Abstract
Background Reactive oxygen species (ROS) are considered to be detrimental to seed viability. However, recent studies have demonstrated that ROS have key roles in seed germination particularly in the release of seed dormancy and embryogenesis, as well as in protection from pathogens.
Scope This review considers the functions of ROS in seed physiology. ROS are present in all cells and at all phases of the seed life cycle. ROS accumulation is important in breaking seed dormancy, and stimulating seed germination and protection from pathogens. However, excessive ROS accumulation can be detrimental. Therefore, knowledge of the mechanisms by which ROS influence seed physiology will provide insights that may not only allow the development of seed quality markers but also help us understand how dormancy can be broken in several recalcitrant species.
Conclusions Reactive oxygen species have a dual role in seed physiology. Understanding the relative importance of beneficial and detrimental effects of ROS provides great scope for the improvement and maintenance of seed vigour and quality, factors that may ultimately increase crop yields.
Keywords: Reactive oxygen species (ROS), seed physiology, germination, seed dormancy, signalling, embryogenesis, programmed cell death (PCD)
INTRODUCTION
Reactive oxygen species (ROS) are natural products of metabolism. They originate from the incomplete or partial reduction of oxygen, which leads to the formation of superoxide (), hydrogen peroxide (H2O2) and ultimately the hydroxyl radical (HO·). In contrast, singlet oxygen (1O2) is produced by direct energy transfer from triplet chlorophyll to oxygen (Apel and Hurt, 2004; Ray et al., 2012; Diaz-Vivancos et al., 2013). It has been assumed for a long time that ROS accumulation is detrimental to seed viability because of oxidative stress imposed during seed desiccation or seed ageing (Bailly, 2004). However, recent studies on seed physiology have shown that ROS also fulfil crucial roles in cell signalling that underpins seed germination, and the breaking of seed dormancy, as well as in protection against pathogens. ROS play important roles at all stages of seed life, from germination (embryogenesis) to cell death [programmed cell death (PCD)]. Evidence from proteomics and transcriptomic studies has disproved the concept that ROS are detrimental to the seed. The dual function of ROS in facilitating cell growth and development at low concentrations while triggering cell death at higher concentrations is of key importance in seed physiology (Moller et al., 2007). Reviews that have discussed the dual role of ROS in seed physiology are listed in Table 1.
Table 1.
Published reviews on the dual role of ROS in seed physiology
| ROS molecule | Physiological trait | Reference |
|---|---|---|
| Hydrogen peroxide (H2O2) | Alleviation of seed dormancy | Wang et al. (1995) |
| Hydrogen peroxide (H2O2) | Somatic embryogenesis | Cui et al. (1999) |
| Superoxide () | Plant defence response | Wisnieewski et al. (1999) |
| Superoxide () | May play roles in survival and germination of recalcitrant seeds | Roach et al. (2010) |
| Hydrogen peroxide (H2O2) | Response to wounding | Oroczo-Cardenas et al. (2001) |
| Hydrogen peroxide (H2O2) | Promoted seed germination by reducing endogenous ABA levels | Barba-Espin et al. (2010) |
| Hydroxyl radical (OH·) | Breakdown of polysaccharides | Fry (1998); Schweikert et al. (2002) |
| Superoxide (), hydroxyl radical (OH·) | Cell growth by auxin | Schopfer and Liszkay (2002) |
| Hydroxyl radical (OH·) | Cell wall loosening during seed germination and seedling growth | Müller et al. (2009) |
| Hydrogen peroxide (H2O2) | Lateral root formation | Chen et al. (2013) |
| Hydrogen peroxide (H2O2) | Promoted seed germination by resurgence of reducing power and the pentose phosphate pathway | Barba-Espin et al. (2011) |
| Hydrogen peroxide (H2O2) | Programmed cell death | de Jong et al. (2002) |
| Superoxide () | Cell death | Doke et al. (1994) |
Seed stages
Seeds are classified into orthodox and recalcitrant types based on their ability to germinate following storage under dry conditions (Roberts, 1973). In orthodox seeds, major changes in moisture content and metabolism occur during the seed life cycle. For example, during embryogenesis, high metabolic activity precedes cell division. While an ample supply of water is indispensable for the translocation of metabolites to the seed during filling, extensive dehydration occurs during maturation and shedding. The low seed moisture content is associated with adaptive mechanisms that are required for seed survival. Orthodox seeds germinate upon subsequent rehydration (El-Maarouf-Bouteau and Bailly, 2008). The desiccative and hydrated stages of seed development require that ROS production and accumulation are tightly controlled to ensure seed survival. ROS act as signalling molecules that break dormancy and facilitate seed germination. Moreover, they act as important signals that trigger cellular defences against biotic and abiotic stresses. At higher concentrations they can trigger molecular genetic programs leading to cell death. Thus the regulation of ROS is indispensable for seed survival (Moradshaban et al., 2013).
