Abstract
Background/Aims:
Nursing home-acquired pneumonia (NHAP) is included under healthcare-associated pneumonia. However, the optimal treatment strategy for NHAP has been controversial in several studies. We evaluated the clinical features of NHAP compared to community-acquired pneumonia (CAP) in elderly patients admitted with pneumonia.
Methods:
This was a retrospective study in elderly patients aged ≥ 65 years with NHAP or CAP who were hospitalized at Jeju National University Hospital between January 2012 and April 2013.
Results:
A total of 209 patients were enrolled, and 58 (27.7%) had NHAP. The patients with NHAP were older, had more frequent central nervous system disorders, and showed worse clinical parameters. Potential drug-resistant pathogens were more frequently detected in the NHAP group (22.4% vs. 9.9%, p = 0.018), and the incidences of Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus were 8.6% and 10.3%, respectively. In-hospital mortality occurred in 13 patients (22.4%) with NHAP and 17 patients (11.2%) with CAP (p = 0.039). In multivariate analyses, only higher pneumonia severity index (PSI) score was associated with increased mortality (p < 0.001), and the PSI score was higher in the NHAP group than that in the CAP group.
Conclusions:
Elderly patients admitted with NHAP showed more severe pneumonia at onset, higher rates of potentially drug-resistant pathogens, and worse clinical outcomes than those with CAP. However, higher in-hospital mortality in those with NHAP seemed to be related to the PSI score reflecting host factors and severity of pneumonia rather than the type of pneumonia or the presence of drug-resistant pathogens.
Keywords: Nursing care, Pneumonia, Antibiotics, Mortality
INTRODUCTION
The average age of the Korean population as well as that in Western countries is increasing. The elderly population > 65 years of age in Korea will be as high as 14.3% in 2019 [1]. The remarkable development of medical science has contributed to prolong life expectancy, and the number of elderly patients residing in nursing homes is increasing. In 2011, pneumonia ranked sixth in terms of the cause-specific mortality rate in Korea, with a recorded mortality rate of 17.2 per 100,000 [1]. The mortality rate for pneumonia increases each year, and the high mortality rate for pneumonia seems to be associated with the increased number of elderly patients [1]. Pneumonia is the fifth highest contributor to the cause-specific mortality rate in patients ≥ 65 years and has increased the mortality rate to 143.2 per 100,000 [1]. Therefore, to determine the appropriate empirical antibiotics, it is important to evaluate the clinical features of pneumonia in elderly patients associated with residing in long-term care facilities, such as nursing homes.
Pneumonia was generally classified as either community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP) prior to 2005. In 2005, the American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) first introduced the concept of healthcare-associated pneumonia (HCAP) [2]. According to the 2005 ATS/IDSA HCAP guidelines, nursing home-acquired pneumonia (NHAP), which develops in residents in nursing homes or extended care facilities, is included as a HCAP category. Such patients with NHAP should receive broad-spectrum empirical antimicrobial therapy directed at potentially drug-resistant (PDR) pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa [2]. Therefore, treatment strategies based on this distinction between NHAP and CAP are important for patients with pneumonia.
However, the 2009 British Thoracic Society (BTS) CAP guidelines did not distinguish the treatment strategies between NHAP and CAP [3]. In addition, although several NHAP studies published in Europe and Asia reported higher mortality in patients with NHAP, PDR pathogens were not frequently isolated in the NHAP group [4-8]. Interregional differences in NHAP clinical characteristics seem to exist, which might be related to the healthcare systems of each country.
Several HCAP studies have been reported recently. However, because HCAP includes heterogeneous subgroups, further individual analysis for each group is needed. Therefore, additional data regarding NHAP among HCAP subgroups needs to be collected and evaluated. Based on recent NHAP results from Europe and Asia, we suggested that the clinical characteristics of the NHAP group would be more similar to those with CAP than HAP [4-8]. We compared clinical characteristics between NHAP and CAP in elderly patients. In addition, we examined whether NHAP was a predictive factor for in-hospital mortality in elderly patients with and without pneumonia.
METHODS
Study design
This retrospective observational study was performed at the Jeju National University Hospital (580-bed hospital in Jeju, South Korea). Elderly patients aged ≥ 65 years diagnosed with NHAP (NHAP group) or CAP (CAP group), who were hospitalized between January 2012 and April 2013, were investigated using medical records. We compared clinical manifestations, underlying diseases, pneumonia severity, pathogens identified, antibiotics, and clinical outcomes between the two groups. Pneumonia severity was assessed via the pneumonia severity index (PSI) and the CURB-65 score [9,10]. The PSI was calculated using age, sex, complications, vital sign abnormalities, laboratory findings, blood gas analysis, and radiographic parameters [9]. The CURB-65 uses a 6-point score, with 1 point added for each of the following criteria: new onset confusion; urea > 7 mmol/L or blood urea nitrogen > 19 mg/dL; respiratory rate ≥ 30 breaths per minute; systolic blood pressure < 90 mmHg or diastolic blood pressure ≤ 60 mmHg; and age ≥ 65 years [10]. This study protocol was approved by the Ethical Review Committee of Jeju National University Hospital (approval number: 2013-12-015). Informed consent was waived because of the retrospective nature of the study.
