 2002. Protection against heat stress-induced oxidative damage in arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiology 128: 682–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Hall JD, Knight MR, Vierling E. 2005. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiology 138: 882–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee HJ, Jung JH, Park CM. 2015. The Arabidopsis thaliana RNA-binding protein FCA regulates thermotolerance by modulating the detoxification of reactive oxygen species. New Phytologist 205: 555–569. [DOI] [PubMed] [Google Scholar]
- Li WM, Wei ZW, Qiao ZH, Wu ZN, Cheng LX, Wang YY. 2013. Proteomics analysis of alfalfa response to heat stress. Plos One 8:e82725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GT, Wang JF, Cramer G, et al. 2012. Transcriptomic analysis of grape (Vitis vinifera L.) leaves during and after recovery from heat stress. BMC Plant Biology 12: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Huang BR. 2000. Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Science 40: 503–510. [Google Scholar]
- Lobell DB, Schlenker W, Costa-Roberts J. 2011. Climate trends and global crop production since 1980. Science 333: 616–620. [DOI] [PubMed] [Google Scholar]
- Locato V, Gadaleta C, De Gara L, de Pinto MC. 2008. Production of reactive species and modulation of antioxidant network in response to heat shock: a critical balance for cell fate. Plant, Cell and Environment 31: 1606–1619. [DOI] [PubMed] [Google Scholar]
- Locato V, de Pinto MC, De Gara L. 2009. Different involvement of the mitochondrial, plastidial and cytosolic ascorbate–glutathione redox enzymes in heat shock responses. Physiologia Plantarum 135: 296–306. [DOI] [PubMed] [Google Scholar]
- Lv WT, Lin B, Zhang M, Hua XJ. 2011. Proline accumulation is inhibitory to arabidopsis seedlings during heat stress. Plant Physiology 156: 1921–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YH, Ma FW, Zhang JK, Li MJ, Wang YH, Liang D. 2008. Effects of high temperature on activities and gene expression of enzymes involved in ascorbate–glutathione cycle in apple leaves. Plant Science 175: 761–766. [Google Scholar]
- Marsoni M, Cantara C, de Pinto MC, et al. 2010. Exploring the soluble proteome of Tobacco Bright Yellow-2 cells at the switch towards different cell fates in response to heat shocks. Plant, Cell and Environment 33: 1161–75. [DOI] [PubMed] [Google Scholar]
- Meehl GA, Tebaldi C. 2004. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305: 994–997. [DOI] [PubMed] [Google Scholar]
- Millar AH, Mittova V, Kiddle G, et al. 2003. Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiology 133: 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Mittler R. 2006. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Annals of Botany 98: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, et al. 2009. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Science Signaling 2: ra45. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, et al. 2011. ROS signaling: the new wave? Trends in Plant Science 16: 300–309. [DOI] [PubMed] [Google Scholar]
- Mittler R, Finka A, Goloubinoff P. 2012. How do plants feel the heat? Trends in Biochemical Sciences 37: 118–125. [DOI] [PubMed] [Google Scholar]
- Moon JC, Jang HH, Chae HB, et al. 2006. The C-type Arabidopsis thioredoxin reductase ANTR-C acts as an electron donor to 2-Cys peroxiredoxins in chloroplasts. Biochemical and Biophysical Research Communications 348: 478–484. [DOI] [PubMed] [Google Scholar]
- Nishizawa A, Yabuta Y, Yoshida E, Maruta T, Yoshimura K, Shigeoka S. 2006. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. The Plant Journal 48: 535–547. [DOI] [PubMed] [Google Scholar]
- Panchuk II, Volkov RA, Schoffl F. 2002. Heat stress- and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis. Plant Physiology 129: 838–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchuk II, Zentgraf U, Volkov RA. 2005. Expression of the Apx gene family during leaf senescence of Arabidopsis thaliana. Planta 222: 926–932. [DOI] [PubMed] [Google Scholar]
- Perez-Ruiz JM, Spinola MC, Kirchsteiger K, Moreno J, Sahrawy M, Cejudo FJ. 2006. Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. The Plant Cell 18: 2356–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pinto MC, Paradiso A, Leonetti P, De Gara L. 2006. Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defence and cell death. The Plant Journal 48: 784–795. [DOI] [PubMed] [Google Scholar]
- de Pinto MC, Locato V, Sgobba A, et al. 2013. S-Nitrosylation of ascorbate peroxidase is part of programmed cell death signaling in tobacco Bright Yellow-2 cells. Plant Physiology 163: 1766–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin D, Wu H, Peng H, et al. 2008. Heat stress-responsive transcriptome analysis in heat susceptible and tolerant wheat (Triticum aestivum L.) by using wheat genome array. BMC Genomics 9: 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed R, Wahid A, Farooq M, Hussain I, Basra SMA. 2011. Role of proline and glycinebetaine pretreatments in improving heat tolerance of sprouting sugarcane (Saccharum sp.) buds. Plant Growth Regulation 65: 35–45. [Google Scholar]
