Abstract
Background and Aims Loss of seed viability has been associated with deteriorative processes that are partly caused by oxidative damage. The breaking of dormancy, a seed trait that prevents germination in unfavourable seasons, has also been associated with oxidative processes. It is neither clear how much overlap exists between these mechanisms nor is the specific roles played by oxygen and reactive oxygen species.
Methods Antioxidant profiles were studied in fresh (dormant) or after-ripened (non-dormant) sunflower (Helianthus annuus) embryos subjected to controlled deterioration at 40 °C and 75 % relative humidity under ambient (21 %) or high O2 (75 %). Changes in seed vigour and viability, dormancy, protein carbonylation and fatty acid composition were also studied.
Key Results After-ripening of embryonic axes was accompanied by a shift in the thiol-based cellular redox environment towards more oxidizing conditions. Controlled deterioration under high O2 led to a faster loss of seed dormancy and significant decreases in glutathione reductase and glutathione peroxidase activities, but viability was lost at the same rate as under ambient O2. Irrespective of O2 concentration, the overall thiol-based cellular redox state increased significantly over 21 d of controlled deterioration to strongly oxidizing conditions and then plateaued, while viability continued to decrease. Viability loss was accompanied by a rapid decrease in glucose-6-phosphate-dehydrogenase, which provides NADPH for reductive processes such as required by glutathione reductase. Protein carbonylation, a marker of protein oxidation, increased strongly in deteriorating seeds. The lipid-soluble tocochromanols, dominated by α-tocopherol, and fatty acid profiles remained stable.
Conclusions After-ripening, dormancy-breaking during ageing and viability loss appeared to be associated with oxidative changes of the cytosolic environment and proteins in the embryonic axis rather than the lipid environment. High O2 concentrations accelerated dormancy alleviation but, surprisingly, did not accelerate the rate of viability loss.
Keywords: Helianthus annuus, sunflower, after-ripening, dormancy, seed ageing, oxygen, glutathione, reactive oxygen species, ROS, stress, seed viability, peroxidase, tocopherol, tocochromanols, fatty acid, antioxidant enzymes
INTRODUCTION
The longevity of orthodox (desiccation-tolerant) seed increases with decreasing seed water content (WC) and storage temperatures (Roberts, 1972; Bewley et al., 2013). Seed storage under non-optimal conditions or long-term storage results in increasingly more oxidized intracellular conditions, and oxidative modifications of macromolecules (Bailly et al., 1996; Job et al., 2005; Kranner et al., 2006, 2011). Reactive oxygen species (ROS) such as the superoxide anion (O2·−), hydrogen peroxide (H2O2) and the hydroxyl radical (·OH) have been associated with such processes, and lead to viability loss. However, ROS are also important signalling molecules involved in plant growth, development and stress response (Foyer and Noctor, 2005; Bailly et al., 2008). In seeds, ROS play a role in dormancy alleviation (Müller et al., 2009; Oracz et al., 2009; Leymarie et al., 2012). Dormancy is a seed trait that restricts germination during unfavourable seasons and is maintained by abscisic acid (ABA), whereas gibberellins and ethylene promote germination (Finch-Savage and Leubner-Metzger, 2006). ROS are intricately linked to hormonal regulation of dormancy breaking, and in certain time windows during imbibition, high ROS concentrations are required to break dormancy (reviewed by El-Maarouf-Bouteau and Bailly, 2008). Dry after-ripening of sunflower seeds to break dormancy has also been associated with increases in H2O2 contents in non-imbibed embryonic axes (Bailly et al., 2008). Enzymes, such as respiratory burst oxidase homologues and extracellular peroxidases, produce O2·− and H2O2 both before and after germination (Müller et al., 2009; Kranner et al., 2010; Roach and Kranner, 2011). H2O2 can cross membranes and is thought to oxidize proteins and RNA as part of dormancy release (Oracz et al., 2007; Bazin et al., 2011). Hence, ROS are important to dormancy breaking and germination, but their potentially damaging oxidative nature means that control by antioxidants is required.
The cellular redox state is governed by the redox state of the water-soluble low-molecular-weight (LMW) thiol antioxidant glutathione (GSH) (Schafer and Buettner, 2001), especially in orthodox seeds (Kranner et al., 2006), which have low or undetectable amounts of ascorbate (Tommasi et al., 1999). Besides GSH, cells contain other LMW thiols, including cysteine and the dipeptides γ-glutamyl-cysteine and cysteinyl-glycine, which are by-products of GSH synthesis and degradation (Zagorchev et al., 2013). Due to the nucleophilic nature of their cysteine residues, LMW thiols can be converted to disulphides (e.g. GSH to GSSG). When the regeneration of disulphides, such as GSSG, is restricted the intracellular redox state can shift significantly towards more oxidizing conditions, which at a certain point triggers cell death (Schafer and Buettner, 2001). In many plant species and tissues, the glutathione half-cell reduction potential (EGSSG/2GSH) has been correlated with cell viability. In seeds, total germination typically declines to 50 % when EGSSG/2GSH rises to between –180 and –160 mV (Kranner et al., 2006). The enzyme glutathione reductase (GR) requires NADPH to convert GSSG to GSH. In non-photosynthetic tissues, NADPH is provided by the oxidative pentose phosphate pathway, of which glucose-6-phosphate dehydrogenase (G6PDH) is the key enzyme. Glutathione is also a substrate of glutathione peroxidase (GPx), which breaks down H2O2, as well as toxic organic peroxides. Other ROS-processing enzymes include catalase, which efficiently scavenges H2O2 at high concentrations. In imbibed sunflower seeds catalase activity has been suggested to maintain dormancy (Oracz et al., 2007), but has also been shown to be important for pre-germination repair of non-dormant seeds (Kibinza et al., 2011).
Oxidative modification to lipids may also contribute to seed viability loss. Lipoxygenase metabolizes poly-unsaturated fatty acids (PUFAs) to precursors of the oxylipin signalling pathway, and may contribute to seed longevity and germination. For example, in rice (Oryza sativa) over-expressing or knocking-down the LOX2 enzyme accelerates germination rates and increases seed longevity, respectively (Huang et al., 2014). Tocochromanols, the vitamin E family, are lipid-soluble antioxidants, and Arabidopsis thaliana mutants that lack all tocopherols have reduced seed longevity (Sattler et al., 2004). However, seed viability loss is not always associated with a loss in tocochromanols (Seal et al., 2010a). Once oxidized, the tocopheroxyl and tocotrienoxyl radicals can be regenerated by glutathione (Munné-Bosch and Alegre, 2002). Besides their function as ROS scavengers, tocochromanols are needed for germination and plant growth (Falk and Munné-Bosch, 2010).
Anoxic environments during seed storage have been used to examine the role of ROS in seed ageing. Lowering the O2 concentrations during storage extended the longevity of Salix nigra (Roqueiro et al., 2010), celery and celeriac (Apium graveolens) seeds (Groot et al., 2015) and a more pronounced affect was seen in extending the longevity of primed lettuce (Latuca sativa) seeds (Ibrahim et al., 1983; Schwember and Bradford, 2011). On the other hand, there is less evidence that elevated O2 decreases seed viability, except in a few studies that combined O2 treatment under very high pressures (Groot et al., 2012), which also exerts physical stress. Seed after-ripening could also be viewed as an early stage of ageing and both processes may share common oxidative pathways. Therefore, dormancy release could be accelerated by ageing seeds under high O2. Furthermore, although there is some evidence that in A. thaliana seed dormancy is negatively correlated with longevity (Nguyen et al., 2012), more research is needed to confirm a link between after-ripening and ageing. Here, we aged dormant and after-ripened (hereafter non-dormant) sunflower seeds under ambient oxygen and elevated oxygen to study how oxygen concentrations affect redox control, and potential downstream effects of seed dormancy alleviation and ageing.
