Abstract
Background and Aims The importance of the alternative oxidase (AOX) pathway, particularly AOX1A, in optimizing photosynthesis during de-etiolation, under elevated CO2, low temperature, high light or combined light and drought stress is well documented. In the present study, the role of AOX1A in optimizing photosynthesis was investigated when electron transport through the cytochrome c oxidase (COX) pathway was restricted at complex III.
Methods Leaf discs of wild-type (WT) and aox1a knock-out mutants of Arabidopsis thaliana were treated with antimycin A (AA) under growth-light conditions. To identify the impact of AOX1A deficiency in optimizing photosynthesis, respiratory O2 uptake and photosynthesis-related parameters were measured along with changes in redox couples, reactive oxygen species (ROS), lipid peroxidation and expression levels of genes related to respiration, the malate valve and the antioxidative system.
Key Results In the absence of AA, aox1a knock-out mutants did not show any difference in physiological, biochemical or molecular parameters compared with WT. However, after AA treatment, aox1a plants showed a significant reduction in both respiratory O2 uptake and NaHCO3-dependent O2 evolution. Chlorophyll fluorescence and P700 studies revealed that in contrast to WT, aox1a knock-out plants were incapable of maintaining electron flow in the chloroplastic electron transport chain, and thereby inefficient heat dissipation (low non-photochemical quenching) was observed. Furthermore, aox1a mutants exhibited significant disturbances in cellular redox couples of NAD(P)H and ascorbate (Asc) and consequently accumulation of ROS and malondialdehyde (MDA) content. By contrast, WT plants showed a significant increase in transcript levels of CSD1, CAT1, sAPX, COX15 and AOX1A in contrast to aox1a mutants.
Conclusions These results suggest that AOX1A plays a significant role in sustaining the chloroplastic redox state and energization to optimize photosynthesis by regulating cellular redox homeostasis and ROS generation when electron transport through the COX pathway is disturbed at complex III.
Keywords: Alternative oxidase pathway, AOX, antimycin A, antioxidants, Arabidopsis thaliana, non-photochemical quenching, NPQ, photosynthesis, redox, respiration, reactive oxygen species, ROS
INTRODUCTION
The mitochondrial electron transport chain (mETC) of higher plants has unique features to transport electrons from reduced ubiquinone to molecular oxygen through the cyanide-insensitive alternative oxidase (AOX) pathway in parallel with the common cyanide-sensitive cytochrome c oxidase (COX) pathway. Electron flow through the COX pathway generates an electrochemical proton gradient across the inner mitochondrial membrane which drives the ATP synthase to convert ADP to ATP. By contrast, electron flow through the AOX pathway is non-phosphorylating as it bypasses two proton translocating complexes and the energy is dissipated as heat (Siedow and Umbach, 2000; Millenaar and Lambers, 2003). Thus, the AOX pathway may appear to be a wasteful process, but based on cellular energy demand, the COX and AOX pathways together maintain the cellular energy balance by regulating their relative distribution.
The role of the AOX pathway in heat generation in thermogenic plants to attract insects as pollinators was identified by Meeuse (1975) and later its significance in floral development was recognized (Wagner et al., 2008; Miller et al., 2011). Furthermore, its engagement was demonstrated in growth and development of higher plants including Salsola divaricata, a C3–C4 intermediate plant (Feng et al., 2007; Gandin et al., 2014b; Garmash et al., 2015). In addition, the role of AOX has become evident in various biotic and abiotic stress conditions such as combat against bacterial pathogens (Cvetkovska and Vanlerberghe, 2012), low oxygen (Clifton et al., 2005), ozone (Tosti et al., 2006), nutrient limitation (Noguchi and Terashima, 2006), salinity (Wang et al., 2010), metal toxicity (Tan et al., 2010), high/low temperature (Murakami and Toriyama, 2008; Wang et al., 2011), high light (HL) and/or drought (Giraud et al., 2008; Vassileva et al., 2009; Zhang et al., 2010; Yoshida et al., 2011a; Zhang et al., 2012) and high CO2 (Gandin et al., 2012).
Under abiotic stress conditions, the contribution of AOX-catalysed respiration becomes significant. It dissipates excess energy in the form of heat and thereby prevents over-reduction of the ubiquinone (UQ) pool, which in turn inhibits the generation of reactive oxygen species (ROS) (Vassileva et al., 2009; Yoshida et al., 2011a). The role of AOX is also recognized in alleviation of reactive nitrogen species, particularly of NO levels in mitochondria, which are induced by reducing equivalents (Gupta et al., 2012; Cvetkovska et al., 2014; Igamberdiev et al., 2014). The role of AOX is not limited to combating stress and was also proposed to be triggered by a variety of signals and acts as a buffer which determines the threshold for the induction of programmed cell death (Van Aken et al., 2009). In breeding studies, AOX was suggested as a marker to bring about efficient cell reprogramming during growth and development under stressful conditions (Arnholdt-Schmitt et al., 2006).
In addition, the physiological role of AOX is well accepted in optimizing photosynthesis. Several studies have demonstrated that any interference in the COX or AOX pathway (using mutants or chemicals) caused a significant decrease in photosynthetic O2 evolution in mesophyll protoplasts or leaf tissue (Padmasree and Raghavendra, 1999a; Yoshida et al., 2006; Strodtkötter et al., 2009; Dinakar et al., 2010a; Zhang et al., 2012; Vishwakarma et al., 2014). However, during stress conditions (drought, HL, high CO2 and temperature) engagement of the AOX pathway was much higher compared with the COX pathway (Vassileva et al., 2009; Dinakar et al., 2010b; Gandin et al., 2012, 2014b; Zhang et al., 2014).
During photosynthesis, reducing equivalents are generated via photoreduction of NADP+ catalysed by the ferredoxin-NADP+ reductase (FNR). The regeneration of NADP+, the terminal electron acceptor, is essential for the avoidance of ROS production, otherwise this can lead to damage of the thylakoid membrane (Niyogi, 1999; Raghavendra and Padmasree, 2003). CO2 assimilation in the Calvin cycle and the malate valve along with malate dehydrogenase (MDH) are essential in NADP+ regeneration, allowing for continued ATP production via the photosynthetic electron transport chain.
Given that plant membranes are broadly impermeable to NAD(P)+ and NAD(P)H, plant cells possess specific translocators enabling the direct and indirect exchange of reducing equivalents. On the one hand, NAD(P)+ can directly be shuttled between subcellular compartments via NAD+-carrier proteins localized in mitochondria as well as in plastids. However, these transporters exhibit a low affinity for NADH and NADPH (Palmieri et al., 2009). On the other hand, plant cells possess specific translocators for the exchange of malate and oxaloacetate (malate–OAA shuttle) enabling the indirect transport of reducing equivalents between different subcellular compartments. Accordingly, malate–OAA shuttles together with MDHs (malate valve) act as a powerful system for balancing the ATP/NAD(P)H ratio and maintaining redox homeostasis in plant cells.
The chloroplastic NADP-dependent MDH (NADP-MDH) uses excess NADPH generated via the photosynthetic electron transport chain to convert OAA to malate, regenerating the electron acceptor NADP+ (Scheibe, 2004). The resulting malate generated through the activity of NADP-MDH in chloroplasts is subsequently translocated to the cytosol via the malate–OAA shuttle, where the interconversion of malate to OAA with concomitant reduction of NAD+ to NADH catalysed by the redox-regulated cytosolic NAD-dependent MDH takes place (Hara et al., 2006). This mechanism allows chloroplastic reducing equivalents to be indirectly transferred to the cytosol and other subcellular compartments, for instance mitochondria, to avoid (1) imbalances in the generation and utilization of reducing equivalents by photochemical reactions and the Calvin cycle and (2) depletion of chloroplast energy carriers which would lead to oxidative stress. A concomitant rise in AOX protein and its respiratory capacity in parallel with an increase in the activity of various enzymes (NADP-malate dehydrogenase, NAD-malate dehydrogenase, NAD-malic enzyme, NADP-isocitrate dehydrogenase) involved in the transport of these reducing equivalents suggests a role of AOX in modulating the cellular redox state and thereby the photosynthetic performances under HL conditions (Fernie et al., 2004; Scheibe et al., 2005; Yoshida et al., 2007, 2008; Xu et al., 2011). The rise in cellular levels of pyruvate under HL conditions emphasized the role of AOX in dissipating excess chloroplastic reducing equivalents (Dinakar et al., 2010b). Thus, the function of AOX is tightly coupled with photosynthesis, as it has a direct impact on the supply and demand of ATP, NAD(P)H and carbon intermediates (Vanlerberghe, 2013). Cooperation between the AOX pathway and assimilation to maintain optimal rates of photosynthesis by regulating the accumulation of reducing equivalents and the over-reduction of the chloroplastic electron transport chain was shown using T-DNA insertion mutants for AOX1A (Gandin et al., 2014a). The co-expression of AOX with other non-phosphorylating genes of the mitochondrial electron transport chain such as NDB52, UCP1 and UCP2 is well known under HL treatment to prevent an over-reduction of the plastoquinone (PQ) and UQ pools (Yoshida et al., 2008, 2011a).
In higher plants, AOX is encoded by two discrete subfamilies. AOX1 is widely known to be induced by stress stimuli and is present in both monocot and dicot plants. By contrast, AOX2 is developmentally expressed and confined to eudicots (Considine et al., 2002). In Arabidopsis, AOX1A and AOX1D are highly stress-responsive amongst hundreds of known genes encoding mitochondrial proteins (Clifton et al., 2006), indicating the essentiality of these genes to cope with stress conditions.
Apart from mitochondrial respiration, plants possess several strategies such as photorespiration and antioxidative systems to adapt to stress conditions. Interestingly, these metabolic pathways were found to be modulated upon constraints at AOX (Strodtkötter et al., 2009; Voss et al., 2013; Vishwakarma et al., 2014). Ascorbate (Asc) is an antioxidant molecule which is abundantly present in all subcellular compartments of plant tissues and occurs at as high as 20 mm in chloroplasts (Smirnoff and Wheeler, 2000). It plays an important role in the detoxification of H2O2 during stress conditions. Furthermore, AOX1A overexpression lines showed higher rates of Asc production when compared with wild-type (WT) in Arabidopsis leaves (Bartoli et al., 2006). Various mitochondrial retrograde regulation (MRR) signals are known to stimulate the expression of AOX1A (Rhoads and Subbaiah, 2007; Suzuki et al., 2012). In rice, AOX1A and AOX1B were expressed during abiotic stress conditions through MRR signalling mediated by radicals (Li et al., 2013). The deficiency of AOX1A during the restriction of the COX pathway caused a significant reduction in photosynthesis, increase in ion leakage and ROS generation, which ultimately led to wilting and necrosis of the plant in spite of the increase in photorespiration (Strodtkötter et al., 2009; Voss et al., 2013).
In the present study, we used both chemical and reverse genetic approaches together to examine (1) the physiological importance of AOX1A in modulating the redox state of chloroplastic electron transport carriers and non-photochemical quenching (NPQ) to sustain photosynthesis, and (2) the impact of AOX1A in regulating the malate valve and the antioxidative systems to maintain the cellular redox state and ROS for optimal photosynthetic performance when complex III of the COX pathway is not functional in the presence of antimycin A (AA).
