Abstract
The aim of the present study was to investigate the effects of paroxetine on the spatial memory and expression level of protein kinase C (PKC) in a rat model of depression. Rat models of depression were established by chronic unpredictable mild stress. The spatial learning and memory function of the rats were assessed by the Morris water maze test. The expression levels of PKC in the hippocampus were detected by western blotting. The results showed that, compared with the control group, the escape latency was prolonged and the percentage of time in the target quadrant and the number of times the rats crossed the platform were reduced in the model group; however, the impaired spatial learning and memory function in these rat models could be restored by paroxetine, almost to a level comparable with that in the normal control animals. In addition, the expression of PKC in the model group was significantly decreased compared with that in the control group, and the expression could also be elevated by paroxetine treatment. These results suggest an association between PKC levels and the pathogenesis of depression. The application of paroxetine can improve the spatial memory and reverse the changes in PKC levels in the hippocampus in the rat model of depression. The present findings have enhanced the understanding of the pathogenesis of depression, and provide experimental evidence for the treatment of depression with paroxetine.
Keywords: depression, paroxetine, spatial memory, protein kinase C, Morris water maze
Introduction
Depression, a mental disorder with a high incidence, has been clinically characterized by mental retardation, anhedonia, a lack of energy and numerous other physical symptoms (1). Depression brings severe stress and places a burden on the patients themselves, their families and society as a whole. The etiology and pathogenesis of depression represent challenging issues in scientific and medical research. Progress has been made in understanding the occurrence and development of the disease, which involves several response elements in a second messenger pathway, including G proteins, adenylyl cyclase and protein kinase C (PKC) (2–4). In particular, studies have shown that the expression and function of PKC are altered in patients or animal models with depression (5,6). Furthermore, the impairment of neurons in the brain has been shown to contribute to the onset and pathogenesis of depression. The modulation of the PKC-cAMP response element binding protein (CREB) signaling pathway may improve the plasticity and function of neurons, contributing to the protection against depression (7,8).
Currently, antidepressant drugs are the major treatment for depression in the clinic. Paroxetine, a type of highly selective 5-hydroxytryptamine (5-HT)-reuptake inhibitor, can inhibit 5-HT reuptake in synapses and increase the nerve conduction rate of 5-HT, exerting antidepressant effects (9). Due to its rapid action and few adverse side effects, paroxetine has been widely used in the clinical treatment of depression; however, the effects of paroxetine on PKC in depression have not been fully addressed.
In the present study, rat models of depression were established by the application of chronic unpredictable mild stress (CUMS), and the effects of paroxetine on the spatial memory and PKC expression in these models were investigated. The findings provide an in-depth understanding of the pathogenesis of depression and the treatment of the disease with antidepressant drugs.
Materials and methods
Experimental animals and grouping
Thirty healthy adult male Sprague Dawley rats (supplied by Qinglongshan Experimental Animal Breeding Farm, Nanjing, China), weighing 200±20 g, were randomly divided into control, model and paroxetine-treated groups (n=10 per group). No significant differences in body weight, age and basal metabolic values were found among the groups. The animal experiments were conducted according to the ethical guidelines of Yan'an University (Yan'an, China).
Animal modeling and drug administration
A rat model of depression was established by CUMS, as previously described (10). In brief, the model rats were subjected to one of the following treatments: Restraint through binding for 2 h, water deprivation for 24 h, fasting for 24 h, reversed day/night cycle for 24 h, forced swimming for 5 min, heat stress at 50°C for 5 min, ice swimming at 4°C for 5 min, tail clip for 1 min and foot shock (1 mA) for 10 sec. The models experienced one stimulus on each day and different stimuli on neighboring days. The procedures lasted for 4 weeks.
Rats in the control group were treated with saline by gavage (5 ml/kg). The model group was also treated with saline by gavage (5 ml/kg) prior to the modeling. In the paroxetine-treated group, the rats were treated with paroxetine (Yangzhou IL-YANG Pharmaceutical Co., Ltd., Yangzhou, China) by gavage (10 mg/kg with the dose volume of 5 ml/kg) prior to modeling.
Morris water maze (MWM)
The spatial learning and memory function of the rat models were assessed by the MWM assay. A circular pool was divided into four quadrants, and a circular black platform was placed in the target quadrant, 2 cm beneath the water surface. Markers in different shapes and colors were posted on the white curtain around the pool for navigation. Each rat was allowed to swim for 90 sec to find the hidden platform (11). The swimming paths of these rats were recorded by a video capture system (THDB-D5M, Terasic Technologies, Inc., Taiwan), and time spent in the target quadrant and the number of times the rats crossed the platform were calculated accordingly.