MATERIALS AND METHODS
Seed material and after-ripening
All experiments were carried out with field-grown sunflower seeds (Helianthus annuus ‘LG5665’) harvested in September 2012. The dormant batch was stored at –20 °C to keep dormancy and the non-dormant batch was after-ripened for 3 months at 20 °C and 75 % relative humidity (RH) before transfer to –20 °C. The use of dormancy here refers to embryos that had restricted germination at 10 °C before controlled deterioration (CD) (Corbineau et al., 1990). The pericarp was removed and visually damaged seeds were discarded before CD.
Controlled deterioration and germination
Embryos (dormant and non-dormant) with a mean WC of 4·15 ± 0·15 % on a fresh weight (f. wt) basis were equilibrated for 48 h over unsaturated LiCl solution at 70 % RH at 4 °C. The seeds were then subjected to CD at 40 °C and 75 % RH using saturated NaCl solution, which was chosen to maintain 4·2 % WC. Replicates were aged in separate plastic boxes, under either ambient levels of O2 or high O2 (75 ± 0·69 %). The O2 concentrations were monitored and readjusted daily using an oxygen dipping probe (Fibox 3; Presens, Regensburg, Germany). Embryos were removed after 0, 21, 24, 27 and 31 d.
Embryos were germinated in darkness at 10 °C, which restricted germination of dormant embryos, or at 25 °C, enabling non-dormant and dormant embryos to germinate. Twenty embryos per replicate (n = 4 and 3 for dormant and non-dormant embryos, respectively) were placed in a closed plastic box on a layer of germination paper (grade 3644; Whatmann/GE Healthcare, Little Chalfont, UK) moistened with 40 mL dH2O. A seed was considered germinated when the radicle extended over 3 mm, and scored until all embryos had either germinated or became mouldy. The distinction between normal and abnormal germination was made according to the International Rules for Seed Testing (ISTA, 2014).
Biochemical analyses
All biochemical analyses were carried out using H. annuus embryonic axes that were isolated from freeze-dried embryos just before grinding. Each replicate (n = 4) contained 50 axes. Material was ground in a 5-mL liquid nitrogen-cooled Teflon capsule with an agate ball at 2800 r.p.m. for 20 s using a Mikro-Dismembrator S (B. Braun, Biotech International, Germany).
HPLC analysis of LMW thiols and disulphides
Ninety minutes prior to extraction, 25 mg polyvinylpolypyrrolidone (PVPP) was imbibed in 0·1 m ice-cold HCl. To this 25 mg of freeze-dried embryonic axes was added and vortexed at full speed for 15 s then centrifuged at 27 000 g for 20 min at 4 °C. Then, 700 µL of supernatant was transferred with a needle avoiding the PVPP pellet below and congealed lipids above and further centrifuged at 27 000 g for 10 min before filtering the extract (Phenex™-RC syringe filter pore size: 0·45 µm; Phenomenex, Macclesfield, UK). Then, 120 µL of this extract was used for the determination of total LMW thiols and 400 µL for disulphides according to Kranner (1998), using an Agilent 1100 HPLC system. Briefly, thiols were fluorescently labelled by monobromobimane (mBBr). ‘Total’ thiol and disulphide concentrations (e.g. GSH + GSSG) were determined after reduction of disulphides by dithiothreitol (DTT). For determination of disulphides, thiol groups were blocked with N-ethylmaleimide (NEM). Excess NEM was removed and the remaining disulphides were reduced with DTT and analysed as above. The mBBr derivatives were separated using a reversed-phase column (250 × 4·6 mm, 3 µm particle size, ODS - Hypersil; Bischoff Chromatography, Leonberg, Germany) and detected by fluorescence (excitation: 380 nm; emission: 480 nm).
The half-cell reduction potential of LMW thiols was calculated from the concentrations of thiols and disulphides considering the WC and taking into account deviations from standard conditions in terms of pH and temperature using the Nernst equation, as described by Birtic et al. (2011) and shown in the equation below, where R is the gas constant (8·314 J K–1 mol–1), T the temperature during CD (313 K), n represents the number of transferred electrons (2GSH → GSSG +2H+ + 2e−) and F stands for the Faraday constant (9·6485 × 104 C mol–1).
HPLC analysis of tocochromanols
Tocochromanols were analysed with an isocratic HPLC method as described by Bagci et al. (2004) using 20 mg of freeze-dried and ground plant material extracted three times in 1 mL of ice-cold heptane by vortexing for 15 s and centrifugation at 13 000 g for 20 min at 4 °C. The combined supernatants were re-centrifuged at 13 000 g for 20 min at 4 °C prior to separation on a reversed-phase column (LC-Diol, 250 × 4·6 mm i.d., 5 µm particle size, Supelcosil™; Supelco Analytical). To quantify abundant and low abundant tocochromanols, 10 and 70 µL of extract was injected, respectively, that were identified and quantified against α- and β-tocopherol and tocotrienol standards.
Enzyme activities
Protein extraction was carried out in a cold room at 4–7 °C. Lipids and lipid-soluble components were first removed from 50 mg of freeze-dried and ground embryonic axes by briefly vortexing in 1 mL hexane. The homogenate was centrifuged at 6000 g for 15 min at 4 °C and the supernatant was discarded. The pellet was dried for 5 min in a SpeedVac (Savant SPD111VP2; Thermo Scientific, Waltham, MA, USA) at room temperature and the pellet was extracted in 2 mL of 0·1 m Sorenseńs phosphate buffer (pH 7·8) containing 50 mg PVPP, 2 mm DTT, 0·1 mm ethylenediaminetetraacetic acid (EDTA), 1·25 mm polyethylene glycol 4000 (PEG 4000) and a protease inhibitor cocktail (cOmplete ULTRA Tablets, EDTA-free; Roche, Indianapolis, IN, USA). The mix was homogenized and incubated for 15 min before centrifugation at 16 000 g for 15 min at 4 °C. The supernatant was desalted with a Sephadex PD-10 Desalting Column (GE Healthcare) following the manufacturer’s gravitational protocol and the resulting protein extracts were used immediately. Enzyme kinetics were recorded in 0·1 m Sorenson’s buffer at pH 7·8, unless otherwise stated, following optimization from procedures given by Bailly and Kranner (2011) using a Perkin Elmer (Waltham, MA, USA) spectrophotometer (Lambda 800 UV/VIS spectrometer) with a cuvette holder maintained at 25 °C. Data were normalized to protein content as quantified by the Bradford assay.