MATERIALS AND METHODS
Plant materials and growth conditions
All experiments were conducted using Columbia (WT) and aox1a T-DNA-insertion (SALK_084897) lines of Arabidopsis thaliana. We have previously shown that the T-DNA-insertion lines were homozygous for AOX1A through northern blot (Strodtkötter et al., 2009) and RT-PCR analysis (Vishwakarma et al., 2014). Seeds were sown on soilrite mix with half-strength Murashige and Skoog medium (MS medium), followed by stratification at 2–4 °C in the dark for 3–4 d before transferring them to the growth chamber. Plants were watered on alternate days and half-strength MS medium was given twice a week. The plants were maintained in a growth chamber at 22–24 °C with 8-h photoperiod and photosynthetic photon flux density (PPFD) of 50–60 µmol photons m−2 s−1.
Antimycin A treatment
Leaf discs (approx. 0·25 cm2) were prepared from the leaves of 10- to 12-week-old plants with a sharp paper punch under water from either side of the midrib. Leaf discs were placed in a series of Petri dishes containing different concentrations (5, 10, 20, 30 and 40 µm) of AA in 0·01 % Tween-20 and were illuminated for 6 h at 50 µmol photons m−2 s−1 (growth light). Controls were treated with only 0·01 % Tween-20 in water, but the effect was negligible. The treated leaf discs were directly used to monitor changes in respiration, photosynthesis, chlorophyll fluorescence and ROS levels. Leaf discs were frozen at −80 °C and subsequently analysed for non-protein redox couples (NADH, NADPH and Asc), malondialdehyde (MDA) content and transcript levels.
Measurement of respiration and photosynthesis
The rates of respiratory O2 uptake and photosynthetic O2 evolution of leaf discs were monitored using a Clark-type oxygen electrode (LD-2, Hansatech Instruments, King’s Lynn, UK). Twenty leaf discs were placed on a topmost nylon mesh, which was homogeneously sprinkled with 200 µL 1·0 m bicarbonate buffer (pH 9·0) to generate approx. 5 % (v/v) CO2 in the electrode chamber (Walker and Walker, 1987). The levels of O2 in the electrode chamber were calibrated as per the manufacturer’s instructions before measurement of respiratory and photosynthetic rates for each sample. After calibration, the traces of O2 uptake were monitored for 5 min in the dark and subsequently the traces of O2 evolution were monitored for 10 min at a PPFD of 250 µmol photons m−2 s−1, which is optimal for A. thaliana leaves (Vishwakarma et al., 2014).
Chlorophyll fluorescence and P700 measurement
Chlorophyll fluorescence and P700 absorbance were measured with a pulse amplitude modulation (PAM) fluorometer (Dual-PAM-100, Heinz Walz GmbH, Effeltrich, Germany). After treatment with AA (20 µm) in the light, the leaf discs were incubated in the dark for 30 min to allow all reaction centres to attain their oxidized state. Chlorophyll fluorescence and P700 parameters were measured for 5 min at 25 °C using the repetitive saturation pulse (SP) method as described by Vishwakarma et al. (2014). The intensity and duration of the SP were 3000 µmol photons m−2 s−1 and 800 ms, respectively. Actinic light (AL) was maintained at 126 µmol photons m−2 s−1. The P700-redox state was measured at two different wavelengths (875 and 830 nm) as described by Klughammer and Schreiber (2008). Maximal quantum yield of photosystem II (PSII) (Fv/Fm), effective quantum yield of PSII [Y(II)], photochemical quenching (qP), NPQ, electron transport rate of PSII [ETR(II)], quantum yield of PSI [Y(I)], reduced P700 (P700red), acceptor-side limitation [Y(NA)] and donor-side limitation [Y(ND)] were automatically calculated by the Dual-PAM-100 software.
NAD(H) and NADP(H) measurements
The total cellular pools of NAD(H) and NADP(H) were measured according to Queval and Noctor (2007). The reduced and oxidized forms of pyridine nucleotides are distinguished by preferential destruction in acid/base. Therefore, leaf discs (100 mg) were finely ground in liquid nitrogen and extracted either in 1·0 mL of 0·2 m HCl for measuring NAD+ and NADP+ or 0·2 m NaOH for measuring NADH and NADPH, respectively. Pyridine nucleotides were quantified by monitoring the phenazine methosulfate-catalysed reduction of dichlorophenolindophenol. For the assay of NAD+ and NADH, the reaction was started by the addition of ethanol in the presence of alcohol dehydrogenase. Similarly, for the assay of NADP+ and NADPH, the reaction was started by addition of glucose 6-phosphate dehydrogenase in the presence of glucose 6-phosphate. In both cases, the decrease in A600 was monitored for 3 min, and concentrations of the corresponding pyridine nucleotides were calculated from calibration curves with the relevant standards (0–40 pmol).
Measurement of ascorbate and dehydroascorbate levels
Asc and dehydroascorbate (DHA) contents were measured according to Foyer et al. (1983). Leaf discs (150 mg) were ground in 3 mL of 2·5 m HClO4 on ice using a mortar and pestle. The extract was adjusted to pH 5·6 by stepwise addition of 1·25 m K2CO3. The resulting precipitate was removed by centrifugation at 8000 g for 10 min at 4 °C. The supernatant was used to estimate the Asc and DHA content and Asc/DHA. An aliquot of 100 µL of the supernatant was added to 900 µL of 0·1 m sodium phosphate buffer (pH 5·6) and the absorbance was measured at 265 nm (A). Ascorbate oxidase (2·5 U, Roche Applied Science, Mannheim, Germany) was added to the above mixture and the absorbance at 265 nm was measured (B). A fresh aliquot containing 100 µL of supernatant was incubated with 10 mm reduced glutathione prepared in 0·1 m Tricine–KOH buffer (pH 8·5) for 15 min at room temperature. The volume was then filled up to 1 mL with 0·1 m sodium phosphate buffer (pH 5·6) and the absorbance was measured at 265 nm (C). Asc was measured as the difference of A minus B, while DHA was measured as the difference of C minus A.
Detection of ROS
After AA treatment, leaf discs were vacuum-infiltrated with 25 µm 2,7-dichlorofluorescein diacetate (H2DCF-DA) at pH 7·4 in 10 mm Tris-HCl (Vishwakarma et al., 2014). The non-polar H2DCF-DA is converted to membrane-impermeable polar H2DCF by cellular esterases and is rapidly oxidized to highly fluorescent DCF by intracellular H2O2. Excess H2DCF-DA was removed by repeated washing steps of the leaf discs, and the samples were immediately monitored for ROS under a laser-scanning confocal microscope (TCSSP-2, AOBS 4 channel UV and visible; Leica, Wetzlar, Germany) at λex 488 nm and λem 525 nm. Total cellular ROS was monitored as green DCF fluorescence, whereas chloroplastic ROS was detected as orange–yellow fluorescence which is formed due to superimposition of chlorophyll autofluorescence (red) and DCF fluorescence (green).
Measurement of H2O2 and lipid peroxidation
Leaf tissue (100 mg) was homogenized in 0·1 % trichloroacetic acid (TCA) on ice. The homogenate was centrifuged at 12 000 g for 15 min at 4 °C. The supernatant obtained was used for quantification of both H2O2 and lipid peroxidation. Total cellular H2O2 was estimated according to Velikova et al. (2000), while lipid peroxidation was quantified in terms of MDA content according to Heath and Packer (1968). For the assay of H2O2, the supernatant (250 µL) was added to an assay medium containing 250 µL 10 mm potassium phosphate buffer (pH 7·0) and 500 µL 1·0 m KI. Absorbance was measured immediately at 390 nm and the concentration of H2O2 was calculated using the relevant standard (0–100 µmol H2O2). For the assay of lipid peroxidation, 1·5 mL 20 % TCA containing 0·5 % thiobarbituric acid (TBA) was added to 500 µL supernatant and boiled in a water bath for 30 min. The reaction was stopped by chilling and centrifuged for 5 min at 12 000 g at 4 °C. The absorbance of the supernatant was measured at 532 nm and corrected for non-specific absorbance at 600 nm. The MDA content was calculated using the extinction coefficient of the MDA–TBA complex (ε = 155 mm−1 cm−1).
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from leaf discs using TRI Reagent (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer’s instructions. One microgram of total RNA was used for first-strand cDNA synthesis using the iScript™ cDNA synthesis kit (BioRad, Hercules, CA, USA), which was performed as indicated in the manufacturer’s instructions. Transcript levels were measured using a 7500 fast real-time PCR machine (Applied Biosystems, Foster City, CA, USA). Two microlitres of cDNA (50 ng) was amplified in the presence of 10 µL 2× SYBR Green PCR Master Mix (Takara Bio, Shiga, Japan), 0·5 µL specific primers (1·0 pm) and 7·0 µL of sterilized water. PCR conditions were 95 °C for 10 min, 40 cycles of 95 °C for 15 s followed by 60 °C for 1 min. The primers used in the present study are listed in Supplementary Data Table S1. Cycle threshold (CT) values were obtained from the exponential phase of PCR amplification. The comparative CT method was used to analyse the relative mRNA expression as described by Livak and Schmittgen (2001). In this method, genes of interest (GOI) were normalized against UBQ5 (housekeeping gene) expression, generating a ΔCT value (ΔCT = GOI CT−UBQ5 CT). The relative expression was then calculated according to the equation 2−ΔΔCT and WT without AA was used as a calibrator.
Statistical analysis
Data presented are means (±s.e.) from three to four independent experiments conducted on different days. Triplicates were run in each independent experiment. The differences between treatments were analysed by one-way ANOVA and Tukey’s test of multiple comparison analysis using Sigma Plot 11.0 software (San José, CA, USA).
RESULTS
Effect of AA on total respiratory O2 uptake and photosynthetic O2 evolution in WT plants and AOX1A T-DNA mutants (aox1a)
The rates of total respiratory O2 uptake and bicarbonate-dependent photosynthetic O2 evolution, the latter an indicator of photosynthetic carbon assimilation (Calvin cycle activity), were monitored in leaf discs of WT and aox1a mutants in the presence of AA, a metabolic inhibitor of complex III, to examine the significance of the AOX pathway in optimizing photosynthesis. The inhibition of electron transport through the COX pathway simulates a metabolic state of oxidative stress. In controls, the rates of respiratory O2 uptake and photosynthetic O2 evolution varied marginally between WT and aox1a mutants. However, the rates of respiratory O2 uptake and photosynthetic O2 evolution varied significantly between WT and aox1a plants when the leaf discs were exposed to increasing concentrations of AA (Fig. 1A, B). When the concentration of AA was increased from zero to 40 µm, WT leaf discs showed 54 % inhibition in rates of respiratory O2 uptake and 60 % inhibition in rates of photosynthetic O2 evolution when compared with their controls without AA. In contrast to WT, in the presence of 40 µm AA, aox1a mutants showed 84 % inhibition in rates of respiratory O2 uptake and 81 % inhibition in rates of photosynthetic O2 evolution. Thus, these results suggest that disturbances in the mitochondrial electron transport through the COX pathway compromised the photosynthetic carbon assimilation of chloroplasts more seriously in aox1a than in WT. As AA is also known to inhibit cyclic electron transport, a low concentration of AA (20 µm) was used in further experiments. At this concentration, AA caused marginal decreases in rates of respiration and photosynthesis in WT, while the rates of respiration and photosynthesis were drastically reduced in aox1a mutants (Fig. 1A, B). These results suggest that 20 µm AA can be applied for restriction of the COX pathway to reveal the importance of AOX1A in optimizing photosynthetic carbon assimilation.
