Abstract
Background/Aims:
Newer P2Y12 inhibitors, such as prasugrel and ticagrelor, have greater antiplatelet efficacy but may increase the risk of bleeding. In this study, we compared the pharmacodynamic efficacy of prasugrel and ticagrelor in East Asian patients with acute coronary syndrome (ACS).
Methods:
We selected 83 ACS patients undergoing percutaneous coronary intervention who were discharged with 90 mg ticagrelor twice daily (n = 24), 10 mg prasugrel daily (n = 39) or 5 mg prasugrel daily (n = 20). After 2 to 4 weeks, on-treatment platelet reactivity (OPR) was assessed in terms of P2Y12 reaction units (PRUs) using the VerifyNow P2Y12 assay (Accumetrics). We compared East Asian (85 < PRU ≤ 275) and Caucasian (85 < PRU ≤ 208) criteria for assessing the therapeutic window of OPR.
Results:
OPR was lowest in the ticagrelor group, followed by the 10 mg prasugrel and 5 mg prasugrel groups (49.1 ± 29.9 vs. 83.7 ± 57.1 vs. 168.5 ± 60.8, respectively; p < 0.001). The 5 mg prasugrel group had the highest proportion of patients with OPR values within the therapeutic window, followed by the 10 mg prasugrel and ticagrelor groups (90.0% vs. 46.2% vs. 12.5%, respectively; p < 0.001 for East Asian criteria; 60.0% vs. 43.6% vs. 12.5%, respectively; p < 0.001 for Caucasian criteria).
Conclusions:
Short-term administration of 5 mg prasugrel facilitated maintenance within the therapeutic window of OPR compared with the 10 mg prasugrel and ticagrelor groups. Thus, 5 mg prasugrel daily may be the optimal antiplatelet regimen for stabilized East Asian ACS patients.
Keywords: Prasugrel, Ticagrelor, Platelet function test
INTRODUCTION
Newer P2Y12 inhibitors, such as prasugrel and ticagrelor, have greater antiplatelet efficacy than clopidogrel [1,2]. Recent randomized control trials have shown that fewer ischemic events occur in acute coronary syndrome (ACS) patients treated with these newer P2Y12 inhibitors compared to patients treated with clopidogrel. However, these agents have been associated with higher bleeding rates [3,4] and increased response in East Asian patients [5,6], raising concern over potential bleeding complications in this population. In particular, excess bleeding complications have occurred during the maintenance phase [7,8]. It is thus necessary to reconsider the safety and efficacy of prolonged administration of such potent antiplatelet agents, especially in East Asian patients who may be more vulnerable to bleeding complications.
The point-of-care VerifyNow P2Y12 test (Accumetrics, San Diego, CA, USA) is commonly used to assess residual platelet reactivity following administration of a P2Y12 inhibitor. Many studies have demonstrated its clinical utility for the prediction of future ischemic and bleeding events in coronary artery disease patients receiving P2Y12 inhibitors following percutaneous coronary intervention (PCI) [9-11]. The international working group on on-treatment platelet reactivity (OPR) has proposed the following classification based on P2Y12 reaction units (PRUs): low on-treatment platelet reactivity (LPR), PRU ≤ 85; high on-treatment platelet reactivity (HPR), 208 < PRU; and OPR within the therapeutic window, 85 < PRU ≤ 208 [12]. However, the therapeutic window may vary according to ethnicity, elapsed time and clinical setting, as well as the presence of ACS [11,13,14]. Cut-off HPR values for the prediction of adverse cardiac events are consistently higher in East Asian samples (PRU = 275) compared with Caucasian samples (PRU = 208 to 240) [13,15-17]. Moreover, limited data are available describing the effects of newer P2Y12 inhibitors on platelet reactivity in the Korean population. To maximize drug efficacy and avoid potential bleeding risks, we need to assess and manage the antiplatelet efficacy of these agents, especially during the maintenance period in patients who present with ACS. Therefore, we evaluated the pharmacodynamic efficacy of 90 mg ticagrelor twice daily, 10 mg prasugrel daily (one a day) or 5 mg prasugrel daily (one a day) on platelet reactivity using the VerifyNow P2Y12 assay in stabilized ACS patients who underwent PCI.