Western blot analysis
Following the behavioral test, the rats were decapitated. The brains were removed and the hippocampi were isolated and stored at −80°C. The samples were homogenized with lysis buffer, containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40 and 0.5% Na-deoxycholate, and the supernatant was collected following centrifugation at 20,000 × g at 4°C for 20 min. Total protein concentrations were determined using BCA assay. The expression levels of PKC were detected by western blotting, as previously described (12–14). Mouse anti-rat monoclonal antibodies against PKC (1:200; sc-17769) and β-actin (1:5,000; sc-8432) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Goat anti-mouse HRP-conjugated IgG (sc-47047; Santa Cruz Biotechnology, Inc.) was used. The bound antibodies were determined by the Pierce enhanced chemiluminescence system (Pierce Biotechnology Inc., Rockford, IL, USA). The experiments were performed >3 times. The mean optical density value of each protein band relative to that of the β-actin band from the same sample was calculated.
Statistical analysis
Data are expressed as the mean ± standard deviation. Statistical analyses were performed using SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA). The t-test and χ2 test were used for data comparison. P<0.05 was considered to indicate a statistically significant difference.
Results
Paroxetine improves spatial learning and memory function in a rat model of depression
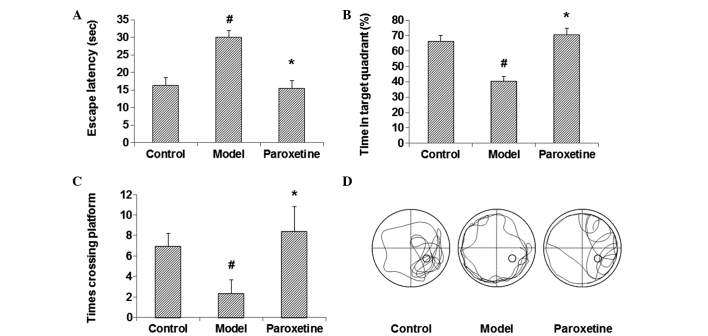
To investigate the effects of paroxetine on the spatial learning and memory function in depression models, an MWM behavioral assay was performed. The results showed that, compared with the control group, the escape latency was significantly prolonged, and the percentage of time in the target quadrant and the number of times the rats crossed the platform were notably reduced in the model group, all with statistical significance (P<0.05) (Fig. 1). Following treatment with paroxetine, however, the escape latency was evidently shortened and the percentage of time in the target quadrant and the number of times the rats crossed the platform were significantly elevated (P<0.05), with results comparable to those of the control group (Fig. 1). These findings suggest that paroxetine can improve the spatial learning and memory function in rat models of depression, almost to a level comparable with that of the normal control animals.
Figure 1.
Paroxetine improves the spatial learning and memory function in rat models of depression. The Morris water maze test was performed to assess the spatial learning and memory function in the rats. (A-C) Results for the (A) escape latencies, (B) percentage of time in the target quadrant and (C) number of times the rats crossed the platform are shown for the rats from the control, model and paroxetine-treated groups (n=10 per group). Results are presented as the mean ± standard deviation. #P<0.05 compared with the control group; *P<0.05 compared with the model group. (D) Representative navigation path recording of the behavioral test.
Paroxetine upregulates the PKC expression level in the hippocampus in rat models of depression
PKC and the associated signaling pathways have been closely linked with the mental processes in the brain and the pathogenesis of mental disorders, including depression. To investigate the mechanism through which paroxetine improves spatial learning and memory function in these depression models, the expression levels of PKC in the hippocampus were assessed by western blot analysis. The results showed that, compared with the control group, the hippocampal PKC content in the model group was notably decreased (P<0.05) (Fig. 2A and B). By contrast, the PKC expression level in the paroxetine-treated group was significantly increased compared with that in the model group (P<0.05) (Fig. 2A and 2B). These results suggest that paroxetine can elevate the expression levels of PKC in the hippocampus in rat models of depression, which may contribute to the improvements in the spatial learning and memory function in these model rats.
Figure 2.
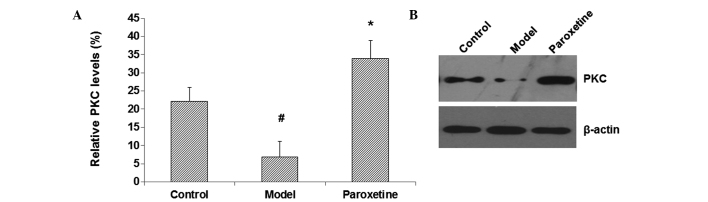
Paroxetine upregulates the expression level of PKC in the hippocampus in rat models of depression. Western blotting was used to detect the protein expression level of PKC in the hippocampus in rats from the control, model and paroxetine-treated groups. (A) Relative optical densities of the protein bands. Results are presented as the mean ± standard deviation. #P<0.05 compared with the control group; *P<0.05 compared with the model group. (B) Representative blots. Experiments were repeated >3 times. PKC, protein kinase C.