GR was measured by the decrease in absorbance at 340 nm (ε = 6·22 mm–1 cm–1) in the presence of 0·2 mm NADPH, 1·7 mm GSSG, 2·5 mm MgCl2 and 400 µL of protein extract in a total reaction volume of 630 µL. G6PDH activity was measured by the decrease in absorbance at 340 nm with 0·7 mm NADP+, 2·6 mm glucose-6-phosphate, 2·6 mm MgCl2 and 370 µL protein extract in a total reaction volume of 630 µL. GPx activity was indirectly determined by following GR activity from the GSSG simultaneously formed by GPX activity in the presence of 1·6 U mL–1 GR, 0·2 mm NADPH, 2·4 mm GSH, 2·4 mm cumene hydroperoxide and 0·8 mm NaN3 and 300 µL protein extract in a total reaction volume of 630 µL. Measurements were started after 2 min by the addition of cumene hydroperoxide to allow all GSSG already present in the extract to be consumed. Lipoxygenase (LOX) activity was measured by following the formation of lipid peroxides at 236 nm (ε = 23 mm–1 cm–1) in the presence of 40 µL protein extract and 80 µL linoleic acid stock in a total reaction volume of 2 mL. The linoleic acid stock (1 mL) contained 15·7 µL linoleic acid, 1·5 % (v/v) Tween 20 and 1·3 % (v/v) 10 m NaOH. Catalase activity was determined by following the decrease in absorbance of H2O2 at 240 nm (ε = 39·4 mm–1 cm–1) in the presence of 15 mm H2O2 and 200 µL protein extract in a total reaction volume of 1·65 mL.
Protein carbonylation
The detection of carbonylated protein was performed using the ‘Oxyblot protein oxidation detection kit’ (Merck Millipore, Billerica, MA, USA) as previously described (Job et al., 2005). Briefly, total soluble proteins were extracted from the freeze dried powder obtained from embryonic axes and incubated with 2,4-dinitrophenylhydrazine (DNP-hydrazine) to derivatize the carbonyl groups to DNP-hydrazone. After separation by SDS-PAGE, proteins were transferred to nitrocellulose membranes and DNP adducts were immunologically detected using a monoclonal anti-DNP antibody. For densitometry analysis, scanned blots were imported into ImageJ (http://rsbweb.nih.gov/ij/) and the resulting band intensity was normalized to the 20-kDa bands of a Coomassie-stained gel run in parallel.
GC-MS analysis of fatty acids
Fatty acids were derivatized to fatty acid methyl esters (FAMEs) as described by Li-Beisson et al. (2013). Briefly, around 10 mg of freeze-dried seed powder was treated with 2·64 mL of methanol/toluene/sulfuric acid [10 : 3 : 0·25 (v/v/v), containing 0·01 % (w/v) butylated hydroxytoluene]. In total, 200 µg of heptadecanoic acid (C17:0, dissolved in hexane) was added simultaneously as an internal standard. Samples were incubated at 80 °C for 90 min with constant agitation before adding 1 mL of hexane and 3 mL of 0·9 % NaCl (w/v). Samples were vigorously mixed before centrifugation for 10 min at 3000 g. The supernatant was transferred to autosampler vials and kept at −20 °C if not analysed immediately.
FAMEs were separated using a Trace 1300 gas chromatograph (Thermo-Scientific) on a 30 m FAMEWAX column (Restek #12497, Bellefonte, PA, USA) and detected using a TSQ 8000 triple quadrupole detector (Thermo-Scientific) operated in full scan mode (50–550 m/z). One microlitre of sample was injected in split mode (split ratio 1 : 100) in a split/splitless inlet heated at 230 °C and containing a Mini-Lam 4-mm split liner (Thermo-Scientific #453A2009). The temperature gradient was first set to 120 °C for 1 min, then increased by 16 °C min–1 up to 190 °C, then by 5 °C min–1 up to 220 °C and finally increased by 2 °C min–1 up to 235 °C, which was held for 7 min with a carrier gas flow of 1·2 mL min−1 of helium. The ion source temperature was set to 250 °C and the transfer line to 240 °C.
A commercial FAME mix (ref. 18919; Sigma Aldrich, St Louis, MO, USA) was used to confirm the identities of the FAs. External standards of palmitic, stearic, oleic, linoleic and linolenic acid were used in combination with the internal standard to estimate the total amount of each fatty acid. Data analysis was performed using the Xcalibur software (Thermo-Scientific).
Statistical analysis
Statistical analysis was carried out with the software package IBM SPSS Statistics 20. When the requirement of an asymptotic significance <0·05 in a Kruskal–Wallis test was fulfilled, data were analysed for significance (P < 0·05), either using one-way analysis of variance (ANOVA) in combination with least significant difference (LSD) post-hoc comparison of means or using an independent t-test for testing statistical differences of protein carbonylation. Total germination data (%) were arcsine-transformed prior to Kruskal–Wallis tests and ANOVA. Replicates with Cook’s distance values greater than 0·1 were considered as outliers and excluded from the data set.
RESULTS
Impacts of after-ripening and high O2 during controlled deterioration on seed physiology and the thiol redox environment
The germination kinetics of non-aged non-dormant and dormant embryos was almost identical at 25 °C. However, 15 % of non-dormant embryos showed abnormal germination, whereas less than 3 % of dormant embryos showed abnormal germination (Fig. 1). Within 5 d, over 90 % of non-dormant embryos had germinated at 10 °C. In contrast, the germination of dormant seeds was delayed, reaching only 75 % total germination even after 180 d (Fig. 1). This shows that the germination of dormant embryos was severely restricted at 10 °C, and that dormancy was slowly broken during prolonged imbibition. Sunflower seeds show thermodormancy at time of harvest (Corbineau et al., 1990), narrowing the temperature range for germination.
Fig. 1.
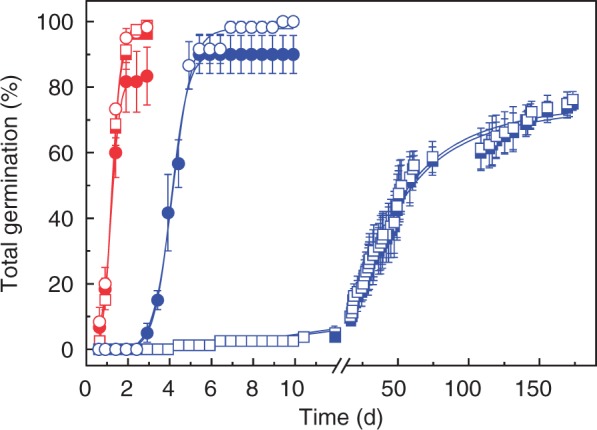
Germination of dormant (squares) and non-dormant (circles) embryos at 25 °C (red) or at 10 °C (blue) before controlled deterioration. Normal germination and total germination, including abnormal seedlings, are shown by closed and open symbols, respectively. No further germination took place after the last time point shown, n = 4 and 3 replicates of 20 embryos for dormant and non-dormant embryos, respectively ± s.e.