Fig.1.
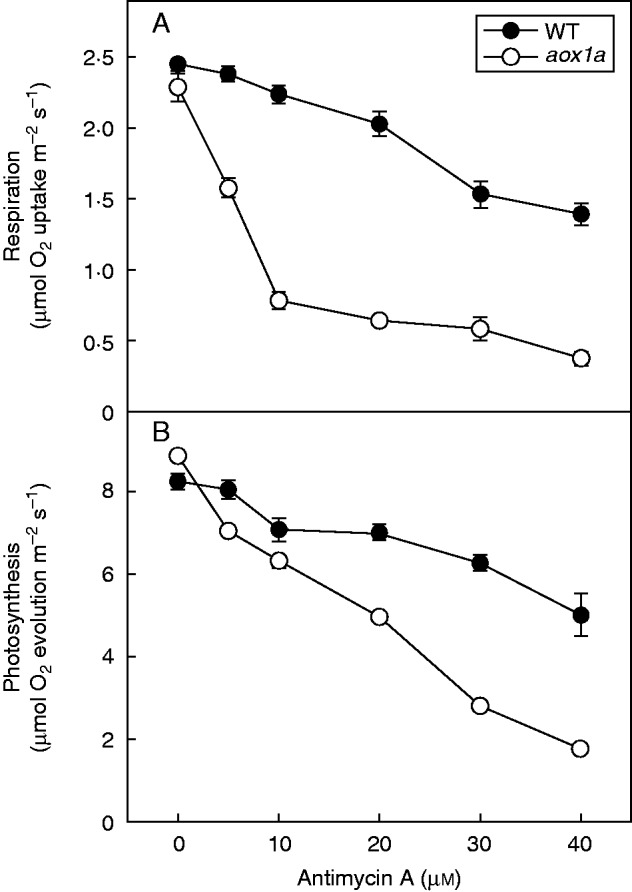
The rates of respiratory O2 uptake (A) and photosynthetic O2 evolution (B) in leaf discs of WT and aox1a mutants after pre-illumination (6 h) at a PPFD of 50 µmol m−2 s−1 at 25 °C in the absence and presence of AA. After pre-illumination, leaf discs were incubated in the dark for 10–15 min during electrode calibration. Subsequently, respiration was measured in the dark for 5 min followed by photosynthesis measured in the light for 10 min at 250 µmol m−2 s−1 as NaHCO3-dependent (approx. 5 %, v/v) O2 evolution.
Effect of AOX1A deficiency on chlorophyll fluorescence and P700 parameters in the presence of AA
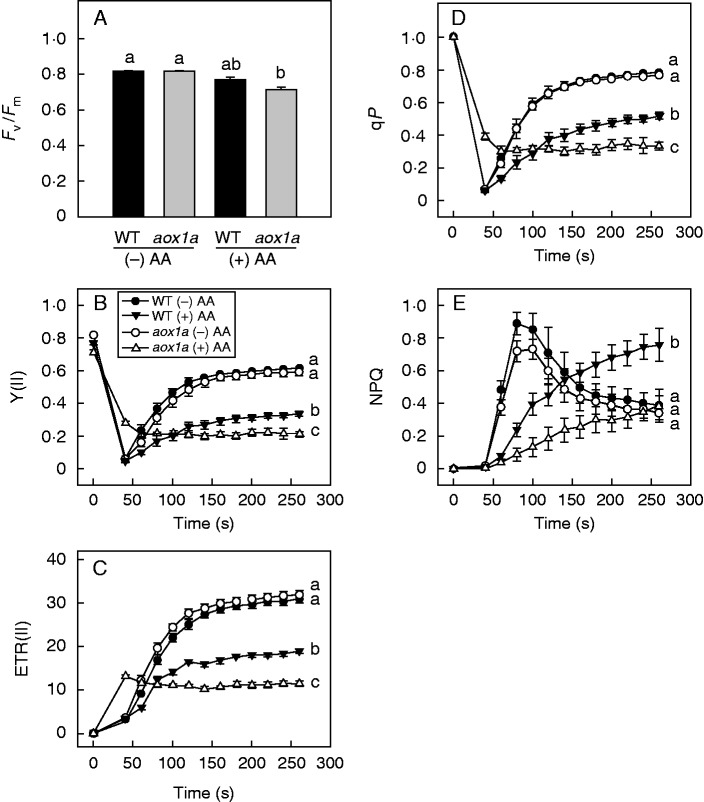
The changes occurring in chloroplastic reaction centres and the electron transport chain were monitored to examine the importance of AOX1A in keeping up photochemical reactions and thermal dissipation, which play significant roles in photoprotection and meeting the energy demands of the photosynthetic carbon reduction cycle during oxidative stress due to restriction of electron transport through the COX pathway. The maximum quantum yield of PSII (Fv/Fm), which indicates the maximum efficiency of PSII, was measured after applying the initial SP. In the absence of AA, both WT and aox1a knock-out plants showed no significant differences in their Fv/Fm ratio. After AA treatment, the Fv/Fm ratio decreased in both genotypes but to a larger extent in aox1a mutants than in WT (Fig. 2A). These results suggest the importance of the AOX pathway in maintaining PSII efficiency at their maximum levels. After measurement of the Fv/Fm ratio, the AL was switched off (40 s) to allow the reaction centres of PSII to attain their oxidized state. Subsequently, various parameters related to chlorophyll fluorescence and P700 were recorded with every SP given up to 260 s at regular intervals of 20 s. In the absence of AA, a similar pattern of Y(II), ETR(II), qP, NPQ, Y(I), P700red, Y(ND) and Y(NA) was observed for WT and aox1a plants (Figs 2B–E and 3). However, after AA treatment Y(II), ETR(II) and qP decreased significantly in aox1a compared with WT (Fig. 2B–D). By contrast, NPQ, an indicator of thermal dissipation, was increased in WT but remained unchanged in aox1a mutants (Fig. 2E). Similarly, Y(I) and the reduction state of P700 decreased drastically in aox1a compared with WT upon AA treatment (Fig. 3A, B). Y(ND) increased in both genotypes but to a larger extent in aox1a mutants. However, such an increase in Y(NA) was not observed in both WT and aox1a plants, irrespective of AA treatment (Fig. 3C, D). These results indicate that AOX1A plays a crucial role in maintaining the electron flow between PSII and PSI, and consequently ΔpH and thereby NPQ to dissipate excess energy in the form of heat.
Fig.2.
Characteristics of chlorophyll fluorescence parameters in WT and aox1a from control (Tween-20) and treated (20 µm AA in Tween-20) leaf discs pre-illuminated (6 h) at a PPFD of 50 µmol m−2 s−1 at 25 °C: (A) maximum quantum yield of PSII (Fv/Fm), (B) relative quantum yield of PSII [Y(II)], (C) electron transport rate of photosystem II (ETRII), (D) photochemical quenching (qP), (E) non-photochemical quenching (NPQ). Other details for measurement of chlorophyll fluorescence parameters were as described in Materials and methods. Different lower-case letters indicate statistically significant differences (P < 0·05).
Fig.3.
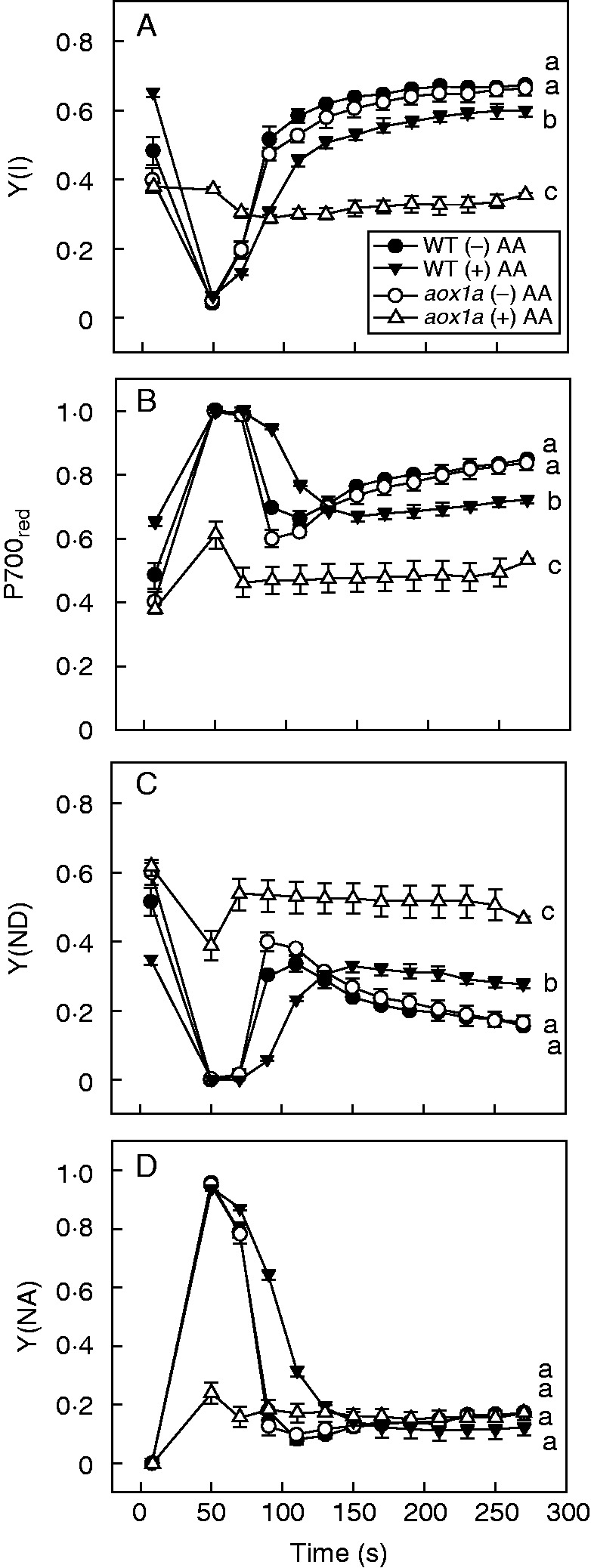
Characteristics of P700 parameters in WT and aox1a from control (Tween-20) and treated (20 µm AA in Tween-20) leaf discs pre-illuminated (6 h) at a PPFD of 50 µmol m−2 s−1 at 25 °C: (A) quantum yield of PSI [Y(I)], (B) reduced P700 (P700red), (C) limitation at the acceptor side of PSI [Y(NA)], (D) limitation at the donor side of PSI [Y(ND)]. Other details for measurement of P700 parameters were as described in Materials and methods. Different lower-case letters indicate statistically significant differences (P < 0·05).
