Abstract
In recent genome-wide association studies (GWAS), 11 risk loci were identified in patients with familial and sporadic Parkinson's disease (PD) in different populations. The LRRK2 gene was found to be a mutation hot spot in European and Asian populations. The aim of the present study was to investigate the incidence of G2019S and R1441C mutations in the LRRK2 gene in individuals from the Xinjiang region of China, and to explore the associations between LRRK2 gene single nucleotide mutations and susceptibility to PD in the Uyghur and Han populations of Xinjiang. A case-control study was conducted with a group of 312 patients with PD, including 130 Uyghur and 182 Han individuals. The control group comprised 359 subjects, including 179 Uyghur and 180 Han individuals. Polymerase chain reaction-restriction fragment length polymorphism and DNA sequencing methods were used to detect the G2019S and R1441C mutations in the LRRK2 gene in the Uyghur and Han populations. No known mutations or new hybrids were found. Thus, there was no evidence that Uyghur and Han patients with PD possess the G2019S or R1441C mutations of the LRRK2 gene. This does not exclude the possibility of the presence other LRRK2 gene mutations that are associated with PD in the Uyghur and Han populations. In the future, the association of the LRRK2 gene with PD development in different regions and populations requires further study, in addition to the regulatory effects of the G2019S and R1441C mutations on gene expression.
Keywords: Uyghur, Parkinson's disease, G2019S, R1441C, mutation
Introduction
Parkinson's disease (PD) is a common degenerative disease of the central nervous system in the elderly, which is primarily associated with environmental and genetic factors. There are a number of genes that have been established to be associated with PD, which include LRRK2. Mutations of LRRK2 are considered to be the most prevalent in the pathogenesis of PD; in studies of North American and European PD patients, 5% had a mutation of LRRK2 and a family history of PD, while 1–2% had sporadic PD (1–4). The LRRK2 gene is the causative gene of the autosomal dominant hereditary type 8 PD (5,6). It is composed of five structural domains, namely, the ankyrin repeat (ANK), leucine-rich repeat (LRR), Ras of complex proteins (Roc) C-terminal of Roc (COR), mitogen activated kinase kinase kinase (MAPKKK) and WD40 regions (7–9). Major mutations of LRRK2 include R1441C, R1441G, R1441H, R1514Q, Y1699C, G2019S, I2020T, I2012T and G2385R (10–12). Mutations of LRRK2 have been associated with a number of diseases, in particular with familial PD and sporadic PD, and the G2019S mutation is one of the most common mutations in PD (13). Clear racial and regional differences exist in the incidence of PD.
Xinjiang is an autonomous region located in Central Asia, which has two predominant populations with different genetic backgrounds, namely, the Uyghur and Han populations. The current case-control study selected PD patients and healthy individuals from the Uyghur and Han populations of the Xinjiang region for the analysis of LRRK2 gene mutations. To the best of our knowledge, this is the first time that the association between the G2019S and R1441C mutations of the LRRK2 gene and PD susceptibility has been investigated in different ethnicities and regions.
Subjects and methods
Diagnostic criteria and study subjects
From June 2010 to April 2013, 312 patients with PD (all sporadic) visiting the specialist neurology clinic of the First Affiliated Hospital of Xinjiang Medical University (Urumqi, China) were enrolled in the study. The diagnosis was in line with the UK Brain Bank diagnostic criteria for PD. Cerebrovascular disease, encephalitis, trauma, drug-induced Parkinson's syndrome, Parkinson's plus syndrome and other severe systemic diseases were excluded. The control group consisted of 359 volunteers from the same region, who had no family history or clinical manifestations of PD. Gender, age and ethnicity were matched between the two groups. The study was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University, and all subjects provided informed consent.
DNA extraction
A sample of blood (2 ml) was collected from each subject, subsequent to the provision of informed consent. Following EDTA anticoagulation, a non-centrifugal-type DNA Extraction kit (Shanghai Tiangen Biotech Co., Ltd., Shanghai, China) was used to extract the genomic DNA Tris-borate buffer (Sangon Biotech Co., Ltd., Shanghai, China) was added and the DNA was maintained at −80°C.
