Abstract
Background/Aims:
To investigate abnormalities in blood electrolyte levels during severe hypoglycemia in Korean patients with type 2 diabetes mellitus (T2DM) in a clinical setting.
Methods:
Blood electrolyte levels in adult T2DM patients during severe hypoglycemia were collected from January 1, 2008 to December 31, 2012. Patients who maintained normal serum creatinine and blood urea nitrogen levels were utilized in the study. Severe hypoglycemia was defined as a condition requiring medical assistance, such as administering carbohydrates when serum glucose levels less than 70 mg/dL were observed, in conjunction with other symptoms of hypoglycemia.
Results:
A total of 1,068 patients who visited the emergency room with severe hypoglycemia were screened, of which 219 patients were included in this study. The incidence of abnormal levels for any electrolyte was 47%. Hypokalemia (< 3.5 mmol/L) was the most common type of electrolyte disturbance observed at 21.9%. A decrease in serum potassium levels was associated with decreases in blood glucose levels (r = 0.151, p = 0.025). During severe hypoglycemia, median blood glucose levels, incidence of tachycardia (> 100 beats per minute) and severe hypertension (≥ 180/120 mmHg) were 30 mg/dL (range, 14 to 62) and 35 mg/dL (range, 10 to 69; p = 0.04), 18.8% and 7.2% (p = 0.02), and 20.8% and 10.2% (p = 0.05) in the hypokalemia and normokalemia groups, respectively.
Conclusions:
During severe hypoglycemia, hypokalemia occurred in 21.9% of T2DM patients and was associated with tachycardia and severe hypertension. Therefore, the results suggest that severe hypoglycemia may increase cardiovascular events in T2DM.
Keywords: Severe hypoglycemia; Electrolytes; Hypokalemia; Diabetes mellitus, type 2
INTRODUCTION
Although previous studies have demonstrated that intensive glycemic control in diabetes mellitus is important to prevent micro- and macrovascular complications [1,2], the risk of hypoglycemia should not be overlooked. Severe hypoglycemia can cause serious conditions such as loss of consciousness, seizures, and encephalopathy and is associated with increased risks of vascular events and death [3,4]. A plausible mechanism of increased cardiovascular mortality during severe hypoglycemia might be associated with fatal arrhythmias induced during hypoglycemia [5]. Several experimental studies have observed relationships between hypokalemia and prolonged corrected QT (QTc) interval, which causes fatal cardiac arrhythmias and sudden death [6-8]. It has been well described that a decrease in serum potassium during insulin induced hypoglycemia is caused both by insulin and adrenaline [9,10].
Furthermore, a relationship between insulin induced hypoglycemia and hypokalemia during hypoglycemia in diabetes has been reported. Recently, one study reported alterations of all electrolytes during insulin induced hypoglycemia in patients with type 1 diabetes. This study suggested hypoglycemia causes increases in serum sodium and chloride levels and decreases in serum potassium levels [11].
However, one study reported altered serum sodium and chloride levels during severe hypoglycemia in diabetes rare, whereas alterations of all electrolyte levels during severe hyperglycemia, such as diabetic ketoacidosis and a hyperglycemic hyperosmolar state, have been well established [12]. Incidences of hypokalemia during severe hypoglycemia in patients with type 2 diabetes appear to be lacking in the documents.
The aim of this study was to evaluate abnormalities in blood electrolyte levels and to assess individuals’ vital signs during severe hypoglycemia in Korean patients with type 2 diabetes in real-life conditions.
METHODS
Research design and methods
Medical records for adult type 2 diabetic patients who experienced hypoglycemia and were treated with glucose were collected from the Emergency Department in the Saint Carollo Hospital from 1 January, 2008 to 31 December, 2012. The criteria for patient inclusion were the diagnosis of type 2 diabetic mellitus and a serum glucose level < 70 mg/dL. Serum electrolyte, creatinine, blood urea nitrogen (BUN) and glucose levels were recorded prior to the administration of carbohydrates by the Emergency Department. The exclusion criteria included patients under 20 years old, patients with abnormal serum creatinine or BUN levels, and patients with alternative causes of electrolyte disturbances or vital signs’ changes such as severe diarrhea, chronic alcoholism, liver cirrhosis, malignancy, infection, and adrenal insufficiency. Severe hypoglycemia was defined as a condition requiring active medical assistance, such as administering carbohydrate when the serum glucose level was less than 70 mg/dL, and with symptoms of hypoglycemia [13].