ROS production and signalling pathways in seeds
The production and accumulation of ROS depends on the metabolic and physiological state of the seed. In the hydrated state (that occurs following imbibition), mitochondria, glyoxysomes and plasma membrane NADPH oxidases act as major sources for ROS, whereas in dry seeds ROS production may result from Amadori and/or Maillard reactions and associated lipid peroxidation mechanisms (Zhang et al., 1995; Lamb and Dixon, 1997; Mc Donald, 1999; Leubner-Metzger, 2005). Unlike ·O2 and H2O2, hydroxyl radicals (·OH) and singlet oxygen (1O2), are highly reactive and cause damage to DNA and proteins (Sharma et al., 2012).
The ROS signalling pathway in seeds is not yet fully understood. An intriguing question concerns how seeds sense the ROS in the hydrated (germination) and desiccated (maturation, shedding) states. During germination, seeds imbibe water, which results in a low cytoplasmic viscosity and free water availability. Due to differences in the reactivity of different ROS, some ROS (·OH) can react only with surrounding molecules but others (H2O2) may move over longer distances in the cell (Moller, 2001). Some ROS forms can activate established signal transduction pathways such as mitogen-activated protein kinase (MAPK) cascades, or inhibit protein phosphatases, and activate Ca2+ channels or Ca2+-binding proteins. This interplay between redox and other signalling pathways may form the basis of coordinated regulation (Apel and Hurt, 2004; Mittler et al., 2004; Brock et al., 2010; Xu et al., 2010; Barba-Espin et al., 2011; Diaz-Vivancos et al., 2013). In contrast, in the dry state, the cytoplasmic viscosity is high and the absence of free water availability may hinder the mobility of the less reactive ROS forms such as H2O2. Several hypotheses have been proposed to describe ROS mobility and their sensing in desiccated seeds. One hypothesis considers that desiccated or dry seeds have restricted hydrated zones, which allow a limited mobility of H2O2, which can participate in the cell signalling in hydrated areas. Another hypothesis suggests that H2O2 can accumulate during the dry stage and then, upon imbibition, this pool acts to signal changes in gene expression (El-Maarouf-Bouteau and Bailly, 2008).
Several reviews to date have concerned ROS production, signalling and physiological functions in abiotic stress responses (Gill and Tuteja, 2010) during seed germination and dormancy (Bailly, 2004; El-Maarouf-Bouteau and Bailly et al., 2008; Diaz-Vivancos et al., 2013). However, few comprehensive reviews have considered the dual roles of ROS in seed physiology because of the perception of ROS only in cytotoxic roles. Hence, the aim of this review is to consider the dual roles of ROS in seed physiology: (1) as positive signalling molecules in seed germination, the alleviation of seed dormancy and in pathogen-induced protection; and (2) as cytotoxic or detrimental molecules.