- Rhee SG, Chae HZ, Kim K. 2005. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radical Biology and Medicine 38: 1543–1552. [DOI] [PubMed] [Google Scholar]
- Rhoads DM, Umbach AL, Subbaiah CC, Siedow JN. 2006. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiology 141: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera M, Valon C, Fenzi F, Giraudat J, Leung J. 2005. The genetics of adaptive responses to drought stress: abscisic acid-dependent and abscisic acid-independent signalling components. Physiologia Plantarum 123: 111–119. [Google Scholar]
- Rikhvanov EG, Gamburg KZ, Varakina NN, et al. 2007. Nuclear–mitochondrial cross-talk during heat shock in Arabidopsis cell culture. The Plant Journal 52: 763–778. [DOI] [PubMed] [Google Scholar]
- Rouhier N, Couturier J, Jacquot JP. 2006. Genome-wide analysis of plant glutaredoxin systems. Journal of Experimental Botany 57: 1685–1696. [DOI] [PubMed] [Google Scholar]
- Schoffl F, Prandl R, Reindl A. 1998. Regulation of the heat-shock response. Plant Physiology 117: 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrato AJ, Perez-Ruiz JM, Spinola MC, Cejudo FJ. 2004. A novel NADPH thioredoxin reductase, localized in the chloroplast, which deficiency causes hypersensitivity to abiotic stress in Arabidopsis thaliana. Journal of Biological Chemistry 279: 43821–43827. [DOI] [PubMed] [Google Scholar]
- Sgobba A, Paradiso A, Dipierro S, De Gara L, de Pinto MC. 2015. Changes in antioxidants are critical in determining cell responses to short- and long-term heat stress. Physiologia Plantarum 153: 68–78. [DOI] [PubMed] [Google Scholar]
- Shaikhali J, Heiber I, Seidel T, et al. , 2008. The redox-sensitive transcription factor Rap2.4a controls nuclear expression of 2-Cys peroxiredoxin A and other chloroplast antioxidant enzymes. BMC Plant Biology 8: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikhali J, Noren L, Barajas-Lopez JD, et al. 2012. Redox-mediated mechanisms regulate DNA binding activity of the G-group of basic region leucine zipper (bZIP) transcription factors in Arabidopsis. Journal of Biological Chemistry 287: 27510–27525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WM, Muramoto Y, Ueda A, Takabe T. 2001. Cloning of peroxisomal ascorbate peroxidase gene from barley and enhanced thermotolerance by overexpressing in Arabidopsis thaliana. Gene 273: 23–27. [DOI] [PubMed] [Google Scholar]
- Snider JL, Oosterhuis DM, Kawakami EM. 2010. Genotypic differences in thermotolerance are dependent upon prestress capacity for antioxidant protection of the photosynthetic apparatus in Gossypium hirsutum. Physiologia Plantarum 138: 268–277. [DOI] [PubMed] [Google Scholar]
- Storozhenko S, De Pauw P, Van Montagu M, Inzé D, Kushnir S. 1998. The heat-shock element is a functional component of the Arabidopsis APX1 gene promoter. Plant Physiology 118: 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio A, Dreos R, Aparicio F, Maule AJ. 2009. The cytosolic protein response as a subcomponent of the wider heat shock response in Arabidopsis. The Plant Cell 21: 642–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram S, Rathinasabapathi B. 2010. Transgenic expression of fern Pteris vittata glutaredoxin PvGrx5 in Arabidopsis thaliana increases plant tolerance to high temperature stress and reduces oxidative damage to proteins. Planta 231: 361–369. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Mittler R. 2006. Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiologia Plantarum 126: 45–51. [Google Scholar]
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. 2011. Respiratory burst oxidases: the engines of ROS signaling. Current Opinion in Plant Biology 14: 691–699. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Salazar C, et al. 2013a. Temporal–spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. The Plant Cell 25: 3553–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Sejima H, Harper J, Mittler R. 2013b. Enhanced seed production under prolonged heat stress conditions in Arabidopsis thaliana plants deficient in cytosolic ascorbate peroxidase 2. Journal of Experimental Botany 64: 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tron AE, Bertoncini CW, Chan RL, Gonzalez DH. 2002. Redox regulation of plant homeodomain transcription factors. Journal of Biological Chemistry 277: 34800–34807. [DOI] [PubMed] [Google Scholar]