Patients’ characteristics including age, gender, height, weight, the duration of diabetes and hypertension, glucose lowering agents, anti-hypertensive medications, smoking history, alcohol consumption, the inducing factor for hypoglycemia, co-morbidities (coronary artery disease and cerebral vascular disease) and vital signs obtained upon hospital arrival were examined for this study. The vital signs were monitored hourly for all patients and were checked more frequently for patients whose vital signs remained unstable. We also assessed hemoglobin A1c (HbA1c), plasma C-peptide levels, blood glucose levels, plasma insulin levels, and serum chemistry profiles obtained during severe hypoglycemia before carohydrates were administered.
Normal ranges for the analyzed electrolyte groups (Ortho Clinical Diagnostics, Raritan, NJ, USA) are as follows: sodium (Na) 135 to 148 mmol/L, chloride (Cl) 95 to 110 mmol/L, potassium (K) 3.5 to 5.3 mmol/L. Levels below the normal range are defined as hypo- and levels above the normal range are defined as hyper-.
The patients’ blood pressure, heart rate, respiratory rate, and body temperature were monitored both during the severe hypoglycemic stage and the recovery stage. The recovery stage was defined as the patients’ condition upon recovery from severe hypoglycemia and being discharged from the emergency room. These individuals were not treated with antihypertensive or vasopressor drugs during this period. Tachycardia was defined as a heart rate of > 100 beats per minute. Severe hypertension is a systolic blood pressure of ≥ 180 mmHg or a diastolic blood pressure of ≥ 120 mmHg [14]. The patient’s body temperature was measured via tympanic membrane and hypothermia was defined by a body temperature of < 35°C [15].
Statistical analysis
The data were analyzed using the SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Normally distributed data are presented as the mean ± standard deviation and non-normally distributed data are presented as the median range. Continuous variables were compared using a Mann-Whitney U test or paired t test. Categorical variables were compared using chi-square tests. Correlations were evaluated using Pearson correlation coefficient or Spearman rank correlation coefficient. The p value < 0.05 was considered statistically significant.
RESULTS
Of the 1,068 patients who visited the emergency room with severe hypoglycemia and were screened, 219 patients were analyzed for this study, 90 of whom were male. The mean patient age was 71.0 ± 10.7 years. The median duration of diabetes was 10 years (range, 0.08 to 40). The median duration of hypertension was 7 years (range, 0.33 to 40) and 66.2% of the subjects had a medical history of hypertension. The mean HbA1c levels were 6.6% ± 1.7%, while the median blood glucose levels were 34 mg/dL (range, 6 to 69). Treatments for type 2 diabetes including oral glucose-lowering agents alone, insulin therapy alone and a combination of both therapies were 79.0%, 12.1%, and 8.9%, respectively. The treatment methods for type 2 diabetes were not significantly different for those with hypokalemia (hypo-K) and normokalemia (normo-K; p = 0.57) (Table 1). The majority of patients treated with oral glucose-lowering agents were also taking sulfonylureas (88.3%).
Table 1.