SIGNALLING ROLE OF ROS IN SEED PHYSIOLOGY
Seed germination
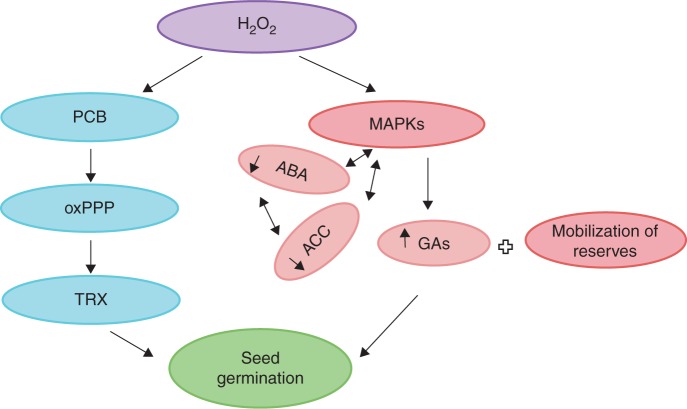
The process of seedling emergence from the seed embryonic axis is known as germination (Bewley and Black, 1994). Seed germination is a complex system regulated by extrinsic and intrinsic factors. Several reports have described ROS involvement in the early imbibition period, together with their signalling roles during seed germination. For example, H2O2, ·O2 and·OH are involved in seed germination (Puntarulo et al., 1991; Caro and Puntarulo, 1999; Schopfer et al., 2001). The probable site of ROS production might be either the mitochondria or the NADPH oxidases of the plasma membrane. It is proposed that the inhibition of NADPH oxidase leads to delayed germination, which indicates a putative function for the NADPH oxidases (Sarath et al., 2007). It is suggested that after imbibition, the resurgence of mitochondrial respiration in the seed might result in electron donation to oxygen as an electron acceptor, leading to ROS production (El-Maarouf-Bouteau and Bailly, 2008). Accumulation of H2O2 may increase the rates of protein carbonylation and protein turnover, as well as decreasing the electron pressure in the mitochondrial electron transport chain, allowing the provision of reducing equivalents (NADPH) to the thioredoxin (Trx) system (via the pentose phosphate pathway), which is involved in the regulation of seed germination and seedling development (Lozano et al., 1996; Job et al., 2005; Oracz et al., 2007; Barba-Espin et al., 2011). In addition, H2O2 accumulation may influence the hormone balance by increasing gibberellins (GAs) and decreasing abscisic acid (ABA) and ethylene via 1-aminocyclopropane-1-carboxylic acid (ACC). This re-modelling of hormone signalling may lead to the recommencement of metabolic activity that is essential for seed germination and seedling emergence (Barba-Espin et al., 2010, 2011; Bahin et al., 2011; Diaz-Vivancos et al., 2013). A schematic model of the signalling role of H2O2 during seed germination is provided in Fig. 1.
Fig. 1.
Signalling role of H2O2 in the seed germination process. During the process of germination, H2O2 triggers seed storage protein carbonylation (PCB) that induces the oxidative pentose phosphate pathway (oxPPP) which supplements NADPH to thioredoxin (TRX) for seed germination. Furthermore, it stimulates an increase in GAs and a decrease of abscisic acid (ABA) and 1-aminocyclopropane-1-carboxylic acid (ACC) through mitogen-activated protein kinase (MAPK) (Lozano et al., 1996; Job et al., 2005; Oracz et al., 2007; Barba-Espin et al., 2010, 2011; Bahin et al., 2011; Diaz-Vivancos et al., 2013). Upon mobilization of reserves, seed germination takes place with resurgence of metabolism. For dormancy alleviation, the ABA content should be high to maintain the dormant state after fruit ripening and, in the non-dormant state, GAs could be high, leading to germination (Liu et al., 2010).
Endosperm weakening
In some seed species (arabidopsis, tobacco), seed germination is hindered by the micropylar endosperm. In order to germinate, the mechanical barrier imposed by the endosperm has to be weakened. Plant growth regulators such as GA3 and ABA regulate hydrolases that in turn regulate endosperm loosening. Several reports have shown that ROS are involved in endosperm loosening (Müller et al., 2009; Barba-Espin et al., 2011). It is suggested that ·OH is generated from ·O2 produced by NADPH oxidases, and H2O2 in Fenton reactions in the cell walls of growing organs. Since cell walls have little or no antioxidant buffering capacity, ·OH produced by Fenton chemistry can break polysaccharides by oxidative scission, resulting in weakening of the endosperm. In addition, it is probable that the physiological role of auxin in promoting cell division and growth) is highly dependent on its ability to promote the generation of ·O2 radicals (Kawano, 2003).