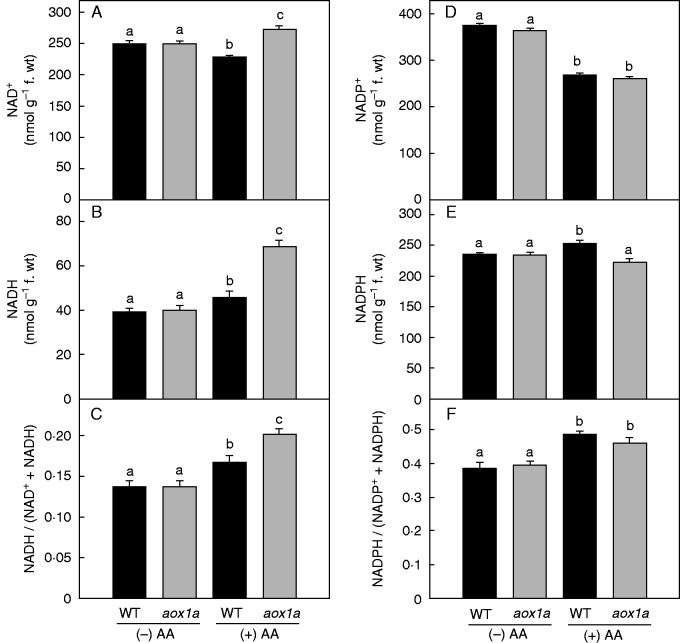
Effect of AOX1A deficiency on cellular redox couples in the presence of AA
The role of the AOX pathway in regulating cellular redox homeostasis to optimize photosynthesis during oxidative stress was determined by monitoring changes in total cellular oxidized and reduced pools of redox couples related to NAD(H), NADP(H) and Asc (Figs 4 and 5).
Fig.4.
Changes in total cellular oxidized and reduced pools of redox couples related to NAD(H) and NADP(H) and their redox ratio in WT and aox1a from control (Tween-20) and treated (20 µm AA in Tween-20) leaf discs pre-illuminated (6 h) at a PPFD of 50 µmol m−2 s−1 at 25 °C: (A) NAD+, (B) NADH, (C) NADH/(NAD++NADH) ratio, (D) NADP+, (E) NADPH and (F) NADPH/(NADP++NADPH) ratio. Pyridine nucleotide contents were calculated with their corresponding standards. Other details for measurement of NAD(P) and NAD(P)H were as described in Materials and methods. Different lower-case letters indicate statistically significant differences (P < 0·05).
Fig.5.
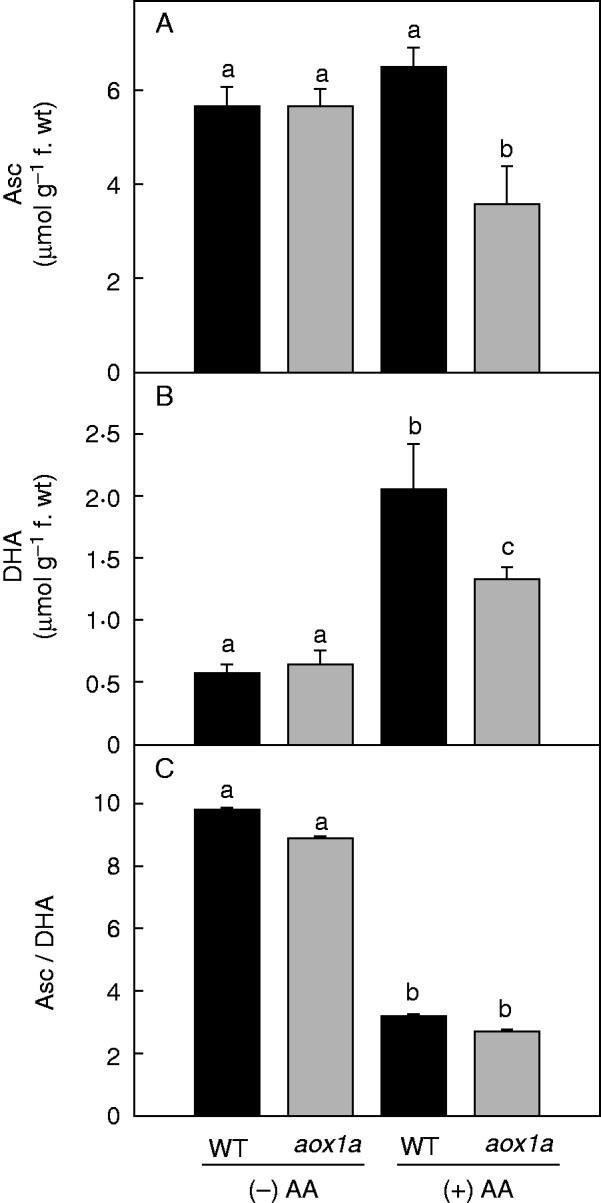
Changes in total cellular oxidized and reduced pools of Asc redox couple in WT and aox1a from control (Tween-20) and treated (20 µm AA in Tween-20) leaf discs pre-illuminated (6 h) at a PPFD of 50 µmol m−2 s−1 at 25 °C: (A) Asc, (B) DHA and (C) Asc/DHA ratio. Other details for measurement of Asc were as described in Materials and methods. Different lower-case letters indicate statistically significant differences (P < 0·05).
In control samples, levels of NAD(P)+ and NAD(P)H and the ratio of NAD(P)H to the total cellular pools of NAD(P)+ plus NAD(P)H were similar in both WT and aox1a mutants (Fig. 4). Upon AA treatment, the increase in the ratio of NADH to the total cellular NAD+ plus NADH pool was significant in aox1a mutants when compared with WT plants (Fig. 4C). In contrast, the redox ratio of NADPH to the total cellular NADP+ plus NADPH pools increased significantly in both genotypes (Fig. 4F). In control samples, the cellular levels of Asc and its oxidized form DHA did not vary between WT and aox1a, while the redox ratios (Asc/DHA) were modulated marginally (Fig. 5). Upon AA treatment, Asc levels increased in WT but decreased in aox1a plants compared with their respective controls (Fig. 5A). By contrast, levels of DHA increased in both genotypes compared with their respective controls (Fig. 5B). Also, the Asc/DHA ratio decreased significantly in both genotypes in the presence, but not in the absence, of AA (Fig. 5C).
These results suggest that the deficiency of AOX1A did not alter the cellular redox state under control conditions. However, upon restriction of the COX pathway, the cellular redox homeostasis was disturbed in both WT and aox1a genotypes. The imbalance in the cellular redox state was more prominent in aox1a than WT, indicating the important role of AOX1A in regulating cellular redox homeostasis and thereby photosynthetic performance.
Effect of AOX1A deficiency on intracellular ROS accumulation and membrane damage in the presence of AA
The role of AOX1A in alleviating cellular ROS and minimizing membrane damage as well as the optimization of photosynthetic performance of leaves during oxidative stress was evaluated by monitoring changes in cellular ROS levels as DCF fluorescence as well as H2O2 and MDA content (Figs 6 and 7). Furthermore, changes in chloroplastic ROS were differentiated from other cellular ROS by monitoring the pseudo orange/yellow colour formed due to superimposition of chlorophyll autofluorescence and DCF fluorescence. In control leaf discs, DCF fluorescence and orange/yellow fluorescence was marginally higher in aox1a knock-out mutants compared with WT (Fig. 6A). In contrast, after AA treatment, DCF as well as orange/yellow fluorescence increased significantly in aox1a mutants (Fig. 6B). In addition, both genotypes showed an increase in H2O2 levels and MDA content in the presence of AA (Fig. 7). The increase in H2O2 was more pronounced in aox1a than in WT compared with their respective controls (Fig. 7A). These results suggest that the deficiency of AOX1A caused an accumulation of total cellular as well as chloroplastic ROS.
Fig.6.
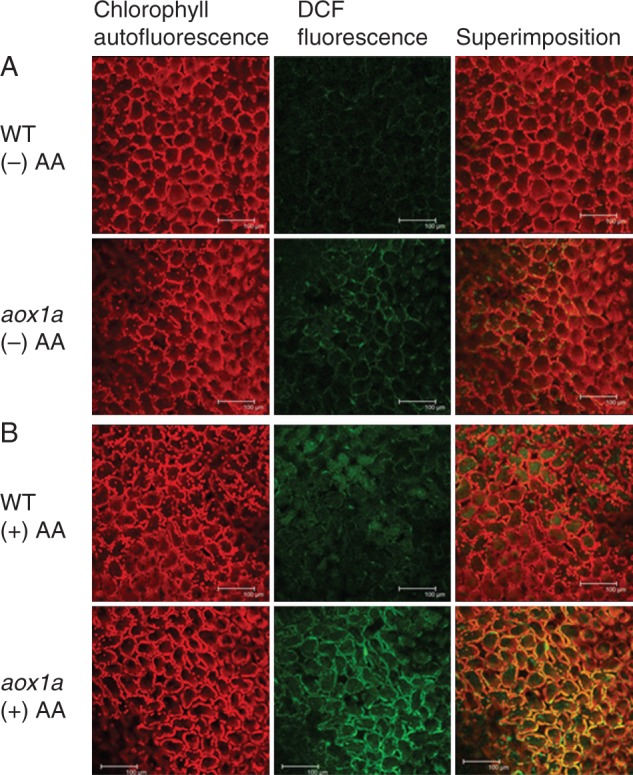
Measurement of ROS formation in WT and aox1a from (A) control (Tween-20) and (B) treated (20 µm AA in Tween-20) leaf discs pre-illuminated (6 h) at a PPFD of 50 µmol m−2 s−1 at 25 °C using confocal laser scanning microscopy. Chlorophyll autofluorescence is shown in red, DCF fluorescence, which represents cellular ROS, is shown in green and superimposed images representing chloroplastic ROS in pseudo orange/yellow. All scale bars = 100 µm.
Fig.7.
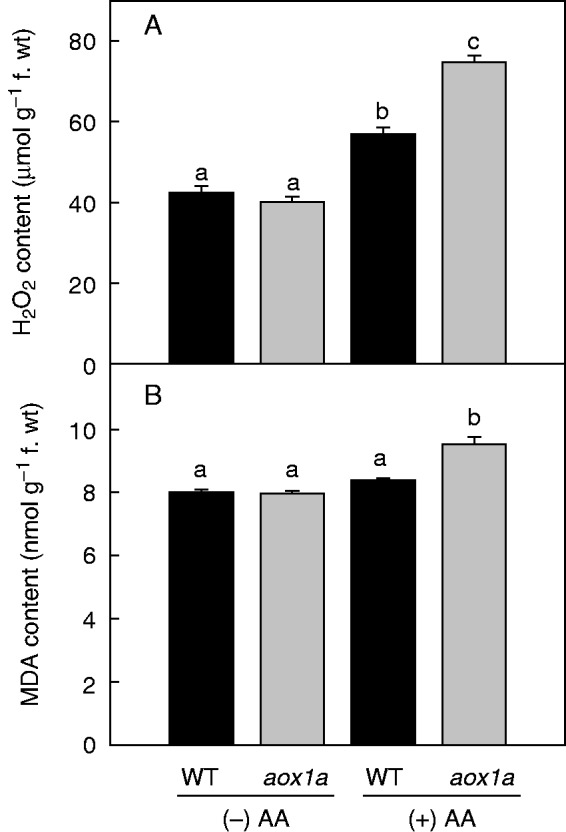
Quantification of H2O2 formation (A) and lipid peroxidation as MDA content (B) in WT and aox1a from control (Tween-20) and treated (20 µm AA in Tween-20) leaf discs pre-illuminated (6 h) at a PPFD of 50 µmol m−2 s−1 at 25 °C. Further details were as described in Materials and methods. Different lower-case letters indicate statistically significant differences (P < 0·05).