Pneumonia definition and categorization
Pneumonia was defined as the presence of a new infiltrate on chest radiography plus at least one of the following: fever (temperature ≥ 38.0°C) or hypothermia (temperature < 35.0°C); new-onset cough with or without sputum; pleuritic chest pain; dyspnea; or altered breath sounds on auscultation. Multilobar involvement was defined as the presence of pneumonic infiltrates in two or more lobes on chest radiography or computed tomography [6].
According to the 2005 ATS/IDSA guidelines, HCAP includes patients with any of the following: (1) residence in a nursing home or long-term care facility; (2) recent history of hospitalization in an acute care hospital for ≥ 2 days in the past 90 days; (3) recent outpatient intravenous therapy (antibiotic or chemotherapy) or wound care within the past 30 days; or (4) attendance at a hospital clinic or dialysis center in the last 30 days [2]. Patients who met (1) were categorized into the NHAP group, and patients who met definitions (2), (3), or (4) were excluded from analysis. CAP was defined as a diagnosis of pneumonia in a patient who did not meet any of the HCAP criteria. And patient who received antibiotics before enrollment or was transferred from other hospital was excluded.
Microbiology
Microorganisms in samples obtained from sputum, tracheal aspirates, bronchial alveolar lavage fluid, or blood within 72 hours after admission were investigated. Sputum samples were cultured in a semi-quantitative manner, and pathogens were identified when a predominant microorganism was detected from group 4 or 5 sputum, according to Geckler’s grading system [11]. Serum samples were evaluated for Mycoplasma pneumoniae or Chlamydia pneumoniae. Serum samples in which particle agglutination antibody titers were > 64 or that were proven to have a 4-fold or greater increase in antibody titer in paired sera was regarded as positive. BinaxNOW (Binax Inc., Scarborough, ME, USA) was used to detect urinary Streptococcus pneumoniae antigens. The BinaxNOW Legionella Urinary Antigen Test (Binax Inc., Scarborough, ME, USA) for Legionella pneumophila serogroup 1 was performed according to the clinical judgment of the attending physicians. Urinary antigen positivity was considered a bacterial infection. The antibiotic sensitivity of all isolates was determined using the disc diffusion method. MRSA, Pseudomonas species, Acinetobacter species, Stenotrophomonas maltophilia, and extended-spectrum β-lactamase (ESBL) producing Enterobacteriaceae were considered PDR pathogens, as reported previously [12].
Clinical outcomes
We compared duration of antibiotic therapy, rate of change of antibiotics, use of inappropriate antibiotics, rate of failure of initial antibiotic therapy, length of hospital stay, and in-hospital mortality rates between each group. Inappropriate antibiotic therapy was defined if the empirical antibiotics were not effective against the pathogens identified based on in vitro susceptibility testing or if clinical stability were not achieved within 72 hours in patients with no identified pathogens. Initial treatment failure was defined as death during initial treatment or a change in antibiotics after 48 hours because of clinical instability including lack of response or worsening of the fever pattern and/or radiographic status, requiring mechanical ventilation and aggressive fluid resuscitation or vasopressors.
Statistical analyses
Data are presented as number (%) or median (range). Continuous variables were compared using Student t test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. Categorical variables were compared using the Pearson chi-square test, and Fisher exact test was used if any cell contained < 5. We performed univariate and multivariate logistic regression analyses to identify independent prognostic factors associated with total in-hospital mortality, as measured by the estimated odds ratio (OR) with 95% confidence intervals (CIs). The p values < 0.05 were considered to indicate statistical significance. Analyses were performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical characteristics
A total of 209 patients were evaluated during the study period. According to the 2005 ATS/IDSA guidelines, 58 patients (27.7%) had NHAP and 151 (72.2%) had CAP (Fig. 1). The numbers of patients in the NHAP group admitted to a nursing home or long-term care hospital were 49 (84.4%) and 9 (15.5%), respectively. The baseline characteristics of each group are presented in Table 1. The median age of the NHAP group was 80 years (interquartile range [IQR], 76 to 86) and was higher than that of the CAP group (77 years; IQR, 71 to 81; p < 0.001). The NHAP group had a higher frequency of risk factors for aspiration and central nervous system disorders. The rate of patients with two or more comorbidities was significantly higher in the NHAP group than that in the CAP group (72.4% vs. 57.6%, p = 0.049).
Figure 1.