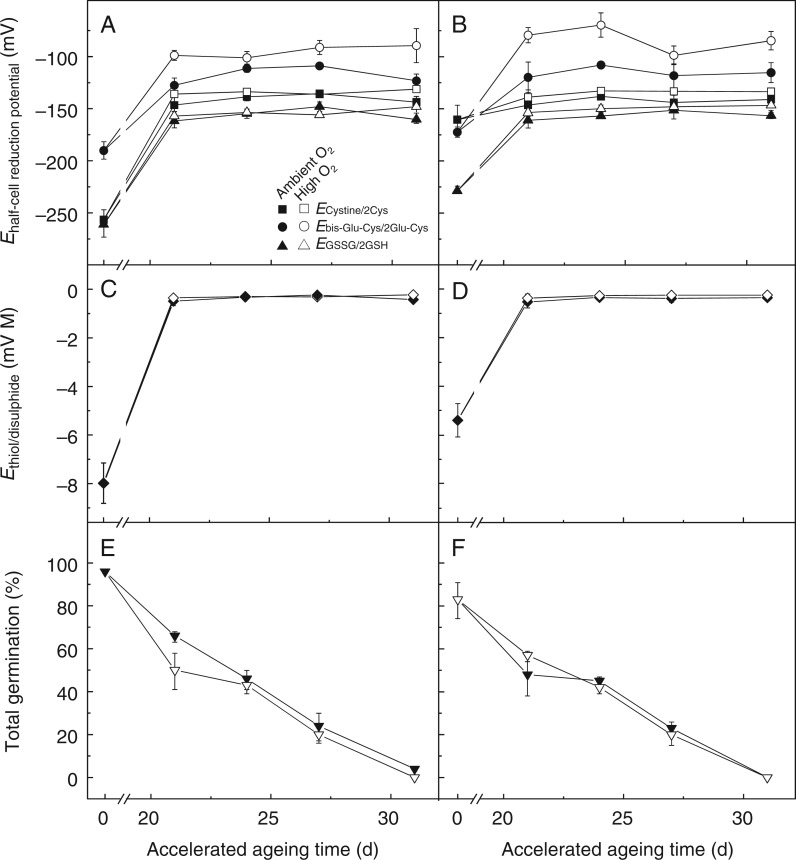
Prior to CD the total glutathione content (GSH + GSSG) of non-dormant embryonic axes was lower than in dormant embryonic axes (1599 and 1753 nmol g–1 d. wt, respectively), and non-dormant axes also contained more GSSG (14·2 and 2·9 %, respectively, Supplementary Data Fig. S1). Therefore, the EGSSG/2GSH of non-dormant embryonic axes was 33 mV less negative than it was in dormant axes (Fig. 2A, B). This increased oxidation state of non-aged non-dormant embryonic axes was also observed for the two next most abundant LMW thiols, γ-glutamyl-cysteine and cysteine, the latter being 100 mV less negative than in dormant axes (Fig. 2A, B). Overall, the Ethiol-disulphide (calculated by combining the individual half-cell reduction potentials of all LMW thiols and disulphide redox couples) was significantly more positive in non-dormant embryos before CD (Fig. 2C, D). In summary, after-ripening was associated with increasingly more oxidizing conditions.
Fig. 2.
Influence of after-ripening and controlled deterioration under high or ambient O2 on the thiol-based cellular redox environment and viability. Embryos were aged for up to 31 d under ambient O2 (filled symbols) or high O2 (open symbols). (A,B) Individual half-cell reduction potentials of glutathione, cysteine and glutamyl-cysteine, as represented by triangles, squares and circles, respectively, in embryonic axes (n = 4 ± s.e.). (C,D) The mathematically combined thiol-based cellular redox environment Ethiol-disulphide. (E,F) Embryo viability, as measured by % of normal germination at 25 °C, n = 4 and 3 replicates of 20 embryos for dormant and non-dormant embryos, respectively, ± s.e. A,C,E and B,D,F represent dormant and non-dormant embryos before CD, respectively.
Within 31 d of CD sunflower embryos lost the ability to germinate, irrespective of dormancy status and O2 concentration (Fig. 2E, F). During CD total glutathione decreased, total cysteine amounts remained rather stable and total γ-glutamyl-cysteine amounts increased (Fig. S1). Controlled deterioration of embryos under high O2 significantly increased the Ebis-glu-cys/2γ-glu-cys after 21 and 24 d of CD for dormant and non-dormant embryos, respectively. Of all the thiol–disulphide redox couples, the effect of elevated O2 was most pronounced for Ebis-glu-cys/2γ-glu-cys (Fig. 2A, B), although this had minor influence on the Ethiol-disulphide (Fig. 2C, D) due to the relatively low abundance of this redox couple (Fig. S1). After 21 d of CD, total germination fell to 48-66 % and total glutathione dropped to around 500 nmol g–1 in both dormant and non-dormant embryonic axes, regardless of the O2 environment, with GSSG representing approx. 75 % of total glutathione (Fig. S1). As a consequence, the EGSSG/2GSH increased to approx. –150 mV (Fig. 2A, B) and the Ethiol-disulphide approached strongly oxidizing conditions (∼ –0·2 mV m) and then plateaued during further CD irrespective of dormancy status or O2 environment (Fig. 2C, D).
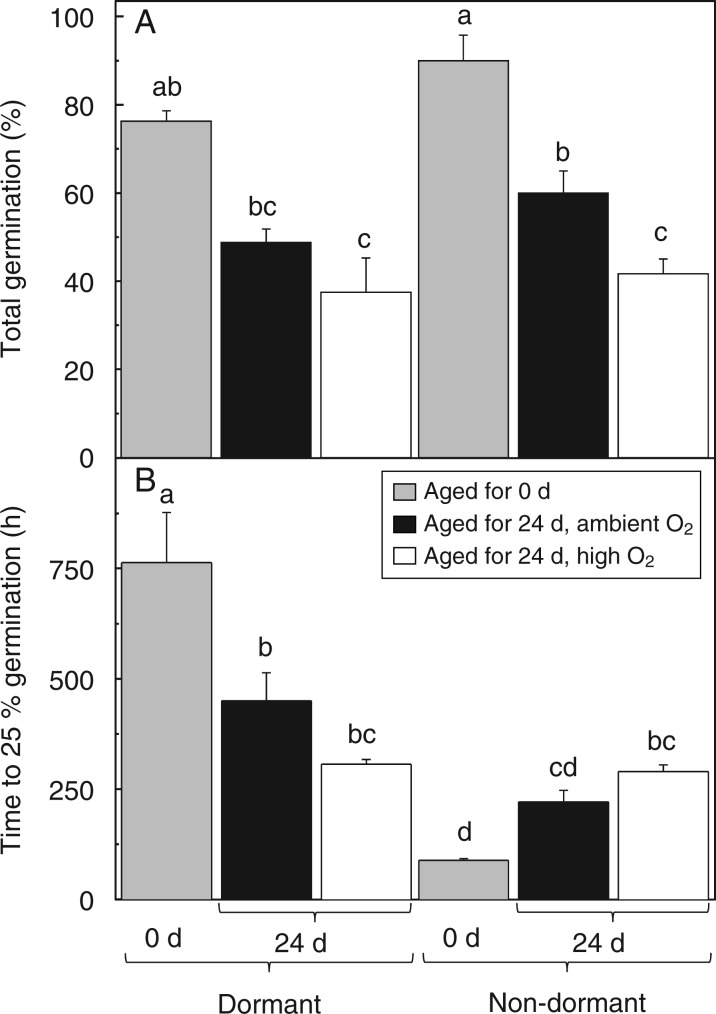
After 24 d of CD under high O2, dormant and non-dormant embryos displayed lower germination than embryos aged under ambient O2, which was significantly lower for non-dormant embryos germinated at 10 °C (Fig. 3A). Dormant and non-dormant embryos aged for 24 d under high O2 required the same time to reach 25 % germination at 10 °C, indicating that both seed lots had equal vigour (Fig. 3B; Supplementary Data Fig. S2). Conversely, a marked difference was observed when embryos were aged under ambient O2, whereby dormant embryos germinated more slowly. This suggests that CD-induced dormancy alleviation occurred faster under high O2.