Effect of AOX1A deficiency on gene expression of antioxidants, the malate–OAA shuttle and respiratory enzymes in the presence of AA
The role of AOX1A in regulating the malate–OAA shuttle and the antioxidative system during oxidative stress was monitored by evaluating changes in transcript levels of genes related to (1) respiratory metabolism: PFK4 – phosphofructokinase, AOX1A – alternative oxidase and COX15 – cytochrome oxidase; (2) malate–OAA shuttle: chlMDH – NADP-dependent chloroplastic malate dehydrogenase, MMDH1 – NAD-dependent mitochondrial malate dehydrogenase and ICDH NADP-dependent chloroplastic/peroxisomal isocitrate dehydrogenase; and (iii) antioxidative enzymes: CSD1 – cytosolic superoxide dismutase, CAT1 – peroxisomal/chloroplastic catalase and sAPX – stromal ascorbate peroxidase (Fig. 8). The expression of AOX1A was notably increased in WT (18-fold) in the presence of AA, whereas no transcript could be detected in aox1a plants, as expected for this knock-out mutant (Fig. 8A). In the absence of AA, the expression of all genes examined in aox1a knock-out mutants was generally similar to WT except sAPX, which is increased in aox1a compared with WT (Fig. 8). Upon AA treatment, the expression of the glycolytic enzyme PFK4 decreased in both genotypes compared with controls without AA (Fig. 8A). In contrast, the expression of COX15 increased in WT, while it remained unchanged in aox1a (Fig. 8A). Similar to PFK4, in the presence of AA, the expression of chlMDH, MMDH1 and ICDH genes related to the malate–OAA shuttle decreased in both genotypes (Fig. 8B). However, the decrease in the expression of MMDH1 and ICDH was more pronounced in aox1a than in WT. By contrast, in the presence of AA, expression of the antioxidative genes CSD1, CAT1 and sAPX increased in WT. These genes showed differential expression in aox1a. Transcript levels of CSD1 increased, while sAPX decreased in leaf discs treated with AA, whereas CAT1 expression was unchanged (Fig. 8C). These results suggest that during COX pathway inhibition, the deficiency of AOX1A not only perturbs respiratory metabolism but also modulates the antioxidative system in order to balance ROS generation and detoxification.
Fig.8.
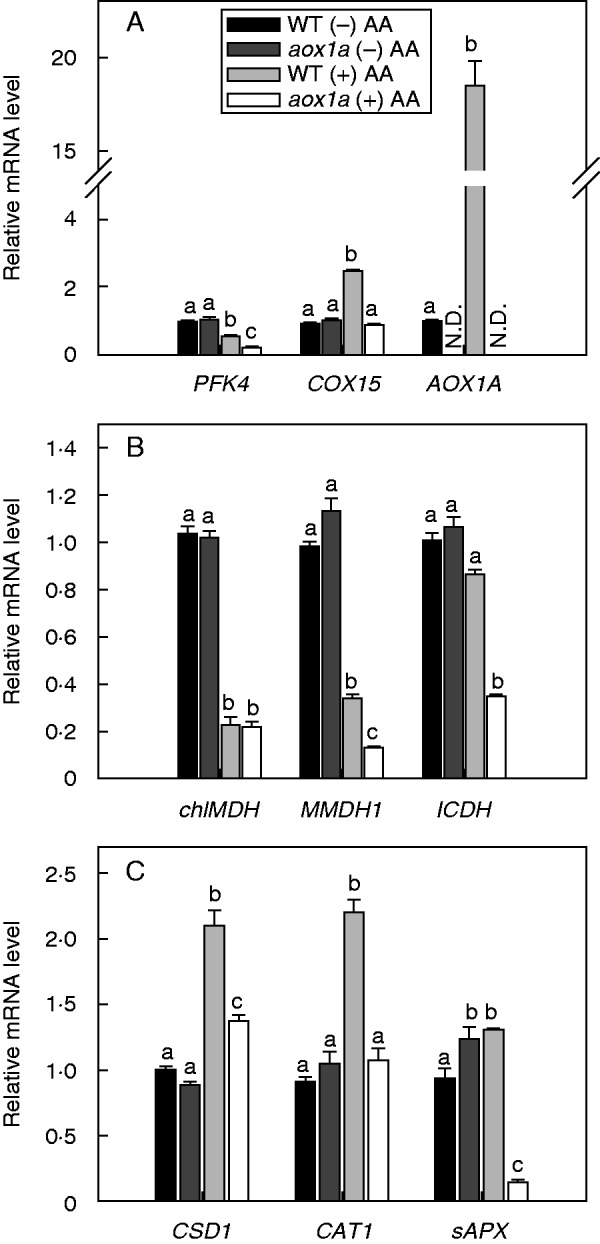
Gene expression profiles in WT and aox1a from control (Tween-20) and treated (20 µm AA in Tween-20) leaf discs pre-illuminated (6 h) at a PPFD of 50 µmol m−2 s−1 at 25 °C: (A) respiratory enzymes (PFK4, COX15 and AOX1A), (B) malate–OAA shuttle enzymes (MMDH1, chlMDH and ICDH) and (C) antioxidative enzymes (CSD1, CAT1 and sAPX). N.D. denotes expression of gene was not detected (A). Transcript abundance was quantified by the ΔΔCT method using UBQ5 as a reference gene. Further details were as described in Materials and methods. Different lower-case letters indicate statistically significant differences (P < 0·05).
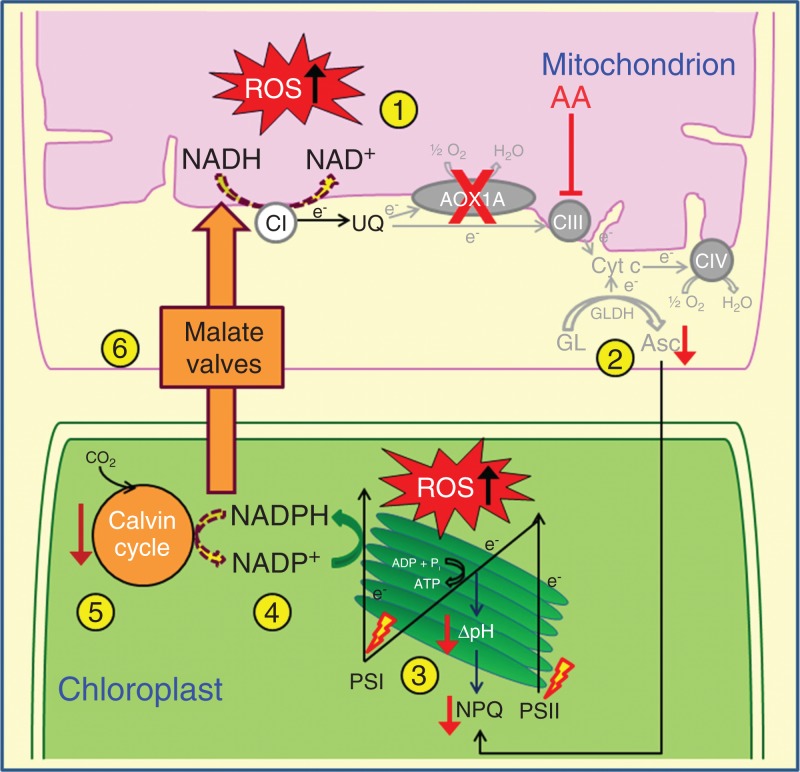
Overall, the results of the present study revealed the impact of deficiency of AOX1A on mitochondrial respiration and chloroplastic photosynthesis when electron transport through the COX pathway is restricted, which is schematically represented in Fig. 9.
Fig.9.
A simple scheme illustrating the impact of AOX1A deficiency on mitochondrial respiration and chloroplastic photosynthesis, when electron transport through the COX pathway is restricted at complex III using AA in leaf discs of Arabidopsis under growth light conditions: (1) over-reduction of mETC and ROS accumulation due to restricted COX and AOX pathways; (2) decrease in Asc biosynthesis; (3) low thylakoid energization; (4) acceptor limitation at PSI; (5) decrease in photosynthetic carbon assimilation; (6) lack of electron sink in mitochondria leads to impairment of photosynthesis. The broken lined arrows indicate a reduction in utilization of reducing equivalents. AA, antimycin A; AOX1A, alternative oxidase 1a; Asc, ascorbate; CI, complex I; CIII, complex III; CIV, complex IV; mETC, mitochondrial electron transport chain; GL, l-galactono-1,4-lactone; GLDH, l-galactono-1,4-lactone dehydrogenase; NPQ, non-photochemical quenching; PSI, photosystem I; PSII, photosystem II; ROS, reactive oxygen species; UQ, ubiquinone.
DISCUSSION
The dependence of chloroplastic photosynthesis on mitochondrial respiration is well documented (Raghavendra et al., 1994; Krömer, 1995; Padmasree et al., 2002; Fernie et al., 2004; Raghavendra and Padmasree, 2003; Noguchi and Yoshida, 2008; Vanlerberghe, 2013). Different components of mitochondrial respiration, including the Krebs cycle, electron transport, oxidative phosphorylation and UCP, were found to be important to sustain photosynthetic performance during normal growth or under various biotic/abiotic stress conditions (Krömer et al., 1993; Padmasree and Raghavendra, 1999a; Sweetlove et al., 2006; Dinakar et al., 2010b; Yoshida et al., 2011a; Araújo et al., 2012, 2014; Gandin et al., 2014a). The relative importance and contribution of the COX and AOX pathways in benefiting different components of photosynthesis such as carbon assimilation, photochemical reactions, photosynthetic induction and light activation of Calvin cycle enzymes was revealed in several model plants using specific metabolic inhibitors as well as transgenic/reverse genetic approaches (Padmasree and Raghavendra, 1999a, b, 2001; Yoshida et al., 2006; Dinakar et al., 2010a; Zhang et al., 2011; Zhang et al., 2014). Furthermore, the significant role of the AOX1A isoform over AOX1D in coordinating the COX pathway to optimize photosynthesis was established in a previous study (Strodtkötter et al., 2009). In the present study, we investigated the physiological role of AOX1A in regulating cellular redox homeostasis and ROS generation to benefit different components of photosynthesis when electron transport through the COX pathway of the mETC is restricted at complex III.