METHODS
Study sample
This retrospective pilot study enrolled all eligible patients who presented to our institution with ACS, underwent PCI with drug-eluting stents, and were discharged with ticagrelor or prasugrel between February 2013 and March 2014 (Fig. 1). Ticagrelor and prasugrel were not used in patients with bradyarrhythmia, history of transient ischemic or cerebrovascular attacks, platelet counts < 150,000/mL, or hematocrit < 30% [3,4]. Prasugrel is also contraindicated in patients with body weight < 60 kg and age ≥ 75 years. Other exclusion criteria were: contraindications to antiplatelet therapy, severe left ventricular dysfunction (ejection fraction < 30%), neoplastic disease, and creatinine clearance rate < 30 mL/min. As this study included all eligible patients, a sample size calculation was not performed. Patients on oral anticoagulants or other antiplatelet agents such as cilostazol were also excluded.
Figure 1.

Selection of the study population. PCI, percutaneous coronary intervention; SAH, subarachnoid hemorrhage.
Clinical, laboratory and angiographic data were collected from 90 Korean patients. Among these patients, six switched from ticagrelor to clopidogrel due to dyspnea (three patients), generalized petechiae (one patient), nasal bleeding (one patient), or generalized rash (one patient) before being tested with the VerifyNow assay. One additional patient could not continue 5 mg prasugrel due to a traumatic subarachnoid hemorrhage. The final analysis included 83 eligible patients. The Institutional Review Board at Wonju Severance Christian Hospital approved this retrospective study and waived the requirement for informed consent (CR314008).
Antithrombotic regimen
All patients received 300 mg aspirin and either 180 mg ticagrelor or 60 mg prasugrel before arriving at the cardiac catheterization laboratory. A bolus of unfractionated heparin (70 U/kg) was administered immediately before coronary angiography through the introducer sheath. A second bolus of unfractionated heparin (70 U/kg) was delivered immediately prior to PCI, and additional heparin was administered to achieve an activated clotting time of 250 to 300 seconds. Aspirin was continued at a dose of 100 mg daily indefinitely following PCI. P2Y12 inhibitors were continued for 1 year after PCI at a dose of 90 mg ticagrelor twice daily, 10 mg prasugrel daily or 5 mg prasugrel daily. The selection of P2Y12 inhibitors depended on physician preference. Intravenous or intracoronary glycoprotein IIb/IIIa inhibitor was also administered at the discretion of the treating physician during PCI.
Measurement of residual platelet reactivity
OPR was assessed using the VerifyNow P2Y12 assay (Accumetrics). Blood samples were obtained within 2 to 4 weeks after discharge (23.2 ± 7.3 days). Each sample was placed in a tube containing 3.2% citrate, and the PRU value was assessed within 2 hours, as previously described [18]. We used two different criteria to define therapeutic windows according to PRU values. The East Asian therapeutic window was defined as 85 < PRU ≤ 275, according to data from Korean observational studies [13,15-17]. The Caucasian therapeutic window was defined as 85 < PRU ≤ 208, according to observational studies from Western countries [12]. HPR was defined by the PRU above the upper limits of each therapeutic window, and LPR was defined by the PRU below the lower limits of the window. The primary endpoint was the OPR measured by the VerifyNow assay, and the secondary endpoint was the proportion of patients within the therapeutic window of OPR.
Statistics
All continuous variables are presented as the mean ± SD and were analyzed using Student t test or one-way analysis of variance (ANOVA). Categorical variables are presented as frequencies (percentage) and were analyzed using the chi-square test or Fisher exact test. Post hoc analyses were performed for parameters with p < 0.05. PRU values in the 180 mg ticagrelor, 10 mg prasugrel, and 5 mg prasugrel groups were compared using ANOVA. Proportions of patients with HPR, LPR, and OPR within the therapeutic window were compared using the chi-square test or Fisher exact test. Statistical significance was defined as p < 0.05. All analyses were performed with the SPSS version 20.0 (IBM Co., Armonk, NY, USA).