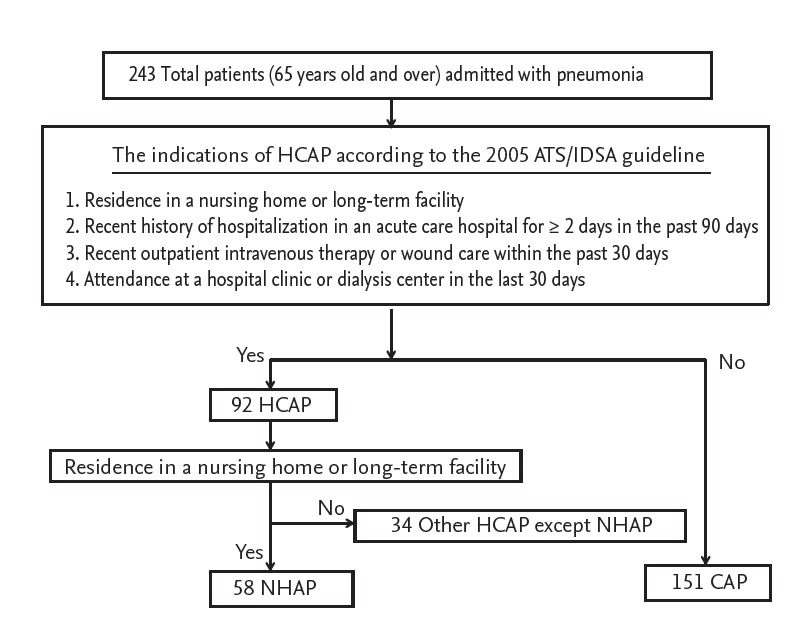
Flow diagram of patient enrollment. HCAP, healthcare-associated pneumonia; ATS/IDSA, The American Thoracic Society/The Infectious Diseases Society of America; NHAP, nursing home-acquired pneumonia; CAP, community-acquired pneumonia.
Table 1.
Baseline characteristics of elderly patients admitted with pneumonia
| Characteristic | NHAP (n = 58) | CAP (n = 151) | p value |
|---|---|---|---|
| Age, yr | 80 (76–86) | 77 (71–81) | < 0.001 |
| Male sex | 28 (48.2) | 97 (64.2) | 0.035 |
| Aspiration tendencya | 55 (94.8) | 47 (31.1) | < 0.001 |
| Tube feeding | 11 (18.9) | 0 | < 0.001 |
| Tracheostomy | 2 (3.4) | 0 | 0.076 |
| Comorbidity | |||
| Malignancy | 7 (12.0) | 11 (7.2) | 0.270 |
| Chronic liver disease | 4 (6.8) | 14 (9.2) | 0.584 |
| Cardiovascular disease | 10 (17.2) | 32 (21.1) | 0.523 |
| Chronic renal disease | 6 (10.3) | 23 (15.2) | 0.360 |
| Diabetes mellitus | 13 (22.4) | 35 (23.1) | 0.906 |
| Chronic lung disease | 12 (20.6) | 46 (30.4) | 0.158 |
| Central nervous system disorders | 53 (91.3) | 47 (31.1) | < 0.001 |
| Immunosuppressive agents | 1 (1.2) | 10 (6.6) | 0.297 |
| Two or more comorbidities | 42 (72.4) | 87 (57.6) | 0.049 |
| Clinical parameters | |||
| Body temperature, °C | 37.1 (36.6–38.1) | 37.5 (36.6–38.3) | 0.174 |
| Altered mentality | 22 (37.9) | 15 (9.9) | < 0.001 |
| Respiratory failureb | 30 (51.7) | 64 (42.3) | 0.224 |
| Severe sepsis or septic shock at onset | 20 (34.4) | 21 (13.9) | 0.001 |
| Intensive care unit admission | 8 (13.7) | 22 (14.5) | 0.886 |
| Need for ventilator | 4 (6.8) | 15 (9.9) | 0.494 |
| Radiological findings | |||
| Multi-lobar involvementc | 49 (84.4) | 110 (72.8) | 0.077 |
| Pleural effusion | 16 (27.5) | 32 (21.1) | 0.325 |
| Laboratory findings | |||
| White blood cells, /mm3 | 10,300 (7,550–14,900) | 11,500 (8,000–14,800) | 0.417 |
| C-reactive protein, mg/dL | 12.43 (5.30–22.48) | 11.21 (5.09–20.21) | 0.467 |
| Procalcitonin, mg/dLd | 0.77 (0.21–4.98) | 0.23 (0.09–0.95) | 0.001 |
| Indices for disease severity | |||
| CURB-65 score | 2 (2–3) | 2 (1–2) | < 0.001 |
| CURB-65 score ≥ 3 | 26 (44.8) | 24 (15.8) | < 0.001 |
| PSI score | 140 (117–171) | 100 (84–125) | < 0.001 |
| PSI class IV and V | 55 (94.8) | 104 (68.8) | < 0.001 |
Values are presented as median (interquartile range) or numbers (%).
NHAP, nursing home-acquired pneumonia; CAP, community-acquired pneumonia; PSI, pneumonia severity index.
Aspiration tendency was defined as having factors predisposing a patient to aspiration, such as a bed-ridden state, central nervous system or oropharyngeal disorders (e.g., malignancy), gastroesophageal disorders (e.g., esophageal diverticulum, achalasia, systemic sclerosis, esophageal cancer, severe reflux esophagitis, or post-gastrectomy), Levin tube inserted state, and subjective and/or observed aspiration/choking/dysphagia/vomiting episode.