Design of primers and amplification of the gene
The primers used for G2019S were based on those used in a previous study by Thaler et al (14) and the sequences were as follows: upstream, 5′-CCTGTGCATTTTCTGGCAGATA-3′ and downstream, 5′-CCTCTGATGTTTTTATCCCCATTC-3′. According to the study by Paisán-Rauíz et al (7), the primer sequences for R1441C were as follows: upstream, 5′-TCAACAGGAATGTGAGCAGG-3′ and downstream 5′-CCCACAATTTTAAGTGAGTTGC-3′. DNA amplification was carried out as follows: The total volume of the quantitative polymerase chain reaction (qPCR) was 20 µl, including 100 ng/µl upstream and downstream primers (0.5 µl), 2X Power Taqman Master Mix (10 µl; Beijing Baitaike Biotechnology Co., Ltd., Beijing, China), 50 ng/µl DNA (3.0 µl) and ddH2O (11 µl). Primers were synthesized by Sangon Biotech Co., Ltd (Shanghai, China). A GeneAmp System 9700 thermal cycler (Applied Biosystems Corporation, Foster City, CA, USA) was used. PCR was performed after the first denaturation at 95°C for 2 min; each cycle consisted of denaturation at 95°C for 20 sec, annealing at 62°C for 20 sec and extension at 72°C for 30 sec. The number of total PCR cycles was 35.
qPCR product detection
Equal volumes of PCR products (7 µl) were taken, sample buffer [2X bromophenol blue; Sangon Biotech (Shanghai) Co., Ltd.] was added and the solution was mixed. The sample was placed on a 4% agarose gel for nucleic acid staining and 4 V/cm electrophoresis was performed for one hour.
Enzyme digestion genotyping
PCR products (8 µl), 10X endonuclease buffer (2 µl; New England Biolabs, Inc., Ipswich, MA, USA) and restriction endonucleases ScfI and BstUl (5 units; New England Biolabs Inc., Ipswich, MA, USA) were added to 20 µl sterile double distilled water and incubated at 37°C overnight (16 h). The digestion products (9 µl) were placed on a 4% agarose gel for ethidium bromide staining, and underwent electrophoresis at 110 V for 1.5 h. A Gel Doc 1000 gel imaging analysis system (Bio-Rad, Hercules, CA, USA) was used to detect the electrophoretic bands (Fig. 1).
Figure 1.
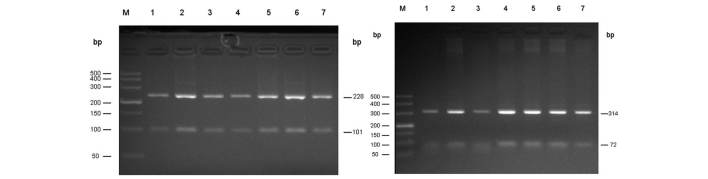
Electrophoresis results of the G2019S mutation in exon 41 of the LRRK2 gene digested with ScfI. Electrophoresis results of the R1441C mutation in exon 31 of the LRRK2 gene digested with BstU1. M, DNA marker 500 bp in length; 1–7, dominant hereditary sporadic PD patients.
Direct sequencing
To determine the accuracy of the results, 10% of the samples were randomly selected (31 from the patient group and 36 from the control group) for direct sequencing, which was performed by Shanghai Invitrogen Biotechnology Co., Ltd. (Shanghai, China).
Statistical methods
The age difference between the two groups was compared using an independent samples t-test; the differences in gender, allele and genotype frequencies between the two groups were compared using a χ2 test. P<0.05 was considered to indicate a statistically significant difference.
Results
qPCR
Using the template DNA, qPCR amplification products of 329 bp were obtained from Uyghur (n=186) and Han (n=127) patients with PD. All the products were digested with ScfI overnight and 228 and 101 bp fragments were obtained as presented in Fig. 1 (left). Following amplification, PCR products of 386 bp were obtained, the resulting products were digested overnight using BstU1, and two fragments of 314 and 72 bp were obtained as presented in Fig. 1 (right).
Sequence analysis
Following random sequencing comparison, no G2019S (Fig. 2) and R1441C (Fig. 3) mutations or novel heterozygotes were found. For G2019S, only the GG genotype and no mutant genotypes were identified in all subjects of the Han and Uygur populations. For R1441C, only one genotype (CC) was identified in the Han and Uygur populations. There were no differences between PD patients and the control group in these two single nucleotide polymorphisms (Tables I and II).
Figure 2.