RESULTS
Baseline characteristics
Baseline characteristics according to type of P2Y12 inhibitor are summarized in Table 1. Age, body mass index, and history of diabetes mellitus, hypertension, hyperlipidemia, and smoking did not differ significantly between the three treatment groups. The highest proportion of male patients was observed in the 10 mg prasugrel group, followed by the 5 mg prasugrel and ticagrelor groups (92.3% vs. 90.0% vs. 62.5%, respectively; p = 0.006). The prevalence of acute myocardial infarction (MI) was the highest in the 10 mg prasugrel group, followed by the 5 mg prasugrel and ticagrelor groups (94.8% vs. 80.0% vs. 33.3%, respectively; p < 0.001).
Table 1.
Baseline characteristics of study participants
| Characteristic | All (n = 83) | Ticagrelor 180 mg (n = 24) | Prasugrel 10 mg (n = 39) | Prasugrel 5 mg (n = 20) | p value |
|---|---|---|---|---|---|
| Age, yr | 57.9 ± 10.1 | 60.1 ± 10.3 | 56.5 ± 9.5 | 58.0 ± 11.0 | 0.392 |
| Male sex | 69 (83.1) | 15 (62.5) | 36 (92.3) | 18 (90.0) | 0.006 |
| Height, cm | 165.7 ± 8.6 | 161.3 ± 9.8 | 169.2 ± 6.0 | 163.9 ± 8.8 | 0.001 |
| Weight, kg | 68.8 ± 11.1 | 65.5 ± 12.4 | 71.8 ± 9.6 | 66.9 ± 11.3 | 0.063 |
| Body mass index, kg/m2 | 24.9 ± 2.5 | 25.0 ± 2.8 | 25.0 ± 2.4 | 24.7 ± 2.5 | 0.909 |
| Systolic blood pressure, mmHg | 130.8 ± 20.5 | 137.1 ± 15.7 | 129.3 ± 20.9 | 126.1 ± 23.6 | 0.164 |
| Diastolic blood pressure, mmHg | 76.5 ± 14.2 | 80.3 ± 15.6 | 75.5 ± 14.6 | 74.0 ± 11.6 | 0.298 |
| Heart rate, bpm | 74.9 ± 14.0 | 76.1 ± 11.7 | 73.8 ± 14.2 | 75.5 ± 16.4 | 0.797 |
| Diagnosis | < 0.001 | ||||
| Unstable angina | 22 (26.5) | 16 (66.7) | 2 (5.1) | 4 (20.0) | |
| Acute myocardial infarction | 61 (73.5) | 8 (33.3) | 37 (94.2) | 16 (80.0) | |
| Past history | |||||
| Hypertension | 31 (37.3) | 12 (50.0) | 12 (30.8) | 7 (35.0) | 0.300 |
| Diabetes | 18 (21.7) | 6 (25.0) | 10 (25.6) | 2 (10.0) | 0.346 |
| Hyperlipidemia | 11 (13.3) | 4 (16.7) | 3 (7.7) | 4 (20.0) | 0.353 |
| Currently smoking | 43 (51.8) | 8 (33.3) | 23 (59.0) | 12 (60.0) | 0.099 |
| Prior myocardial infarction | 2 (2.4) | 1 (4.2) | 1 (2.6) | 0 | 0.666 |
| LVEF, % | 55.8 ± 10.9 | 61.2 ± 10.0 | 52.1 ± 11.1 | 56.5 ± 8.8 | 0.004 |
| Culprit lesion | 0.133 | ||||
| Left main | 1 (1.2) | 1 (4.2) | 0 | 0 | |
| LAD | 40 (48.2) | 10 (41.7) | 22 (56.4) | 8 (40.0) | |
| LCX | 12 (14.5) | 3 (12.5) | 8 (20.5) | 1 (5.0) | |
| RCA | 30 (36.1) | 10 (41.7) | 9 (23.1) | 11 (55.0) | |
| Disease extent | 0.117 | ||||
| 1 VD | 41 (49.4) | 15 (62.5) | 19 (48.7) | 7 (35.0) | |
| 2 VD | 32 (38.6) | 9 (37.5) | 15 (38.5) | 8 (40.0) | |
| 3 VD | 10 (12.0) | 0 | 5 (12.8) | 5 (25.5) | |