- Vacca RA, de Pinto MC, Valenti D, Passarella S, Marra E, De Gara L. 2004. Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco Bright-Yellow 2 cells. Plant Physiology 134: 1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti D, Vacca RA, de Pinto MC, De Gara L, Marra E, Passarella S. 2007. In the early phase of programmed cell death in Tobacco Bright Yellow 2 cells the mitochondrial adenine nucleotide translocator, adenylate kinase and nucleoside diphosphate kinase are impaired in a reactive oxygen species-dependent manner. Biochimica et Biophysica Acta 1767: 66–78. [DOI] [PubMed] [Google Scholar]
- Van Breusegem F, Dat JF. 2006. Reactive oxygen species in plant cell death. Plant Physiology 141: 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderauwera S, Suzuki N, Miller G, et al. 2011. Extranuclear protection of chromosomal DNA from oxidative stress. Proceedings of the National Academy of Sciences, USA 108: 1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini C, Marsoni M, Cantara C, et al. 2012. The soluble proteome of tobacco Bright Yellow-2 cells undergoing H2O2-induced programmed cell death. Journal of Experimental Botany 63: 3137–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov RA, Panchuk II, Mullineaux PM, Schoffl F. 2006. Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Molecular Biology 61: 733–746. [DOI] [PubMed] [Google Scholar]
- Wang L, Guo YJ, Jia LX, et al. 2014. Hydrogen peroxide acts upstream of nitric oxide in the heat shock pathway in Arabidopsis seedlings. Plant Physiology 164: 2184–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LJ, Li SH. 2006. Salicylic acid-induced heat or cold tolerance in relation to Ca 2+ homeostasis and antioxidant systems in young grape plants. Plant Science 170: 685–694. [Google Scholar]
- Wang X, Cai JA, Jiang D, Liu FL, Dai TB, Cao WX. 2011. Pre-anthesis high-temperature acclimation alleviates damage to the flag leaf caused by post-anthesis heat stress in wheat. Journal of Plant Physiology 168: 585–593. [DOI] [PubMed] [Google Scholar]
- Wang X, Cai J, Liu FL, et al. 2012. Pre-anthesis high temperature acclimation alleviates the negative effects of post-anthesis heat stress on stem stored carbohydrates remobilization and grain starch accumulation in wheat. Journal of Cereal Science 55: 331–336. [Google Scholar]
- Wang X, Cai J, Liu FL, et al. 2014. Multiple heat priming enhances thermo-tolerance to a later high temperature stress via improving subcellular antioxidant activities in wheat seedlings. Plant Physiology and Biochemistry 74: 185–192. [DOI] [PubMed] [Google Scholar]
- Wang ZL, Pote J, Huang BR. 2003. Responses of cytokinins, antioxidant enzymes, and lipid peroxidation in shoots of creeping bentgrass to high root-zone temperatures. Journal of the American Society for Horticultural Science 128: 648–655. [Google Scholar]
- Weston DJ, Karve AA, Gunter LE, et al. 2011. Comparative physiology and transcriptional networks underlying the heat shock response in Populus trichocarpa, Arabidopsis thaliana and Glycine max. Plant, Cell and Environment 34: 1488–1506. [DOI] [PubMed] [Google Scholar]
- Wu QY, Lin J, Liu JZ, et al. 2012. Ectopic expression of Arabidopsis glutaredoxin AtGRXS17 enhances thermotolerance in tomato. Plant Biotechnology Journal 10: 945–955. [DOI] [PubMed] [Google Scholar]
- Xu S, Li JL, Zhang XQ, Wei H, Cui LJ. 2006. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environmental and Experimental Botany 56: 274–285. [Google Scholar]
- Yamamoto Y, Aminaka R, Yoshioka M, et al. 2008. Quality control of photosystem II: impact of light and heat stresses. Photosynthesis Research 98: 589–608. [DOI] [PubMed] [Google Scholar]
- Yang W, Sun Y, Chen S, et al. , 2011. The effect of exogenously applied nitric oxide on photosynthesis and antioxidant activity in heat stressed chrysanthemum. Biologia Plantarum 55: 737–740. [Google Scholar]
- Yin H, Chen QM, Yi MF. 2008. Effects of short-term heat stress on oxidative damage and responses of antioxidant system in Lilium longiflorum. Plant Growth Regulation 54: 45–54. [Google Scholar]
- Young LW, Wilen RW, Bonham-Smith PC. 2004. High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility, induces fruit abortion, and disrupts seed production. Journal of Experimental Botany 55: 485–495. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li DM, Sun WJ, Wang XJ, Bai JG. 2012. Exogenous p-hydroxybenzoic acid regulates antioxidant enzyme activity and mitigates heat stress of cucumber leaves. Scientia Horticulturae 148: 235–245. [Google Scholar]
- Zhao XX, Huang LK, Zhang XQ, Li Z, Peng Y. 2014. Effects of heat acclimation on photosynthesis, antioxidant enzyme activities, and gene expression in orchardgrass under heat stress. Molecules 19: 13564–13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Zhou T, Li MX, et al. , 2012. The Arabidopsis J-protein AtDjB1 facilitates thermotolerance by protecting cells against heat-induced oxidative damage. New Phytologist 194: 364–78. [DOI] [PubMed] [Google Scholar]
- Zinta G, AbdElgawad H, Domagalska MA, et al. 2014. Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Global Change Biology 20: 3670–3685. [DOI] [PubMed] [Google Scholar]