Fig. 3.
Influence of after-ripening and controlled deterioration under high or ambient O2 on viability, dormancy and vigour of sunflower embryos germinated at 10 °C. (A) Viability, as measured by % of normal seedling germination of dormant (left) and non-dormant (right) embryos aged for 0 or 24 d under ambient O2 or high O2 (see key). (b) Dormancy and vigour changes, as shown by the time to 25 % germination of the embryo shown in A, n = 4 and 3 replicates of 20 embryos for dormant and non-dormant embryos, respectively ± s.e. Bars labelled with the same letter do not differ significantly at P < 0·05.
Impacts of after-ripening and high O2 during controlled deterioration on antioxidant enzyme activities
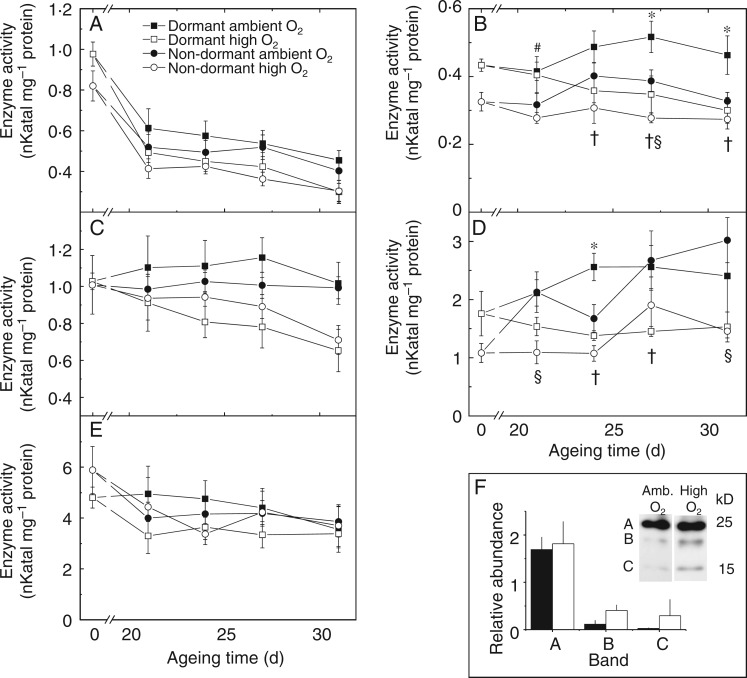
The activity of G6PDH of non-aged dormant and non-dormant embryonic axes was 0·97 ± 0·06 and 0·82 ±0·07 nkat mg–1 protein, respectively, and significantly declined in all scenarios within 21 d of CD (Fig. 4A). Between 21 and 31 d of CD the activity of G6PDH decreased significantly when dormant embryonic axes were aged under high O2, but not when aged under ambient O2. Glutathione reductase activity in non-aged dormant and non-dormant embryonic axes was 0·43 ± 0·02 and 0·33 ± 0·03 nkat mg–1 protein, respectively (Fig. 4B), and remained relatively stable during 31 d of CD under ambient O2. However, it declined significantly by 31 % in dormant axes aged under high O2; GR activity also declined slightly, but insignificantly, in non-dormant embryonic axes under high O2. In both dormant and non-dormant embryonic axes catalase activity remained constant during CD under ambient O2, varying little between the non-aged activity level of 1·03 ± 0·04 and 1·01 ± 0·16 nkat mg–1 protein for dormant and non-dormant axes, respectively. Under high O2 catalase activity decreased to 63 and 71 % of initial enzyme activity in dormant and non-dormant embryonic axes, respectively (Fig. 4C). Glutathione peroxidase activity was 1·76 ± 0·38 and 1·08 ± 0·16 nkat mg–1 protein for non-aged dormant and non-dormant embryonic axes, respectively (Fig. 4D). Controlled deterioration of non-dormant embryos for 21 d under ambient O2 was associated with a significant increase in GPx activity, increasing to three times its initial activity after 31 d of CD. In dormant embryonic axes GPx activity also increased, although less so. Strikingly, the increase in GPx activity was significantly prevented by ageing embryos under high O2 (Fig. 4D). Activity of LOX decreased slightly during CD in dormant and non-dormant embryos (Fig. 4E). Controlled deterioration also led to an increase in protein carbonylation (Supplementary Data Fig. S3), which for an approx. 20-kDa protein was significantly (P = 0·013) increased after 24 d of CD under high O2 (Fig. 4F). Therefore, CD under high O2 was associated to lowered enzyme activities and increases in protein carbonylation.
Fig. 4.
Influence of after-ripening and controlled deterioration under high or ambient O2 on antioxidant enzyme activities and protein carbonylation in sunflower embryonic axes. The activity of (A) glucose-6-phosphate dehydrogenase, (B) glutathione reductase, (C) catalase, (D) glutathione peroxidase and (E) lipoxygenase of axes from embryos that were dormant (squares) or non-dormant (circles) prior to ageing. Embryos were aged under ambient O2 (closed symbols) or high O2 (open symbols) for up to 31 d, n = 4 replicates of 50 embryonic axes ± s.e. Significance difference (P < 0·05) is shown between O2 treatments of dormant (†) and non-dormant axes (§), dormant and non-dormant axes aged under ambient (*) or high (#) O2. (F) Carbonylated proteins in embryonic axes after 24 d ageing under ambient or high O2, as visualized by blotting with anti-DNP after separation by 1-D PAGE. The three bands between 25 and 15 kDa were quantified relative to a Coomassie-stained loading control run in parallel. Significance difference (P < 0·013) was found for band B using an independent t-test.
Impacts of after-ripening and high O2 during controlled deterioration on tocochromanols and fatty acids
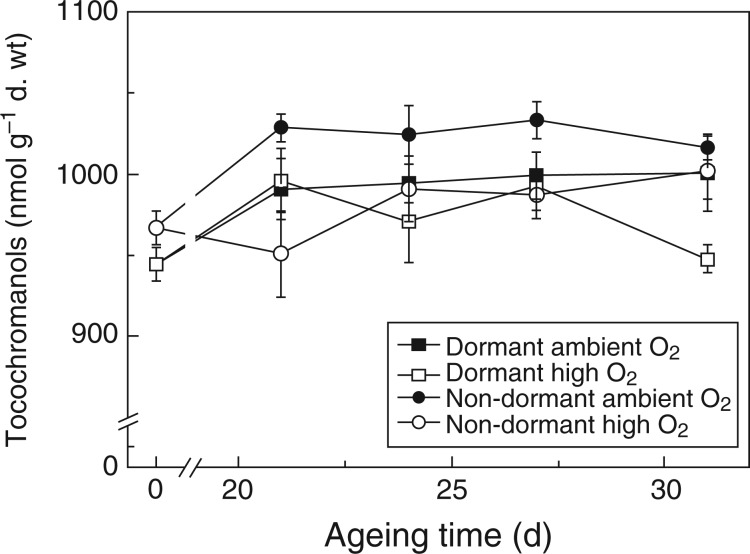
The main tocochromanols detected in both dormant and non-dormant embryonic axes were α-tocopherol, β-tocotrienol, α-tocotrienol and β-tocopherol, representing >95, 1·2, 0·2 and ≤0·2 % of total tocochromanol contents, respectively (Supplementary Data Fig. S4). Total tocochromanol amounts in non-aged dormant and non-dormant embryonic axes were 945 ± 10 and 967 ± 11 nmol g–1 d. wt, respectively, and did not differ significantly. The amount of total tocochromanols increased significantly until 24 d of CD, but not in embryos aged under high O2 (Fig. 5). α-Tocotrienol was the only tocochromanol to decrease during CD and this was most notable in dormant axes, decreasing by up to 17 % after 31 d of CD. In contrast, β-tocotrienol increased in both dormant and non-dormant axes by up to 59 % (Fig. S4). In summary, the total tocochromanol concentration of embryonic axes increased slightly as the embryos lost viability, but there was no such increase when embryos were aged under high O2.