| Stents used | 1.4 ± 0.8 | 1.8 ± 0.9 | 1.3 ± 0.8 | 1.2 ± 0.6 | 0.029 |
| Stent diameter, mm | 3.2 ± 0.7 | 3.3 ± 0.6 | 3.1 ± 0.7 | 3.1 ± 0.9 | 0.776 |
| Stent length, mm | 32.6 ± 19.0 | 40.5 ± 22.5 | 30.9 ± 17.3 | 25.9 ± 13.8 | 0.031 |
| Discharge medication | |||||
| ACEi or ARB | 54 (65.1) | 19 (79.2) | 25 (64.1) | 10 (50.0) | 0.128 |
| β-Blocker | 68 (81.9) | 13 (54.2) | 36 (92.3) | 19 (95.0) | < 0.001 |
| CCB | 4 (4.8) | 3 (12.5) | 0 | 1 (5.0) | 0.080 |
| Statin | 23 (95.8) | 23 (95.8) | 39 (100.0) | 19 (95.0) | 0.397 |
| Periods for PFT, day | 23.1 ± 7.3 | 30.0 ± 2.7 | 17.8 ± 5.5 | 25.6 ± 5.8 | < 0.001 |
Values are presented as mean ± SD or number (%).
bpm, beats per minute; LVEF, left ventricular ejection fraction; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; VD, vessel disease; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; PFT, platelet function test.
On-treatment platelet reactivity
The mean period for conducting the VerifyNow assay after discharge was shorter in the 10 mg prasugrel group (17.8 ± 5.5 days) than in the 180 mg ticagrelor (30.0 ± 2.7 days) and 5 mg prasugrel (25.6 ± 5.8 days) groups. A scatterplot of PRU values according to the different regimens is shown in Fig. 2. The mean OPR was the highest (p < 0.001) in the 5 mg prasugrel group (168.5 ± 60.8), followed by the 10 mg prasugrel (83.7 ± 57.1) and 180 ticagrelor (49.1 ± 29.9) groups. A post hoc analysis showed that the OPR values were significantly different in all groups (p < 0.05). When applying the East Asian criteria for defining the therapeutic window, the proportion of patients within the therapeutic window range was the highest in the 5 mg prasugrel group (90.0%), followed by the10 mg prasugrel (46.2%) and 180 mg ticagrelor groups (12.5%, p < 0.001) (Fig. 3). The majority of patients in the ticagrelor group had LPR (87.5%), whereas only a minority of patients in the 5 mg prasugrel group had LPR (10.0%). HPR was not noted in any group. When the Caucasian criteria for defining the therapeutic window were applied, the proportion of patients within the therapeutic window was also the highest in the 5 mg prasugrel group (60.0%), followed by the 10 mg prasugrel (43.6%) and ticagrelor groups (12.5%, p < 0.001) (Fig. 4). The proportion of HRP was 30% in the 5 mg prasugrel group, while the HPR was noted as 2.5% and 0% in 10 mg prasugrel and ticagrelor groups, respectively.
Figure 2.
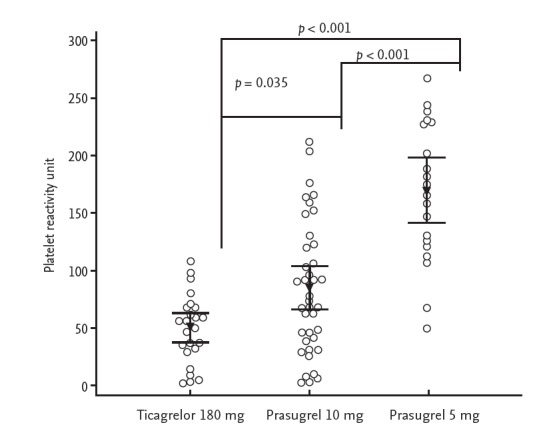
Scatterplot of platelet reactivity unit values grouped by antiplatelet agent. Arrows represent the means and bars represent 95% confidence intervals.