Seed protection from pathogens
Several studies have demonstrated that ROS play key signalling roles in abiotic and biotic stresses (Kotchoni et al., 2006; Gomes and Garcia, 2013). During their development and lifespan, seeds may be exposed to biotic stress. ROS accumulation would safeguard seeds from pathogen attack. It is well known that ROS may directly affect pathogens or act indirectly by prompting the hypersensitive reaction leading to PCD (Grant and Loake, 2000). During infection, ·O2 produced by the activation of plasma membrane NADPH oxidase is converted to H2O2 either by spontaneous dismutation or by superoxide dismutase (SOD) activity (Dietz et al., 2010). The H2O2 produced by this process kills the local infected cells and thus inhibits pathogen entry. In addition, it has a substantial role in resisting the cell infection, implicating a substrate role in cell wall peroxidation (Levine et al., 1994). Moreover, it has been demonstrated that supply of ROS from germinated seeds has an inhibitory role against pathogens (Murphy et al., 1998). In order to validate the hypothesis of pathogen-induced protection through ROS, much more research is needed.
Alleviation of seed dormancy
Seed dormancy is a phenomenon which delays germination even under favourable conditions. Seed dormancy can be imposed either by the seed coat or by any surrounding structures, whereas in embryo dormancy the embryo is inherent and devoid of any influence of the surrounding structures. Seed dormancy is regulated mostly by ABA and GAs, and the alleviation of dormancy results from the alteration of the balance of their ratio. For example, it is suggested that a high ABA/GA ratio culminates in dormancy maintenance whereas a low ABA/GA ratio leads to dormancy release. In other words, GAs favour dormancy release but ABA maintains seed dormancy. In a recent study, where H2O2 was added exogenously, it was observed that the ABA levels were decreased with a subsequent increase of GAs, that triggers the release of seed dormancy (Liu et al., 2010). Recent studies further substantiate that the cross-talk of ROS with GAs, ethylene and ABA demonstrates the pivotal role of ROS in alleviation of seed dormancy (Finkelstein et al., 2008). For instance, it has been demonstrated that the interaction of ROS and ABA or GAs alleviated seed dormancy of barley (Bahin et al., 2011). The probable mechanism of ROS in this process might involve oxidation of reserve proteins during after-ripening, showing that seed dormancy accompanies protein mobilization. To corroborate this hypothesis, dormant sunflower seeds were imbibed and treated with hydrogen cyanide (HCN) which is a dormancy-ameliorating compound. Interestingly, H2O2 was produced in response to cyanide, which triggered the ethylene pathway and protein carbonylation (Oracz et al., 2009). In contrast, a ROS-generating compound, such as methylviologen, activated protein carbonylation with concomitant seed dormancy alleviation (Bailey et al., 2008). These studies corroborate the putative role of ROS and protein oxidation in seed dormancy alleviation which needs further validation with other dormant seed species.
SEED DETERIORATION
Possible routes of seed deterioration during the ageing process
To obtain a higher yield and productivity, viable seed is indispensable. Conventionally, stored seed has been used to produce crops, but improper storage leads to poor germination and loss of seed viability. Seed longevity is a paramount trait playing a significant role in conserving the germplasm (ex situ). Seed ageing, occurring either by natural or accelerated ageing (which can be achieved by increasing the relative humidity and moisture content), has been studied by several researchers. Studies revealed that the ageing process resulted in deterioration of seed viability mainly due to the production and progressive accumulation of ROS. In order to maintain seed viability, regulation of ROS by enzymatic and non-enzymatic (antioxidant activities) and DNA repair mechanisms should be at an appropriate level in the embryo (Dona et al., 2013).