Fig. 5.
Influence of after-ripening and controlled deterioration under high or ambient O2 on the total tocochromanol content of sunflower embryonic axes. Seeds that were dormant (squares) or non-dormant (circles) prior to ageing were aged under ambient O2 (closed symbols) or high O2 (open symbols) for up to 31 d, n = 4 replicates of 50 embryonic axes ± s.e.
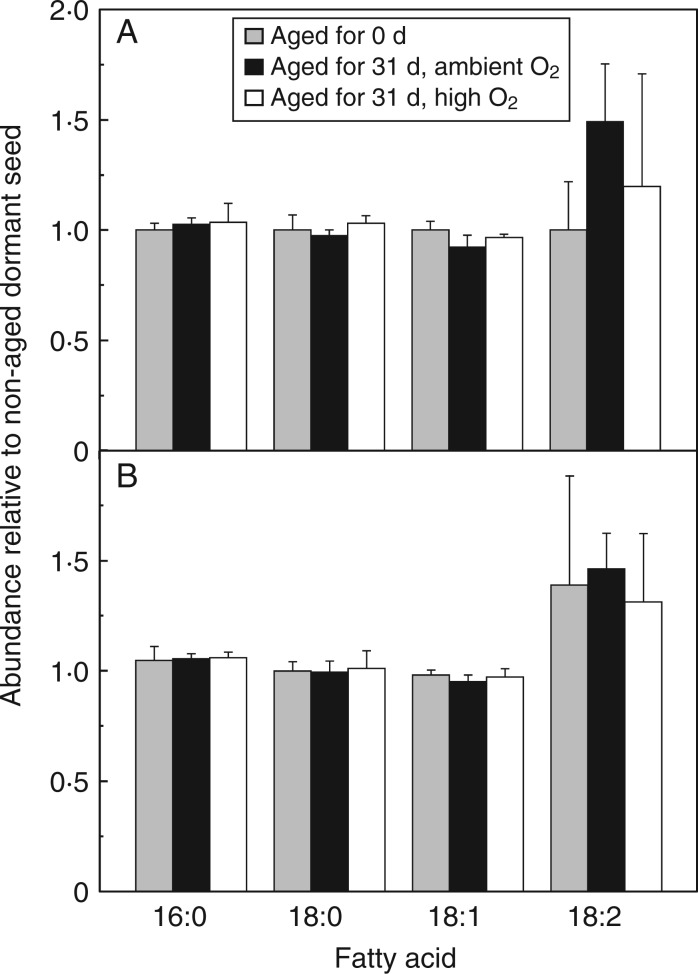
Of the total fatty acids, oleic acid (C18:1) was the most abundant (∼79 %) followed by linoleic (C18:2; ∼11 %), palmitic (C16:0; ∼4·5 %) and stearic acids (C18:0; ∼2·8 %). No significant differences in fatty acid amounts were found between dormant and non-dormant embryonic axes nor between aged and non-aged axes (Fig. 6). Thus, the total fatty acid content and composition was unaffected by CD under the contrasting oxygen atmospheres.
Fig. 6.
Influence of after-ripening and controlled deterioration after 31 d under high or ambient O2 on the relative abundance of the four most abundant fatty acids in embryonic axes. (A) Dormant and (B) non-dormant embryos were aged for 0 or 31 d under ambient O2 or high O2 (see key). 16:0 = palmitic acid, 18:0 = stearic acid, 18:1 = oleic acid and 18:2 = linoleic acid, n = 4 replicates of 50 embryonic axes ± s.e.
DISCUSSION
ROS are thought to contribute to deteriorative processes that result in seed viability loss. ROS also play important signalling roles in embryos and seedlings, and ROS production has been associated with both dormancy release and seed ageing.
After-ripening
After-ripening widened the temperature window in which seeds were able to germinate and led to a slightly higher occurrence of abnormally germinated embryos at 25 °C (Fig. 1), indicating that damage was incurred to a certain proportion of embryos. After-ripening was associated with increasingly more oxidizing conditions as demonstrated by the differences between the Ethiol/disulphide (which is dominated by EGSSG/2GSH) of dormant and that of non-dormant embryos (Fig. 2A, B). An increase in EGSSG/2GSH state during after-ripening was also observed in barley (Hordeum vulgare) seeds (Bahin et al., 2011). Furthermore, increased protein carbonylation has previously been observed in after-ripened sunflower embryonic axes (Oracz et al., 2007). These findings are in agreement with the suggestion of Bailly et al. (2008) that seed germination requires an ‘oxidative window’, postulating that a certain oxidative load is needed to break dormancy.
Lowered catalase activity has been suggested to release dormancy by enabling H2O2 concentrations to rise (Oracz et al., 2009; Leymarie et al., 2012). We observed no difference in catalase activity between dormant and non-dormant embryos, but found higher GPx activity in non-aged dormant embryonic axes (Fig. 4C, D), an enzyme that also breaks down H2O2. Peroxidases have a higher affinity for H2O2 and are therefore more efficient in breaking down H2O2 at low concentrations (Halliwell, 2006). A GPx in A. thaliana has been shown in vitro to bind and lower the activity of a 2C-type protein phosphatase involved in transmitting the ABA signal during germination (Miao et al., 2006). The activities of GR and G6PDH also tended to decrease upon after-ripening (Fig. 4A, B). Taken together, the increased oxidative load indicated by the increase in Ethiol/disulphide and the lower glutathione-related enzyme activities during after-ripening could be viewed as the beginning of the ageing process that also had downstream effects on dormancy status.