Figure 3.
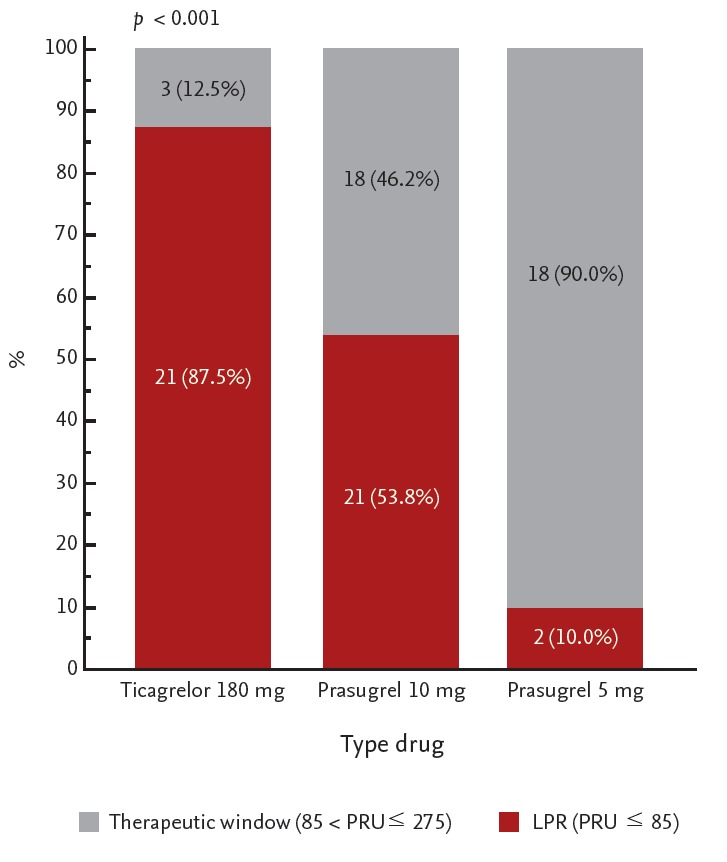
Proportion of the therapeutic window grouped by antiplatelet agent based on East Asian criteria (85 < platelet reactivity unit [PRU] ≤ 275). LPR, low on-treatment platelet reactivity.
Figure 4.

Proportion of the therapeutic window grouped by antiplatelet agent based on Caucasian criteria (85 < platelet reactivity unit [PRU] ≤ 208). HPR, high on-treatment platelet reactivity; LPR, low on-treatment platelet reactivity.
DISCUSSION
The study described herein demonstrates the antiplatelet efficacy of 5 or 10 mg daily prasugrel and 90 mg twice daily ticagrelor in Korean patients with ACS. Our main findings suggest that commonly used doses of ticagrelor and prasugrel excessively inhibit platelet activation, leading to LPR in Korean patients. The highest proportion of patients within the therapeutic window was found in those patients taking 5 mg prasugrel based on East Asian and Caucasian criteria. This suggests that daily administration of 5 mg prasugrel may optimally inhibit platelet reactivity in East Asian patients stabilized after ACS.
HPR is a risk factor for post-PCI stent thrombosis and MI [10,12]. This association is more prominent in patients with ACS compared to those with stable coronary artery disease [13,14,19,20]. Prasugrel and ticagrelor have emerged as alternatives to clopidogrel for resolving HPR [21]. Their anti-ischemic efficacy with respect to reducing MI, death and stent thrombosis was demonstrated in the TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction (TRITON-TIMI 38) and PLATelet inhibition and patient Outcomes (PLATO) trials [3,4]. Thus, European PCI guidelines recommend prasugrel and ticagrelor over clopidogrel as first-line antiplatelet agents in ACS patients [22,23]. However, these trials were conducted primarily in patients of European descent with very few East Asian patients in the cohorts (< 5% in PLATO and < 1% in TRITON-TIMI 38) [3,4,24]. Thus, there may be limitations to generalizing these results to East Asian patients. In a randomized controlled trial of Japanese patients, a dose of 3.75 mg prasugrel was associated with a lower incidence of ischemic events compared to clopidogrel (9.4% vs. 11.8%, respectively; p > 0.05), despite a similar risk of major bleeding [25]. Although these results were not statistically significant, they should be interpreted with caution since doses of prasugrel representing one-third of the conventional dose demonstrated inferior efficacy and safety compared to clopidogrel.