Summary of patients' information collected during a severe hypoglycemic event
| Variable | Total | Hypokalemia | Normokalemia | Hyperkalemia | p value |
|---|---|---|---|---|---|
| No. of individuals | 219 | 48 | 167 | 4 | |
| Age, yr | 71.0 ± 10.7 | 69.4 ± 11.1 | 71.4 ± 10.6 | 71.3 ± 11.8 | 0.13 |
| Sex, male:female | 90:129 | 20:28 | 69:98 | 1:3 | 0.97 |
| Duration of diabetes, yr | 10 (0.08–40.0) | 10 (0.083–40.0) | 10 (0.16–40) | 10 (3–20) | 0.06 |
| Hypertension | 145 (66.2) | 34 (70.8) | 108 (64.7) | 3 (75.0) | 0.43 |
| Duration, yr | 7 (0.33–40) | 10 (0.33–40) | 10 (1–40) | 8 (8–10) | 0.87 |
| ARB/ACE inhibitors | 50 | 11 | 39 | 0 | 0.49 |
| Diuretics | 22 | 5 | 17 | 0 | 0.80 |
| β-Blockers | 13 | 6 | 6 | 1 | 0.07 |
| Calcium channel blockers | 36 | 10 | 25 | 1 | 0.69 |
| Treatment of diabetes | 214 | 47 | 163 | 4 | 0.57 |
| OHA alone | 169 (79.0) | 35 (74.5) | 131 (80.4) | 3 (75.0) | |
| Insulin alone | 26 (12.1) | 6 (12.8) | 19 (11.7) | 1 (25.0) | |
| Combination | 19 (8.9) | 6 (12.8) | 13 (8.0) | 0 | |
| HbA1c, % | 6.6 ± 1.7 | 6.9 ± 1.9 | 6.5 ± 1.7 | 5.7 ± 0.0 | 0.49 |
| Blood glucose, mg/dL | 34 (6–69) | 30 (14–62) | 35 (10–69) | 46 (6–65) | 0.04 |
| Serum sodium, mmol/L | 141.5 ± 4.8 | 141.8 ± 3.7 | 141.4 ± 5.1 | 139.2 ± 3.9 | 0.78 |
| Serum chloride, mmol/L | 103.5 ± 5.1 | 102.6 ± 4.7 | 103.8 ± 5.3 | 102.6 ± 3.8 | 0.12 |
| Serum potassium, mmol/L | 4.0 ± 0.6 | 3.2 ± 0.2 | 4.1 ± 0.4 | 5.7 ± 0.2 | < 0.01 |
Values are presented as number, mean ± SD, median (interquartile range), or number (%). A p value indicates comparison of participant characteristics between hypokalemia and normokalemia groups. Vital signs and laboratory data during severe hypoglycemia were measured upon hospital arrival.
ARB, angiotensin II receptor blockers; ACE, angiotensin-converting enzyme; OHA, oral hypoglycemic agent; HbA1c, hemoglobin A1c.
The occurrence of normal levels for the electrolytes—serum potassium, sodium, and chloride—was 53% (116/219 patients). Of the 47% of patients with electrolyte disturbances, 39.7% (87/219 patients) of patients were abnormal in one electrolyte level and 7.3% (16/219 patients) in two electrolyte levels. A disturbance in all three electrolyte levels was not observed. The most common type of electrolyte disturbance present during severe hypoglycemia was pure hypokalemia at 18.7% (41/219 patients) (Fig. 1).
Figure 1.
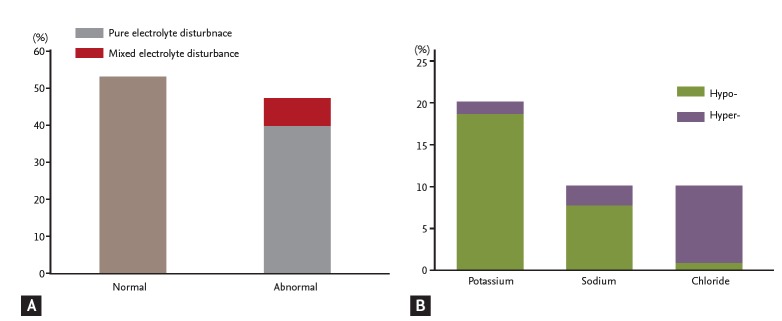
(A) The incidence of normal and abnormal blood electrolytes during severe hypoglycemia. (B) In pure electrolyte disturbances, the incidence of each electrolyte disturbance. The normal ranges for each electrolyte are defined as follows: sodium 135 to 148 mmol/L, chloride 95 to 110 mmol/L, potassium 3.5 to 5.3 mmol/L. Levels below the normal range are defined as hypoand levels above the normal range are defined as hyper-. A pure electrolyte disturbance was defined as an abnormal level in the one electrolyte and a mixed electrolyte disturbance was defined as two or three electrolyte levels being abnormal.