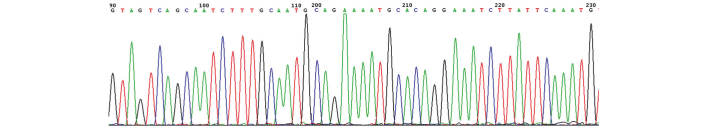
Partial sequencing figure of exon 41 in the LRRK2 gene.
Figure 3.
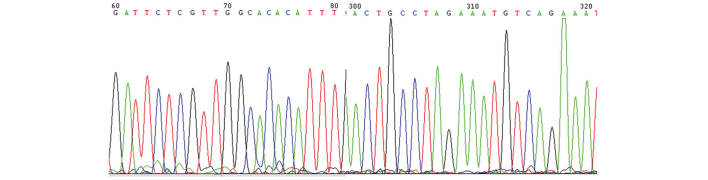
Partial sequencing figure of exon 31 in the LRRK2 gene.
Table I.
G2019S genotype and allele frequency comparison/cases (%) of Parkinson's disease and control groups of Uyghur and Han individuals from Xinjiang.
| Genotype frequency | Allele frequency | Control group | Allele frequency | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethnic group | PD group | GG | GA | AA | G | A | Control group | GG | GA | AA | G | A |
| Uyghur | 130 | 130 (100) | 0 (0) | 0 (0) | 260 (100) | 0 (0) | 179 | 179 (100) | 0 (0) | 0 (0) | 358 (100) | 0 (0) |
| Male | 76 | 76 (100) | 0 (0) | 0 (0) | 152 (100) | 0 (0) | 104 | 104 (100) | 0 (0) | 0 (0) | 208 (100) | 0 (0) |
| Female | 54 | 54 (100) | 0 (0) | 0 (0) | 108 (100) | 0 (0) | 75 | 75 (100) | 0 (0) | 0 (0) | 150 (100) | 0 (0) |
| Han | 182 | 182 (100) | 0 (0) | 0 (0) | 364 (100) | 0 (0) | 181 | 181 (100) | 0 (0) | 0 (0) | 362 (100) | 0 (0) |
| Male | 109 | 109 (100) | 0 (0) | 0 (0) | 218 (100) | 0 (0) | 109 | 109 (100) | 0 (0) | 0 (0) | 218 (100) | 0 (0) |
| Female | 73 | 73 (100) | 0 (0) | 0 (0) | 146 (100) | 0 (0) | 72 | 72 (100) | 0 (0) | 0 (0) | 144 (100) | 0 (0) |
Table II.
R441C genotype and allele frequency comparison/cases (%) of Parkinson's disease and control groups of Uyghur and Han individuals from Xinjiang.
| Genotype frequency | Allele frequency | Control group | Allele frequency | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethnic group | PD group | CC | CT | CG | GT | TT | GG | C | T | Control group | CC | CT | CG | GT | TT | GG | C | T |
| Uyghur | 130 | 130 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 260 (100) | 0 (0) | 179 | 179 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 358 (100) | 0 (0) |
| Male | 76 | 76 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 152 (100) | 0 (0) | 104 | 104 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 208 (100) | 0 (0) |
| Female | 54 | 54 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 108 (100) | 0 (0) | 75 | 75 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 150 (100) | 0 (0) |
| Han | 182 | 182 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 364 (100) | 0 (0) | 181 | 181 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 362 (100) | 0 (0) |
| Male | 109 | 109 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 218 (100) | 0 (0) | 109 | 109 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 218 (100) | 0 (0) |
| Female | 73 | 73 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 146 (100) | 0 (0) | 72 | 72 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 144 (100) | 0 (0) |
Discussion
In recent years, an increasing number of studies have focused on PD, and in particular on the genes associated with PD. By far, the LRRK2 gene is the most frequently mutated gene in autosomal dominant hereditary PD patients, and its incidence gradually increases with age (1,15–17). The LRRK2 gene is located on chromosome 12p11.2-q13.1 and has a total length of 1,441 nucleotides, containing 51 exons and encoding 2,527 amino acids, with a relative molecular weight of 286,000. The product protein LRRK2/dardarin is rich in leucine (18). In the study of the molecular activity of LRRK2, two regions associated with GTP enzymes and kinases have been found. The most common LRRK2 mutations in the two regions affect the enzymatic activity, suggesting that their functionality is important (19). Different LRRK2 mutations have been reported in patients with familial and sporadic PD. Genome-wide association studies (GWAS) have found that common mutations in SNCA, LRRK2, MAPT and HLA are risk factors for PD, and have demonstrated that a loss of chromosome 18 can significantly increase the development of PD (20–33). G2019S is the most common causative mutation of PD, and ~2% of PD cases are caused by the G2019S mutation (34). G2019S is located in exon 41 of the LRRK2 gene; it increases LRRK2 kinase activity and accelerates the phosphorylation of ezrin/radixin/moesin protein (11); however, while it induces neuronal apoptosis it has no effect on the GTP carrier or GTP activity. G2019S reduces the interaction of LRRK2 with the 14-3-3 proteins and increases the aggregation of and interaction with FADD (35). Healy et al (17) found that in 19,376 PD patients across 21 regions, the G2019S mutation was highest in North African populations, and that it accounted for 39% of sporadic PD and 36% of familial PD. In the Jewish population of Northern Europe, G2019S accounted for 10% of cases of sporadic and 28% of cases of familial PD. However, in Asia this locus mutation is very rare, only accounting for 0.1% of LRRK2 mutations. In Japan, India, Singapore, China, Taiwan and the mainland, Gly2019Ser and Arg1441Cys/Gly mutations were not found in PD patients during LRRK2 gene detection.