Respiratory failure was defined when PaO2 ≤ 60 mmHg or when the PaO2/FiO2 ratio ≤ 300 mmHg.
Multi-lobar involvement was defined as the presence of pneumonic infiltrates in two or more lobes on chest radiography or computed tomography.
The procalcitonin test was performed in 49 patients with NHAP and 117 with CAP.
The NHAP group had worse clinical parameters, such as altered mental status and severe sepsis or septic shock at onset compared to those in the CAP group; however, the rate of intensive care unit (ICU) admission and the need for mechanical ventilation was not difference between the two groups. The rates of multi-lobar involvement and pleural effusion between the groups were not different. The initial median procalcitonin level was higher in the NHAP group than that in the CAP group (0.77 vs. 0.23, p = 0.001), and the PSI and CURB-65 scores, as pneumonia severity indices, were higher in the NHAP group than those in the CAP group. The rates of patients with CURB-65 scores ≥ 3 (44.8% vs. 15.8%, p < 0.001) and PSI class IV or more (94.8% vs. 68.8%, p < 0.001) were higher in the NHAP group than those in the CAP group.
Microbiological etiology
The distributions of pathogens isolated from each group are shown in Table 2. An etiological diagnosis was possible for 35 patients (60.3%) in the NHAP group and 62 (41.0%) in the CAP group (p = 0.012). S. pneumoniae was the most frequent pathogen in both groups, followed by S. aureus.
Table 2.
Microorganisms identified in elderly patients admitted with pneumonia
| Microorganism | NHAP (n = 58) | CAP (n = 151) | p value |
|---|---|---|---|
| Gram-positive bacteria | |||
| Streptococcus pneumoniae | 14 (24.1) | 29 (19.2) | 0.430 |
| Staphylococcus aureus | 7 (12.0) | 9 (5.9) | 0.152 |
| MSSA | 1 (1.2) | 2 (1.3) | 1.000 |
| MRSA | 6 (10.3) | 7 (4.6) | 0.196 |
| Other gram-positive species | 2 (3.4) | 5 (3.3) | 1.000 |
| Gram-negative bacteria | |||
| Pseudomonas aeruginosa | 5 (8.6) | 4 (2.6) | 0.119 |
| Haemophilus influenza | 0 | 2 (1.3) | 1.000 |
| Klebsiella pneumoniae | |||
| ESBL (+) | 0 | 2 (1.3) | 1.000 |
| ESBL (–) | 5 (8.6) | 7 (4.6) | 0.320 |
| Acinetobacter species | 3 (5.1) | 1 (0.6) | 0.066 |
| Moraxella catarrhalis | 0 | 0 | 1.000 |
| Other gram-negative speciesa | 0 | 1 (0.6) | 1.000 |
| Mycoplasma pneumonia | 6 (10.3) | 5 (3.3) | 0.076 |
| Unknown | 23 (39.6) | 89 (58.9) | 0.012 |
| Polymicrobial pathogens | 5 (8.6) | 2 (1.3) | 0.141 |
| Potentially drug-resistant pathogensb | 13 (22.4) | 15 (9.9) | 0.018 |
Values are presented as number (%).
NHAP, nursing home-acquired pneumonia; CAP, community-acquired pneumonia; MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; ESBL, extended-spectrum β-lactamase.
Other gram-negative species included Escherichia coli, Enterobacter sp., Serratia marcescens, and Legionella pneumophila.
Potentially drug-resistant pathogens included MRSA, Pseudomonas sp., Acinetobacter sp., Stenotrophomonas maltophilia, and ESBL-producing Enterobacteriaceae.
The frequency of PDR pathogens was significantly higher in the NHAP group than that in the CAP group (22.4% vs. 9.9%, p = 0.018); however, the isolation rates of P. aeruginosa (8.6% vs. 2.6%, p = 0.119), MRSA (10.3% vs. 4.6%, p = 0.196), ESBL-producing Klebsiella pneumoniae (0% vs. 1.3%, p = 1.000), and Acinetobacter sp. (5.1% vs. 0.6%, p = 0.066) were not different between the groups.
Initial antibiotic treatment
Table 3 shows the initial antimicrobial regimens. Thirty-six patients (62.0%) in the NHAP group and 125 (82.7%) in the CAP group (p = 0.001) initially received combination therapy. The most frequent regimen in both groups was combination therapy with a third-generation cephalosporin and macrolide (29.3% vs. 62.9%, p < 0.001). Anti-pseudomonal agents were more frequently used in the NHAP group than that in the CAP group (51.7% vs. 13.2%, p < 0.001), and anti-MRSA agents were rarely used in either group (1.7% vs. 1.3%, p = 1.000).
Table 3.