The median serum potassium levels during severe hypoglycemia were 3.92 mmol/L (range, 2.61 to 5.86). Serum potassium and blood glucose levels were positively correlated during severe hypoglycemia (r = 0.151, p = 0.025) (Fig. 2A) and also showed significant correlation when adjusted by age (r = 0.167, p = 0.013). However, no correlations between serum potassium and sodium levels (r = –0.075, p = 0.270) or serum potassium and chloride levels (r = 0.071, p = 0.296) were observed during a hypoglycemic event (Fig. 2B). The incidences of hypo-K (n = 48), normo-K (n = 167), and hyperkalemia (hyper-K, n = 4) were 21.9%, 76.3%, and 1.8 %, respectively (Fig. 3A). In the hypo-K and normo-K groups, the median blood glucose levels were 30 mg/dL (range, 14 to 62) and 35 mg/dL (range, 10 to 69), respectively. Blood glucose levels were significantly lower in the hypo-K group when compared to the normo-K group (p = 0.04). The prevalence of preexisting hypertension, the duration of hypertension and the use of antihypertensive agents were not significantly different between the hypo-K and normo-K groups. The patients’ age and serum potassium levels during hypoglycemia were positively correlated (r = 0.175, p = 0.009) (Fig. 4). However, no significant difference in patient age was observed between the hypo-K and normo-K groups (69.4 ± 11.1 years vs. 71.4 ± 10.6 years, p = 0.13). Furthermore, there were no significant differences in HbA1c, serum sodium and serum chloride levels between the hypo-K and normo-K groups (Table 1).
Figure 2.
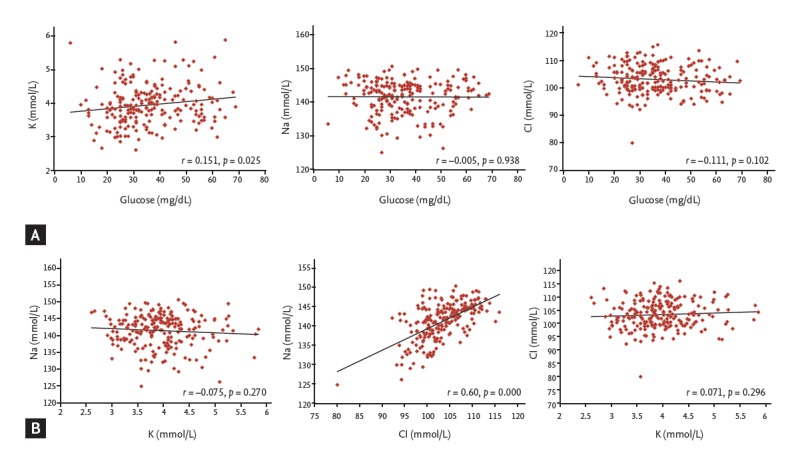
(A) Correlation between the blood glucose and each electrolyte during severe hypoglycemia. (B) Correlation between the blood electrolyte levels during severe hypoglycemia.
Figure 3.
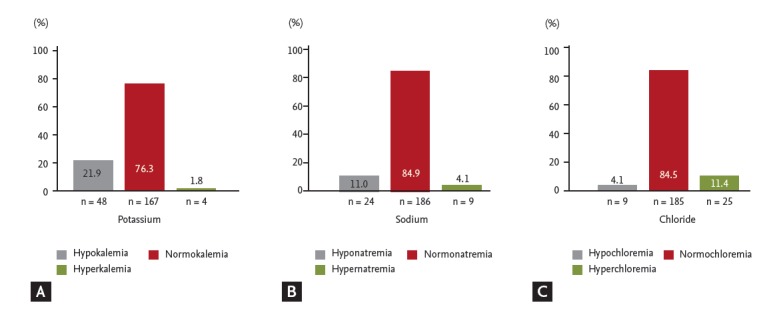
The incidence of each electrolyte during severe hypoglycemia. (A) Potassium, (B) sodium, and (C) chloride are shown.
Figure 4.