R1441C is another common mutation locus in LRRK2, it is located in exon 31 of the LRRK2 gene, and it induces neuronal apoptosis. It has no kinase activity, or at least the effect is negligible; however, it stabilizes the LRRK2 dimer, reduces GTP activity and participates in FADD aggregation (35). R1441G, found in northern Spain, has the same codon as R1441H, which primarily occurs in the Caucasian population (36). Of 304 patients with PD in Belgium, 18.1% had familial PD, and the R1441C mutation accounted for 10.7% (37). R1441C is in the Roc functional domain, with GTP enzyme sequences to participate in regulatory activities, including signal transduction, cell differentiation and cell growth (38). R1441C impairs dopamine neurotransmission and D2 receptor function, leading to degeneration of the dopaminergic nervous system in patients with PD (38,39). To the best of our knowledge, there has been no relevant report of the mutation in Han PD patients.
In the current study, the G2019S and RL441C mutations of the LRRK2 gene, or any novel variant of these, were not found to be present in PD patients from the Han and Uyghur populations. This is consistent with previous studies concerning the Chinese population (40,41). These mutations may not be hot spot mutations in PD; or the small sample size of this study may explain why the mutations were not found. Future studies of these populations may investigate recent-onset PD patients, larger sample sizes and other mutations of the LRRK2 gene.
References
- 1.Kachergus J, Mata IF, Hulihan M, et al. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am J Hum Genet. 2005;76:672–680. doi: 10.1086/429256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kay DM, Zabetian CP, Factor SA, et al. Parkinson's disease and LRRK2: frequency of a common mutation in U.S. movement disorder clinics. Mov Disord. 2006;21:519–523. doi: 10.1002/mds.20751. [DOI] [PubMed] [Google Scholar]
- 3.Aasly JO, Toft M, FernandezMata I, et al. Clinical features of LRRK2-associated Parkinson's disease in central Norway. Ann Neurol. 2005;57:762–765. doi: 10.1002/ana.20456. [DOI] [PubMed] [Google Scholar]
- 4.Gilks WP, AbouSleiman PM, Gandhi S, et al. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- 5.Gatto EM, Parisi V, Converso DP, et al. The LRRK2 G2019S mutation in a series of Argentinean patients with Parkinson's disease: Clinical and demographic characteristic. Neurosci Lett. 2013;14:1–5. doi: 10.1016/j.neulet.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Zhang ZX, Anderson DW, Huang JB, et al. Prevalence of Parkinson's disease and related disorders in the elderly population of greater Beijing, China. Mov Disord. 2003;7:764–772. doi: 10.1002/mds.10445. [DOI] [PubMed] [Google Scholar]
- 7.Paisán-Ruíz C, Jain S, Evans EW, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Zimprich A, Biskup S, Leitner P, Lichtner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorpific pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Funayama M, Hasegawa K, Kowa H, et al. A new locus for Parkinson's disease (PARK8) maps to chromosome 12p11. 2-q13.1. Ann Neurol. 2002;51:296–301. doi: 10.1002/ana.10113. [DOI] [PubMed] [Google Scholar]
- 10.Toft M, Mata IF, Ross OA, Kachergus J, et al. Pathogenicity of the Lrrk2 R1514Q substitution in Parkinson's disease. Mov Disord. 2007;22:389–392. doi: 10.1002/mds.21217. [DOI] [PubMed] [Google Scholar]
- 11.Punia S, Behari M, Govindappa ST, Swaminath PV, Jayaram S, Goyal V, Muthane UB, Juyal RC, Thelma BK. Absence/rarity of commonly reported LRRK2 mutations in Indian Parkinson's disease patients. Neurosci Lett. 2006;409:83–88. doi: 10.1016/j.neulet.2006.04.052. [DOI] [PubMed] [Google Scholar]