Discussion
In the present study, a rat model of depression was established by CUMS, effectively simulating the major symptoms of depression in human patients: Anhedonia, reduced autonomous behavior, declined social communication abilities, and impaired learning and memory function. A number of studies have shown that abnormalities in the hippocampus are closely associated with the occurrence and development of depression (15–18). Based on these results, the effects of paroxetine on spatial learning and memory function and the expression levels of PKC in the hippocampus were investigated in a rat model of depression in the present study.
As a selective 5-HT reuptake inhibitor, paroxetine can rapidly increase the level of 5-HT in the brain, suggesting that paroxetine may target the post-receptor cell signal transduction pathway (19,20). PKC, a main regulator of the phospholipase C/Ca2+ system, plays an important role in cell signal transduction (21). Through the PKC/CREB signaling pathway, PKC regulates the excitability of nerve cells and affects the synthesis and release of monoamine neurotransmitters, with a relatively high content in the hippocampus (22). Autopsy has shown that there are lower levels of CREB in the brains of patients with depression, and CREB levels are elevated in those patients who have been using antidepressants prior to mortality, indicating that antidepressants may act on the PKC/CREB signaling pathway (23,24). Liu and Xu (25) have suggested that the expression levels of PKCβII on cell membranes in the cerebral cortex are significantly decreased in rat models of depression, and that the changes in PKC concentration and/or activity are associated with the etiology of the disease. In the present study, the PKC content in the model group was evidently decreased compared with that in the control group. Furthermore, compared with the model group, the expression levels of PKC in the paroxetine-treated group were significantly increased. These results indicate that the changes in PKC expression levels may be associated with the pathogenesis of depression. Paroxetine is able to reverse the changes in PKC levels in the hippocampus, and exert antidepressant effects, most likely through the PKC/CREB signaling pathway. Zheng et al (26) have suggested that the long-term use of paroxetine may restore the phosphorylation of CREB to normal levels in the hippocampus in rat models of depression, and increase the concentration of phosphorylated CREB. The longer the duration of the treatment, the more reversal effects due to paroxetine would be observed.
In conclusion, the present results showed that paroxetine improved the spatial learning and memory function in rat models of depression, and the antidepressant effect may have been associated with the PKC/CREB signaling pathway. These findings not only enhance the understanding of the pathogenesis of depression but also provide experimental evidence for the treatment of depression with such antidepressant drugs as paroxetine.
Acknowledgements
This study was supported by the High-Level University Construction Project of Shaanxi, China (grant no. 2013SXTS02).
References
- 1.Burton C, McKinstry B, Szentagotai Tătar A, SerranoBlanco A, Pagliari C, Wolters M. Activity monitoring in patients with depression: A systematic review. Affect Disord. 2013;145:21–28. doi: 10.1016/j.jad.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Wu F, Kong LT, Tang YQ. Effects of fluoxetine and tianeptine on protein kinase C expression in hippocampus in rat models of chronic stress. Zhong Guo Quan Ke Yi Xue. 2012;15:1375–1377. (In Chinese) [Google Scholar]
- 3.Vorhees CV, Morford LR, Graham DL, Skelton MR, Williams MT. Effects of periadolescent fluoxetine and paroxetine on elevated plus-maze, acoustic startle, and swimming immobility in rats while on and off-drug. Behav Brain Funct. 2011;7:41. doi: 10.1186/1744-9081-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Z, Li HF, Liu JM. Study on the expression of central protein kinase C and adenylate cyclase in depression rats with and without antidepressant treatment. Zhong Guo Shen Jing Jing Shen Ji Bing Za Zhi. 2010;36:225–228. (In Chinese) [Google Scholar]
- 5.Hahn CG, Umapathy, Wang HY, et al. Lithium and valproic acid treatments reduce PKC activation and receptor-G protein coupling in platelets of bipolar manic patients. J Psychiatr Res. 2005;39:355–363. doi: 10.1016/j.jpsychires.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Liu J. Change of cyclic adenosine monophosphate concentration and PKC expression in behavioral deficit-induced depression rat brain. Zhong Hua Jing Shen Ke Za Zhi. 2002;35:173–176. (In Chinese) [Google Scholar]