Generally, it is acknowledged that seeds stored for a longer duration are prone to oxidative damage due to the cumulative increase of ROS. ROS act as a messenger at lower concentrations, and a threshold increase will damage the polyunsaturated fatty acids of phospholipids, DNA and proteins. Subsequently, loss of seed viability and reduced seed germination are the repercussions of ageing (Kranner and Colville, 2010). Hence, an important observation is that under stress conditions ROS were generated in the cell, but at the same time the cellular machinery combats the ROS by enzymatic and non-enzymatic mechanisms (antioxidants) to circumvent the problem. Therefore, a pre-requisite event for seed deterioration under ageing is generation of ROS coupled with decline of the antioxidant potential of the cell (He and Kermode, 2010; Kibinza et al., 2011). For instance, ROS formed during ageing were scavenged by glutathione S-hydrolase (GSH) which leads to an increase in EGSSG/2GSH. This, in turn, splits Trx (TRX-H;3) from apoptosis signal-regulating kinase 1 (ASK 1). ASK 1 activates a MAPK kinase cascade that results in gene expression changes which could lead to nucleic acid damage and breakdown of structural and nuclear proteins (Chen et al., 2013).
Programmed cell death (PCD) by cytochrome c (apoptogenic proteins)
Another possible mechanism for PCD is through calcium-mediated release of cytochrome c (apoptogenic proteins) from the mitochondria. Under the seed ageing process, calcium levels in the cytosol increase simultaneously with the release of cytochrome c by activation of the permeability transition pore (PTP) through the adenine nucleotide translocator (ANT) and voltage-dependent anion channel (VDAC). The released cytochrome c activates the caspases and downstream proteolytic cascade involved in PCD. Metacaspases of plants share similar structure and sequence with animal caspases but the latter are devoid of caspase-like activity. Cysteine proteases such as RD21 and papain are the main proteases activated in the seed ageing process whose function is to degrade the nuclear and structural proteins (Lam and Pozo, 2000). In addition, the proteasome also possesses caspase-like activity. For example, the proteasome may activate the caspase cascade for the induction of PCD and degrades the misfolded proteins (ER stress) by the ubiquitin–proteasome pathway (Chen et al., 2013).
An important event involved in the seed ageing phenomenon consists of endoplasmic reticulum (ER) stress. Oxidative stress perturbs the protein folding environment in the lumen of the ER, which results in accumulation of misfolded and unfolded proteins. Furthermore, the findings were corroborated by the upregulation of binding protein (BiP) which is a chaperone whose main function is to restore the misfolded and unfolded proteins (Adamakis et al., 2011). Further studies have revealed the involvement of ER stress, PCDh (DNA laddering indicative of PCD) and the ubiquitin–proteasome pathways in the seed ageing process.
On the other hand, plants possess combating enzymatic and non-enzymatic (antioxidants) mechanisms to ameliorate the oxidative damage caused by increasing ROS concentrations. The ROS act as signalling molecules in response to pathogen attack, stomatal opening and PCD (Apel and Hurt, 2004). From recent gene profiling studies, it is reasoned that DNA repair mechanisms are activated at an early stage of seed imbibition (Macovei et al., 2010; Balestrazzi et al., 2012). Hence, the paramount feature is to withstand stress by expressing the ROS scavenging potential, concomitantly increasing seed viability and germination. Therefore, it is generally hypothesized that cells possess antioxidant systems to combat ROS attack, but oxidative injury takes place when ROS production exceeds the cell antioxidant machinery (Liu et al., 2007).
CONCLUSIONS
Seeds are essential for sustainable crop production. It is widely believed that stored seeds are prone to oxidative damage because of ROS accumulation, which results in poor germination and loss of seed viability. Traditionally, farmers particularly in poor countries store seed in order to raise further generations. The accumulation of oxidative damage during storage has long been considered to be a major cause of decreased seed quality. However, a greater understanding of the functions of ROS in the processes that determine seed longevity and seed ageing is required because recent studies have shown that seed deterioration may be caused by pathways other than the accumulation of oxidative damage that lead to PCD. Conversely, the beneficial effects of ROS have generated increasing interest because of their functions in the regulation of the cellular redox state, and their putative roles in the control of seed dormancy, seed germination, and in mediating phytohormone cross-talk, particularly between GAs and ABA. It is clear that the presumption that ‘ROS are detrimental’ has to be revisited and needs further substantiation.