Initial antibiotic treatment in elderly patients admitted with pneumonia
| Treatment regimen | NHAP (n = 58) | CAP (n = 151) | p value |
|---|---|---|---|
| Monotherapy | 22 (37.9) | 26 (17.2) | 0.001 |
| 3rd cepha | 1 (1.7) | 1 (0.6) | 0.479 |
| Fluoroquinolone | 6 (10.3) | 18 (11.9) | 0.749 |
| Anti-pseudomonal agenta | 15 (25.8) | 7 (4.6) | < 0.001 |
| Combination therapy | 36 (62.0) | 125 (82.7) | 0.001 |
| 3rd cepha + macrolide | 17 (29.3) | 95 (62.9) | < 0.001 |
| 3rd cepha + fluoroquinolone | 4 (6.8) | 6 (3.9) | 0.469 |
| 3rd cepha + clindamycin | 0 | 3 (1.9) | 0.562 |
| 3rd cepha + macrolide + clindamycin | 3 (5.1) | 8 (5.2) | 1.000 |
| Anti-pseudomonal agent + macrolide | 1 (1.7) | 3 (1.9) | 1.000 |
| Anti-pseudomonal agent + fluoroquinolone | 8 (13.7) | 7 (4.6) | 0.033 |
| Anti-pseudomonal agent + clindamycin | 2 (3.4) | 1 (0.6) | 0.187 |
| Vancomycin + anti-pseudomonal agent | 1 (1.7) | 2 (1.3) | 1.000 |
| Use of anti-pseudomonal agent | 30 (51.7) | 20 (13.2) | < 0.001 |
| Use of anti-MRSA agent | 1 (1.7) | 2 (1.3) | 1.000 |
Values are presented as number (%).
NHAP, nursing home-acquired pneumonia; CAP, community-acquired pneumonia; 3rd cepha, third-generation cephalosporin; MRSA, methicillin-resistant Staphylococcus aureus.
Anti-pseudomonal agents included cefepime, piperacillin/tazobactam, and carbapenem.
Clinical outcomes
Table 4 shows the clinical outcomes of patients with pneumonia. The duration of antibiotic administration (12 days vs. 10 days, p = 0.378), rates of antibiotic change (34.4% vs. 25.8%, p = 0.213), use of inappropriate antibiotics (15.5% vs. 9.9%, p = 0.929), and failure of initial antibiotic therapy (37.9% vs. 25.1%, p = 0.068) were not different between the groups; however, the median length of hospital stay was longer in the NHAP group than that in the CAP group (11 days vs. 7 days, p = 0.018). The NHAP group had higher all cause 3-day mortality, 30-day mortality, and total in-hospital mortality rates than those in the CAP group (8.6% vs. 1.3%, p = 0.019; 22.4% vs. 9.9%, p = 0.018; and 22.4% vs. 11.2%, p = 0.039, respectively) (Fig. 2A). However, the pneumonia-related mortality rate did not differ between the two groups (12.0% vs. 8.6%, p = 0.446). Additionally, patients in the NHAP group admitted to a long-term care hospital tended to have a higher total in-hospital mortality rate than those admitted to a nursing home, although the difference was not significant (33.3% vs. 20.4%, p = 0.404) (Fig. 2B).
Table 4.
Clinical outcomes of elderly patients admitted with pneumonia
| Clinical outcome | NHAP (n = 58) | CAP (n = 151) | p value |
|---|---|---|---|
| Duration of antibiotic therapy, day | 12 (8–17) | 10 (8–14) | 0.378 |
| Change of antibiotics | 20 (34.4) | 39 (25.8) | 0.213 |
| Use of inappropriate antibiotics | 9 (15.5) | 15 (9.9) | 0.929 |
| Failure of initial antibiotics therapy | 22 (37.9) | 38 (25.1) | 0.068 |
| Length of hospital stay, day | 11 (7–17) | 7 (5–12) | 0.018 |
| Pneumonia related to mortality rate | 7 (12.0) | 13 (8.6) | 0.446 |
| Total in-hospital mortality rate | 13 (22.4) | 17 (11.2) | 0.039 |
| 3-Day in-hospital mortality rate | 5 (8.6) | 2 (1.3) | 0.019 |
| 30-Day in-hospital mortality rate | 13 (22.4) | 15 (9.9) | 0.018 |
Values are presented as median (interquartile range) or numbers (%).
NHAP, nursing home-acquired pneumonia; CAP, community-acquired pneumonia.
Figure 2.