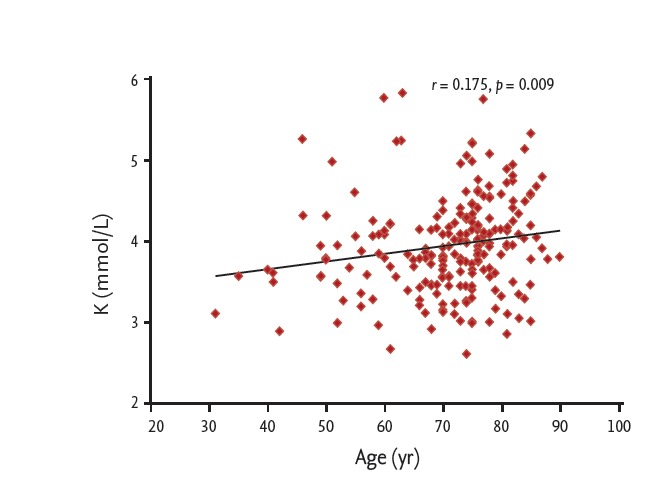
Correlation between patient age and serum potassium levels during severe hypoglycemia.
The median sodium and chloride levels during severe hypoglycemia upon emergency room arrival were 142.3 mmol/L (range, 124.9 to 150.5) and 103.2 mmol/L (range, 80.1 to 116.0), respectively. No correlation between blood glucose levels and both serum sodium (r = –0.005, p = 0.938) and serum chloride levels (r = –0.111, p = 0.102) was observed. However, serum sodium levels and chloride levels were highly correlated (r = 0.60, p = 0.000) (Fig. 2). The presence of hyponatremia (n = 24), normonatremia (n = 186), and hypernatremia (n = 9) were 11.0%, 84.9%, and 4.1%, respectively (Fig. 3B). The incidences of hypochloremia (n = 9), normochloremia (n = 185), and hyperchloremia (n = 25) were 4.1%, 84.5%, and 11.4%, respectively (Fig. 3C). No additional clinical parameters or subgroups were classified by the serum sodium and chloride levels.
Heart rate, systolic, and diastolic blood pressure were significantly different during the severe hypoglycemia stage, as compared to the recovery stage (Table 2). However, during severe hypoglycemia, heart rate, systolic, and diastolic blood pressure were not significantly different among the electrolyte subgroups (Table 3), nor did these parameters differ significantly when examining blood glucose levels.
Table 2.
A comparison of blood pressure and heart rate between the severe hypoglycemic stage and the recovery stage
| Variable | Hypoglycemia (n = 219) | Recovered stage (n = 213) | p value |
|---|---|---|---|
| Heart rate, beats/min | 81.3 ± 14.1 | 77.3 ± 12.8 | 0.000 |
| Tachycardiaa | 21 (9.6) | 10 (4.7) | 0.049 |
| Systolic blood pressure, mmHg | 146.6 ± 25.5 | 135.5 ± 19.3 | 0.000 |
| Diastolic blood pressure, mmHg | 84.6 ± 13.2 | 80.4 ± 12.0 | 0.000 |
| Severe hypertensionb | 29 (13.2) | 4 (1.9) | 0.000 |
Values are presented as means ± SD or number (%).
Tachycardia was defined as a heart rate of > 100 beats per minute.
Severe hypertension was defined as a systolic blood pressure of ≥ 180 mmHg or a diastolic blood pressure of ≥ 120 mmHg.
Table 3.
The comparisons of blood pressure and heart rate between each subgroup of electrolytes analyzed during a severe hypoglycemic event
| Variable | Potassium (normal range, 3.5–5.3 mmol/L) |
Sodium (normal range, 135–148 mmol/L) |
Chloride (normal range, 95–110 mmol/L) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Hypokalemia | Normokalemia | p value | Hyponatremia | Normonatremia | p value | Normochloremia | Hyperchloremia | p value | |
| Number | 48 | 167 | 24 | 186 | 185 | 25 | |||
| Hear rate, beats/min | 84.0 ± 16.0 | 80.8 ± 13.6 | 0.20 | 79.1 ± 12.2 | 81.7 ± 14.6 | 0.32 | 81.5 ± 13.7 | 79.0 ± 14.7 | 0.42 |
| Tachycardiaa | 9 (18.8) | 12 (7.2) | 0.02 | 2 (8.3) | 19 (10.2) | 0.77 | 18 (9.7) | 1 (4.0) | 0.35 |
| SBP, mmHg | 146.3 ± 29.7 | 146.5 ± 24.0 | 0.67 | 140.8 ± 25.9 | 147.3 ± 25.8 | 0.26 | 146.9 ± 25.3 | 142.0 ± 24.0 | 0.30 |
| DBP, mmHg | 84.6 ± 14.3 | 84.6 ± 13.0 | 0.89 | 84.6 ± 12.5 | 84.8 ± 13.5 | 0.92 | 84.6 ± 13.5 | 83.6 ± 11.5 | 0.62 |
| Severe hypertensionb | 10 (20.8) | 17 (10.2) | 0.05 | 2 (8.3) | 27 (14.5) | 0.41 | 25 (13.5) | 3 (12.0) | 0.83 |
Values are presented as means ± SD or number (%).