LITERATURE CITED
- Adamakis I-DS, Panteris E, Eleftheriou EP. 2011. The fatal effect of tungsten on Pisum sativum L. root cells: indications for endoplasmic reticulum stress induced programmed cell death. Planta 234: 21–34. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55: 373–399. [DOI] [PubMed] [Google Scholar]
- Bahin E, Bailly C, Sotta B, Kranner I, Corbineau F, Leymarie J. 2011. Crosstalk between reactive oxygen species and hormonal signalling pathway regulates grain dormancy in barley. Plant, Cell and Environment 34: 980–993. [DOI] [PubMed] [Google Scholar]
- Bailly C. 2004. Active oxygen species and antioxidants in seed biology. Seed Science Research 14: 93–107. [Google Scholar]
- Bailly C, El-Maarouf-Bouteau H., Corbineau F. 2008. From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. Comptes Rendus Biologies 331: 806–814. [DOI] [PubMed] [Google Scholar]
- Balestrazzi A, Confalonieri M, Dona‘ M, Carbonera D. 2012. Genotoxic stress, DNA repair, and crop productivity. In: Tuteja N, Gill SS, eds. Crop improvement under adverse conditions. Berlin: Springer-Verlag, 153–169. [Google Scholar]
- Barba-Espin G, Diaz-Vivancos P, Clemente-Moreno MJ, et al. 2010. Interaction between hydrogen peroxide and plant hormones during germination and the early growth of pea seedlings. Plant, Cell and Environment 33: 981–994. [DOI] [PubMed] [Google Scholar]
- Barba-Espín G, Diaz-Vivancos P, Job D, Belghazi M, Job C, Hernandez JA. 2011. Understanding the role of H2O2 during pea seed germination: a combined proteomic and hormone profiling approach. Plant, Cell and Environment 34: 1907–1919. [DOI] [PubMed] [Google Scholar]
- Bewley JD, Black M. 1994. Seeds. Physiology of development and germination, 2nd edn New York: Plenum Press. [Google Scholar]
- Brock AK, Willmann R, Kolb D, et al. 2010. The Arabidopsis mitogen-activated protein kinase phosphatase PP2C5 affects seed germination, stomatal aperture, and abscisic acid-inducible gene expression. Plant Physiology 153: 1098–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro A, Puntarulo S. 1999. Nitric oxide generation by soybean embryonic axes. Possible effect on mitochondrial function. Free Radical Research 31: S205–S212. [DOI] [PubMed] [Google Scholar]
- Chen H, Osuna D, Colville L, Lorenzo O, Graeber K. 2013. Transcriptome-wide mapping of pea seed ageing reveals a pivotal role for genes related to oxidative stress and programmed cell death. PLoS One 8: e78471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui K, Xing G, Liu X, Xing G, Wang Y. 1999. Effect of hydrogen peroxide on somatic embryogenesis of Lycium barbarum L. Plant Science 146: 9–16. [Google Scholar]
- Diaz-Vivancos P, Barba-Espin G, Hernandez JA. 2013. Elucidating hormonal/ROS networks during seed germination: insights and perspectives. Plant Cell Reports 32: 1491–1502. [DOI] [PubMed] [Google Scholar]
- Dietz K, Jacquot J, Harris G. 2010. Hubs and bottlenecks in plant molecular signalling networks. New Phytologist 188: 919–938. [DOI] [PubMed] [Google Scholar]
- Doke N, Miura Y, Sanchez LM, Kawakita K. 1994. Involvement of superoxide in signal transduction: responses to attack by pathogens, physical and chemical shocks and UV irradiation. In: Foyer CH, Mullineaux P, eds. Causes of photooxidative stress and amelioration of defense systems in plants. Boca Raton, FL: CRC Press, 177–218. [Google Scholar]
- Dona M, Balestrazzi A, Mondoni A, et al. 2013. DNA profiling, telomere analysis and antioxidant properties as tools for monitoring ex situ seed longevity. Annals of Botany 111: 987–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Maarouf-Bouteau, Bailly C. 2008. Oxidative signaling in seed germination and dormancy. Plant Signaling and Behaviur 3: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD. 2002. Abscisic acid signaling in seeds and seedlings. The Plant Cell 14: S15–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC. 1998. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochemical Journal 332: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48: 909–930. [DOI] [PubMed] [Google Scholar]