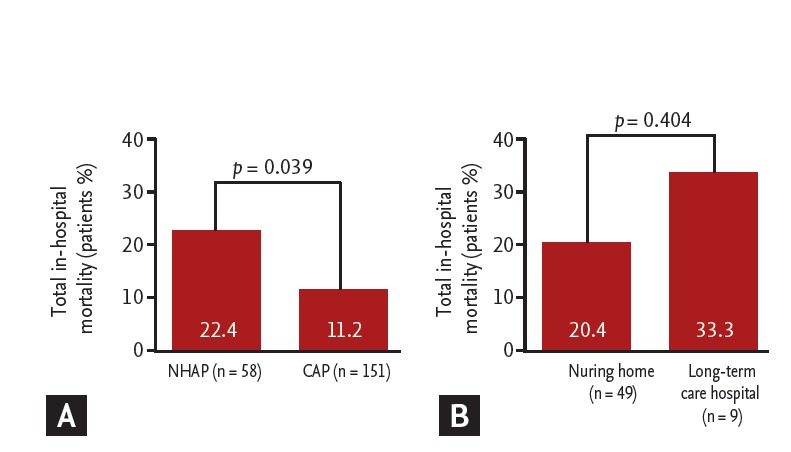
Comparison of total in-hospital mortality rate (A) between nursing home-acquired pneumonia (NHAP) and community-acquired pneumonia (CAP) and (B) between patients admitted to a nursing home and a long-term care hospital in the NHAP group.
In the univariate analysis for risk factors related to mortality, the presence of NHAP, PSI score, and CURB-65 score were associated with increased mortality in elderly patients (≥ 65 years) admitted with pneumonia. Otherwise, age and the presence of two or more comorbidities and PDR pathogens were not significantly associated with total in-hospital mortality (Table 5); however, only the PSI score was independently associated with increased total in-hospital mortality in the multivariate logistic regression analysis (OR, 1.042; 95% CI, 1.021 to 1.065; p < 0.001) (Table 5).
Table 5.
Risk factors associated with total in-hospital mortality in elderly patients admitted with pneumonia
| Predictive factor | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age | 1.050 | 0.996–1.108 | 0.072 | 1.005 | 0.936–1.079 | 0.892 |
| Two or more comorbidities | 2.316 | 0.944–5.678 | 0.066 | 1.117 | 0.347–3.598 | 0.852 |
| PDR pathogens | 2.466 | 0.983–6.189 | 0.054 | 1.219 | 0.354–4.196 | 0.753 |
| NHAP | 2.277 | 1.026–5.054 | 0.043 | 0.358 | 0.107–1.198 | 0.095 |
| PSI score | 1.041 | 1.027–1.055 | < 0.001 | 1.042 | 1.021–1.065 | < 0.001 |
| CURB-65 score | 3.166 | 2.071–4.839 | < 0.001 | 1.176 | 0.583–2.372 | 0.650 |
OR, odds ratio; CI, confidence interval; PDR, potentially drug-resistant; NHAP, nursing home-acquired pneumonia; PSI, pneumonia severity index.
DISCUSSION
Before the 2005 ATS/IDSA HCAP guidelines, NHAP was included as a CAP category [13]; however, because some studies performed in the early 2000s reported high isolation rates of PDR pathogens in patients with NHAP, there was a disagreement over the NHAP classification [14-16]. Because nursing home or long-term care facility residents have several medical diseases and more functional disabilities, they have an increased risk for PDR pathogens. Therefore, it had been suggested that patients with NHAP might require empirical antibiotic treatment distinct from those with CAP.
The 2005 ATS/IDSA guidelines first introduced the concept of HCAP [2]. These guidelines stated that patients with HCAP have a higher rate of PDR pathogens, such as MRSA or P. aeruginosa and show higher mortality rates compared to those with CAP [2]. Therefore, the guidelines recommended that patients with HCAP including those with NHAP should receive PDR-targeted antibiotic treatment, including anti-MRSA agents and/or anti-pseudomonal agents, similar to patients with HAP [2]. Major studies from the United States published after establishment of the guidelines supported this statement [17-19]. However, the PDR-targeted antibiotic recommendations in the 2005 ATS/IDSA guidelines were based almost entirely on experience with severe NHAP that required management in an ICU [14,15]. Moreover, previous HCAP studies from the United States did not separate the clinical features of patients with NHAP from those with HCAP [17-19].
Recent NHAP studies show poor adherence to the 2005 ATS/IDSA antibiotic guidelines, and the controversy has increased regarding how NHAP should be treated between CAP and HAP [13]. The 2009 BTS CAP guidelines state that there is no difference in the distribution of causative pathogens between patients with NHAP and elderly patients with CAP [3]. A recent prospective United Kingdom cohort study demonstrated that the increased mortality caused by HCAP reported in the 2005 ATS/IDSA definitions was primarily related to underlying patient-related factors rather than the presence of antibiotic-resistant pathogens [20]. That study did not establish a clear indication to change current prescribing practices in a United Kingdom cohort. Two studies from Germany also showed that the microbiological and mortality data of patients with NHAP were more similar to those with CAP [4,6]. Recent studies performed in Asia have also reported poor outcomes in patients with NHAP, but the isolation rate of PDR pathogens was relatively low and similar to that of CAP [7,8]. These findings from Europe and Asia suggest that it is difficult to reach a consensus regarding treatment of patients with NHAP based on the 2005 ATS/IDSA HCAP antibiotic guidelines. In addition, the increased use of PDR-targeted antibiotic regimens has raised concerns about antibiotic resistance, and evidence for positive clinical outcomes was limited.