LPR has also been associated with increased bleeding complications [10,11]. Campo et al. [11] suggested a cutoff PRU value of < 85 for predicting bleeding episodes. In the present study, 87.5% of patients in the ticagrelor group and 53.8% in the 10 mg prasugrel group had LPR compared to 10% patients in the 5 mg prasugrel group with LPR. Ischemic, as well as hemorrhagic complications are strongly associated with all-cause mortality following PCI [10,26]. Premature termination of antiplatelet therapy and platelet activation by blood transfusion are potential causes for higher mortality risk [12]. Thus, clinicians must pay careful attention when initiating prolonged antiplatelet therapy with conventional dosages of these potent P2Y12 inhibitors, particularly in East Asian patients. In the present study, most patients were switched from prasugrel or ticagrelor to clopidogrel following the platelet function tests. No adverse ischemic or bleeding events occurred during the period from discharge to platelet function testing.
Despite the high prevalence of CYP2C19 polymorphisms and HPR in East Asians, the incidence of ischemic events is paradoxically lower in this population compared to patients of European descent [27]. We hypothesize that this may be due to a reduced contribution of HPR in the occurrence of ischemic events in East Asian patients compared to those of European descent. Several factors also contribute to the development of adverse cardiac events following PCI, including underlying risk factors, inflammation and coagulation [28-30]. Lower levels of the inflammatory marker C-reactive protein in East Asians have been observed [31], and patients of Asians/Pacific Islander descent have a 70% reduction in the prevalence of venous thromboembolism compared to Europeans [32]. Moreover, bleeding events occur more frequently in East Asian patients treated with warfarin [33] or clopidogrel [34]. This low thrombogeneity could partly explain the low incidence of ischemic events in East Asian patients. Population-based, personalized antithrombotic therapy is necessary to achieve a balance between the reduction in ischemic events and excessive bleeding [35]. When we considered the decreased risk of thrombotic events and increased risk of bleeding in East Asian patients, we found that 5 mg prasugrel, rather than 90 mg ticagrelor twice daily or 10 mg prasugrel daily, was more appropriate for Korean patients with ACS to reach the therapeutic window of OPR during maintenance periods.
The present study has several limitations. First, it was performed retrospectively in a single center with a small study sample, and the selection of antiplatelet agents was dependent upon the operator. We thus had to minimize the exclusion of patients rather than perform propensity matching for the adjustment of covariates. As a result, selection bias may affect our findings and careful interpretation is required. Second, prasugrel is contraindicated in patients with a body weight < 60 kg and age ≥ 75 years, leading to a higher proportion of males in the prasugrel group. Third, HPR is not likely a stable phenomenon, and intra-individual variability in PRU has been noted previously [11]. Therefore, single measurements of PRU cannot ensure steady state platelet reactivity. Fourth, the median follow-up period for the VerifyNow assay was shorter in the 10 mg prasugrel group than in the other groups. However, a period of 2 to 4 weeks may be sufficient to show stabilized platelet reactivity in patients with ACS [36]. Fifth, the cut-off value for LPR and the prognostic impact of LPR has not been evaluated in the Korean population, and thus we used the Caucasian cut-off value of LPR to define the therapeutic window. The clinical impact of LPR should be evaluated through a large-scale observational study in East Asian populations. Finally, we tested platelet reactivity only using the VerifyNow P2Y12 assay, which is a well-validated test for the measurement of platelet reactivity and widely used around the world [12].
In conclusion, the recommended doses of prasugrel (10 mg daily) and ticagrelor (90 mg twice daily) for reducing platelet reactivity in patients of European descent may be excessive for Korean patients, resulting in increased risk of bleeding. Therefore, 5 mg prasugrel daily could be the most appropriate regimen to prevent increased bleeding risk and maintain antiplatelet efficacy in Korean patients during the maintenance phase. Further randomized controlled trials powered for clinical and safety outcomes are warranted to test this hypothesis.