SBP, systolic blood pressure; DBP, diastolic blood pressure.
Tachycardia was defined as a heart rate of > 100 beats per minute.
Severe hypertension was defined as a systolic blood pressure of ≥ 180 mmHg or a diastolic blood pressure of ≥ 120 mmHg.
The incidences of tachycardia and severe hypertension during severe hypoglycemia were significantly higher than those of the recovery stage (9.6% vs. 4.7%, p = 0.049) (13.2% vs. 1.9%, p = 0.000) (Table 2). In the hypo-K and normo-K groups, the incidences of tachycardia during severe hypoglycemia were 18.8% and 7.2%, respectively. The presence of tachycardia was significantly higher in the hypo-K group than in the normo-K group during severe hypoglycemia (p = 0.02). Although the incidence of severe hypertension during severe hypoglycemia did not significantly differ between the hypo-K and normo-K groups (p = 0.05), the incidence of severe hypertension of the hypo-K group was twice that of the normo-K group (20.8% vs. 10.2%). However, for the serum electrolyte subgroups, sodium, and chloride, the incidences of tachycardia and severe hypertension during severe hypoglycemia were not significantly different (Table 3).
No significant differences in body temperature between the severe hypoglycemic stage and the recovery stage were observed (36.14°C ± 0.25°C vs. 36.14°C ± 0.26°C, p = 0.87), and no cases of hypothermia were recorded.
DISCUSSION
This study is the first to examine electrolyte profiles during severe hypoglycemia in patients with type 2 diabetes in clinical practice. The results demonstrated that 47% of patients with severe hypoglycemia had electrolyte disturbances, with pure hypokalemia being the most common type. Furthermore, a positive relationship between a decrease in serum potassium and a decrease in plasma glucose was observed, and hypokalemia was associated with tachycardia and severe hypertension. Therefore, this study suggests that hypokalemia associated with hypoglycemia may be associated with the increased risks of cardiovascular events in type 2 diabetes during severe hypoglycemia.
Previous epidemiological studies have shown that the incidence of severe hypoglycemia due to efforts to intensively control glucose has increased [16,17]. Severe hypoglycemia could lead to arrhythmias, cardiovascular events, dementia, and death [18,19]. It is well accepted that the prolongation of QTc intervals related to ventricular arrhythmias and sudden death is induced by severe hypoglycemia in patients with both type 1 and type 2 diabetes [20]. Although hypoglycemia itself without hypokalemia might cause the prolongation of QTc interval in patients with type 2 diabetes [21], hypoglycemia with hypokalemia could augment the prolongation of the QTc interval [6,20]. Moreover, hypokalemia-associated with hypoglycemia is known to be associated with hyperinsulinemia and the increased secretion of catecholamines [20,22].
One previous study reported that during severe hypoglycemia, the incidence of hypokalemia was 42.4% and 36.3% in type 1 and type 2 diabetes, respectively [19]. They suggested hypokalemia shows another threat associated with severe hypoglycemia [19]. The present study demonstrated the incidence of hypokalemia was 21.9% in type 2 diabetes during severe hypoglycemia and also found that serum potassium and blood glucose levels were positively correlated during severe hypoglycemia. In the present study, the presence of hypokalemia was lower than that of the aforementioned study, most likely due to a lower number of patients treated with insulin therapy (21.0% vs. 51.0%). Additionally, hypothermia was not observed in this study while the previous study reported the incidence of hypothermia as 22.6%. Therefore it is speculated that hypokalemia might be associated with relative hyperinsulinemia and hypothermia during severe hypoglycemia.