Essentiality of AOX1A for optimal photosynthetic performance and chloroplastic heat dissipation
The use of aox1a mutants along with AA treatment allowed us to analyse the state of COX pathway restriction in the background of an impaired AOX pathway, whereas the treatment of WT with AA resulted only in COX pathway restriction. Restriction of the electron transport through complex III was ascertained by monitoring the decrease in rates of respiratory O2 uptake with increasing concentrations of AA (Fig. 1A). Interference of the mETC through complex III caused a drastic reduction in both photosynthetic carbon assimilation and photochemical reactions in aox1a mutants compared with WT (Figs 1B, 2 and 3). Strodtkötter et al. (2009) showed that induction of AOX1D compromised the function of AOX1A in T-DNA insertion mutants of aox1a. However, in spite of the expression of AOX1D, aox1a mutants could neither maintain respiration nor their photosynthetic performance compared with WT plants when electron transport through the COX pathway is inhibited.
AA is well known to bind to the Qi site of the cytbc1 complex in mETC (Xia et al., 1997). However, its effect on cyclic electron flow around PSI (CEF-PSI) was also reported (Endo et al., 1998; Munekage et al., 2004). Such interference of AA on CEF-PSI might be small or negligible in the present study for the following reasons: (1) the concentration of AA required to inhibit CEF-PSI is 10–100 times higher than that required to in hibit the COX pathway (Taira et al., 2013); (2) the effects of AA on respiration and photosynthesis were found to be biphasic in the present study. For instance, the treatment of WT leaf discs with ≤20 µm AA caused only <18 % decrease in rates of both respiration and photosynthesis, while treatment with ≥20 µm AA caused a drastic decrease of ≤70 % in their rates (Fig. 1); (3) the total cellular levels of ATP as well as the ATP/ADP ratio were higher in aox1a plants (with and without AA treatment) compared with WT (without AA) in both protoplasts and leaf discs (Supplementary Data Fig. S1; Strodtkötter et al., 2009); and (4) the effects of AA on most of the parameters examined in the present study were always less pronounced in WT than in aox1a plants (Figs 1–8). Unlike the protoplasts or cyanobacteria (Yeremenko et al., 2005) where AA is easily permeable through plasma/cell membranes, leaf discs are less permeable to AA. Hence, effective concentrations of AA that ultimately reach chloroplasts at the cellular level are expected to be much less than 20 µm. Moreover, the effect of 20 µm AA observed in respiratory and photosynthetic rates of aox1a leaf discs in the present study corroborated well with the effects examined on mesophyll protoplasts of aox1a knock-out mutants (Fig. 1; Strodtkötter et al., 2009). Thus, AA was used at 20 µm in all further studies to exemplify the physiological role of AOX1A in optimizing photosynthesis.
Linear electron flow and CEF-PSI are coupled to proton pumping across the thylakoid membrane, resulting in acidification of the thylakoid lumen, thereby generating a proton gradient (ΔpH). This ΔpH is utilized for ATP synthesis and NPQ induction (Shikanai, 2007, 2014). The impact of the proton gradient on NPQ induction was evident through an Arabidopsis pgr5 (proton gradient regulation) mutant (defective in CEF), which showed a lower NPQ at high irradiance (Yoshida et al., 2011b). Although the role of AOX1A in modulating NPQ was not shown in their study, several other studies have demonstrated that the lack of AOX caused a significant decrease in quantum yield of PSII, ETR and NPQ under HL conditions (Zhang et al., 2010; Zhang et al., 2012; Vishwakarma et al., 2014). Furthermore, aox1a pgr5 double mutants showed a significant decrease in CEF-PSI compared with pgr5, indicating the synergetic function of AOX1A and CEF-PSI (Yoshida et al., 2011b). In Arabidopsis, the targeting of AOX1A and AOX2 into chloroplasts using its own transit peptide resulted in induction of NPQ and also suggested that these isoforms have the ability to substitute the function of plastid terminal oxidase (PTOX, an analogue of AOX), which mediates the electron flow from PQ to O2 and thus prevent the over reduction of PQ during excess light (McDonald et al., 2011; Fu et al., 2012). In the present study, although PTOX activity was not measured directly, a significant decrease in ETR(II) and NPQ in aox1a mutants compared with WT plants suggests that PTOX could not compensate for the lack of AOX1A. Furthermore, an increase in Y(ND) in aox1a mutants indicates the down-regulation of PSII due to donor-side limitation of PSI, as evident by a decrease in ETR(II), Y(II) and qP (Figs 2B–D and 3C). Taken together, these results demonstrate that AOX1A plays a crucial role in maintaining the balanced electron flow between PSI and PSII, and thereby ΔpH and NPQ induction, which protect photosynthesis from photoinhibition when electron flow through the COX pathway is restricted.
AOX1A maintains the redox balance between photochemical reactions and the Calvin cycle by regulating the malate valve to dissipate excess chloroplastic reducing equivalents
During active photosynthesis, photochemical reactions generate reducing equivalents at much higher magnitudes than their demands in the Calvin cycle. Under such conditions, excess reducing equivalents are transported to peroxisomes or mitochondria via the cytosol through the malate valve, which allows the exchange of malate for OAA across the chloroplast membrane via specific transporters (Noguchi and Yoshida, 2008; Weber and Linka, 2011). The mitochondrial electron transport chain oxidizes these reducing equivalents by external/internal NAD(P)H dehydrogenases and AOX (Rasmusson et al., 2004; Noguchi and Yoshida, 2008). The activity of the enzymes NADP-MDH, NAD-MDH, citrate synthase (CS), NADP-ICDH and NAD-ME, which are known to actively coordinate the malate–OAA shuttle, were significantly increased in aox1a knock-out plants compared with WT under HL conditions in order to prevent an over-reduction of chloroplastic electron transport carriers (Zhang et al., 2010; Xu et al., 2011). Furthermore, the aox1a mutants exhibited an increase in NAD(P)H levels and redox ratios of their corresponding redox couples under HL conditions when compared with WT plants. This indicates the significance of AOX1A in oxidizing excess reducing equivalents (Vishwakarma et al., 2014). Similarly, in the present study aox1a plants showed an excess NADH level compared with WT when the COX pathway is restricted (Fig. 4B). By contrast, NADPH levels did not further increase in aox1a mutants compared with WT plants as expected (Fig. 4E). This is because, as aox1a is more prone to oxidative damage caused by AA compared with WT, which is evident by the increase in H2O2 and MDA content (Fig. 7), it might exhibit metabolic adjustments to oxidize/utilize excess NADPH in the following reactions: (1) generation of by NADPH oxidase (Sagi and Fluhr, 2006; Andronis et al., 2014); (2) photorespiration, as evident by an increase in the glycine decarboxylase P-protein in aox1a plants after treatment with AA (Strodtkötter et al., 2009; Voss et al., 2013); and (3) ascorbate–glutathione cycle or glutathione cycle to scavenge H2O2 by ascorbate peroxidase or glutathione peroxidase (Foyer and Noctor, 2011; Gill et al., 2013). However, a pronounced increase in the ratio of the NADH redox couple as well as the decrease in transcript levels of MMDH1 and ICDH in aox1a mutants implicated the importance of AOX1A in relieving excess reductive pressure on chloroplastic redox carriers by regulating the malate valve particularly when the COX pathway is compromised (Figs 4C and 8B).
Significance of AOX1A in regulating cellular ROS and antioxidative system
The AOX pathway plays a significant role in the dissipation of chloroplastic reducing equivalents through the malate valve and prevents the over-reduction of mETC carriers. Such a role was hypothesized earlier by using salicylhydroxamic acid (SHAM), an inhibitor of the AOX pathway, and was further confirmed in the present study by using aox1a mutants (Padmasree and Raghavendra, 1999c; Yoshida et al., 2006; Figs 4 and 8B). Therefore, the likelihood of electron leakage from mETC is expected to be higher in AA-treated aox1a mutants (Møller, 2001; Strodtkötter et al., 2009; Yoshida et al., 2011a). When single electrons react with molecular oxygen, ROS (OH−, and H2O2) are generated which can influence the expression of the genes related to antioxidative strategies by modifying transcription factors through retrograde regulatory mechanisms (Apel and Hirt, 2004; Choudhury et al., 2013; Li et al., 2013). These include biosynthesis of carotenoids and flavonoids, the xanthophyll cycle, water–water cycle, NPQ, photorespiration and ROS scavenging enzymes (Niyogi, 2000; Murchie and Niyogi, 2011). If cellular ROS generation exceeds the scavenging capacity of the above mentioned defence strategies, it leads to oxidative stress and ROS can damage various cellular structures including DNA, proteins, lipids or cell walls (Foyer and Noctor, 2005; Gill and Tuteja, 2010; del Río, 2015). During salt stress, the activity and transcript levels of antioxidative enzymes were shown to be significantly increased to scavenge excess ROS (Wang et al, 2010). In agreement with this, in the present study, AA treatment induced the expression of antioxidative genes in WT plants, and hence ROS accumulation was less, which is not the case in aox1a mutants (Figs 6B, 7A and 8C). In aox1a mutants, AA treatment resulted in an accumulation of total cellular ROS including chloroplastic ROS, as indicated by localization of DCF fluorescence in those areas which contain high chlorophyll autofluorescence (Fig. 6B). Furthermore, the increase in membrane damage under these conditions was evident from the increase in MDA content (Fig. 7B). The expression of antioxidative genes was much lower in AA-treated aox1a mutants which could be due to partial impairment of antioxidative systems. This may explain AOX1A signalling in the induction of antioxidative enzymes and/or synergistic expression of AOX1A and antioxidative genes (Fig. 8A).
The cellular redox state also plays an important role in mediating the signalling linked to developmental processes or environmental changes (Foyer and Noctor, 2005; Suzuki et al., 2012). NAD+, NADP+, glutathione and Asc, the key players of the ascorbate–glutathione cycle, interact strongly with ROS to maintain cellular redox homeostasis (Noctor, 2006). Among ROS, H2O2 is more stable than and 1O2. Asc, the most abundant antioxidant in plant cells, is oxidized by H2O2 to monodehydroascorbate (MDHA) in the presence of ascorbate peroxidase (APX) and is efficiently recycled back by the action of monodehydroascorbate reductase (MDHAR) or dehydroascorbate reductase (DHAR) (Smirnoff, 2000). Thus, Asc plays an important role in the detoxification of ROS under various stress conditions. In the present study, the pronounced decrease in Asc and sAPX levels along with reduction in respiratory O2 uptake or photosynthetic O2 evolution in aox1a mutants upon treatment with AA as opposed to WT plants indicate the relative importance of AOX1A over the COX pathway in regulating the antioxidative system to optimize photosynthesis (Figs 1, 5A and 8C; Yabuta et al., 2007; Dinakar et al., 2010a). Furthermore, the increase in Asc levels parallels an increase in COX15 in WT but not in aox1a plants, suggesting a role of the AOX pathway in regulating Asc biosynthesis (Figs 5A and 8A; Bartoli et al., 2000). Moreover, Asc is a cofactor of violaxanthin de-epoxidase, which activates the zeaxanthin-dependent qE (energy-dependent quenching) and thus induces NPQ (Tóth et al., 2013). In the present study, after restriction of complex III, a decrease in NPQ in aox1a mutants could also be due to low Asc levels (Figs 2E and 5A). The importance of ROS, antioxidative metabolites and antioxidative enzymes in mediating beneficial interactions between mitochondrial respiration and chloroplastic photosynthesis was shown in Pisum sativum (Dinakar et al., 2010a).