KEY MESSAGE
1. In East Asian populations, conventional dosages of prasugrel (10 mg daily) and ticagrelor (90 mg twice daily) excessively inhibit platelet reactivity.
2. A daily dose of 5 mg prasugrel may be the optimum regimen for East Asian patients with acute coronary syndrome during the maintenance period.
Acknowledgments
The authors are indebted to Dr. Olivia Y. Hung for her editorial suggestions.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Wallentin L, Varenhorst C, James S, et al. Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur Heart J. 2008;29:21–30. doi: 10.1093/eurheartj/ehm545. [DOI] [PubMed] [Google Scholar]
- 2.Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 4.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 5.Small DS, Kothare P, Yuen E, et al. The pharmacokinetics and pharmacodynamics of prasugrel in healthy Chinese, Japanese, and Korean subjects compared with healthy Caucasian subjects. Eur J Clin Pharmacol. 2010;66:127–135. doi: 10.1007/s00228-009-0737-1. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Butler K, Yang L, Yang Z, Teng R. Pharmacokinetics and tolerability of single and multiple doses of ticagrelor in healthy Chinese subjects: an open-label, sequential, two-cohort, single-centre study. Clin Drug Investig. 2012;32:87–97. doi: 10.2165/11595930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol. 2008;51:2028–2033. doi: 10.1016/j.jacc.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Becker RC, Bassand JP, Budaj A, et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2011;32:2933–2944. doi: 10.1093/eurheartj/ehr422. [DOI] [PubMed] [Google Scholar]
- 9.Sibbing D, Steinhubl SR, Schulz S, Schomig A, Kastrati A. Platelet aggregation and its association with stent thrombosis and bleeding in clopidogrel-treated patients: initial evidence of a therapeutic window. J Am Coll Cardiol. 2010;56:317–318. doi: 10.1016/j.jacc.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 10.Stone GW, Witzenbichler B, Weisz G, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. 2013;382:614–623. doi: 10.1016/S0140-6736(13)61170-8. [DOI] [PubMed] [Google Scholar]
- 11.Campo G, Parrinello G, Ferraresi P, et al. Prospective evaluation of on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J Am Coll Cardiol. 2011;57:2474–2483. doi: 10.1016/j.jacc.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 12.Tantry US, Bonello L, Aradi D, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62:2261–2273. doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 13.Ahn SG, Lee SH, Yoon JH, et al. Different prognostic significance of high on-treatment platelet reactivity as assessed by the VerifyNow P2Y12 assay after coronary stenting in patients with and without acute myocardial infarction. JACC Cardiovasc Interv. 2012;5:259–267. doi: 10.1016/j.jcin.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Park DW, Ahn JM, Song HG, et al. Differential prognostic impact of high on-treatment platelet reactivity among patients with acute coronary syndromes versus stable coronary artery disease undergoing percutaneous coronary intervention. Am Heart J. 2013;165:34–42.e1. doi: 10.1016/j.ahj.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Ko YG, Suh JW, Kim BH, et al. Comparison of 2 point-of-care platelet function tests, VerifyNow Assay and Multiple Electrode Platelet Aggregometry, for predicting early clinical outcomes in patients undergoing percutaneous coronary intervention. Am Heart J. 2011;161:383–390. doi: 10.1016/j.ahj.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 16.Park KW, Jeon KH, Kang SH, et al. Clinical outcomes of high on-treatment platelet reactivity in Koreans receiving elective percutaneous coronary intervention (from results of the CROSS VERIFY study) Am J Cardiol. 2011;108:1556–1563. doi: 10.1016/j.amjcard.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Jin HY, Yang TH, Kim DI, et al. High post-clopidogrel platelet reactivity assessed by a point-of-care assay predicts long-term clinical outcomes in patients with ST-segment elevation myocardial infarction who underwent primary coronary stenting. Int J Cardiol. 2013;167:1877–1881. doi: 10.1016/j.ijcard.2012.04.154. [DOI] [PubMed] [Google Scholar]