As a result, physicians were faced with the difficulty in determining the appropriate empirical antibiotics for NHAP between the 2005 ATS/IDSA antibiotic guidelines for HCAP and the 2009 BTS antibiotic guidelines for CAP. CAP-targeted narrow-spectrum antibiotic therapy may not cover PDR pathogens, whereas PDR pathogen-targeted treatment may further produce antibiotic resistance.
The present study is the first associated with NHAP performed in elderly patients aged ≥ 65 years in Korea, and we enrolled a greater number of patients compared to previous Korean studies. Korea has no guidelines regarding NHAP. Although several HCAP studies in Korea have been reported, the NHAP data are limited [21,22]. The first Korean study of NHAP (n = 49) compared to HAP (n = 81) was reported in 2010 [21]. The clinical manifestations of the NHAP group were similar to those of the HAP group [21]. PDR pathogens were less frequently isolated in the NHAP group (16.3% vs. 43.2%, p < 0.002), and the isolation rates of P. aeruginosa and MRSA in the NHAP group were 8.2% and 4.1%, respectively [21]. An additional NHAP Korean study demonstrated that patients with NHAP had more underlying diseases and showed worse clinical parameters compared to those with CAP [22]. Furthermore, although PDR pathogens were more frequently isolated in the NHAP group, the isolation rates of P. aeruginosa and MRSA were low (3.0% and 4.5%, respectively) [22]. However, that study was limited in that the distribution of the NHAP group (66/110 patients, 60%) was different from that of other hospitals in Korea.
After establishment of the 2005 ATS/IDSA antibiotic guidelines for HCAP in Korea, many Korean physicians included anti-pseudomonal and/or anti-MRSA agents in the initial regimens to treat patients with NHAP. In our study, anti-pseudomonal agents were administered to 51.7% of the patients in the NHAP group and to 13.2% in the CAP group (p < 0.001); however, anti-MRSA agents were administered to only 1.7% in the NHAP group and 1.3% in the CAP group (p = 1.000). We found significant differences in PDR pathogens between the NHAP and CAP groups (22.4% vs. 9.9%, p = 0.018). The rates of isolating P. aeruginosa and MRSA in the NHAP group were 8.6% and 10.3%, which was higher than that reported in other European and Asian studies. However, the most common pathogen in the NHAP group was S. pneumoniae, as in the CAP group. These results suggest that routine empirical regimens to cover PDR pathogens, such as P. aeruginosa or MRSA, might be excessive in some patients admitted with NHAP. Therefore, guidelines for accurate identification of PDR pathogens in patients with NHAP are needed.
Although the NHAP group had worse clinical parameters at admission, including altered mental status and severe sepsis/septic shock, the rate of ICU admission and the need for mechanical ventilation did not differ between the two groups. These findings probably resulted from families of patients not wanting ICU treatment given to patients in a bed-ridden status, with poor performance status, low likelihood of recovery, and extreme old age.
In the present study, the NHAP group showed a longer duration of hospital stay and higher in-hospital mortality rate than those in the CAP group. Because there are no specific indices for evaluating NHAP or HCAP severity, we used the PSI and CURB-65 scores to predict in-hospital mortality between the NHAP and CAP groups. The pneumonia related mortality rate did not differ between the groups, whereas the all-cause mortality rate was higher in the NHAP group than that in the CAP group. Several variables, such as age, two or more comorbidities, presence of PDR pathogens, and pneumonia type, were not independent factors associated with all-cause mortality in the multivariate analysis. The PSI score, which comprehensively reflects host factors and pneumonia severity, including age, co-morbidities, physical examination, and selected laboratory and radiographic findings was the only factor that predicted all-cause mortality.
Our study had several limitations. Firstly, the study was retrospective at a single center; thus, the results should be interpreted carefully, as the findings could differ from those of other Korean hospitals. Because the enrolled patients were elderly and had several co-morbidities, there was a possibility of receiving antibiotic treatment within weeks or months. Thus, patients could develop PDR pathogens and the oral microbial flora could have changed. However, as the study was retrospective, we could not investigate whether patients received previous antibiotic therapy or not. Second, we included patients from long-term care hospitals and nursing homes. According to the current guidelines, patients admitted to these facilities are equally categorized into the NHAP group [2]. In the present study, patients admitted to a long-term care hospital tended to show a higher total in-hospital mortality rate than the others. Subgroup analyses for clinical outcomes in patients with NHAP are needed in future studies. Third, although the ATS/IDSA 2005 guidelines recommend semi-quantitative or quantitative cultures to identify causative microorganisms in patients with HCAP [2], we did not perform these cultures tests for most patients. Therefore, the microorganisms identified could be oropharyngeal colonizers and may not have been the definite causes of pneumonia.
In conclusion, elderly patients admitted with NHAP showed a twofold increase in the frequency of PDR pathogens and a twofold increase in total in-hospital mortality compared to those with CAP. Thus, NHAP may be a useful concept to differentiate patients with poor clinical outcomes from those patients with CAP. However, the risk of in-hospital mortality in elderly patients admitted with pneumonia was not related to the presence of NHAP, and the PSI score was predictive of total in-hospital mortality. Further studies are needed to assess factors that predict PDR pathogens to determine empirical antibiotic regimens in patients with NHAP.