- 12.Dächsel JC, Farrer MJ. LRRK2 and Parkinson Disease. Arch Neurol. 2010;67:542–547. doi: 10.1001/archneurol.2010.79. [DOI] [PubMed] [Google Scholar]
- 13.Thaler A, Ash E, GanOr Z, OrrUrtreger A, Giladi N. The LRRK2 G2019S mutation as the cause of Parkinson's disease in Ashkenazi Jews. J Neural Transm. 2009;116:1473–1482. doi: 10.1007/s00702-009-0303-0. [DOI] [PubMed] [Google Scholar]
- 14.Thaler A, Mirelman A, Gurevich T, et al. Lower cognitive performance in healthy G2019S LRRK2 mutation carriers. Neurology. 2012;79:1027–1032. doi: 10.1212/WNL.0b013e3182684646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldwurm S, Zini M, Mariani L, Tesei S, Miceli R, Sironi F, Clementi M, Bonifati V, Pezzoli G. Evaluation of LRRK2 G2019S penetrance: relevance for genetic counselling in Parkinson disease. Neurology. 2007;68:1141–1143. doi: 10.1212/01.wnl.0000254483.19854.ef. [DOI] [PubMed] [Google Scholar]
- 16.Haugarvoll K, Rademakers R, Kachergus JM, et al. LrrK2 R1441C parkinsonism is clinically similar to sporadic Parkinson disease. Neurology. 2008;70:1456–1460. doi: 10.1212/01.wnl.0000304044.22253.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healy DG, Falchi M, O'Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW. International LRRK2 Consortium: Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paisán-Ruíz C, Lang AE, Kawari T, et al. LRRK2 gene in Parkinson disease: Mutation analysis and case control association study. Neurology. 2005;65:696–700. doi: 10.1212/01.WNL.0000167552.79769.b3. [DOI] [PubMed] [Google Scholar]
- 19.Taymans JM. The GTPase function of LRRK2. Biochem Soc Trans. 2012;40:1063–1069. doi: 10.1042/BST20120133. [DOI] [PubMed] [Google Scholar]
- 20.Simón-Sánchez J, Schulte C, Bras JM, Sharma M, et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M, Nakamura Y, Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet. 2009:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 22.Pankratz N, Beecham GW, DeStefano AL, Dawson TM, Doheny KF, Factor SA, Hamza TH, Hung AY, Hyman BT, Ivinson AJ, Krainc D, Latourelle JC, Clark LN, Marder K, Martin ER, Mayeux R, Ross OA, Scherzer CR, Simon DK, Tanner C, Vance JM, Wszolek ZK, Zabetian CP, Myers RH, Payami H, Scott WK, Foroud T. Meta-analysis of Parkinson's disease: identification of a novel locus, RIT2. Ann Neurol. 2012;71:370–384. doi: 10.1002/ana.22687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pankratz N, Wilk JB, Latourelle JC, DeStefano AL, Halter C, Pugh EW, Doheny KF, Gusella JF, Nichols WC, Foroud T, Myers RH. PSG-PROGENI, Gene PD and Investigators, Coordinators and Molecular Generic Laboratries: Genome wide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet. 2009;124:593–605. doi: 10.1007/s00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, Beecham GW, Wang L, Züchner S, Konidari I, Wang G, Singer C, Nahab F, Scott B, Stajich JM, Pericak-Vance M, Haines J, Vance JM, Martin ER. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet. 2010;74:97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson's disease. Nat Genet. 2010;42:781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saad M, Lesage S, SaintPierre A, Corvol JC, Zelenika D, Lambert JC, Vidailhet M, Mellick GD, Lohmann E, Durif F, Pollak P, Damier P, Tison F, Silburn PA, Tzourio C, Forlani S, Loriot MA, Giroud M, Helmer C, Portet F, Amouyel P, Lathrop M, Elbaz A, Durr A, Martinez M, Brice A. French Parkinson's Disease Genetics Study Group: Genome-wide association study confirms BST1 and suggests a locus on 12q24 as the risk loci for Parkinson's disease in the European population. Hum Mol Genet. 2011;20:615–627. doi: 10.1093/hmg/ddq497. [DOI] [PubMed] [Google Scholar]