Thus, the present study suggests the physiological importance of AOX1A in performing mitochondrial and extramitochondrial functions to optimize chloroplastic photosynthesis when electron transport through the COX pathway is restricted (Fig. 9). In mitochondria, AOX1A plays a role: (1) in the maintenance of mETC redox homeostasis; (2) preventing accumulation of reducing equivalents and thereby ROS generation; and (3) regulation of Asc biosynthesis. On the other hand, AOX1A performs the following functions outside the mitochondria: (1) sustaining photosynthetic carbon assimilation; (2) induction of NPQ; (3) operation of the malate–OAA shuttle to dissipate excess chloroplastic reducing equivalents and recycle redox carriers; (4) maintenance of redox homeostasis by preventing the over-reduction of chloroplastic ETC carriers and ROS generation; and (5) coordinate with antioxidative systems to maintain cellular ROS at optimal levels.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: Details of primers used for real-time PCR. Figure S1: Measurement of total content of ATP, ADP and their corresponding ratio in WT and aox1a from control and treated leaf discs.
ACKNOWLEDGEMENTS
This work was supported by grants to K.P. (PI) and S.D.T. (Co-PI) from the Department of Biotechnology, New Delhi (No. BT/PR10272/GBD/27/85/2007), while the concept was initiated during the stays (2005–2006 and 2007) of K.P. as an AvH Research Fellow in the laboratory of R.S. J.S. thanks the University of Osnabrück for Research Fund, and A.V. is grateful to CSIR for a Senior Research Fellowship. We thank Prof. A. S. Raghavendra from the University of Hyderabad for critical suggestions while implementing the experiments. We thank Ms Nalini and Mrs Leena for their technical assistance with confocal microscopy and real-time PCR, respectively. The departmental/school facilities of the University of Hyderabad were supported by grants from DST-FIST, UGC-SAP-CAS, DBT-CREBB, DST-PURSE, UPE and UGC-DRS, all from New Delhi, India. No conflict of interest is declared.
LITERATURE CITED
- Andronis EA, Moschou PN, Toumi I, Roubelakis-Angelakis KA. 2014. Peroxisomal polyamine oxidase and NADPH-oxidase cross-talk for ROS homeostasis which affects respiration rate in Arabidopsis thaliana. Frontiers in Plant Science 5: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55: 373–99. [DOI] [PubMed] [Google Scholar]
- Araújo WL, Nunes-Nesi A, Nikoloski Z, Sweetlove LJ, Fernie AR. 2012. Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environment 35: 1–21. [DOI] [PubMed] [Google Scholar]
- Araújo WL, Nunes-Nesi A, Fernie AR. 2014. On the role of plant mitochondrial metabolism and its impact on photosynthesis in both optimal and sub-optimal growth conditions. Photosynthesis Research 119: 141–156. [DOI] [PubMed] [Google Scholar]
- Arnholdt-Schmitt B, Costa JH, de Melo DF. 2006. AOX–a functional marker for efficient cell reprogramming under stress? Trends in Plant Science 11: 281–287. [DOI] [PubMed] [Google Scholar]
- Bartoli CG, Pastori GM, Foyer CH. 2000. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiology 123: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli CG, Yu J, Gómez F, Fernández L, McIntosh L, Foyer CH. 2006. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. Journal of Experimental Botany 57: 1621–1631. [DOI] [PubMed] [Google Scholar]
- Choudhury S, Panda P, Sahoo L, Panda SK. 2013. Reactive oxygen species signaling in plants under abiotic stress. Plant Signaling and Behavior 8: e23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton R, Lister R, Parker KL, et al. 2005. Stress-induced co-expression of alternative respiratory chain components in Arabidopsis thaliana. Plant Molecular Biology 58: 193–212. [DOI] [PubMed] [Google Scholar]
- Clifton R, Millar AH, Whelan J. 2006. Alternative oxidases in Arabidopsis: a comparative analysis of differential expression in the gene family provides new insights into function of non-phosphorylating bypasses. Biochimica et Biophysica Acta 1757: 730–741. [DOI] [PubMed] [Google Scholar]
- Considine MJ, Holtzapffel RC, Day DA, Whelan J, Millar AH. 2002. Molecular distinction between alternative oxidase from monocots and dicots. Plant Physiology 129: 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetkovska M, Vanlerberghe GC. 2012. Coordination of a mitochondrial superoxide burst during the hypersensitive response to bacterial pathogen in Nicotiana tabacum. Plant Cell Environment 35: 1121–1136. [DOI] [PubMed] [Google Scholar]
- Cvetkovska M, Dahal K, Alber NA, Jin C, Cheung M, Vanlerberghe GC. 2014. Knockdown of mitochondrial alternative oxidase induces the ‘stress state’ of signaling molecule pools in Nicotiana tabacum, with implications for stomatal function. New Phytologist 203: 449–461. [DOI] [PubMed] [Google Scholar]
- del Río LA. 2015. ROS and RNS in plant physiology: an overview. Journal of Experimental Botany 66: 2827–2837. [DOI] [PubMed] [Google Scholar]
- Dinakar C, Abhaypratap V, Yearla SR, Raghavendra AS, Padmasree K. 2010a. Importance of ROS and antioxidant system during the beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Planta 231: 461–474. [DOI] [PubMed] [Google Scholar]
- Dinakar C, Raghavendra AS, Padmasree K. 2010b. Importance of AOX pathway in optimizing photosynthesis under high light stress: role of pyruvate and malate in activating AOX. Physiologia Plantarum 139: 13–26. [DOI] [PubMed] [Google Scholar]
- Endo T, Shikanai T, Sato F, Asada K. 1998. NAD(P)H dehydrogenase-dependent, antimycin A-sensitive electron donation to plastoquinone in tobacco chloroplasts. Plant Cell Physiology 39: 1226–1231. [Google Scholar]
- Feng H, Li H, Li X, et al. 2007. The flexible interrelation between AOX respiratory pathway and photosynthesis in rice leaves. Plant Physiology and Biochemistry 45: 228–235. [DOI] [PubMed] [Google Scholar]
- Fernie AR, Carrari F, Sweetlove LJ. 2004. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Current Opinion in Plant Biology 7: 254–261. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. 2005. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17: 1866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. 2011. Ascorbate and glutathione: the heart of the redox hub. Plant Physiology 155: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C, Rowell J, Walker D. 1983. Measurement of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta 157: 239–244. [DOI] [PubMed] [Google Scholar]
- Fu A, Liu H, Yu F, Kambakam S, Luan S, Rodermel S. 2012. Alternative oxidases (AOX1a and AOX2) can functionally substitute for plastid terminal oxidase in Arabidopsis chloroplasts. Plant Cell 24: 1579–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandin A, Duffes C, Day DA, Cousins AB. 2012. The absence of alternative oxidase AOX1A results in altered response of photosynthetic carbon assimilation to increasing CO2 in Arabidopsis thaliana. Plant Cell Physiology 53: 1627–1637. [DOI] [PubMed] [Google Scholar]
- Gandin A, Denysyuk M, Cousins AB. 2014a. Disruption of the mitochondrial alternative oxidase (AOX) and uncoupling protein (UCP) alters rates of foliar nitrate and carbon assimilation in Arabidopsis thaliana. Journal of Experimental Botany 65: 3133–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandin A, Koteyeva NK, Voznesenskaya EV, Edwards GE, Cousins AB. 2014b. The acclimation of photosynthesis and respiration to temperature in the C3-C4 intermediate Salsola divaricata: induction of high respiratory CO2 release under low temperature. Plant Cell Environment 37: 2601–2612. [DOI] [PubMed] [Google Scholar]
- Garmash EV, Grabelnych OI, Velegzhaninov IO, et al. 2015. Light regulation of mitochondrial alternative oxidase pathway during greening of etiolated wheat seedlings. Journal of Plant Physiology 174: 75–84. [DOI] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48: 909–930. [DOI] [PubMed] [Google Scholar]
- Gill SS, Anjum NA, Hasanuzzaman M, et al. 2013. Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations. Plant Physiology and Biochemistry 70: 204–212. [DOI] [PubMed] [Google Scholar]
- Giraud E, Ho LH, Clifton R, et al. 2008. The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiology 147: 595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta KJ, Igamberdiev AU, Mur LA. 2012. NO and ROS homeostasis in mitochondria: a central role for alternative oxidase. New Phytologist 195: 1–3. [DOI] [PubMed] [Google Scholar]
- Hara S, Motohashi K, Arisaka F, et al. 2006. Thioredoxin-h1 reduces and reactivates the oxidized cytosolic malate dehydrogenase dimer in higher plants. Journal of Biological Chemistry 281: 32065–32071. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics 125: 189–198. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Ratcliffe RG, Gupta KJ. 2014. Plant mitochondria: source and target for nitric oxide. Mitochondrion 19: 329–333. [DOI] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U. 2008. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Application Notes 1: 11–14. [Google Scholar]
- Krömer S. 1995. Respiration during photosynthesis. Annual Review of Plant Biology 46: 45–70. [Google Scholar]
- Krömer S, Malmberg G, Gardeström P. 1993. Mitochondrial contribution to photosynthetic metabolism: a study with barley (Hordeum vulgare L.) leaf protoplasts at different light intensities and CO2 concentrations. Plant Physiology 102: 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Liang DD, Li J, et al. 2013. Unravelling mitochondrial retrograde regulation in the abiotic stress induction of rice ALTERNATIVE OXIDASE 1 genes. Plant Cell Environment 36: 775–788. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- McDonald AE, Ivanov AG, Bode R, Maxwell DP, Rodermel SR, Hüner NP. 2011. Flexibility in photosynthetic electron transport: the physiological role of plastoquinol terminal oxidase (PTOX). Biochimica et Biophysica Acta - Bioenergetics 1807: 954–967. [DOI] [PubMed] [Google Scholar]
- Meeuse BJ. 1975. Thermogenic respiration in aroids. Annual Review of Plant Physiology 26: 117–126. [Google Scholar]
- Millenaar F, Lambers H. 2003. The alternative oxidase: in vivo regulation and function. Plant Biology 5: 2–15. [Google Scholar]
- Miller RE, Grant NM, Giles L, et al. 2011. In the heat of the night-alternative pathway respiration drives thermogenesis in Philodendron bipinnatifidum. New Phytologist 189: 1013–1026. [DOI] [PubMed] [Google Scholar]
- Møller IM. 2001. Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annual Review of Plant Physiology and Plant Molecular Biology 52: 561–591. [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hashimoto M, Miyake C, et al. 2004. Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429: 579–582. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Toriyama K. 2008. Enhanced high temperature tolerance in transgenic rice seedlings with elevated levels of alternative oxidase, OsAOX1a. Plant Biotechnology 25: 361–364. [Google Scholar]
- Murchie EH, Niyogi KK. 2011. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiology 155: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK. 1999. Photoprotection revisited: genetic and molecular approaches. Annual Review of Plant Biology 50: 333–359. [DOI] [PubMed] [Google Scholar]
- Niyogi KK. 2000. Safety valves for photosynthesis. Current Opinion in Plant Biology 3: 455–460. [DOI] [PubMed] [Google Scholar]