KEY MESSAGE
1. Patients with nursing home-acquired pneumonia showed poorer outcomes and higher rates of potentially drug-resistant pathogens compared to those with community-acquired pneumonia.
2. Only the pneumonia severity index score was predictive of in-hospital mortality in elderly patients with pneumonia.
Acknowledgments
This study was supported by a research grant from Jeju National University in 2014.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Statistics Korea . Daejeon (KR): Statistics Korea; c2013. Korean Statistical Information Service [Internet] [cited 2013 Sep 18]. Available from: http://kostat.go.kr/portal/korea/index.action. [Google Scholar]
- 2.American Thoracic Society; Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 3.Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64 Suppl 3:iii1–iii55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 4.Ewig S, Klapdor B, Pletz MW, et al. Nursing-home-acquired pneumonia in Germany: an 8-year prospective multicentre study. Thorax. 2012;67:132–138. doi: 10.1136/thoraxjnl-2011-200630. [DOI] [PubMed] [Google Scholar]
- 5.Ma HM, Wah JL, Woo J. Should nursing home-acquired pneumonia be treated as nosocomial pneumonia? J Am Med Dir Assoc. 2012;13:727–731. doi: 10.1016/j.jamda.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Polverino E, Dambrava P, Cilloniz C, et al. Nursing home-acquired pneumonia: a 10 year single-centre experience. Thorax. 2010;65:354–359. doi: 10.1136/thx.2009.124776. [DOI] [PubMed] [Google Scholar]
- 7.Liapikou A, Polverino E, Cilloniz C, et al. A worldwide perspective of nursing home-acquired pneumonia compared with community-acquired pneumonia. Respir Care. 2014;59:1078–1085. doi: 10.4187/respcare.02788. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa N, Saito Y, Sasaki M, Tsuda Y, Mochizuki H, Takahashi H. Comparison of clinical profile in elderly patients with nursing and healthcare-associated pneumonia, and those with community-acquired pneumonia. Geriatr Gerontol Int. 2014;14:362–371. doi: 10.1111/ggi.12110. [DOI] [PubMed] [Google Scholar]
- 9.Fine MJ, Medsger AR, Stone RA, et al. The hospital discharge decision for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 1997;157:47–56. [PubMed] [Google Scholar]
- 10.Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geckler RW, Gremillion DH, McAllister CK, Ellenbogen C. Microscopic and bacteriological comparison of paired sputa and transtracheal aspirates. J Clin Microbiol. 1977;6:396–399. doi: 10.1128/jcm.6.4.396-399.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trouillet JL, Chastre J, Vuagnat A, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998;157:531–539. doi: 10.1164/ajrccm.157.2.9705064. [DOI] [PubMed] [Google Scholar]
- 13.El-Solh AA. Nursing home acquired pneumonia: approach to management. Curr Opin Infect Dis. 2011;24:148–151. doi: 10.1097/QCO.0b013e328343b6cc. [DOI] [PubMed] [Google Scholar]
- 14.El-Solh AA, Sikka P, Ramadan F, Davies J. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med. 2001;163(3 Pt 1):645–651. doi: 10.1164/ajrccm.163.3.2005075. [DOI] [PubMed] [Google Scholar]
- 15.El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650–1654. doi: 10.1164/rccm.200212-1543OC. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Moragon E, Garcia Ferrer L, Serra Sanchis B, Fernandez Fabrellas E, Gomez Belda A, Julve Pardo R. Community-acquired pneumonia among the elderly: differences between patients living at home and in nursing homes. Arch Bronconeumol. 2004;40:547–552. doi: 10.1016/s1579-2129(06)60373-x. [DOI] [PubMed] [Google Scholar]
- 17.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 18.Zilberberg MD, Shorr AF, Micek ST, Mody SH, Kollef MH. Antimicrobial therapy escalation and hospital mortality among patients with health-care-associated pneumonia: a single-center experience. Chest. 2008;134:963–968. doi: 10.1378/chest.08-0842. [DOI] [PubMed] [Google Scholar]
- 19.Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51:3568–3573. doi: 10.1128/AAC.00851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalmers JD, Taylor JK, Singanayagam A, et al. Epidemiology, antibiotic therapy, and clinical outcomes in health care-associated pneumonia: a UK cohort study. Clin Infect Dis. 2011;53:107–113. doi: 10.1093/cid/cir274. [DOI] [PubMed] [Google Scholar]
- 21.Yoon WK, Kim M, Kim YY, et al. The clinical and microbial characteristics of healthcare-associated pneumonia. Korean J Med. 2010;78:709–716. [Google Scholar]
- 22.Cho YJ, Jung BK, Ahn JS. A comparative study of nursing home-acquired pneumonia with community-acquired pneumonia. Tuberc Respir Dis. 2011;70:224–234. [Google Scholar]