Lower doses of thiazide or thiazide-like diuretics can cause a significant decrease in serum potassium levels [23]. Conversely, angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors can induce hyperkalemia [24]. Several experimental studies have demonstrated that the β-adrenoreceptor blockade attenuates the decrease in serum potassium during insulin-induced hypoglycemia [9,10]. However, this study could not find any antihypertensive agents associated with hypokalemia during severe hypoglycemia due to multidrug regimens and limited data.
Only one previous experimental study has reported that insulin-induced hypoglycemia provokes increases in serum sodium and serum chloride levels, but decreases serum potassium levels during hypoglycemia. They suggested these changes in blood electrolyte levels during hypoglycemia could be explained by the effect of insulin and catecholamines on the Na+/K+ pump [11]. It was this study that first described the disturbance of serum sodium and serum chloride levels during severe hypoglycemia in a clinical setting. However, contrary to our expectations, this study demonstrated that serum potassium levels were not associated with serum sodium and serum chloride levels during severe hypoglycemia. Additionally, this study suggested that the serum sodium and serum chloride disturbances during severe hypoglycemia may not negatively affect heart rates and blood pressure. However, this could not be confirmed as the reported change of serum sodium and serum chloride levels with or without the change of serum potassium levels during severe hypoglycemia could not be located.
Several studies have reported that sympathoadrenal activation response to hypoglycemia resulted in increases in heart rates and blood pressure [19,25,26]. Similarly, this study also observed significant increases in heart rates and blood pressure during severe hypoglycemia, as compared to the recovery stage. Incidences of tachycardia and severe hypertension were significantly different during severe hypoglycemia, as compared to the recovery stage. This study is the first to demonstrate that the incidences of tachycardia and severe hypertension were higher in the hypo-K group than in the normo-K group during severe hypoglycemia. This further supports the theory that adrenalin release during hypoglycemia drives potassium into the cell as suggested in previous studies [22,27].
A few limitations were observed with this study. Firstly, due to a limited sample size, the association between electrolyte disturbances and insulin and C-peptide levels could not be examined. Secondly, records of counter-regulatory hormones responding to hypoglycemia could not be located; however, they are checked routinely in a clinical setting. This study provides important clinical information about severe hypoglycemia; however, further studies involving larger sample sizes from multiple centers are needed to confirm these results.
In summary, this study showed that electrolyte disturbances occurred in type 2 diabetes patients with severe hypoglycemia, and hypokalemia was the most common type. Furthermore, hypokalemia-associated with hypoglycemia provoked tachycardia and severe hypertension during severe hypoglycemia.
KEY MESSAGE
1. A total of 47% of type 2 diabetic patients with severe hypoglycemia exhibited electrolyte disturbances, with pure hypokalemia being the most common type.
2. A positive relationship between a decrease in serum potassium and a decrease in plasma glucose during severe hypoglycemia was observed.