- 27.Spencer CC, Plagnol V, Strange A, et al. Dissection of the genetics of Parkinson's disease identifies an additional association 5′ of SNCA and multiple associated haplotypes at 17q21. Hum Mol Genet. 2011;20:345–353. doi: 10.1093/hmg/ddq469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simón-Sánchez J, Schulte C, Lesage S, Sveinbjörnsdóttir S, Stefánsson K, Martinez M, Hardy J, Heutink P, Brice A, Gasser T, Singleton AB, Wood NW. Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377:641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plagnol V, Nalls MA, Bras JM, et al. A two-stage meta-analysis identifies several new loci for Parkinson's disease. PLoS Genet. 2011;7:e1002142. doi: 10.1371/journal.pgen.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U, Mountain JL, Goldman SM, Tanner CM, Langston JW, Wojcicki A, Eriksson N. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson's disease. PLoS Genet. 2011;7:e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Cheng R, Verbitsky M, Kisselev S, Browne A, MejiaSanatana H, Louis ED, Cote LJ, Andrews H, Waters C, Ford B, Frucht S, Fahn S, Marder K, Clark LN, Lee JH. Genome-wide association study identifies candidate genes for Parkinson's disease in an Ashkenazi Jewish population. BMC Med. 2011;12:104. doi: 10.1186/1471-2350-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pihlstrøm L, Axelsson G, Bjørnarå KA, Dizdar N, Fardell C, Forsgren L, Holmberg B, Larsen JP, Linder J, Nissbrandt H, Tysnes OB, Ohman E, Dietrichs E, Toft M. Supportive evidence for 11 loci from genome-wide association studies in Parkinson's disease. Neurobiol Aging. 2012;34:1708.e7–1708.e13. doi: 10.1016/j.neurobiolaging.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Sharma M, Ioannidis JP, Aasly JO, et al. Large-scale replication and heterogeneity in Parkinson disease genetic loci. Neurology. 2012;79:659–667. doi: 10.1212/WNL.0b013e318264e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin I, Dawson VL, Dawson TM. The impact of genetic research on our understanding of Parkinson's disease. Prog. Brain Res. 2010;183:21–41. doi: 10.1016/S0079-6123(10)83002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rideout HJ, Stefanis L. The neurobiology of LRRK2 and its role in the pathogenesis of Parkinson's disease. Neurochem Res. 2014;39:576–592. doi: 10.1007/s11064-013-1073-5. [DOI] [PubMed] [Google Scholar]
- 36.Rideout HJ, Stefanis L. The neurobiology of LRRK2 and its role in the pathogenesis of Parkinson's disease. Neurochem Res. 2014;39:576–592. doi: 10.1007/s11064-013-1073-5. [DOI] [PubMed] [Google Scholar]
- 37.Mata IF, Wedemeyer WJ, Farrer MJ, Taylor JP, Gallo KA. LRRK2 in Parkinson's disease: Protein domains and functional insights. Trends Neurosci. 2006;29:286–293. doi: 10.1016/j.tins.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Paisán-Ruíz C, Jain S, Evans EW, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Tong Y, Pisani A, Martella G, Karouani M, Yamaguchi H, Pothos EN, Shen J. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci USA. 2009;106:14622–14627. doi: 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan EK, Peng R, Teo YY, et al. Multiple LRRK2 variants modulate risk of Parkinson disease: a Chinese multicenter study. Hum Mutat. 2010;31:561–568. doi: 10.1002/humu.21225. [DOI] [PubMed] [Google Scholar]
- 41.Fung HC, Chen CM, Hardy J, et al. Lack of G2019S LRRK2 mutation in a cohort of Taiwanese with sporadic Parkinson's disease. Mov Disord. 2006;21:880–881. doi: 10.1002/mds.20814. [DOI] [PubMed] [Google Scholar]