- 18.Price MJ. Bedside evaluation of thienopyridine antiplatelet therapy. Circulation. 2009;119:2625–2632. doi: 10.1161/CIRCULATIONAHA.107.696732. [DOI] [PubMed] [Google Scholar]
- 19.Marcucci R, Gori AM, Paniccia R, et al. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation. 2009;119:237–242. doi: 10.1161/CIRCULATIONAHA.108.812636. [DOI] [PubMed] [Google Scholar]
- 20.Parodi G, Marcucci R, Valenti R, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215–1223. doi: 10.1001/jama.2011.1332. [DOI] [PubMed] [Google Scholar]
- 21.Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–1105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 22.Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 23.Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Steg PG, James SK, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 24.Ge J, Zhu J, Hong BK, et al. Prasugrel versus clopidogrel in Asian patients with acute coronary syndromes: design and rationale of a multi-dose, pharmacodynamic, phase 3 clinical trial. Curr Med Res Opin. 2010;26:2077–2085. doi: 10.1185/03007995.2010.502048. [DOI] [PubMed] [Google Scholar]
- 25.Saito S, Isshiki T, Kimura T, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J. 2014;78:1684–1692. doi: 10.1253/circj.cj-13-1482. [DOI] [PubMed] [Google Scholar]
- 26.Chhatriwalla AK, Amin AP, Kennedy KF, et al. Association between bleeding events and in-hospital mortality after percutaneous coronary intervention. JAMA. 2013;309:1022–1029. doi: 10.1001/jama.2013.1556. [DOI] [PubMed] [Google Scholar]
- 27.Park KW, Lee JM, Kang SH, et al. Safety and efficacy of second-generation everolimus-eluting Xience V stents versus zotarolimus-eluting resolute stents in real-world practice: patient-related and stent-related outcomes from the multicenter prospective EXCELLENT and RESOLUTE-Korea registries. J Am Coll Cardiol. 2013;61:536–544. doi: 10.1016/j.jacc.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Jeong YH. “East asian paradox”: challenge for the current antiplatelet strategy of “one-guideline-fits-all races” in acute coronary syndrome. Curr Cardiol Rep. 2014;16:485. doi: 10.1007/s11886-014-0485-4. [DOI] [PubMed] [Google Scholar]
- 29.Park DW, Lee SW, Yun SC, et al. A point-of-care platelet function assay and C-reactive protein for prediction of major cardiovascular events after drug-eluting stent implantation. J Am Coll Cardiol. 2011;58:2630–2639. doi: 10.1016/j.jacc.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 30.Yang TH, Kim DI, Kim DK, et al. Detection of clopidogrel hyporesponsiveness using a point-of-care assay and the impact of additional cilostazol administration after coronary stent implantation in diabetic patients. Korean J Intern Med. 2011;26:145–152. doi: 10.3904/kjim.2011.26.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley-Hedgepeth A, Lloyd-Jones DM, Colvin A, et al. Ethnic differences in C-reactive protein concentrations. Clin Chem. 2008;54:1027–1037. doi: 10.1373/clinchem.2007.098996. [DOI] [PubMed] [Google Scholar]
- 32.White RH, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res. 2009;123 Suppl 4:S11–S17. doi: 10.1016/S0049-3848(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 33.Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–315. doi: 10.1016/j.jacc.2007.01.098. [DOI] [PubMed] [Google Scholar]
- 34.Mak KH, Bhatt DL, Shao M, et al. Ethnic variation in adverse cardiovascular outcomes and bleeding complications in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study. Am Heart J. 2009;157:658–665. doi: 10.1016/j.ahj.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 35.Jeong YH, Bliden KP, Tantry US, Gurbel PA. Do East Asians have different hypercoagulable states compared with Western population? Am Heart J. 2011;162:e19–e20. doi: 10.1016/j.ahj.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Collet JP, Cuisset T, Range G, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367:2100–2109. doi: 10.1056/NEJMoa1209979. [DOI] [PubMed] [Google Scholar]