- Gomes MP, Garcia QS. 2013. Reactive oxygen species and seed germination. Biologia 68: 351–357. [Google Scholar]
- Grant JJ, Loake GJ. 2000. Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiology 124: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Kermode AR. 2010. Programmed cell death of the megagametophyte during post-germinative growth of white spruce (Picea glauca) seeds is regulated by reactive oxygen species and the ubiquitin-mediated proteolysis system. Plant and Cell Physiology 51: 1707–1720. [DOI] [PubMed] [Google Scholar]
- Job C, Rajjou L, Lovigny Y, Belghazi M, Job D. 2005. Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiology 138: 790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong AJ, Yakimova ET, Kapchina VM, Woltering EJ. 2002. A critical role for ethylene in hydrogen peroxide release during programmed cell death in tomato suspension cells. Planta 214: 537–545. [DOI] [PubMed] [Google Scholar]
- Kawano T. 2003. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Reports 9: 829–837. [DOI] [PubMed] [Google Scholar]
- Kibinza S, Bazin J, Bailly C, Farrant JM, Corbineau F. 2011. Catalase is a key enzyme in seed recovery from ageing during priming. Plant Science 181: 309–315. [DOI] [PubMed] [Google Scholar]
- Kotchoni S, Kuhns C, Ditzer A, Kirch H, Bartels D. 2006. Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant, Cell and Environment 29: 1033–1048. [DOI] [PubMed] [Google Scholar]
- Kranner I, Colville L. 2010. Metals and seeds: biochemical and molecular implications and their significance for seed germination. Environmental and Experimental Botany 72: 93–105. [Google Scholar]
- Lam E, Pozo O. 2000. Caspase-like protease involvement in the control of plant cell death. Plant Molecular Biology 44: 417–428. [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. 1997. The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology 48: 251–275. [DOI] [PubMed] [Google Scholar]
- Leubner-Metzger G. 2005. Beta-1,3-glucanase gene expression in low-hydrated seeds as a mechanism for dormancy release during tobacco after-ripening. The Plant Journal 41: 133–145. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. 1994. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593. [DOI] [PubMed] [Google Scholar]
- Liu X, Xing D, Li L, Zhang L. 2007. Rapid deterioration of seed vigour based on the level of superoxide generation during early imbibition. Photochemical and Photobiological Sciences 6: 767–774. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ye N, Liu R, Chen M, Zhang J. 2010. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. Journal of Experimental Botany 61: 2979–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano RM, Wong JH, Yee BC, Peters A, Kobrehel K, Buchanan BB. 1996. New evidence for a role of thioredoxin h in germination and seedling development. Planta 200: 100–106. [Google Scholar]
- Macovei A, Balestrazzi A, Confalonieri M, Carbonera D. 2010. The Tdp1 (tyrosyl-DNA phosphodiesterase) gene family in Medicago truncatula Gaertn.: bioinformatic investigation and expression profiles in response to copper- and PEG-mediated stress. Planta 232: 393–407. [DOI] [PubMed] [Google Scholar]
- McDonald MB. 1999. Seed deterioration: physiology, repair and assessment. Seed Science and Technology 27: 177–237. [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. 2004. Reactive oxygen gene network of plants. Trends in Plant Science 9: 490–498. [DOI] [PubMed] [Google Scholar]
- Moller IM. 2001. Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annual Review of Plant Physiology and Plant Molecular Biology 52: 561–591. [DOI] [PubMed] [Google Scholar]
- Møller IM., Jensen PE., Hansson A. 2007. Oxidative modifications to cellular components in plants. Annual Review of Plant Biology 58: 459–481. [DOI] [PubMed] [Google Scholar]