3. Hypokalemia-associated with hypoglycemia was associated with tachycardia and severe hypertension during severe hypoglycemia.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 3.Oyer DS. The science of hypoglycemia in patients with diabetes. Curr Diabetes Rev. 2013;9:195–208. doi: 10.2174/15733998113099990059. [DOI] [PubMed] [Google Scholar]
- 4.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 5.Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63:1738–1747. doi: 10.2337/db13-0468. [DOI] [PubMed] [Google Scholar]
- 6.Marques JL, George E, Peacey SR, et al. Altered ventricular repolarization during hypoglycaemia in patients with diabetes. Diabet Med. 1997;14:648–654. doi: 10.1002/(SICI)1096-9136(199708)14:8<648::AID-DIA418>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Landstedt-Hallin L, Englund A, Adamson U, Lins PE. Increased QT dispersion during hypoglycaemia in patients with type 2 diabetes mellitus. J Intern Med. 1999;246:299–307. doi: 10.1046/j.1365-2796.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- 8.Heller SR. Abnormalities of the electrocardiogram during hypoglycaemia: the cause of the dead in bed syndrome? Int J Clin Pract. Suppl 2002(129):27–32. [PubMed] [Google Scholar]
- 9.Petersen KG, Schluter KJ, Kerp L. Regulation of serum potassium during insulin-induced hypoglycemia. Diabetes. 1982;31:615–617. doi: 10.2337/diab.31.7.615. [DOI] [PubMed] [Google Scholar]
- 10.Fisher BM, Thomson I, Hepburn DA, Frier BM. Effects of adrenergic blockade on serum potassium changes in response to acute insulin-induced hypoglycemia in nondiabetic humans. Diabetes Care. 1991;14:548–552. doi: 10.2337/diacare.14.7.548. [DOI] [PubMed] [Google Scholar]
- 11.Caduff A, Lutz HU, Heinemann L, Di Benedetto G, Talary MS, Theander S. Dynamics of blood electrolytes in repeated hyper- and/or hypoglycaemic events in patients with type 1 diabetes. Diabetologia. 2011;54:2678–2689. doi: 10.1007/s00125-011-2210-9. [DOI] [PubMed] [Google Scholar]
- 12.Kitabchi AE, Nyenwe EA. Hyperglycemic crises in diabetes mellitus: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinol Metab Clin North Am. 2006;35:725–751. doi: 10.1016/j.ecl.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Workgroup on Hypoglycemia. American Diabetes Association Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 15.Brown DJ, Brugger H, Boyd J, Paal P. Accidental hypothermia. N Engl J Med. 2012;367:1930–1938. doi: 10.1056/NEJMra1114208. [DOI] [PubMed] [Google Scholar]
- 16.Kim JT, Oh TJ, Lee YA, et al. Increasing trend in the number of severe hypoglycemia patients in Korea. Diabetes Metab J. 2011;35:166–172. doi: 10.4093/dmj.2011.35.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson ES, Koepsell TD, Reiber G, Stergachis A, Platt R. Increasing incidence of serious hypoglycemia in insulin users. J Clin Epidemiol. 2002;55:253–259. doi: 10.1016/s0895-4356(01)00479-6. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Choi Y, Jun C, et al. Neurocognitive changes and their neural correlates in patients with type 2 diabetes mellitus. Endocrinol Metab (Seoul) 2014;29:112–121. doi: 10.3803/EnM.2014.29.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsujimoto T, Yamamoto-Honda R, Kajio H, et al. Vital signs, QT prolongation, and newly diagnosed cardiovascular disease during severe hypoglycemia in type 1 and type 2 diabetic patients. Diabetes Care. 2014;37:217–225. doi: 10.2337/dc13-0701. [DOI] [PubMed] [Google Scholar]
- 20.Heller SR, Robinson RT. Hypoglycaemia and associated hypokalaemia in diabetes: mechanisms, clinical implications and prevention. Diabetes Obes Metab. 2000;2:75–82. doi: 10.1046/j.1463-1326.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- 21.Beom JW, Kim JM, Chung EJ, et al. Corrected QT interval prolongation during severe hypoglycemia without hypokalemia in patients with type 2 diabetes. Diabetes Metab J. 2013;37:190–195. doi: 10.4093/dmj.2013.37.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen TF, Baekgaard M, Dideriksen JL, et al. A physiological model of the effect of hypoglycemia on plasma potassium. J Diabetes Sci Technol. 2009;3:887–894. doi: 10.1177/193229680900300436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukete BN, Rosendorff C. Effects of low-dose thiazide diuretics on fasting plasma glucose and serum potassium-a meta-analysis. J Am Soc Hypertens. 2013;7:454–466. doi: 10.1016/j.jash.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Raebel MA. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 2012;30:e156–e166. doi: 10.1111/j.1755-5922.2010.00258.x. [DOI] [PubMed] [Google Scholar]
- 25.Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care. 2011;34 Suppl 2:S132–S137. doi: 10.2337/dc11-s220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev. 2008;24:353–363. doi: 10.1002/dmrr.865. [DOI] [PubMed] [Google Scholar]
- 27.Brown MJ, Brown DC, Murphy MB. Hypokalemia from beta2-receptor stimulation by circulating epinephrine. N Engl J Med. 1983;309:1414–1419. doi: 10.1056/NEJM198312083092303. [DOI] [PubMed] [Google Scholar]