Effects of O2 concentration during controlled deterioration on seed dormancy and viability
The rate of viability loss during CD was the same, irrespective of dormancy status and, unexpectedly, O2 concentration (Fig. 2E, F), although some differences between the two O2 concentrations were observed during earlier CD intervals under suboptimal germination conditions (Fig. 3). The loss of two-thirds of total glutathione within 21 d of CD led to a strong shift in Ethiol-disulphide towards more positive values (i.e. oxidizing conditions), which did not change with further CD, in both dormant and non-dormant embryonic axes irrespective of O2 concentration during CD (Fig. 2C, D). In conjunction with the strongly oxidizing conditions reached within 21 d, protein carbonylation, a marker of protein oxidation (Davies et al., 1999), increased during CD (Supplementary Data Fig. S3) in agreement with previous studies of seed ageing in arabidopsis (e.g. Job et al., 2005). In the present study, strongly oxidizing conditions, as viewed through Ethiol-disulphide, which occurred early in CD could be associated with ensuing viability loss. As elevated O2 concentrations during CD neither impacted Ethiol-disulphide nor exacerbated the rate of viability loss, it appears that critical O2-induced damage was already occurring under ambient O2 concentrations. However, CD under high O2 alleviated dormancy faster (Fig. 3B) and increased protein carbonylation (Fig. 4F), highlighting the complexity of the processes that affect viability and dormancy status in relation to redox environment. In comparison with CD under ambient O2, the accelerated loss of dormancy during CD under high O2 could have been related to lowered GPx activity (Fig. 4D), allowing H2O2 levels to increase, which is associated with dormancy release in sunflower seeds (Bailly et al., 2008). GR activity also increased when embryos were aged under ambient O2, whereas CD of dormant embryos under elevated O2 significantly restricted this increase (Fig. 4B). Enzyme activity was recently confirmed to occur in a desiccation-tolerant moss at the same RH (75 %) used for CD in this study (Fernández-Marín et al., 2013). G6PDH activity decreased rapidly within 21 d of CD irrespective of the O2 concentration during CD (Fig. 4A). In non-photosynthetic tissues, the oxidative pentose phosphate shunt is the only pathway that provides NADPH for various processes (Bowsher et al., 1992; Pleite et al., 2005), including the GR-catalysed reduction of GSSG to GSH. The significant decline in G6PDH activity after 21 d of CD, alongside the shift in Ethiol-disulphide towards more oxidizing conditions, supports that NADPH production is essential to maintain cellular redox control during CD of sunflower embryos.
The lipid environment during controlled deterioration
Seed ageing has been associated with lipid peroxidation in lipid-rich seeds (Stewart and Bewley, 1980; Bailly et al., 1996) and was reported to be accelerated in arabidopsis mutants deficient in tocopherols (Sattler et al., 2004). Here, while significant changes during CD were observed in the cytosolic environment and to proteins, total fatty acids remained stable and the contents of two tocochromanols increased only slightly. The relatively high abundance (79 %) of the monounsaturated acid oleic acid rather than PUFAs could be part of an avoidance mechanism of sunflower embryonic axes to evade lipid peroxidation. Furthermore, sunflower cotyledons suffered more lipid peroxidation than embryonic axes (Bailly et al., 1996), which could also be attributed to the greater antioxidant protection of the axes (Torres et al., 1997). The onset of CD under ambient O2 was accompanied by an increase in α-tocopherol, the most abundant tocochromonanol (Supplementary Data Fig. S4), together with the increase in GPX and GR activities discussed earlier. Controlled deterioration under high O2 restricted an increase in α-tocopherol as it did with GPX and GR activities, but total tocochromonal amounts did not fall (Fig. 4). No tocochromanol breakdown was observed during CD of Vernonia galamensis seeds (Seal et al., 2010a). In lipid-rich Suadea maritima seeds α-tocopherol was lost upon long-term storage at 4 °C, but not during CD (Seal et al., 2010b). Taken together, viability loss during CD in sunflower seeds could not be attributed to changes in the lipid environment and tocochromanols in the embryonic axes.
CONCLUSIONS
After-ripening of sunflower embryos was associated with a significantly increased oxidative load, indicated by the increase in Ethiol/disulphide, which could be interpreted as the start of the ageing process. In both dormant and non-dormant embryos CD led to a further increase in Ethiol/disulphide up to strongly oxidizing conditions, where it plateaued. Initially, this was accompanied by a decline in G6PDH activity, and was followed by progressive viability loss. Viability loss occurred at the same rates regardless of O2 concentration and dormancy status. However, CD under elevated O2 alleviated dormancy faster. Finally, most of the described changes during CD were found in water-soluble metabolites and enzymes, showing that the cytosolic environment and proteins were more sensitive to oxidation than the lipid environment. In conclusion, it appears that dormancy alleviation during after-ripening may overlap with oxidative processes that induce ageing, but more work is needed to disentangle them.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure SF1: influence of after-ripening and high or ambient O2 during controlled deterioration of embryos on contents of glutathione, cysteine, cystine, bis-γ-glutamyl-cystine and γ-glutamyl-cystiene of embryonic axes. Figure SF2: germination curves at 25 or 10 °C of dormant (squares) and non-dormant (circles) sunflower embryos after 24 d of controlled deterioration. Figure SF3: protein carbonylation in embryonic axes during ageing of non-dormant embryos under ambient O2. Figure SF4: influence of after-ripening and high or ambient O2 during controlled deterioration of embryos on the contents of α-tocopherol, β-tocotrienol, α-tocotrienol and β-tocopherol of embryonic axes.
ACKNOWLEDGMENTS
This work was supported by funding from the Seventh Framework Programme of the European Union, grant 311840 ‘EcoSeed’ (Impacts of Environmental Conditions on Seed Quality). Ines Bauer (University of Innsbruck) is thanked for conducting the carbonylation assay.
LITERATURE CITED
- Bagci E, Vural M, Dirmenci T, Bruehl L, Aitzetmuller K. 2004. Fatty acid and tocochromanol patterns of some Salvia L. species. Zeitschrift für Naturforschung C-a Journal of Biosciences 59: 305–309. [DOI] [PubMed] [Google Scholar]
- Bahin E, Bailly C, Sotta B, Kranner I, Corbineau F, Leymarie J. 2011. Crosstalk between reactive oxygen species and hormonal signalling pathways regulates grain dormancy in barley. Plant Cell and Environment 34: 980–993. [DOI] [PubMed] [Google Scholar]
- Bailly C, Kranner I. 2011. Analyses of reactive oxygen species and antioxidants in relation to seed longevity and germination. In: Kermode AR, ed. Seed dormancy: methods and protocols, methods in molecular biology. Berlin: Springer Science + Business Media, 343–367. [DOI] [PubMed] [Google Scholar]
- Bailly C, Benamar A, Corbineau F, Come D. 1996. Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiologia Plantarum 97: 104–110. [Google Scholar]
- Bailly C, El-Maarouf-Bouteau H, Corbineau F. 2008. From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. Comptes Rendus Biologies 331: 806–814. [DOI] [PubMed] [Google Scholar]
- Bazin J, Batlla D, Dussert S, El-Maarouf-Bouteau H, Bailly C. 2011. Role of relative humidity, temperature, and water status in dormancy alleviation of sunflower seeds during dry after-ripening. Journal of Experimental Botany 62: 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Bradford K, Hilhorst H, Nonogaki H. 2013. Seeds: physiology of development, germination and dormancy. New York: Springer. [Google Scholar]