- MoradShaban, Abasalt RA, Ghorban DM, Tahmine B. 2013. Review on dual role of reactive oxygen species in seed physiology and germination. International Journal of Agriculture and Crop Sciences 5: 2390–2393. [Google Scholar]
- Müller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Luebner-Metzger G. 2009. In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiology 150: 1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TM, Asard H, Cross AR. 1998. Possible sources of reactive oxygen during the oxidative burst in plants. In: Asard H, Berczi A, eds. Plasma membrane redox systems and their role in biological stresses and disease. Dordrecht: Kluwer Academic Publishers, 215–246. [Google Scholar]
- Oracz K, El Maarouf-Bouteau H, Farrant JM, et al. 2007. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. The Plant Journal 50: 452–465. [DOI] [PubMed] [Google Scholar]
- Oracz K, El-Maarouf-Bouteau H, Kranner I, Bogatek R, Corbineau F, Bailly C. 2009. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiology 150: 494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cardenas ML, Narvaez-Vasquez J, Ryan CA. 2001. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. The Plant Cell 13: 179–191. [PMC free article] [PubMed] [Google Scholar]
- Puntarulo S, Galleano M, Sanchez RA, Boveris A. 1991. Superoxide anion and hydrogen peroxide metabolism in soybean embryonic axes during germination. Biochimica et Biophysica Acta 1074: 277–283. [DOI] [PubMed] [Google Scholar]
- Ray PD, Huang B-W, Tsuji Y. 2012. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular Signaling 24: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach T, Beckett RP, Minibayeva FV, et al. 2010. Extracellular superoxide production, viability and redox poise in response to desiccation in recalcitrant Castanea sativa seeds. Plant, Cell and Environment 33: 59–75. [DOI] [PubMed] [Google Scholar]
- Roberts EH. 1973. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants predicting the storage life of seeds. Seed Science and Technology 1: 499–514. [Google Scholar]
- Sarath G, Hou G, Baird LM, Mitchell RB. 2007. Reactive oxygen species, ABA and nitric oxide interactions on the germination of warm-season C (4)-grasses. Planta 226: 697–708. [DOI] [PubMed] [Google Scholar]
- Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A. 2002. Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 214: 821–828. [DOI] [PubMed] [Google Scholar]
- Schopfer P, Plachy C, Frahry G. 2001. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiology 125: 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweikert C, Liszkay A, Schopfer P. 2002. Scission of polysaccharides by peroxidase-generated hydroxyl radicals. Phytochemistry 53: 565–570. [DOI] [PubMed] [Google Scholar]
- Sharma NL, Kuniyal JC, Singh M, et al. 2012. Atmospheric aerosol characteristics retrieved using ground based solar extinction studies at Mohal in the Kullu valley of northwestern Himalayan region. Indian Journal of Earth System Science 121: 221–235. [Google Scholar]
- Smith RD, Dickie JD, Linington SH, Pritchard HW, Probert RJ. 2003. Seed conservation: turning science into practice. London: Royal Botanic Gardens, Kew. [Google Scholar]
- Wang M, Heimovaara-Dijkstra S, Van Duijn B. 1995. Modulation of germination of embryos isolated from dormant and nondormant barley grains by manipulation of endogenous abscisic levels. Planta 195: 586–592. [Google Scholar]
- Wisnieewski JP, Cornille P, Agnel JP, Montillet JL. 1999. The extensin multigene family responds differentially to superoxide or hydrogen peroxide in tomato cell cultures. FEBS Letters 447: 264–268. [DOI] [PubMed] [Google Scholar]
- Xu HN, Li KZ, Yang FJ, Shi Q, Wang X. 2010. Overexpression of CsNMAPK in tobacco enhanced seed germination under salt and osmotic stresses. Molecular Biology Reports 37: 3157–3163. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yajima H, Umezawa Y, Nakagawa Y, Esashi Y. 1995. GC-MS identification of volatile compounds evolved by dry seeds in relation to storage conditions. Seed Science Technology 23: 59–68. [Google Scholar]