- 7.Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/S0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 8.Wierda KD, Toonen RF, de Wit H, Brussaard AB, Verhage M. Interdependence of PKC-dependent and PKC-independent pathways for presynaptic plasticity. Neuron. 2007;54:275–290. doi: 10.1016/j.neuron.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Purgato M, Papola D, Gastaldon C, Trespidi C, Magni LR, Rizzo C, Furukawa TA, Watanabe N, Cipriani A, Barbui C. Paroxetine versus other anti-depressive agents for depression. Cochrane Database Syst Rev. 2014;4:CD006531. doi: 10.1002/14651858.CD006531.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai YF, Wang Y, Liu XD. Changes of glial fibrillary acidic protein and c-fos levels in rat models of chronic stress depression. Zhong Guo Zhi Ye Yi Xue. 2010;37:458–461. (In Chinese) [Google Scholar]
- 11.Keck ME, Sartori SB, Welt T, Müller MB, Ohl F, Holsboer F, Landgraf R, Singewald N. Differences in serotonergic neurotransmission between rats displaying high or low anxiety/depression-like behaviour: Effects of chronic paroxetine treatment. J Neurochem. 2005;92:1170–1179. doi: 10.1111/j.1471-4159.2004.02953.x. [DOI] [PubMed] [Google Scholar]
- 12.Tyeryar KR, Vongtau HO, Undieh AS. Diverse antidepressants increase CDP-diacylglycerol production and phosphatidylinositide resynthesis in depression-relevant regions of the rat brain. BMC Neurosci. 2008;9:12. doi: 10.1186/1471-2202-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X. Effect of cAMP/PKA/CREB signaling pathway and regulatory proteins PDE-4 and ERK on learning and memory function. Yi Xue Zong Shu. 2011;17:2241–2243. (In Chinese) [Google Scholar]
- 14.Zheng L, Wang YM. Changes in serum neurotrophic factor 3 after paroxetine treatment in depression. Gui Yang Yi Xue Yuan Xue Bao. 2013;38:347–350. (In Chinese) [Google Scholar]
- 15.Hu Y, Yin WG, Lin R, Li W. Comparison of brain-derived neurotrophic factor levels in hippocampus and serum in two rat models of depression. Zhong Guo Lao Nian Xue Za Zhi. 2009;29:2188–2190. (In Chinese) [Google Scholar]
- 16.Wei KL, Cheng YM, Sang WH, et al. Comparative study of duloxetine and paroxetine in treating depression with different symptoms. Zhongguo Lin Chuang Yao Li Xue Za Zhi. 2011;27:252–254. (In Chinese) [Google Scholar]
- 17.Yang M, Wen SY, Wu MC. Improvement in negative emotion and inflammatory factor levels in chronic heart failure patients after paroxetine treatment. Zhong Guo Yao Fang. 2013;24:3433–3435. (In Chinese) [Google Scholar]
- 18.Fei HZ, Wang H, Hu XY, et al. Improvement in oxidative stress, HPA axis function, and hippocampal brain-derived neurotrophic factor expression after paroxetine treatment. Zhong Guo Lin Chuang Yao Li Xue Za Zhi. 2012;17:1137–1142. (In Chinese) [Google Scholar]
- 19.Meng X. Effects of paroxetine combined with mental intervention on post-stroke depression. Pract Prev Med. 2011;18:491–493. [Google Scholar]
- 20.Ni GH, Shao B, Fan H. Effects of restraint stress and paroxetine in post-stroke rats. Chongqing Yi Xue Za Zhi. 2010;39(1038):1033–1035. (In Chinese) [Google Scholar]
- 21.Abrial E, Lucas G, Scarna H, Haddjeri N, Lambás-Señas L. A role for the PKC signaling system in the pathophysiology and treatment of mood disorders: Involvement of a functional imbalance? Mol Neurobiol. 2011;44:407–419. doi: 10.1007/s12035-011-8210-4. [DOI] [PubMed] [Google Scholar]
- 22.Réus GZ, Stringari RB, Ribeiro KF, Ferraro AK, Vitto MF, Cesconetto P, Souza CT, Quevedo J. Ketamine plus imipramine treatment induces antidepressant-like behavior and increases CREB and BDNF protein levels and PKA and PKC phosphorylation in rat brain. Behav Brain Res. 2011;221:166–171. doi: 10.1016/j.bbr.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Ding G, Yu G, Wu Y, et al. Effects of Jiawei Xiaoyao decoction on cAMP, PKA, and PKC levels in hippocampus of depression rats. Zhong Guo Shi Yan Fang Ji Xue Za Zhi. 2012;18:162–164. (In Chinese) [Google Scholar]
- 24.Li Z. Role of brain-derived neurotrophic factor in depression pathogenesis. Shanghai Jiao Tong Da Xue Xue Bao (Yi Xue Ban) 2010;30:651–655. (In Chinese) [Google Scholar]
- 25.Liu X, Xu J. Changes of protein kinase CβII expression in subcellular fractions from stress-induced depression rat brain. Zhong Guo Xing Wei Yi Xue Ke Xue Za Zhi. 2005;14:678–680. (In Chinese) [Google Scholar]
- 26.Zheng H, Ma G, Fu X. Effects of paroxetine on hippocampus-dependent learning and memory and ERK-CREB signaling pathway in rat models of depression. Zhong Guo Yao Xue Za Zhi. 2008;43:1234–1238. (In Chinese) [Google Scholar]