- Birtic S, Colville L, Pritchard HW, Pearce SR, Kranner I. 2011. Mathematically combined half-cell reduction potentials of low-molecular-weight thiols as markers of seed ageing. Free Radical Research 45: 1093–1102. [DOI] [PubMed] [Google Scholar]
- Bowsher CG, Boulton EL, Rose J, Nayagam S, Emes MJ. 1992. Reductant for glutamate synthase in generated by the oxidative pentose phosphate pathway in non-photosynthetic root plastids. The Plant Journal 2: 893–898. [Google Scholar]
- Corbineau F, Bagniol S, Côme D. 1990. Sunflower (Helianthus annuus L.) seed dormancy and its regulation by ethylene. Israel Journal of Botany 39: 313–325. [Google Scholar]
- Davies MJ, Fu SL, Wang HJ, Dean RT. 1999. Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radical Biology and Medicine 27: 1151–1163. [DOI] [PubMed] [Google Scholar]
- El-Maarouf-Bouteau H, Bailly C. 2008. Oxidative signaling in seed germination and dormancy. Plant Signalling Behaviour 3: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk J, Munné-Bosch S. 2010. Tocochromanol functions in plants: antioxidation and beyond. Journal of Experimental Botany 61: 1549–1566. [DOI] [PubMed] [Google Scholar]
- Fernández-Marín B, Kranner I, San Sebastián M, Artetxe U, Laza JM, Vilas JL, Pritchard HW, Nadajaran J, Míguez F, Becerril JM, García-Plazaola JI. 2013. Evidence for the absence of enzymatic reactions in the glassy state. A case study of xanthophyll cycle pigments in the desiccation-tolerant moss Syntrichia ruralis. Journal of Experimental botany 64: 3033–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. 2006. Seed dormancy and the control of germination. New Phytologist 171: 501–523. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. 2005. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17: 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot SPC, Surki AA, de Vos RCH, Kodde J. 2012. Seed storage at elevated partial pressure of oxygen, a fast method for analysing seed ageing under dry conditions. Annals of Botany 110: 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot SPC, de Groot L, Kodde J, van Treuren R. 2015. Prolonging the longevity of ex situ conserved seeds by storage under anoxia. Plant Genetic Resources 13: 18–26. [Google Scholar]
- Halliwell B. 2006. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiology 141: 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Cai M, Long Q, et al. 2014. OsLOX2, a rice type I lipoxygenase, confers opposite effects on seed germination and longevity. Transgenic Research 23: 643–655. [DOI] [PubMed] [Google Scholar]
- Ibrahim AE, Roberts EH, Murdoch AJ. 1983. Viability of lettuce seeds. 2. Survival and oxygen-uptake in osmotically controlled storage. Journal of Experimental Botany 34: 631–640. [Google Scholar]
- ISTA. 2014. The International Rules for Seed Testing, 5.1–5.7. Bassersdorf, Switzerland: ISTA. [Google Scholar]
- Job C, Rajjou L, Lovigny Y, Belghazi M, Job D. 2005. Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiology 138: 790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibinza S, Bazin J, Bailly C, Farrant JM, Corbineau F, El-Maarouf-Bouteau H. 2011. Catalase is a key enzyme in seed recovery from ageing during priming. Plant Science 181: 309–315. [DOI] [PubMed] [Google Scholar]
- Kranner I. 1998. Determination of glutathione, glutathione disulfide and two related enzymes, grutathione reductase and glucose-6-phosphate dehydrogenase, in fungal and plant cells. In: Varma A, ed. Mycorrhiza manual. New York: Springer, 227–241. [Google Scholar]
- Kranner I, Birtic S, Anderson KM, Pritchard HW. 2006. Glutathione half-cell reduction potential: a universal stress marker and modulator of programmed cell death? Free Radical Biology and Medicine 40: 2155–2165. [DOI] [PubMed] [Google Scholar]
- Kranner I, Roach T, Beckett RP, Whitaker C, Minibayeva FV. 2010. Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. Journal of Plant Physiology 167: 805–811. [DOI] [PubMed] [Google Scholar]
- Kranner I, Chen HY, Pritchard HW, Pearce SR, Birtic S. 2011. Inter-nucleosomal DNA fragmentation and loss of RNA integrity during seed ageing. Plant Growth Regulation 63: 63–72. [Google Scholar]
- Leymarie J, Vitkauskaite G, Hoang HH, et al. 2012. Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant and Cell Physiology 53: 96–106. [DOI] [PubMed] [Google Scholar]
- Li-Beisson Y, Shorrosh B, Beisson F, et al. 2013. Acyl-lipid metabolism. Arabidopsis Book 11: e0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Lv D, Wang P, et al. 2006. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18: 2749–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Carstens AC, Linkies A, Torres MA, Leubner-Metzger G. 2009. The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. New Phytologist 184: 885–897. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L. 2002. The function of tocopherols and tocotrienols in plants. Critical Reviews in Plant Sciences 21: 31–57. [Google Scholar]
- Nguyen TP, Keizer P, van Eeuwijk F, Smeekens S, Bentsink L. 2012. Natural variation for seed longevity and seed dormancy are negatively correlated in Arabidopsis. Plant Physiology 160: 2083–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oracz K, El-Maarouf Bouteau H, Farrant JM, et al. 2007. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant Journal 50: 452–465. [DOI] [PubMed] [Google Scholar]
- Oracz K, El-Maarouf-Bouteau H, Kranner I, Bogatek R, Corbineau F, Bailly C. 2009. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiology 150: 494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleite R, Pike MJ, Garcés R, Martínez-Force E, Rawsthorne S. 2005. The sources of carbon and reducing power for fatty acid synthesis in the heterotrophic plastids of developing sunflower (Helianthus annuus L.) embryos. Journal of Experimental Botany 56: 1297–1303. [DOI] [PubMed] [Google Scholar]
- Roach T, Kranner I. 2011. Extracellular superoxide production associated with secondary root growth following desiccation of Pisum sativum seedlings. Journal of Plant Physiology 168: 1870–1873. [DOI] [PubMed] [Google Scholar]
- Roberts EH. 1972. Viability of seeds. London: Chapman & Hall. [Google Scholar]
- Roqueiro G, Facorro GB, Huarte MG, et al. 2010. Effects of photooxidation on membrane integrity in Salix nigra seeds. Annals of Botany 105: 1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D. 2004. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16: 1419–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. 2001. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Biology and Medicine 30: 1191–1212. [DOI] [PubMed] [Google Scholar]
- Schwember AR, Bradford KJ. 2011. Oxygen interacts with priming, moisture content and temperature to affect the longevity of lettuce and onion seeds. Seed Science Research 21: 175–185. [Google Scholar]
- Seal CE, Zammit R, Scott P, Flowers TJ, Kranner I. 2010a. Glutathione half-cell reduction potential and α-tocopherol as viability markers during the prolonged storage of Suaeda maritima seeds. Seed Science Research 20: 47–53. [Google Scholar]
- Seal CE, Zammit R, Scott P, Nyamongo DO, Daws MI, Kranner I. 2010b. Glutathione half-cell reduction potential as a seed viability marker of the potential oilseed crop Vernonia galamensis. Industrial Crops and Products 32: 687–691. [Google Scholar]
- Stewart RRC, Bewley JD. 1980. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiology 65: 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasi F, Paciolla C, Arrigoni O. 1999. The ascorbate system in recalcitrant and orthodox seeds. Physiologia Plantarum 105: 193–198. [Google Scholar]
- Torres M, De Paula M, Perez-Otaola M, Darder M, Frutos G, Martinez-Honduvilla CJ. 1997. Ageing-induced changes in glutathione system of sunflower seeds. Physiologia Plantarum 101: 807–814. [Google Scholar]
- Zagorchev L, Seal CE, Kranner I, Odjakova M. 2013. A central role for thiols in plant tolerance to abiotic stress. International Journal of Molecular Sciences 14: 7405–7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.