- Noctor G. 2006. Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant Cell Environment 29: 409–425. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Terashima I. 2006. Responses of spinach leaf mitochondria to low N availability. Plant Cell Environment 29: 710–719. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Yoshida K. 2008. Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion 8: 87–99. [DOI] [PubMed] [Google Scholar]
- Padmasree K, Raghavendra AS. 1999a. Importance of oxidative electron transport over oxidative phosphorylation in optimizing photosynthesis in mesophyll protoplasts of pea (Pisum sativum L.). Physiologia Plantarum 105: 546–553. [Google Scholar]
- Padmasree K, Raghavendra AS. 1999b. Response of photosynthetic carbon assimilation in mesophyll protoplasts to restriction on mitochondrial oxidative metabolism: metabolites related to the redox status and sucrose biosynthesis. Photosynthesis Research 62: 231–239. [Google Scholar]
- Padmasree K, Raghavendra AS. 1999c. Prolongation of photosynthetic induction as a consequence of interference with mitochondrial oxidative metabolism in mesophyll protoplasts of the pea (Pisum sativum L.). Plant Science 142: 29–36. [Google Scholar]
- Padmasree K, Raghavendra AS. 2001. Consequence of restricted mitochondrial oxidative metabolism on photosynthetic carbon assimilation in mesophyll protoplasts: decrease in light activation of four chloroplastic enzymes. Physiologia Plantarum 112: 582–588. [DOI] [PubMed] [Google Scholar]
- Padmasree K, Padmavathi L, Raghavendra AS. 2002. Essentiality of mitochondrial oxidative metabolism for photosynthesis: optimization of carbon assimilation and protection against photoinhibition. Critical Review of Biochemistry and Molecular Biology 37: 71–119. [DOI] [PubMed] [Google Scholar]
- Palmieri F, Rieder B, Ventrella A, et al. 2009. Molecular identification and functional characterization of Arabidopsis thaliana mitochondrial and chloroplastic NAD+ carrier proteins. Journal of Biological Chemistry 284: 31249–31259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queval G, Noctor G. 2007. A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: application to redox profiling during Arabidopsis rosette development. Analytical Biochemistry 363: 58–69. [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Padmasree K. 2003. Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends in Plant Science 8: 546–553. [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Padmasree K, Saradadevi K. 1994. Interdependence of photosynthesis and respiration in plant cells: interactions between chloroplasts and mitochondria. Plant Science 97: 1–14. [Google Scholar]
- Rasmusson AG, Soole KL, Elthon TE. 2004. Alternative NAD(P)H dehydrogenases of plant mitochondria. Annual Review of Plant Biology 55: 23–39. [DOI] [PubMed] [Google Scholar]
- Rhoads DM, Subbaiah CC. 2007. Mitochondrial retrograde regulation in plants. Mitochondrion 7: 177–194. [DOI] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. 2006. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiology 141: 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R. 2004. Malate valves to balance cellular energy supply. Physiologia Plantarum 120: 21–26. [DOI] [PubMed] [Google Scholar]
- Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S. 2005. Strategies to maintain redox homeostasis during photosynthesis under changing conditions. Journal of Experimental Botany 56: 1481–1489. [DOI] [PubMed] [Google Scholar]
- Shikanai T. 2007. Cyclic electron transport around photosystem I: genetic approaches. Annual Review of Plant Biology 58: 199–217. [DOI] [PubMed] [Google Scholar]
- Shikanai T. 2014. Central role of cyclic electron transport around photosystem I in the regulation of photosynthesis. Current Opinion in Biotechnology 26: 25–30. [DOI] [PubMed] [Google Scholar]
- Siedow JN, Umbach AL. 2000. The mitochondrial cyanide-resistant oxidase: structural conservation amid regulatory diversity. Biochimica et Biophysica Acta, 1459: 432–439. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. 2000. Ascorbic acid: metabolism and functions of a multi-facetted molecule. Current Opinion in Plant Biology 3: 229–235. [PubMed] [Google Scholar]
- Smirnoff N, Wheeler GL. 2000. Ascorbic acid in plants: biosynthesis and function. Critical Reviews in Biochemistry and Molecular Biology 35: 291–314. [DOI] [PubMed] [Google Scholar]
- Strodtkötter I, Padmasree K, Dinakar C, et al. 2009. Induction of the AOX1D isoform of alternative oxidase in A thaliana T-DNA insertion lines lacking isoform AOX1A is insufficient to optimize photosynthesis when treated with antimycin A . Molecular Plant 2: 284–297. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Koussevitzky S, Mittler R, Miller G. 2012. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environment 35: 259–270. [DOI] [PubMed] [Google Scholar]
- Sweetlove LJ, Lytovchenko A, Morgan M, et al. 2006. Mitochondrial uncoupling protein is required for efficient photosynthesis. Proceedings of the National Academy of Sciences U S A 103: 19587–19592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira Y, Okegawa Y, Sugimoto K, Abe M, Miyoshi H, Shikanai T. 2013. Antimycin A-like molecules inhibit cyclic electron transport around photosystem I in ruptured chloroplasts. FEBS Open Biology 3: 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YF, O’Toole N, Taylor NL, Millar AH. 2010. Divalent metal ions in plant mitochondria and their role in interactions with proteins and oxidative stress-induced damage to respiratory function. Plant Physiology 152: 747–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosti N, Pasqualini S, Borgogni A, et al. 2006. Gene expression profiles of O3-treated Arabidopsis plants. Plant Cell Environment 29: 1686–702. [DOI] [PubMed] [Google Scholar]
- Tóth SZ, Schansker G, Garab G. 2013. The physiological roles and metabolism of ascorbate in chloroplasts. Physiologia Plantarum 148: 161–175. [DOI] [PubMed] [Google Scholar]
- Van Aken O, Giraud E, Clifton R, Whelan J. 2009. Alternative oxidase: a target and regulator of stress responses. Physiologia Plantarum 137: 354–361. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC. 2013. Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. International Journal of Molecular Science 14: 6805–6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva V, Simova-Stoilova L, Demirevska K, Feller U. 2009. Variety-specific response of wheat (Triticum aestivum L.) leaf mitochondria to drought stress. Journal of Plant Research 122: 445–454. [DOI] [PubMed] [Google Scholar]
- Velikova V, Yordanov I, Edreva A. 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Science 151: 59–66. [Google Scholar]
- Vishwakarma A, Bashyam L, Senthilkumaran B, Scheibe R, Padmasree K. 2014. Physiological role of AOX1a in photosynthesis and maintenance of cellular redox homeostasis under high light in Arabidopsis thaliana. Plant Physiology and Biochemistry 81: 44–53. [DOI] [PubMed] [Google Scholar]
- Voss I, Sunil B, Scheibe R, Raghavendra AS. 2013. Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biology 15: 713–722. [DOI] [PubMed] [Google Scholar]
- Wagner AM, Krab K, Wagner MJ, Moore AL. 2008. Regulation of thermogenesis in flowering Araceae: the role of the alternative oxidase. Biochimica et Biophysica Acta 1777: 993–1000. [DOI] [PubMed] [Google Scholar]
- Walker D, Walker R. 1987. The use of the oxygen electrode and fluorescence probes in simple measurements of photosynthesis. Sheffield: University of Sheffield Press. [Google Scholar]
- Wang H, Liang X, Huang J, et al. 2010. Involvement of ethylene and hydrogen peroxide in induction of alternative respiratory pathway in salt-treated Arabidopsis calluses. Plant Cell Physiology 51: 1754–1765. [DOI] [PubMed] [Google Scholar]
- Wang J, Rajakulendran N, Amirsadeghi S, Vanlerberghe GC. 2011. Impact of mitochondrial alternative oxidase expression on the response of Nicotiana tabacum to cold temperature. Physiologia Plantarum 142: 339–351. [DOI] [PubMed] [Google Scholar]
- Weber AP, Linka N. 2011. Connecting the plastid: transporters of the plastid envelope and their role in linking plastidial with cytosolic metabolism. Annual Review of Plant Biology 62: 53–77. [DOI] [PubMed] [Google Scholar]
- Xia D, Yu CA, Kim H, et al. 1997. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science 277: 60–66. [DOI] [PubMed] [Google Scholar]
- Xu F, Yuan S, Lin HH. 2011. Response of mitochondrial alternative oxidase (AOX) to light signals. Plant Signaling and Behavior 6: 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuta Y, Mieda T, Rapolu M, et al. 2007. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. Journal of Experimental Botany 58: 2661–2671. [DOI] [PubMed] [Google Scholar]
- Yeremenko N, Jeanjean R, Prommeenate P, et al. 2005. Open reading frame ssr2016 is required for antimycin A-sensitive photosystem I-driven cyclic electron flow in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiology 46: 1433–1436. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Terashima I, Noguchi K. 2006. Distinct roles of the cytochrome pathway and alternative oxidase in leaf photosynthesis. Plant Cell Physiology 47: 22–31. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Terashima I, Noguchi K. 2007. Up-regulation of mitochondrial alternative oxidase concomitant with chloroplast over-reduction by excess light. Plant Cell Physiology 48: 606–614. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe C, Kato Y, Sakamoto W, Noguchi K. 2008. Influence of chloroplastic photo-oxidative stress on mitochondrial alternative oxidase capacity and respiratory properties: a case study with Arabidopsis yellow variegated 2. Plant Cell Physiology 49: 592–603. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe CK, Hachiya T, et al. 2011a. Distinct responses of the mitochondrial respiratory chain to long- and short-term high-light environments in Arabidopsis thaliana. Plant Cell Environment 34: 618–628. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe CK, Terashima I, Noguchi K. 2011b. Physiological impact of mitochondrial alternative oxidase on photosynthesis and growth in Arabidopsis thaliana. Plant Cell Environment 34: 1890–1899. [DOI] [PubMed] [Google Scholar]
- Zhang DW, Xu F, Zhang ZW, et al. 2010. Effects of light on cyanide-resistant respiration and alternative oxidase function in Arabidopsis seedlings. Plant Cell Environment 33: 2121–2131. [DOI] [PubMed] [Google Scholar]
- Zhang DW, Yuan S, Xu F, et al. 2014. Light intensity affects chlorophyll synthesis during greening process by metabolite signal from mitochondrial alternative oxidase in Arabidopsis. Plant Cell Environment doi: 10.1111/pce.12438. [DOI] [PubMed] [Google Scholar]
- Zhang LT, Zhang ZS, Gao HY, et al. 2011. Mitochondrial alternative oxidase pathway protects plants against photoinhibition by alleviating inhibition of the repair of photodamaged PSII through preventing formation of reactive oxygen species in Rumex K-1 leaves. Physiologia Plantarum 143: 396–407. [DOI] [PubMed] [Google Scholar]
- Zhang L-T, Zhang Z-S, Gao H-Y, et al. 2012. The mitochondrial alternative oxidase pathway protects the photosynthetic apparatus against photodamage in Rumex K-1 leaves. BMC Plant Biology 12: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.